Abstract
Definitive immunization guidelines for internationally adopted children are lacking. We examined whether these children had serologic evidence of protection against vaccine-preventable diseases. For children with ≥3 vaccine doses, overall protection was high for diphtheria (85%), tetanus (95%), polio (93%), hepatitis B (77%), and Hib (67%). For children ≥12 months of age with ≥1 dose of measles, mumps, or rubella vaccines, 95%, 72%, and 94% were immune, respectively. Children without immunization documentation had lower immunity. Serologic testing was useful in verifying the immunization status in internationally adopted children with and without documentation of immunizations.
Keywords: International adoption, Immunization, Serology, Vaccine preventable diseases
1. Introduction
In 2008, 17,443 children were internationally adopted by families in the United States (US) and more than 200,000 children were adopted in the past decade [1]. The American Academy of Pediatrics (AAP) recommends that children undergo a comprehensive health assessment shortly after arrival to the US, including an assessment of previously administered vaccines [2]. While the Advisory Committee on Immunization Practices (ACIP) [3], the Infectious Disease Society of America (IDSA) [4], and the AAP [2] provide several options for assuring that internationally adopted children are properly immunized, no clear consensus exists about which strategy is optimal. Written records may be accepted as valid if: the vaccines, dates of administration, numbers of doses, intervals between doses, and patient’s age at the time of immunization are all consistent with the current US or World Health Organization schedule. Serologic testing may also be used to verify the child’s immunizations. Another option provided is to give one dose of vaccine and do serologic testing one month later. If there are concerns about whether vaccines were administered properly or were immunogenic, repeat administration of immunizations is recommended.
The lack of definitive recommendations for immunization decisions in internationally adopted children is likely due to the varied results from previously published studies [5–15]. The interpretation and comparison of results from these studies may differ due to differences in laboratory methods and definitions of protection. In addition, many of these studies have small sample sizes, minimal country-specific data, and evaluated only a few vaccine antigens. Serologic testing has been used in research settings in vaccine development and post-licensure surveillance to verify response to vaccine antigens and to provide a surrogate for protection [16]. In addition, many health care providers, employers, and public health agencies utilize serology in populations such as military recruits, health care workers, and pregnant women to document their immune status and to determine their potential need for immunization [3,4]. Since serum antibody testing is widely accepted as a method to verify immunity [3,4,16], we have routinely used serologic testing to guide our recommendations for immunization in internationally adopted children. To assist in providing data for evidence-based guidelines, the objective of our study was to determine whether internationally adopted children had serologic evidence of protection against vaccine preventable diseases and to determine if documentation of immunization was associated with protective antibody levels.
2. Methods
2.1. Study population
Children evaluated at the International Adoption Center at Cincinnati Children’s Hospital Medical Center (CCHMC) in Cincinnati, OH from November 1999 through June 2004 were eligible for the study if they had serologic testing for any vaccine antigen. They were excluded from the analysis for a specific antigen if they received a vaccine in the US for that antigen prior to serologic testing. In the case of hepatitis B virus (HBV), they were also excluded if they had serologic evidence of past or current infection or hepatitis B virus testing was inconclusive (positive testing for hepatitis B surface antigen and/or hepatitis B core antibody). Children ≥5 months of age could have testing for diphtheria, tetanus, polio, HBV, or Haemophilus influenzae type b (Hib), and those ≥12 months of age could also have testing for measles, mumps, rubella, or varicella. Demographic and clinical data extracted from the child’s record included: birth country, gender, birth date, visit date, documentation of immunizations (type and date), history of measles, mumps, rubella, or varicella disease, and serologic testing (dates and results). In countries with fewer than 10 children, the children were grouped by region as follows: Africa (Ethiopia, Ghana, Liberia, and South Africa), Other Asian (Azerbaijan, Cambodia, Nepal, Philippines and Thailand), Other Eastern European (Albania, Belarus, Moldova and Slovakia), Other Latin American and Caribbean (Bolivia, Colombia, Haiti, and Peru). Detailed country-specific data for individual countries with ≥10 children is provided in the appendices. This study was approved by the CCHMC Institutional Review Board.
