Abstract
The epidermal growth factor receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases. This family includes EGFR/ErbB1/HER1, ErbB2/HER2/Neu ErbB3/HER3, and ErbB4/HER4. For many years it was believed that EGFR plays a minor role in the development and progression of breast malignancies. However, recent findings have led investigators to revisit these beliefs. Here we will review these findings and propose roles that EGFR may play in breast malignancies. In particular, we will discuss the potential roles that EGFR may play in triple-negative tumors, resistance to endocrine therapies, maintenance of stem-like tumor cells, and bone metastasis. Thus, we will propose the contexts in which EGFR may be a therapeutic target.
Keywords: Epidermal growth factor receptor, Resistance to endocrine therapy, Bone metastasis, Triple-negative tumors, Stem-like tumor cells
1. Introduction
The study of breast cancer has provided opportunities to test concepts emerging from basic studies of cell proliferation, signal transduction and developmental biology. One subject of these basic studies is the epidermal growth factor receptor (EGFR) or ErbB family of receptor tyrosine kinases. This family includes EGFR/ErbB1/HER1, ErbB2/HER2/Neu ErbB3/HER3, and ErbB4/HER4. These receptors play distinct roles in breast malignancies [1–15]. ErbB2 is a therapeutic target in breast tumors that overexpress the receptor. In contrast, the roles that ErbB4 plays in breast malignancies remain a subject of opposing views. For many years it was believed that EGFR plays a minor role in the development and progression of breast malignancies. However, recent findings have led investigators to revisit these beliefs. Here we will review these findings and propose roles that EGFR may play in breast malignancies. Thus, we will propose the contexts in which EGFR may be a therapeutic target.
1.1. EGFR Ligands and Signaling
EGFR signaling is stimulated by members of the epidermal growth factor (EGF) family of peptide growth factors, whose roles in stimulating ErbB receptor signaling and coupling to biological responses have been intensively studied [2, 12, 16, 17]. EGFR agonists include the epidermal growth factor (EGF), transforming growth factor alpha (TGF-α), heparin-binding EGF-like growth factor (HB-EGF), amphiregulin (AREG), epiregulin (EPI), epigen (EPG), betacellulin (BTC) and neuregulin (NRG) 2β. These agonists are expressed as integral membrane proteins and are cleaved by metalloproteinases to release soluble, mature ligands. These metalloproteinases are typically members of the ADAM (a disintegrin and metalloproteinase) family of membraneous proteases. For example, ADAM17 (tumor necrosis factor α converting enzyme - TACE) cleaves AREG, EPR, HB-EGF and TGFα [18–22].
Because cleavage of the ligand precursors is required for release of soluble, mature ligands, ligand cleavage represents a potential point in which agonist-induced EGFR signaling can be regulated. However, the transmembrane ligands stimulate EGFR signaling on adjacent cells, apparently through a juxtracrine signaling mechanism that may mediate the stromal-epithelial interactions characteristic of the breast [23–25].
The mechanisms by which EGFR signaling is stimulated by agonist binding have been extensively studied [16, 17, 26, 27]. To summarize, EGFR consists of an extracellular domain, a hydrophobic transmembrane domain, an intracellular catalytic tyrosine kinase domain, and several intracellular tyrosine residues whose phosphorylation is responsible for coupling to downstream effectors. Ligand binding to the extracellular domain stabilizes the EGFR in an extended conformation that is competent for receptor dimerization. Dimerization then enables the cytoplasmic domain of one receptor monomer (the regulatory monomer) to stabilize the tyrosine kinase domain of another monomer (the catalytic monomer) in the active conformation and presents the tyrosine residues of the regulatory monomer to the catalytic site of the catalytic monomer. In this manner EGFR dimerization enables its tyrosine phosphorylation.
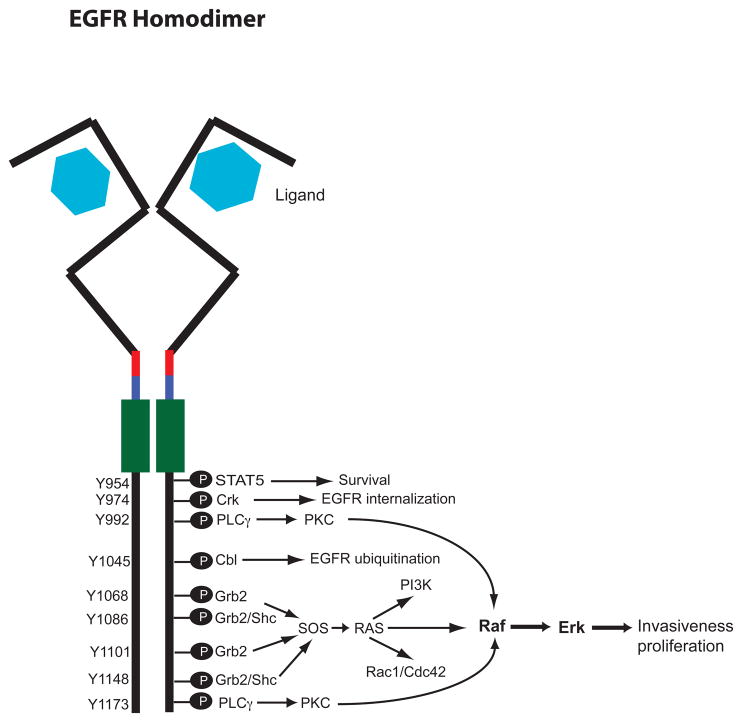
Approximately 10 EGFR tyrosine residues are phosphorylated following ligand engagement and receptor dimerization [17, 28]. These phosphorylation sites bind adapter proteins and other signaling molecules that possess SH2 (Src-homology domain 2) or PTB (phospho-tyrosine binding) motifs. Several of phosphorylated tyrosine residues can bind unique effectors and each EGFR agonist is likely to stimulate EGFR phosphorylation at a unique subset of tyrosine residues. Thus, EGFR agonists typically stimulate EGFR coupling to multiple effectors, including Ras, MAPK, Src, STAT 3/5, PLCγ, PKC, and PI3 kinase [17, 29]. These effectors are typically coupled to increased survival, proliferation, motility and invasiveness displayed by malignant tumor cells.
In contrast, some EGFR agonists also stimulate coupling to downstream molecules that negatively regulate the receptor. For instance, phosphorylation of EGFR Tyr974 triggers EGFR endocytosis and phosphorylation of EGFR Tyr1045 triggers Cbl-dependent EGFR ubiquitination and proteosomal degradation [17, 30]. EGFR phosphorylation also triggers EGFR binding to SHPTP protein tyrosine phosphatases, in which in turn dephosphorylate EGFR [17, 31, 32]. Thus, EGFR agonists also stimulate pathways that negatively regulate EGFR coupling to malignant phenotypes and the balance between these positive and negative regulation of EGFR coupling to malignant phenotypes may be altered in tumor cells.
1.2. EGFR Signaling Specificity
Several factors contribute to EGFR signaling specificity. One is the presence of other ErbB family receptors. For example, ErbB2 can stabilize EGFR in a conformation that is competent for dimerization and tyrosine phosphorylation even in the absence of ligand binding, thereby contributing to ligand-independent EGFR signaling and increased ligand affinity for the EGFR [16, 33, 34]. Furthermore, ErbB2 and ErbB4 heterodimerize with EGFR upon agonist binding to EGFR. This results in phosphorylation of the heterodimerization partner (ErbB2 or ErbB4) and may result in phosphorylation of a different set of EGFR tyrosine residues [16, 33]. The latter mechanism may account for the observation that heterodimerization of ErbB2 with EGFR alters EGFR endocytosis and intracellular trafficking [35–37]. In any event, agonist-induced heterodimerization of EGFR with a partner ErbB receptor alters the consequences of stimulation with a given EGFR ligand by coupling to different signaling pathways and biological responses than EGFR homodimers.