2.2. Serologic testing
Standard serologic assays and methods at commercial laboratories were used. More than 95% of children had testing done for a given vaccine antigen at the same laboratory utilized by CCHMC during the study period. For diphtheria and tetanus, immunoglobulin G (IgG) enzyme-linked immunosorbent assays (ELISA) were done. The definition of protective antibody for diphtheria was >0.10 IU/mL [16–19] and for tetanus was >0.10 IU/mL [16,19–21]. For polio, neutralizing antibody to each polio serotype was performed and the definition of protection was a titer of ≥1:8 for each serotype [16,22–24]. An ELISA for HBV (hepatitis B surface antibody/anti-HBs) was used and the definition of protection was ≥10 mIU/mL [16,25]; the Abbott assay (Chicago, IL) was used in >95% of children [26]. For Hib, an IgG ELISA to polyribosylribitol phosphate (PRP) was used and two definitions of protection were evaluated (≥1.0 and ≥0.15 IU/mL) [16,27,28]. For measles and mumps, immunofluoresent antibody assays were used for 97% of children with cut-off values of ≥1:8 for measles [29–31] and ≥1:16 for mumps [31,32]; the remaining children had immunosorbent assays done. For rubella, all children had immunosorbent assays done; 95% of the assays had an ELISA done with a cut-off value of ≥10 IU/mL as positive [33]. For varicella, 98% of children had an ELISA done with a qualitative cut-off as positive with an OD ratio of ≥1.10 [34].
2.3. Statistical analysis
Our primary outcome of interest was the defined level of protective antibody for each vaccine antigen which served as a surrogate for immunity/protection [16]. Protective antibody was examined by the number of documented vaccine doses and by birth country. For the four countries with the largest number of children (Russia, China, Guatemala, and Kazakhstan), the Cochran–Armitage trend test and the exact test for trends were used to examine the association between protection and an increasing number of doses. For South Korea, very few children were unimmunized; therefore the test for trend could not be done. Chi-square and Fisher’s exact tests were used to examine country-specific differences among the four countries with the largest number of children. The sensitivity and specificity of varicella disease history to predict protective antibodies was calculated. Statistical analyses were performed using SAS® (Version 9.2, SAS Institute, Cary, NC). All tests were two-sided and p-values ≤0.05 or 95% confidence intervals (CI) that did not include one were considered statistically significant. Adjustments were not made for multiple testing. Demographic variables were descriptively summarized as medians and ranges for non-normally distributed variables and as percentages for categorical variables.
3. Results
3.1. Study population characteristics
Overall, 816 children were evaluated at the International Adoption Center at CCHMC from November 1999 through June 2004. After all exclusion criteria were applied, 746 remained eligible. For the HBV analysis, 31 were ineligible due to current (n = 5) or resolved infection (n = 26). Table 1 summarizes the demographic characteristics of the children studied. Consistent with US trends in international adoption [1], the majority of children emigrated from one of five countries: Russia (34%), China (21%), Guatemala (12%), Kazakhstan (7%), and South Korea (6%). Females accounted for 54% of adoptees, however, Chinese children were more likely to be female compared to children from other countries (p < 0.0001). The median ages at adoption and at initial adoption center visit were 14 and 15 months, respectively. Documentation of at least one vaccine from the birth country was available for 89% of the children.
Table 1.
Characteristics of study population.
| Country/region | Total studied |
Female gender |
Age at adoption (in months) |
Age at initial visit (in months) |
Immunization documentation |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | Median | Range | Median | Range | N | % | |
| Russia | 253 | 34 | 107 | 42 | 17 | (5–200) | 18 | (6–201) | 226 | 89 |
| China | 155 | 21 | 142 | 92 | 13 | (8–104) | 14 | (9–131) | 140 | 90 |
| Guatemala | 93 | 12 | 39 | 42 | 9 | (5–118) | 10 | (5–118) | 88 | 95 |
| Kazakhstan | 51 | 7 | 24 | 47 | 12 | (7–173) | 12 | (7–173) | 50 | 98 |
| South Korea | 43 | 6 | 18 | 42 | 8 | (5–27) | 8 | (5–40) | 43 | 100 |
| Ukraine | 29 | 4 | 10 | 34 | 38 | (15–171) | 39 | (15–172) | 29 | 100 |
| Bulgaria | 25 | 3 | 12 | 48 | 29 | (12–163) | 31 | (21–164) | 21 | 84 |
| Romania | 24 | 3 | 10 | 42 | 35 | (10–75) | 37 | (11–118) | 24 | 100 |
| India | 18 | 2 | 10 | 56 | 14 | (5–89) | 14 | (5–89) | 17 | 94 |
| Other Asian/Pacific Rima | 15 | 2 | 9 | 60 | 14 | (6–90) | 15 | (6–152) | 8 | 53 |
| Vietnam | 15 | 2 | 8 | 53 | 12 | (3–82) | 15 | (7–83) | 6 | 40 |
| Africab | 10 | 1 | 7 | 70 | 56 | (5–181) | 57 | (6–182) | 5 | 50 |
| Other Eastern Europec | 8 | 1 | 5 | 63 | 18 | (9–39) | 19 | (9–40) | 5 | 63 |
| Other Latin American/Caribbeand | 7 | 1 | 4 | 57 | 50 | (7–192) | 50 | (7–194) | 4 | 57 |
| Total | 746 | 100 | 405 | 54 | 14 | (3–200) | 15 | (5–201) | 666 | 89 |
Includes Azerbaijan, Cambodia, Nepal, Philippines, and Thailand.
Includes Ethiopia, Ghana, Liberia and South Africa.
Includes Albania, Belarus, Moldova, and Slovakia.
Includes Bolivia, Colombia, Peru and Haiti.
3.2. Protective antibody by vaccine antigen
The number of children with at least one serologic test done and therefore included in the study was 746, however the number of children included for the analysis for a specific vaccine antigen includes only those children with serologic testing done for that antigen. Table 2 provides a summary with all countries combined of the proportion of children with protective antibody by the number of documented immunizations for each vaccine antigen. In Table 3, overall and country-specific data for the five countries with the largest number of children evaluated (Russia, China, Guatemala, Kazakhstan and South Korea) are presented for each vaccine antigen by <3 and ≥3 documented doses of vaccine. To provide detailed country-specific data by the number of documented doses of vaccine, additional tables are provided in Appendices 1–5.
Table 2.
Proportion of children with protective antibody by vaccine antigen and number of vaccine doses.
| Vaccine antigen | Number of vaccine doses |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 doses |
1 dose |
2 dosesa |
3 dosesb |
4 dosesc |
5 dosesd |
Total |
|||||||||||||||
| N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | |
| Diphtheria | 95 | 49 | (39–60) | 33 | 76 | (58–89) | 79 | 89 | (79–95) | 331 | 85 | (81–88) | 106 | 87 | (80–93) | 21 | 90e | (70–99) | 665 | 80 | (77–83) |