Numerous studies indicate that different EGFR ligands induce distinct biological responses and patterns of EGFR coupling to signaling pathways. For example, TGFα and AREG are more effective stimuli of EGFR coupling to biological responses associated with tumor cell metastasis (motility, invasiveness, etc.) than is EGF. These biological differences appear to be due to differences in the sites of agonist-induced EGFR tyrosine phosphorylation. EGF stimulates greater phosphorylation of EGFR Tyr1045 than does AREG. Thus, EGF stimulates greater EGFR ubiquitination and turnover than does AREG, presumably because of increased EGFR coupling to the ubiquitin ligase c-Cbl. Moreover, the duration of EGFR coupling to MAPK and PLCγ signaling is greater following stimulation with AREG than with EGF [38–43].
The mechanism by which different ligands cause phosphorylation of distinct sets of EGFR tyrosine residues is unclear. However, the crystal structure of the EGFR extracellular domain dimer when bound with EGF is distinct from the crystal structure of the EGFR extracellular domain when bound with TGFα. Thus, ligand-specific differences in the juxtapositioning of the receptor monomers within the receptor dimer may lead to differences in receptor tyrosine residue availability to the receptor kinase domain for phosphorylation [17].
2. Manuscript Body
2.1. EGFR and Primary Breast Tumors
The roles that EGFR and its ligands play in breast cancer have been a subject of intensive study and controversy. Some retrospective immunohistochemical studies have indicated that EGFR overexpression in primary tumors is an indicator of poor prognosis [44–47], whereas other similar studies have failed to establish such a link [10, 48]. Collectively, these studies suggest that EGFR is expressed in 18–35% of breast cancers but is not overexpressed relative to the normal breast epithelia [49]. Of course, because increased EGFR signaling is commonly associated with increased EGFR turnover, immunohistochemical analyses of EGFR protein expression may not be ideal for evaluating the role that EGFR may be playing in breast malignancies.
Initial studies have suggested that expression of EGF, TGFα or AREG is associated with larger and more aggressive tumors [9, 50, 51]. However, more extensive studies have failed to link ligand expression to prognosis [49, 52]. This apparent dichotomy may be explained by the fact that immunohistochemical analyses of ligand expression in tumor samples primarily detects the immature, transmembrane form of the ligand, whereas signaling might be driven largely by the mature soluble form of the ligand.
2.1.1. Triple-Negative, Basal Breast Tumors
The development of platforms capable of simultaneously evaluating gene expression from a large portion of the genome has led to the identification of gene expression profiles that classify breast cancers. This has yielded further insights into the roles that EGFR and EGFR ligands may play in breast cancer. Basal-type breast cancers express markers frequently found in cells that are in contact with the basement membrane. Such markers include keratin 5 and 17 (basal keratins), P-cadherin, and troponin [53–56]. Basal-type breast cancers are associated with large size, high tumor grade, poor survival, and increased frequency of distant metastases [56]. These tumors typically lack expression of the estrogen receptor-alpha (ERα), progesterone receptor, and ErbB2. Thus, basal tumors are frequently referred to as “triple-negative” breast tumors [57]. Given the relative aggressiveness of these tumors and the absence of targeted therapeutics for treating these tumors, the identification of targets for treating these tumors is a priority.
Gene expression profiling and immunohistochemical studies have indicated that 50 to 70% of basal breast tumors exhibit EGFR expression [58]. Moreover, our preliminary analyses of breast cancer transcriptome datasets GSE2034 [59], GSE2603 [60], and GSE12276 [61] from the NCBI Gene Expression Omnibus reveal that the EGFR ligand TGFα and the EGFR/ErbB3/ErbB4 ligand NRG2β are expressed at significantly higher levels in ERα-negative tumors than in ERα-positive tumors. Likewise, the expression of ADAMs and MMPs responsible for maturation (cleavage) of EGFR ligands is higher in ERα-negative tumors than in ERα-positive tumors. A low level of EGFR expression in basal tumors correlates with a reduced incidence of metastases [62]. Similarly, EGFR expression in basal tumors correlates with TGFα and ADAM-17 expression [63]. Thus, a sizable fraction of basal breast cancers appear to exhibit autocrine TGFα-EGFR signaling and this may account for the poor prognosis associated with these tumors [63].
In contrast, ERα-positive tumors tend to exhibit elevated AREG expression but no increase in EGFR expression [59–61]. This pattern of expression is similar to that exhibited by the normal mammary epithelia, in which ERα-positive cells exhibit little EGFR expression but do express AREG [64]. The AREG findings are consistent with previous reports from breast cancer cell lines that indicate this ligand is an estrogen regulated gene, but can be activated by several other pathways present in both ERα-positive and negative cancers [65, 66]. One possible interpretation of the molecular profile data is that ERα-positive breast cancer would lack high levels of autocrine EGFR signaling, but could engage in paracrine EGFR signaling with fibroblast-like cells in the microenvironment through AREG.
2.1.2. Resistance to Antiestrogens
The estrogen receptor partial agonist tamoxifen (Tam) is commonly used to treat ERα-positive breast cancer in both pre- and post-menopausal women. However, a significant fraction of ER+ tumors exhibit intrinsic resistance to Tam and in many patients responsiveness of ERα-positive tumors to Tam is of limited duration due to acquired resistance [67, 68]. Indeed, many ERα+ tumors acquire complete resistance to Tam, resulting in a restoration of tumor growth and metastasis [67, 68].
Tam resistance may arise through overexpression or phosphorylation of the ERα co-activator AIB1/SRC-3 (amplified in breast cancer 1/steroid hormone receptor co-activator 3) [67, 68]. This alters the effects of Tam on ERα-mediated gene expression, leading to Tam stimulation of mitogenic signaling pathways [67, 68]. Signaling pathways downstream of several different tyrosine kinases induce phosphorylation of AIB1, suggesting that EGFR signaling may cause Tam resistance via this mechanism [67–69].
Tyrosine phosphorylation of ERα causes tamoxifen resistance by enabling estrogen-independent ERα-mediated gene expression [69]. A number of different tyrosine kinases may catalyze ER tyrosine phosphorylation, including ErbB2 [68, 69]. Because ErbB2 is a common heterodimerization partner of EGFR, ligand-induced EGFR signaling may contribute to ER tyrosine phosphorylation and tamoxifen resistance.
Fulvestrant (Faslodex®; ICI 182,780) triggers rapid ERα degradation via the proteasome and is frequently used to treat receptor positive, tamoxifen-resistant tumors [68]. However, acquired resistance frequently arises, limiting the utility of this approach [68]. Chronic treatment of ERα-positive breast tumor cell lines with fulvestrant leads to clones that display resistance to fulvestrant. These models of acquired resistance typically display a loss of ERα-expression and elevated EGFR or ErbB2 expression and receptor tyrosine phosphorylation [70, 71]. These cell lines also display elevated TGFα expression and retain AREG expression [70, 71]. These data suggest that enhanced autocrine EGFR/ErbB2 signaling may compensate for the loss of ER expression and signaling in fulvestrant-resistant breast tumors. However, this hypothesis has yet to be tested in breast cancer patient samples.