| Tetanus | 94 | 54 | (44–64) | 33 | 85 | (68–95) | 82 | 98 | (91–100) | 330 | 94 | (91–97) | 109 | 96 | (91–99) | 22 | 100e | (85–100) | 670 | 89 | (87–91) |
| Polio | 76 | 80 | (71–89) | 38 | 79 | (63–90) | 60 | 90 | (79–96) | 256 | 91 | (87–94) | 112 | 95 | (89–98) | 98 | 96e | (90–99) | 640 | 90 | (88–92) |
| Serotype 1 | 76 | 84 | (76–92) | 38 | 87 | (72–96) | 60 | 97 | (88–100) | 256 | 91 | (88–95) | 112 | 97 | (92–99) | 98 | 97e | (91–99) | 640 | 93 | (90–95) |
| Serotype 2 | 76 | 88 | (81–95) | 38 | 95 | (82–99) | 60 | 97 | (88–100) | 256 | 95 | (92–97) | 112 | 98 | (94–100) | 98 | 98f | (93–100) | 640 | 95 | (93–97) |
| Serotype 3 | 76 | 86 | (78–93) | 38 | 84 | (69–94) | 60 | 92 | (82–97) | 256 | 95 | (92–97) | 112 | 97 | (92–99) | 98 | 98e | (93–100) | 640 | 94 | (92–95) |
| Hepatitis B | 172 | 27 | (21–34) | 52 | 50 | (36–64) | 106 | 61 | (52–71) | 333 | 77 | (72–81) | 7 | 71e | (29–96) | 670 | 60 | (56–63) | |||
| Hib | 228 | 20 | (15–25) | 17 | 59 | (33–82) | 15 | 100 | (78–100) | 39 | 67e | (52–81) | 299 | 32 | (27–38) | ||||||
| Measles | 114 | 58 | (49–67) | 227 | 94 | (91–97) | 41 | 100e | (91–100) | 382 | 84 | (80–87) | |||||||||
| Mumps | 143 | 31 | (23–38) | 167 | 71 | (64–78) | 11 | 91e | (59–100) | 321 | 54 | (48–59) | |||||||||
| Rubella | 188 | 30 | (23–36) | 112 | 94 | (88–97) | 5 | 100e | (48–100) | 305 | 54 | (49–60) | |||||||||
| Varicella | 375 | 33 | (28–38) | 21 | 38 | (18–62) | 395 | 33 | (29–38) | ||||||||||||
Equal to ≥2 for measles, mumps and rubella.
Equal to ≥3 for Hib.
Equal to ≥4 for hepatitis B.
Equal to ≥5 for diphtheria, tetanus and polio.
Cochran–Armitage/Exact Test for Trend examining trend for an increase in the proportion protected by increasing number of doses: p < 0.001.
Cochran–Armitage/Exact Test for Trend examining trend for an increase in the proportion protected by increasing number of doses: p = 0.003.
Table 3.
Proportion of children with protective antibody by vaccine antigen and number of vaccine doses.
| Vaccine antigen | Country/region |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Russia |
China |
Guatemala |
Kazakhstan |
South Korea |
Overall |
|||||||||||||
| N | % | (95% CI) | N | % | (95% CI) | N | % | (95% CI) | N | % | (95% CI) | N | % | (95% CI) | N | % | (95% CI) | |
| Diphtheria | ||||||||||||||||||
| <3 doses | 100 | 73 | (64–82) | 32 | 38 | (21–54) | 16 | 75 | (48–93) | 10 | 90 | (56–100) | 11 | 100 | (72–100) | 207 | 69 | (62–75) |
| ≥3 doses | 131 | 88 | (82–93)a | 102 | 76 | (68–85)b | 72 | 94 | (86–98)c | 40 | 80 | (64–91) | 27 | 96 | (81–100) | 458 | 85 | (82–89)b |
| Tetanus | ||||||||||||||||||
| <3 doses | 102 | 83 | (76–91) | 32 | 41 | (24–58) | 16 | 94 | (70–100) | 10 | 90 | (56–100) | 11 | 100 | (72–100) | 209 | 76 | (70–82) |
| ≥3 doses | 132 | 95 | (89–98)a | 104 | 90 | (85–96)b | 72 | 99 | (93–100) | 39 | 95 | (83–99) | 27 | 100 | (87–100) | 461 | 95 | (93–97)b |
| Polio | ||||||||||||||||||
| <3 doses | 81 | 83 | (74–91) | 23 | 74 | (52–90) | 13 | 85 | (55–98) | 7 | 86 | (42–100) | 11 | 100 | (72–100) | 174 | 83 | (78–89) |
| ≥3 doses | 138 | 97 | (93–99)d | 108 | 85 | (78–92) | 71 | 90 | (81–96) | 41 | 98 | (87–100) | 25 | 96 | (80–100) | 466 | 93 | (90–95)d |
| Hepatitis B | ||||||||||||||||||
| <3 doses | 156 | 46 | (38–54) | 60 | 38 | (26–51) | 26 | 42 | (23–61) | 14 | 43 | (18–71) | 9 | 33 | (7–70) | 330 | 42 | (37–47) |
| ≥3 doses | 85 | 89 | (81–95)b | 75 | 75 | (65–85)b | 56 | 88 | (76–95)b | 32 | 63 | (46–79) | 29 | 62 | (44–80) | 340 | 77 | (72–81)b |
| Hib | ||||||||||||||||||
| <3 doses | 102 | 20 | (12–27) | 68 | 10 | (4–20) | 21 | 81 | (58–95) | 23 | 22 | (7–44) | 15 | 80 | (52–96) | 260 | 27 | (22–33) |
| ≥3 doses | 0 | – | 0 | – | 33 | 61 | (44–77) | 0 | – | 5 | 100 | (48–100) | 39 | 67 | (52–81)b | |||
| Measles | ||||||||||||||||||
| <1 doses | 49 | 49 | (35–63) | 16 | 56 | (30–80) | 4 | 50 | (7–93) | 6 | 17 | (0–64) | 2 | 50 | (1–99) | 114 | 58 | (49–67) |
| ≥1 doses | 99 | 92 | (85–96)b | 63 | 95 | (87–99)d | 26 | 100 | (87–100)c | 14 | 100 | (77–100)d | 6 | 100 | (54–100) | 268 | 95 | (92–97)b |
| Mumps | ||||||||||||||||||
| <1 doses | 45 | 33 | (20–47) | 51 | 16 | (7–29) | 3 | 67 | (9–99) | 6 | 17 | (0–64) | 2 | 0 | (0–84) | 143 | 31 | (23–38) |
| ≥1 doses | 85 | 69 | (60–79)b | 8 | 38 | (9–76) | 24 | 83 | (63–95) | 13 | 85 | (55–98)a | 6 | 83 | (36–100) | 178 | 72 | (66–79)b |
| Rubella | ||||||||||||||||||
| <1 doses | 75 | 40 | (29–51) | 50 | 10 | (3–22) | 2 | 100 | (16–100) | 17 | 0 | (0–20) | 2 | 50 | (1–99) | 188 | 30 | (23–36) |
| ≥1 doses | 46 | 96 | (85–99)b | 6 | 50 | (12–88)c | 22 | 91 | (71–99) | 2 | 100 | (16–100)a | 6 | 100 | (54–100) | 117 | 94 | (88–98)b |
p < 0.01.
p < 0.0001.
p < 0.05.
p < 0.001.
3.2.1. Diphtheria
Of the 665 children with diphtheria antibody testing done, most (86%) had received at least one dose of a diphtheria vaccine and 69% had ≥3 doses (Appendix 1). The overall proportion of children with protective antibody was 80% (Table 2). Overall, the proportion with protection was significantly higher in children with ≥3 doses (85%) compared to children with <3 doses (69%) (p < 0.001) (Table 3). Immunity was also significantly higher with ≥3 doses compared to <3 doses in Russian (p < 0.01), Chinese (p < 0.001) and Guatemalan (p < 0.05) children. As the number of documented doses increased, protection also increased for children with all countries combined (p < 0.0001) (Table 2), and specifically for Russia (p < 0.001), China (p < 0.001) and Guatemala (p < 0.05) (Appendix 1).
3.2.2. Tetanus
Of the 670 children with tetanus antibody testing done, most (86%) had received at least one dose of a tetanus vaccine and 69% had ≥3 doses (Appendix 1). The overall proportion of children with protective antibody to tetanus was 89% (Table 2). Overall, as well as for Russian and Chinese adoptees, children with ≥3 doses were significantly more likely to be protected (95%) compared to children with <3 doses (76%) (p < 0.001) (Table 3). The proportion of children with immunity against tetanus was greater with increasing number of doses, overall (p < 0.0001) and in children from Russia (p < 0.001) and China (p < 0.001) (Appendix 1).