2.1.3. Breast Cancer Stem Cells
Solid tumors typically consist of a heterogeneous mix of cellular phenotypes that include poorly differentiated cells that undergo rapid cell division, differentiated cells that are incapable of cell division, and quiescent cells that possess the capacity for self-renewal and can give rise to the other types of tumor cells. This self-renewal and pluripotency have led this category of cells to be called cancer stem cells or stem-like cancer cells [72, 73].
Breast cancer cells that have been isolated from pleural effusions exhibit a high level of CD44 expression and a low level of CD24 expression [74]. While these cells display a homogenous phenotype, they are extraordinarily efficient at forming phenotypically heterogeneous tumors in immunocompromised mice. Moreover, these cells readily form colonies in suspension cultures and exhibit very aggressive behaviors in metastasis and invasion assays [74]. Thus, these CD44+/CD24− breast tumor cells exhibit characteristics of tumor stem cells. ALDH1 has also emerged as a marker of tumor cells that exhibit stem-like characteristics [75, 76].
There is no direct evidence indicating that EGFR and its ligands are involved in the establishment or maintenance of breast tumor stem cells. However, stem-like tumor cells are much more rare in ERα-positive breast tumors and breast cell lines (which typically have little EGFR and ErbB2 expression) than in triple-negative breast tumors and breast cell lines (which typically exhibit elevated EGFR expression) [76]. Ligand-induced EGFR signaling is required for stem-like breast tumor cells (including those derived from DCIS tumors) to form colonies in semi-solid medium [77]. Overexpression of ErbB2 in mammary epithelial cells and breast cancer cell lines increases the fraction of cells that display stem-like properties [78]. Finally, a preliminary report from a small clinical trial indicates that the dual specificity EGFR/ErbB2 tyrosine kinase inhibitor lapatinib reduces the number of CD44+/CD24− cells found in breast tumor specimens [79]. These reports provide intriguing hints that the ligand-induced EGFR/ErbB2 signaling may play a substantial role in establishing and maintaining breast cancer stem-like cells. Nonetheless, additional direct experimentation is necessary to evaluate this hypothesis.
2.2. EGFR and Bone Metastasis
The most common metastasis site of breast cancer is the bone [80]. Nearly 70% of invasive breast cancer cases result in metastasis to the bone and generate severe pain and disability in the patient [80]. Destruction of bone matrix is responsible for the fractures and bone pain associated with advanced breast cancer [81]. The majority of tumors that metastasize to bone are ERα-positive [82], but there are a fraction of ERα-negative tumors that also metastasize to this location [83]. Bone metastases were largely refractory to the traditional systemic approaches (radiation therapy and chemotherapy) used to treat advanced breast cancer [80, 81, 84]. Recently, the integration of the fields of basic bone cell biology and cancer biology has produced insights that have generated new and partially effective therapeutic approaches to this devastating form of metastasis. Agents such as bisphosphonates reduce bone destruction and tumor growth by targeting the bone microenvironment rather than the tumor [84]. Recently, EGFR signaling has come into focus as a potential microenvironment target that could be exploited to reduce the morbidity associated with this form of metastasis.
Metastasis to any organ features invasion of cancer cells through normal tissue into the blood stream (initiation), extravasation and infiltration of a distant tissue (progression), and growth of a destructive colony within the new context (virulence) [85]. The genes that mediate these events are likely to be dispensable for primary tumor initiation and growth and may or may not be part of gene expression profiles exhibited by the primary tumor [85]. We have analyzed breast cancer transcriptome datasets from the NCBI Gene Expression Omnibus to compare the patterns of ErbB receptor and ligand in primary tumors that ultimately produced bone metastasis to the patterns found in tumors that failed to metastasize or produced metastases to other sites [59–61]. We have also compared ErbB receptor expression in a small set of bone metastasis samples with ErbB receptor expression in breast cancer samples removed from the lung, brain and liver [83]. ErbB2 expression was lower in those ERα-negative tumors that produced bone metastases than in tumors that did not metastasize to bone, which suggests that tumors that overexpress ErbB2 typically metastasize to visceral sites [86]. Surprisingly, AREG expression was significantly lower in ERα-negative tumors that ultimately metastasized to bone than in other ERα-negative tumors. However, we found little additional evidence for differential expression of ErbB family receptors. These findings suggest that EGFR signaling may be dysregulated in bone metastases through post-transcriptional events. As indicated below, several emerging lines of evidence involving ligand-activating proteases support a role for the EGFR signaling in bone metastasis.
2.2.1. Latent Bone Colonization by Breast Tumor Cells
Frequently, bone metastasis arise in breast cancer patients years after the identification and treatment of the primary tumor [87]. This implies that breast cancer cells remain dormant or indolent within the body. Over the past two decades methodology has been developed to identify dormant/latent tumor cells within patients. Individual or small groups of tumor cells found in the bone marrow of patients who lack discernable bone metastases are termed disseminated tumor cells (DTCs) [87]. The presence of DTCs in the bone marrow is predictive of metastatic disease both in the bone and at other sites [87–89]. The vast majority of DTCs present in the bone marrow of breast cancer patients are CD44+/CD24−, making them reminiscent of stem-like breast cancer cells [90]. However, elevated EGFR and ErbB2 are also markers for DTCs [91, 92]. This suggests that ErbB receptors play a role in the establishment or maintenance of stem-like breast cancer cells, but there is no further information regarding potential function of ErbB receptors in the infiltration of breast cancer cells into of bone, or regarding their possible impact on latency/indolence [93].
2.2.2. Bone Metastasis: A Vicious Cycle
Much of the advances in the understanding of breast cancer colonization of bone has stemmed from studies of the MDA-MB-231 ERα-negative breast cancer cell line in bone xenografts. MDA-MB-231 cells possess a basal phenotype [94] and various bone-seeking sublines have been developed to dissect the molecular and cellular regulators of osteolytic growth of this cell line [95, 96]. On the basis of these studies, the concept of “the vicious cycle” of tumor cell growth linked to bone destruction has been developed [96]. This model holds that breast cancer cells direct the resident cells of bone to uncouple the physiological linkage between bone matrix destruction and new bone formation [96]. The MDA-MB-231 cells produce cytokines and growth factors that engage in paracrine signaling with osteoclasts, cells that dissolve bone matrix, and osteoblasts, which are responsible for bone formation [96, 97]. Osteoclast formation is mediated mainly through RANK (receptor activator of nuclear factor β-ligand) and its agonist RANKL (RANK ligand), the latter of which is produced by osteoblasts and bone marrow stromal cells [93, 96]. Osteoblastic cells also produce a soluble RANKL sink called osteoprotegrin (OPG) [80, 93]. Thus, osteoclast formation is influenced by the balance between RANKL and OPG in the bone microenvironment [96]. In addition, osteoblasts produce colony-stimulating factor (CSF-1), which recruits monocytes from bone marrow progenitors that ultimately can be differentiated into osteoclasts in the presence of high levels of RANKL [96] [98, 99]. In the MDA-MB-231 xenograft models, the breast cancer cells produce several growth factors and cytokines that perturb the RANKL/OPG ratio and increase the number of monocytes that can be differentiated to osteoclasts [95, 97, 99]. The osteoclast-mediated destruction of bone releases growth factors embedded in the bone matrix. These stimulate their cognate receptors on the cancer cell, resulting in increased tumor cell proliferation and production of cytokines that skew the RANKL/OPG ratio toward increased osteoclastogenesis, thereby propagating a vicious cycle of tumor cell proliferation and bone destruction [97, 100]. It should be noted that this model is based on the activities of the ERα-negative MDA-MB-231 breast tumor cell line, and it is unclear whether all of the specific molecules and cellular interactions apply to the more common form of disease progression that arise from ERα-positive breast tumors.
2.2.3. EGFR and Osteolysis
There is growing evidence suggesting that EGFR signaling in osteoblasts directly contributes to osteolysis or bone resorption. EGFR is expressed by cultured osteoblasts, but not osteoclasts or monocytes [101, 102]. Furthermore, EGF, TGFα, and MDA-MB-231 cells (which express various ErbB ligands) stimulate bone turnover and osteoclastogenesis in various model systems [103–106]. This osteoclastogenesis is accompanied by decreased OPG expression and minimal change in RANKL expression by the bone cells [106]. EGFR TKIs inhibit CSF-1 and RANKL production from human bone marrow stromal cells and osteoclast formation in vitro [107]. These studies clearly support the concept that EGFR signaling within the osteoblast promotes osteoclastogenesis through perturbation of the RANKL/OPG balance.
2.2.4. EGFR and Osteoblast Function
Studies of bone biology suggest additional roles for EGFR ligands in the pathogenesis of osteolytic lesions. Parathyroid hormone (PTH), the main serum calcium regulator, stimulates AREG gene transcription 10 to 20-fold and stimulates more modest increases in transcription of the TGFα and HB-EGF genes [108, 109]. The PTH receptor, like other serpentine G-protein-coupled receptors (GPCRs), appears to be coupled to proteases (such as ADAM-17) that cleave ErbB receptor ligand precursors and enable the release of the mature, soluble ligands [110].
Exogenous EGFR ligands stimulate the proliferation of osteoblasts, inhibit their differentiation, and decrease their mineralization [109]. Moreover, 4-week-old transgenic mice lacking AREG expression exhibit less trabecular bone in the tibia than do wild-type littermates [109]. Thus, EGFR signaling may mediate the impact of PTH on the recruitment and expansion of cells committed to the osteoblast lineage, whereas excessive ligand signaling could prevent these cells from undergoing terminal differentiation and forming mineralized bone [109]. The uncoupling of bone formation from the accelerated bone resorption would be a key feature of disease states like breast cancer-induced osteolysis.
2.2.5. EGFR and PTHrP
In the MDA-MB-231 model, PTH receptor signaling is one of the key events in regulating the vicious cycle of breast cancer osteolysis and colonization [111]. MDA-MB-231 cells express parathyroid hormone-related peptide (PTHrP), another PTH receptor agonist that stimulates RANKL expression and inhibits OPG expression in cells of the osteoblast lineage [111]. The pattern of PTHrP expression by breast cancers at various stages of progression resembles that displayed by metastasis virulence factors [85]. PTHrP expression is lower in primary breast cancers that ultimately metastasize to bone than in other primary breast tumors; however, PTHrP expression is very high among metastatic tumor cells within the bone microenvironment [112–115]. PTHrP gene expression in these metastatic tumor cells appears to be stimulated by TGFβ released from the bone matrix via osteoclast activity [96, 100]. Nonetheless, the signaling between the PTHrP and the EGFR system is not simply directed from cancer cell to the microenvironment. In many epithelial cells EGFR is coupled to PTHrP gene expression [116–118]. In fact, an autocrine loop of AREG-EGFR signaling activates PTHrP transcription in the MDA-MB-231 line in vitro [119]. Thus, autocrine EGFR stimulation in breast cancer cells may contribute to the release of cytokines, such as PTHrP, that directly perturb the RANK/OPG balance and indirectly stimulate EGFR signaling within cells of the osteoblast lineage.
2.2.6. EGFR Ligands and Activating Proteases as Bone Metastasis Virulence Factors
Analysis of MDA-MB-231 subclones identified 11 genes whose overexpression is specific to clones that readily colonize the bone and form aggressive osteolytic lesions [95]. Moreover, combinations of 3 of these genes are sufficient to induce osteolytic growth by parental MDA-MB-231 cells. Thus, these 11 genes appear to influence distinct events in the process of bone metastasis. These 11 genes include IL-11, which alters the RANKL/OPG balance, and connective tissue factor, which stimulates osteoblast proliferation. These 11 genes also include the proteases MMP1 and ADAMTS-1 (a disintegrin and metalloproteinase with thrombospondin motifs), whose roles in bone metastasis were not readily apparent [95].
Overexpression of MMP1 and ADAMTS-1 in MDA-MB-231 cells dramatically increased AREG shedding and resulted in a cell line that formed more aggressive osteolytic lesions in the bone. Conditioned medium from the MDA-MB-231/ADAMTS-1/MMP1 cells altered the RANKL/OPG balance in a primary mouse bone cell culture and enhanced osteoclastogenesis. This enhanced osteoclastogenesis could be inhibited by the EGFR TKI gefitinib or by the anti-EGFR antibody cetuximab. Moreover, these agents (gefitinib 100 mg/kg daily or cetuximab 100 mg/kg weekly) prevented MDA-MB-231/ADAMTS-1/MMP1 cells from stimulating the formation of osteolytic lesions in the bone of immunocompromised mice injected with these cells [120]. These findings suggest that EGFR ligands or the proteases that regulate their availability can serve as breast cancer metastasis virulence factors and that metastasis could be blocked by EGFR antagonists that have no apparent direct effect on the breast tumor cells themselves.
This finding that AREG expression is necessary but not sufficient for MDA-MB-231 cells to colonize the bone is consistent with the observation that AREG expression is lower in ERα-negative breast tumors that ultimately metastasized to bone than in ERα-negative breast tumor that failed to metastasize to bone. Presumably, differences in the ability of breast tumor cells to colonize bone is regulated by proteases cleave AREG and enable it to stimulate EGFR signaling. Indeed, elevated expression of ADAMTS-1 and MMP1 is observed in primary breast cancer tumors that ultimately metastasize to bone [63]. Furthermore, given that various GPCRs are coupled to increased activity of MMPs and ADAMs, we speculate that increased signaling by GPCRs on tumor cells in the bone microenvironment may contribute to bone colonization by coupling to increased activity MMPs and ADAMs [121, 122].
To summarize, the complex post-transcriptional regulation of EGFR ligand processing and receptor interactions provides mechanisms through which EGFR coupling to bone colonization may be enhanced. Thus, numerous gene products that contribute to EGFR signaling in breast tumor cells or osteoblasts may function as bone metastasis virulence factors. (1) The combination of an EGFR ligand (such as AREG) and an active shedding protease (such as MMP1 or ADAMTS-1) in breast tumor cells could activate paracrine EGFR signaling in osteoblasts, resulting in reduced OPG expression, increased osteoclastogenesis and decreased bone mineralization. (2) Autocrine EGFR signaling in the tumor cell could couple to PTHrP expression and release by tumor cells, leading to increased RANKL and decreased OPG expression in osteoblasts. (3) PTHrP released by tumor cells could also stimulate AREG expression and ADAM17 activity in osteoblasts, leading to increased EGFR signaling in the osteoblasts. Thus, PTHrP could play a central role in two pathways that independently lead to a robust alteration of the RANKL/OPG balance to favor osteoclast formation and osteolytic activity.
The multiple mechanisms by which MDA-MB-231 cells can stimulate EGFR coupling to osteolytic effects in the bone microenvironment indicate that this pathway may be a major component of the pathogenesis of osteolytic lesions triggered by this ERα-negative breast cancer line. Moreover, AREG transcription is positively regulated by ERα in the mouse mammary gland and breast cancer cells [64, 66]. Thus, deregulated signaling through the AREG-EGFR pathway may be a general mechanism by which multiple types of breast cancer form osteolytic bone metastases.
Small-molecule EGFR tyrosine kinase inhibitors and antagonistic anti-EGFR antibodies have exhibited little effect on primary tumor growth or patient outcome in breast cancer monotherapy clinical trials. One possibility is that anti-EGFR agents will be effective against bone metastases, but will have little effect on the primary tumor [97, 120, 123–125]. The other possibility is that these agents may be effective only as part of combination therapy regimens. Indeed, emerging data appear to support this possibility, particularly in advanced ERα-positive breast cancers [126–128].
Figure 1. The liganded EGFR homodimer possess multiple sites of tyrosine phosphorylation and couples to multiple signaling effectors.
A schematic representation of the liganded EGFR homodimer is shown. The light blue hexagons represent the ligand. EGFR is depicted by a black line. Red and blue overlays represent the transmembrane and juxtamembrane domains, respectively. Green boxes represent the tyrosine kinase domains. Sites of cytoplasmic tyrosine (Y) phosphorylation are indicated, as are cytosolic effector proteins that bind to these phosphorylated tyrosine residues and some of the effector signaling pathways.
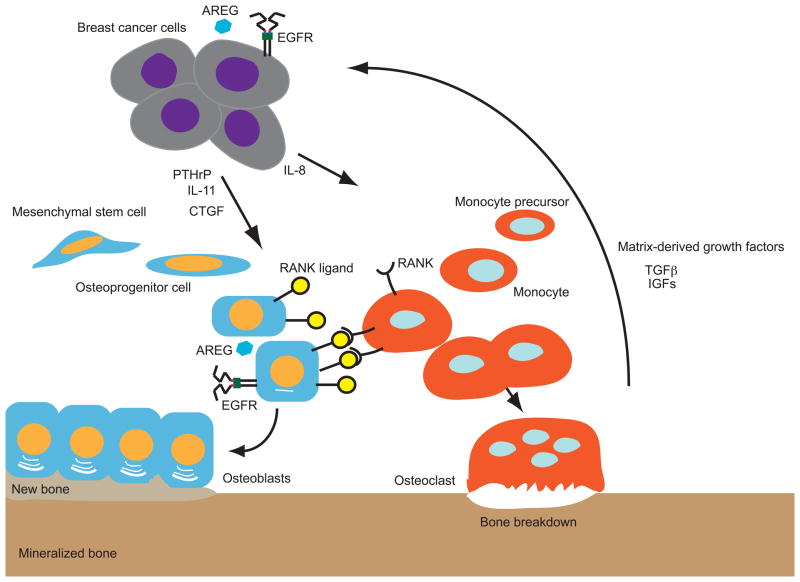
Figure 2. Complex interactions of tumor and bone cells regulate bone biosynthesis and breakdown.
Breast cancer cells express PTHrP, IL-11, and CTGF, which stimulate RANK ligand (RANKL) expression by cells of the osteoblast lineage. RANKL binding to RANK on monocytes stimulates their differentiation to active osteoclasts and consequent bone breakdown. Breast cancer cells also express IL-8, which directly stimulates monocyte production and leads to increased osteoclast formation. Breakdown of the bone matrix by osteoclasts releases TGFβ and IGFs, which stimulate tumor cell survival, proliferation, and release of osteolytic factors. Both breast cancer cells and cells of the osteoblast lineage express EGFR and the EGFR ligand AREG.
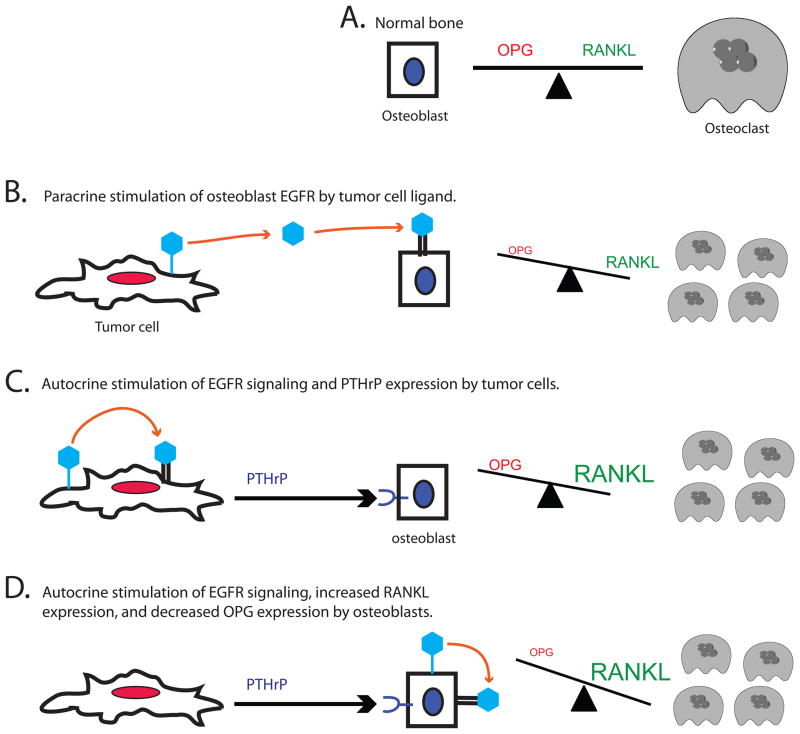
Figure 3. EGFR may play multiple roles in breast cancer-induced osteolysis.
(A) In normal bone RANKL stimulation of osteoclast-mediated bone turnover and is balanced by the OPG antagonist of RANKL. (B) An EGFR ligand (light blue hexagon) expressed and shed by tumor cells may stimulate paracrine signaling by EGFR (double black bars) expressed by osteoblasts. This would inhibit OPG expression by osteoblasts, leading to increased RANKL stimulation of RANK expressed by osteoclasts and increased osteoclast-mediated bone turnover. (C) An EGFR ligand expressed and shed by tumor cells may stimulate autocrine signaling by EGFR expressed by the tumor cells, leading to PTHrP expression by these tumor cells. This stimulates RANKL expression and inhibits OPG expression by osteoblasts, again leading to increased RANKL stimulation of RANK expressed by osteoclasts and increased osteoclast-mediated bone turnover. (D) PTHrP expressed by tumor cells can also stimulate expression of an EGFR ligand by osteoblasts, leading to autocrine EGFR signaling and coupling to increased RANKL expression and decreased OPG expression in osteoblasts. Again, this leads to increased RANKL stimulation of RANK expressed by osteoclasts and increased osteoclast-mediated bone turnover.
Acknowledgments
The authors acknowledge support of NIH grants to DJR (R01CA114209) and the Purdue University Center for Cancer Research (P30CA023168).
Abbreviations
- ADAM
A disintegrin and metalloproteinase
- ADAMTS
ADAM with thrombospondin motif
- AREG
Amphiregulin
- CSF-1
Colony-stimulating factor -1
- DTC
Disseminated tumor cell
- EGFR
Epidermal Growth Factor Receptor
- ER
Estrogen receptor
- OPG
Osteoprotegrin
- PTH
Parathyroid hormone
- PTHrP
Parathyroid hormone-related protein
- RANK
Receptor activator of nuclear factor β-ligand
- RANKL
RANK ligand
- TACE
Tumor necrosis factor alpha converting enzyme
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carney W, Leitzel K, Ali S, Neumann R, Lipton A. HER-2 therapy. HER-2/neu diagnostics in breast cancer. Breast Cancer Res. 2007;9:207. doi: 10.1186/bcr1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 3.Jones FE. HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:247–58. doi: 10.1007/s10911-008-9076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donovan N, Crown J. EGFR and HER-2 antagonists in breast cancer. Anticancer Res. 2007;27:1285–94. [PubMed] [Google Scholar]
- 5.Roskoski R., Jr The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 6.Spector N, Xia W, El-Hariry I, Yarden Y, Bacus S. HER2 therapy. Small molecule HER-2 tyrosine kinase inhibitors. Breast Cancer Res. 2007;9:205. [Google Scholar]
- 7.Stern D. ERBB3/HER3 and ERBB2/HER2 Duet in Mammary Development and Breast Cancer. J Mammary Gland Biol Neoplasia. 2008;13:215–23. doi: 10.1007/s10911-008-9083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundvall M, Iljin K, Kilpinen S, Sara H, Kallioniemi OP, Elenius K. Role of ErbB4 in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:259–68. doi: 10.1007/s10911-008-9079-3. [DOI] [PubMed] [Google Scholar]
- 9.Suo Z, Risberg B, Karlsson MG, Villman K, Skovlund E, Nesland JM. The expression of EGFR family ligands in breast carcinomas. Int J Surg Pathol. 2002;10:91–9. doi: 10.1177/106689690201000202. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71:67–75. doi: 10.1023/a:1013397232011. [DOI] [PubMed] [Google Scholar]
- 11.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–84. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 12.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–84. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaczek A, Brandt B, Bielawski KP. The diverse signaling network of EGFR, HER2, HER3 and HER4 tyrosine kinase receptors and the consequences for therapeutic approaches. Histol Histopathol. 2005;20:1005–15. doi: 10.14670/HH-20.1005. [DOI] [PubMed] [Google Scholar]
- 14.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res. 2010;16:1373–83. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 16.Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–8. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ., 2nd Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther. 2009;122:1–8. doi: 10.1016/j.pharmthera.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mill CP, Chester JA, Riese DJ. EGFR may couple moderate alcohol consumption to increased breast cancer risk. Breast Cancer (London) 2009;2009:31–8. doi: 10.2147/bctt.s6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 20.Shah BH, Catt KJ. TACE-dependent EGF receptor activation in angiotensin-II-induced kidney disease. Trends Pharmacol Sci. 2006;27:235–7. doi: 10.1016/j.tips.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Bhola NE, Grandis JR. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci. 2008;13:1857–65. doi: 10.2741/2805. [DOI] [PubMed] [Google Scholar]
- 22.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–60. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 23.Tada H, Sasada R, Kawaguchi Y, Kojima I, Gullick WJ, Salomon DS, et al. Processing and juxtacrine activity of membrane-anchored betacellulin. J Cell Biochem. 1999;72:423–34. [PubMed] [Google Scholar]
- 24.Dobashi Y, Stern DF. Membrane-anchored forms of EGF stimulate focus formation and intercellular communication. Oncogene. 1991;6:1151–9. [PubMed] [Google Scholar]
- 25.Dong J, Opresko LK, Chrisler W, Orr G, Quesenberry RD, Lauffenburger DA, et al. The membrane-anchoring domain of epidermal growth factor receptor ligands dictates their ability to operate in juxtacrine mode. Mol Biol Cell. 2005;16:2984–98. doi: 10.1091/mbc.E04-11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riese DJ, 2nd, Gallo RM, Settleman J. Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis. Bioessays. 2007;29:558–65. doi: 10.1002/bies.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemmon MA, Schlessinger J. Cell Signaling by Receptor Tyrosine Kinases. Cell. 2009;141:1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005–0008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemmon MA, Schlessinger J. Cell Signaling by Receptor Tyrosine Kinases. Cell. 2010;141:1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–84. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- 31.Keilhack H, Tenev T, Nyakatura E, Godovac-Zimmermann J, Nielsen L, Seedorf K, et al. Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J Biol Chem. 1998;273:24839–46. doi: 10.1074/jbc.273.38.24839. [DOI] [PubMed] [Google Scholar]
- 32.Tenev T, Keilhack H, Tomic S, Stoyanov B, Stein-Gerlach M, Lammers R, et al. Both SH2 domains are involved in interaction of SHP-1 with the epidermal growth factor receptor but cannot confer receptor-directed activity to SHP-1/SHP-2 chimera. J Biol Chem. 1997;272:5966–73. doi: 10.1074/jbc.272.9.5966. [DOI] [PubMed] [Google Scholar]
- 33.Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat. 1995;35:115–32. doi: 10.1007/BF00694752. [DOI] [PubMed] [Google Scholar]
- 34.Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61:1339–47. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 35.Gulliford TJ, Huang GC, Ouyang X, Epstein RJ. Reduced ability of transforming growth factor-alpha to induce EGF receptor heterodimerization and downregulation suggests a mechanism of oncogenic synergy with ErbB2. Oncogene. 1997;15:2219–23. doi: 10.1038/sj.onc.1201595. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Zhang L, Yeung TK, Chen X. Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol Biol Cell. 1999;10:1621–36. doi: 10.1091/mbc.10.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendriks BS, Wiley HS, Lauffenburger D. HER2-mediated effects on EGFR endosomal sorting: analysis of biophysical mechanisms. Biophys J. 2003;85:2732–45. doi: 10.1016/s0006-3495(03)74696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilmore JL, Scott JA, Bouizar Z, Robling A, Pitfield SE, Riese DJ, 2nd, et al. Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res Treat. 2008;110:493–505. doi: 10.1007/s10549-007-9748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–74. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern KA, Place TL, Lill NL. EGF and amphiregulin differentially regulate Cbl recruitment to endosomes and EGF receptor fate. Biochem J. 2008;410:585–94. doi: 10.1042/BJ20071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willmarth NE, Baillo A, Dziubinski ML, Wilson K, Riese DJ, 2nd, Ethier SP. Altered EGFR localization and degradation in human breast cancer cells with an amphiregulin/EGFR autocrine loop. Cell Signal. 2009;21:212–9. doi: 10.1016/j.cellsig.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmore JL, Gonterman RM, Menon K, Lorch G, Riese DJ, 2nd, Robling A, et al. Reconstitution of amphiregulin-epidermal growth factor receptor signaling in lung squamous cell carcinomas activates PTHrP gene expression and contributes to cancer-mediated diseases of the bone. Mol Cancer Res. 2009;7:1714–28. doi: 10.1158/1541-7786.MCR-09-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joslin EJ, Opresko LK, Wells A, Wiley HS, Lauffenburger DA. EGF-receptor-mediated mammary epithelial cell migration is driven by sustained ERK signaling from autocrine stimulation. J Cell Sci. 2007;120:3688–99. doi: 10.1242/jcs.010488. [DOI] [PubMed] [Google Scholar]
- 44.Garcia Castro C, Ravina M, Castro V, Salido EC. Expression of epidermal growth factor receptor (proto-oncogene c-erbB-1) and estrogen receptor in human breast carcinoma. An immunocytochemical study of 70 cases. Arch Gynecol Obstet. 1993;252:169–77. doi: 10.1007/BF02426354. [DOI] [PubMed] [Google Scholar]
- 45.Martinazzi M, Crivelli F, Zampatti C, Martinazzi S. Epidermal growth factor receptor immunohistochemistry in different histological types of infiltrating breast carcinoma. J Clin Pathol. 1993;46:1009–10. doi: 10.1136/jcp.46.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newby JC, A’Hern RP, Leek RD, Smith IE, Harris AL, Dowsett M. Immunohistochemical assay for epidermal growth factor receptor on paraffin-embedded sections: validation against ligand-binding assay and clinical relevance in breast cancer. Br J Cancer. 1995;71:1237–42. doi: 10.1038/bjc.1995.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder W, Biesterfeld S, Zillessen S, Rath W. Epidermal growth factor receptor-immunohistochemical detection and clinical significance for treatment of primary breast cancer. Anticancer Res. 1997;17:2799–802. [PubMed] [Google Scholar]
- 48.Ferrero JM, Ramaioli A, Largillier R, Formento JL, Francoual M, Ettore F, et al. Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-up. Ann Oncol. 2001;12:841–6. doi: 10.1023/a:1011183421477. [DOI] [PubMed] [Google Scholar]
- 49.Pawlowski V, Revillion F, Hebbar M, Hornez L, Peyrat JP. Prognostic value of the type I growth factor receptors in a large series of human primary breast cancers quantified with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000;6:4217–25. [PubMed] [Google Scholar]
- 50.Ma L, de Roquancourt A, Bertheau P, Chevret S, Millot G, Sastre-Garau X, et al. Expression of amphiregulin and epidermal growth factor receptor in human breast cancer: analysis of autocriny and stromal-epithelial interactions. J Pathol. 2001;194:413–9. doi: 10.1002/path.902. [DOI] [PubMed] [Google Scholar]
- 51.Soares R, Pereira MB, Silva C, Amendoeira I, Wagner R, Ferro J, et al. Expression of TGF-alpha and EGFR in Breast Cancer and its Relation to Angiogenesis. Breast J. 2000;6:171–7. doi: 10.1046/j.1524-4741.2000.98046.x. [DOI] [PubMed] [Google Scholar]
- 52.Desruisseau S, Palmari J, Giusti C, Romain S, Martin PM, Berthois Y. Clinical relevance of amphiregulin and VEGF in primary breast cancers. Int J Cancer. 2004;111:733–40. doi: 10.1002/ijc.20312. [DOI] [PubMed] [Google Scholar]
- 53.van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 54.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 55.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Da Silva L, Clarke C, Lakhani SR. Demystifying basal-like breast carcinomas. J Clin Pathol. 2007;60:1328–32. doi: 10.1136/jcp.2006.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korsching E, Jeffrey SS, Meinerz W, Decker T, Boecker W, Buerger H. Basal carcinoma of the breast revisited: an old entity with new interpretations. J Clin Pathol. 2008;61:553–60. doi: 10.1136/jcp.2008.055475. [DOI] [PubMed] [Google Scholar]
- 58.Burness ML, Grushko TA, Olopade OI. Epidermal growth factor receptor in triple-negative and basal-like breast cancer: promising clinical target or only a marker? Cancer J. 2010;16:23–32. doi: 10.1097/PPO.0b013e3181d24fc1. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 60.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A, et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–28. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 63.Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117:337–45. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A. 2007;104:5455–60. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rho JY, Wada-Kiyama Y, Onishi Y, Kiyama R, Sakuma Y. Expressional regulation of neuronal and cancer-related genes by estrogen in adult female rats. Endocr Res. 2004;30:257–67. doi: 10.1081/erc-120039579. [DOI] [PubMed] [Google Scholar]
- 66.Britton DJ, Hutcheson IR, Knowlden JM, Barrow D, Giles M, McClelland RA, et al. Bidirectional cross talk between ERalpha and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res Treat. 2006;96:131–46. doi: 10.1007/s10549-005-9070-2. [DOI] [PubMed] [Google Scholar]
- 67.Fan P, Yue W, Wang JP, Aiyar S, Li Y, Kim TH, et al. Mechanisms of resistance to structurally diverse antiestrogens differ under premenopausal and postmenopausal conditions: evidence from in vitro breast cancer cell models. Endocrinology. 2009;150:2036–45. doi: 10.1210/en.2008-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jordan VC, O’Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25:5815–24. doi: 10.1200/JCO.2007.11.3886. [DOI] [PubMed] [Google Scholar]
- 69.Santen RJ, Fan P, Zhang Z, Bao Y, Song RX, Yue W. Estrogen signals via an extra-nuclear pathway involving IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer cells. Steroids. 2009;74:586–94. doi: 10.1016/j.steroids.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 70.Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66:11954–66. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 71.Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, et al. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat. 2009;114:263–75. doi: 10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 73.Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27:44–6. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- 74.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99:616–27. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 78.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt C. Lapatinib study supports cancer stem cell hypothesis, encourages industry research. J Natl Cancer Inst. 2008;100:694–5. doi: 10.1093/jnci/djn168. [DOI] [PubMed] [Google Scholar]
- 80.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 81.Ali SM, Harvey HA, Lipton A. Metastatic breast cancer: overview of treatment. Clin Orthop Relat Res. 2003;415:S132–7. doi: 10.1097/01.blo.0000092981.12414.7b. [DOI] [PubMed] [Google Scholar]
- 82.Clark GM, Sledge GW, Jr, Osborne CK, McGuire WL. Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol. 1987;5:55–61. doi: 10.1200/JCO.1987.5.1.55. [DOI] [PubMed] [Google Scholar]
- 83.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–63. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 86.Emi Y, Kitamura K, Shikada Y, Kakeji Y, Takahashi I, Tsutsui S. Metastatic breast cancer with HER2/neu-positive cells tends to have a morbid prognosis. Surgery. 2002;131:S217–21. doi: 10.1067/msy.2002.119580. [DOI] [PubMed] [Google Scholar]
- 87.Riethdorf S, Wikman H, Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- 88.Fehm T, Mueller V, Marches R, Klein G, Gueckel B, Neubauer H, et al. Tumor cell dormancy: implications for the biology and treatment of breast cancer. Apmis. 2008;116:742–53. doi: 10.1111/j.1600-0463.2008.01047.x. [DOI] [PubMed] [Google Scholar]
- 89.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumour dormancy. Apmis. 2008;116:754–70. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 90.Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–21. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 91.Braun S, Schlimok G, Heumos I, Schaller G, Riethdorf L, Riethmuller G, et al. ErbB2 overexpression on occult metastatic cells in bone marrow predicts poor clinical outcome of stage I–III breast cancer patients. Cancer Res. 2001;61:1890–5. [PubMed] [Google Scholar]
- 92.Pantel K, Schlimok G, Braun S, Kutter D, Lindemann F, Schaller G, et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst. 1993;85:1419–24. doi: 10.1093/jnci/85.17.1419. [DOI] [PubMed] [Google Scholar]
- 93.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–8. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 94.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 96.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 97.Guise TA. Breaking down bone: new insight into site-specific mechanisms of breast cancer osteolysis mediated by metalloproteinases. Genes Dev. 2009;23:2117–23. doi: 10.1101/gad.1854909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z, Schem C, Shi Y, Medina D, Zhang M. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clin Exp Metastasis. 2007;25:389–400. doi: 10.1007/s10585-007-9117-3. [DOI] [PubMed] [Google Scholar]
- 99.Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002;62:5571–9. [PubMed] [Google Scholar]
- 100.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davideau JL, Sahlberg C, Thesleff I, Berdal A. EGF receptor expression in mineralized tissues: an in situ hybridization and immunocytochemical investigation in rat and human mandibles. Connect Tissue Res. 1995;32:47–53. doi: 10.3109/03008209509013705. [DOI] [PubMed] [Google Scholar]
- 102.Ng KW, Partridge NC, Niall M, Martin TJ. Epidermal growth factor receptors in clonal lines of a rat osteogenic sarcoma and in osteoblast-rich rat bone cells. Calcif Tissue Int. 1983;35:298–303. doi: 10.1007/BF02405050. [DOI] [PubMed] [Google Scholar]
- 103.Guise TA, Yoneda T, Yates AJ, Mundy GR. The combined effect of tumor-produced parathyroid hormone-related protein and transforming growth factor-alpha enhance hypercalcemia in vivo and bone resorption in vitro. J Clin Endocrinol Metab. 1993;77:40–5. doi: 10.1210/jcem.77.1.8325957. [DOI] [PubMed] [Google Scholar]
- 104.Ibbotson KJ, D’Souza SM, Ng KW, Osborne CK, Niall M, Martin TJ, et al. Tumor-derived growth factor increases bone resorption in a tumor associated with humoral hypercalcemia of malignancy. Science. 1983;221:1292–4. doi: 10.1126/science.6577602. [DOI] [PubMed] [Google Scholar]
- 105.Ibbotson KJ, D’Souza SM, Smith DD, Carpenter G, Mundy GR. EGF receptor antiserum inhibits bone resorbing activity produced by a rat Leydig cell tumor associated with the humoral hypercalcemia of malignancy. Endocrinology. 1985;116:469–71. doi: 10.1210/endo-116-1-469. [DOI] [PubMed] [Google Scholar]
- 106.Zhu J, Jia X, Xiao G, Kang Y, Partridge NC, Qin L. EGF-like ligands stimulate osteoclastogenesis by regulating expression of osteoclast regulatory factors by osteoblasts: implications for osteolytic bone metastases. J Biol Chem. 2007;282:26656–64. doi: 10.1074/jbc.M705064200. [DOI] [PubMed] [Google Scholar]
- 107.Normanno N, De Luca A, Aldinucci D, Maiello MR, Mancino M, D’Antonio A, et al. Gefitinib inhibits the ability of human bone marrow stromal cells to induce osteoclast differentiation: implications for the pathogenesis and treatment of bone metastasis. Endocr Relat Cancer. 2005;12:471–82. doi: 10.1677/erc.1.00956. [DOI] [PubMed] [Google Scholar]
- 108.Qin L, Qiu P, Wang L, Li X, Swarthout JT, Soteropoulos P, et al. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem. 2003;278:19723–31. doi: 10.1074/jbc.M212226200. [DOI] [PubMed] [Google Scholar]
- 109.Qin L, Partridge NC. Stimulation of amphiregulin expression in osteoblastic cells by parathyroid hormone requires the protein kinase A and cAMP response element-binding protein signaling pathway. J Cell Biochem. 2005;96:632–40. doi: 10.1002/jcb.20550. [DOI] [PubMed] [Google Scholar]
- 110.Cole JA. Parathyroid hormone activates mitogen-activated protein kinase in opossum kidney cells. Endocrinology. 1999;140:5771–9. doi: 10.1210/endo.140.12.7173. [DOI] [PubMed] [Google Scholar]
- 111.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–9. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Southby J, Kissin MW, Danks JA, Hayman JA, Moseley JM, Henderson MA, et al. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Res. 1990;50:7710–6. [PubMed] [Google Scholar]
- 113.Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51:3059–61. [PubMed] [Google Scholar]
- 114.Henderson M, Danks J, Moseley J, Slavin J, Harris T, McKinlay M, et al. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J Natl Cancer Inst. 2001;93:234–7. doi: 10.1093/jnci/93.3.234. [DOI] [PubMed] [Google Scholar]
- 115.Vargas SJ, Gillespie MT, Powell GJ, Southby J, Danks JA, Moseley JM, et al. Localization of parathyroid hormone-related protein mRNA expression in breast cancer and metastatic lesions by in situ hybridization. J Bone Miner Res. 1992;7:971–9. doi: 10.1002/jbmr.5650070814. [DOI] [PubMed] [Google Scholar]
- 116.Cho YM, Lewis DA, Koltz PF, Richard V, Gocken TA, Rosol TJ, et al. Regulation of parathyroid hormone-related protein gene expression by epidermal growth factor-family ligands in primary human keratinocytes. J Endocrinol. 2004;181:179–90. doi: 10.1677/joe.0.1810179. [DOI] [PubMed] [Google Scholar]
- 117.Lorch G, Gilmore JL, Koltz PF, Gonterman RM, Laughner R, Lewis DA, et al. Inhibition of epidermal growth factor receptor signalling reduces hypercalcaemia induced by human lung squamous-cell carcinoma in athymic mice. Br J Cancer. 2007;97:183–93. doi: 10.1038/sj.bjc.6603828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Richard V, Rosol TJ, Foley J. PTHrP gene expression in cancer: do all paths lead to Ets? Crit Rev Eukaryot Gene Expr. 2005;15:115–32. doi: 10.1615/critreveukaryotgeneexpr.v15.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gilmore JL, Scott JA, Bouizar Z, Robling A, Pitfield SE, Riese DJ, 2nd, et al. Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, et al. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009;23:1882–94. doi: 10.1101/gad.1824809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.MacLeod JR, Yano S, Chattopadhyay N, Brown EM. Extracellular calcium-sensing receptor transactivates the epidermal growth factor receptor by a triple-membrane-spanning signaling mechanism. Biochem Biophys Res Commun. 2004;320:455–60. doi: 10.1016/j.bbrc.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 122.Gschwind A, Hart S, Fischer OM, Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. Embo J. 2003;22:2411–21. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Normanno N, Morabito A, De Luca A, Piccirillo MC, Gallo M, Maiello MR, et al. Target-based therapies in breast cancer: current status and future perspectives. Endocr Relat Cancer. 2009;16:675–702. doi: 10.1677/ERC-08-0208. [DOI] [PubMed] [Google Scholar]
- 124.Albain KS, Elledge R, Gradishar WJ, Hayes DF, Rowinsky E, Hudis C, et al. Open-label, phase II, multicenter trial of ZD1839 (‘Iressa’) in patients with advanced breast cancer. Breast Cancer Res Treat. 2002;76:S33. [Google Scholar]
- 125.von Minckwitz G, Jonat W, Fasching P, du Bois A, Kleeberg U, Luck HJ, et al. A multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005;89:165–72. doi: 10.1007/s10549-004-1720-2. [DOI] [PubMed] [Google Scholar]
- 126.Cristofanilli M, Valero V, Mangalik A, Royce M, Rabinowitz I, Arena FP, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–14. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 127.Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, et al. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16:1486–97. doi: 10.1158/1078-0432.CCR-09-1764. [DOI] [PubMed] [Google Scholar]
- 128.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]