3.2.3. Polio
Of the 640 children with polio antibody testing done, most (88%) had received at least one dose of polio vaccine and 73% had ≥3 doses (Appendix 2). The overall proportion of children with protective antibody was 90% and 83% for those with <3 doses and 93% for those with ≥3 doses (Table 2). The proportion immune was greater as the number of doses increased, for all countries combined (p < 0.0001) and specifically, for Russia (p < 0.01) (Appendix 2a). Immunity against each serotype was similar among serotypes 1–3 (93%, 95%, and 94%, respectively) (Appendix 2b–d). Using a more conservative titer (≥1:32), 84% of children were immune overall while for serotypes 1–3, 87%, 93%, and 90%, were protected, respectively.
3.2.4. Hepatitis B (HBV)
Of the 670 children with hepatitis B serology done, most (74%) had documentation of receiving at least one dose of HBV vaccine and 51% had ≥3 doses (Appendix 3). Overall, 60% of children had protective antibody for HBV (Table 2). Children with ≥3 doses were significantly more likely to be immune (77%) compared to those with <3 doses (42%) (p < 0.001) (Table 2). The proportion of children with immunity increased with an increasing number of documented doses overall (for all countries combined) and specifically for Russia, China and Guatemala (all p < 0.0001) (Appendix 3). For children who had received 3 HBV vaccine doses, the interval from the third dose to serologic testing was significantly shorter in children with protective antibody compared to those without (median 162 vs 231 days p = 0.002). There was no difference in the proportion with protective antibody who had received their third dose prior to 6 months of age versus after 6 months of age (p = 0.12). Boosting was examined in those 54 children who had initial non-protective antibody levels and then had a HBV vaccine in the US. For children with 0, 1, 2 and 3 doses in their birth county, 43%, 50%, 65% and 82% respectively had protective antibody levels after a US dose when retested.
3.2.5. Haemophilus influenzae type b (Hib)
Testing for Hib began in 2002. Our study included 299 children with Hib test results. Only 24% of these children had documentation of Hib vaccine; 61% were from Guatemala and 25% were from South Korea (Appendix 4). Using a conservative definition of protection (>1.00 IU/mL), the overall proportion of children with protective antibody was 32% while for children with 0 doses and ≥3 doses 20% and 67% were protected, respectively (Table 2). When the definition of protection was ≥0.15 IU/mL, as expected, the overall proportion of children with protective antibody was 53% and for children with 0 doses and ≥3 doses the proportion protected was 43% and 77%, respectively (Appendix 4). In the 26 children ≥5 years of age without documentation of Hib vaccine, 50% had protection at the ≥1.0 level and 85% had protection at the ≥0.15 level.
3.2.6. Measles
Of the 382 children ≥12 months of age with serologic testing, 70% had documentation of a measles-containing vaccine (Appendix 5). Overall, 84% of children were immune (Table 2). Protection increased as the number of doses increased; the proportion with immunity was 58% for children with no documented doses, 94% with one dose and 100% with ≥2 doses (p < 0.0001) (Table 3). This trend was also significant when examined by country for children from Russia, China, Guatemala, and Kazakhstan (p < 0.0001, p = 0.0003, p = 0.014, p = 0.0004, respectively). In children with only one documented dose of vaccine, there were no significant differences in the proportion protected by country and there was no significant difference in the proportion protected in children with one versus two doses of vaccine.
When the data were examined by age at vaccination (<9 months, 9–11 months and ≥12 months, there were 41, 183 and 144 children with testing done after 12 months of age eligible for analysis, respectively. There was no significant difference in the proportion of children with protective antibody to measles by age of immunization. For children immunized <9 months of age, 95% had protective levels of antibody, while 94% of children immunized at 9–11 months and ≥12 months of age were immune.
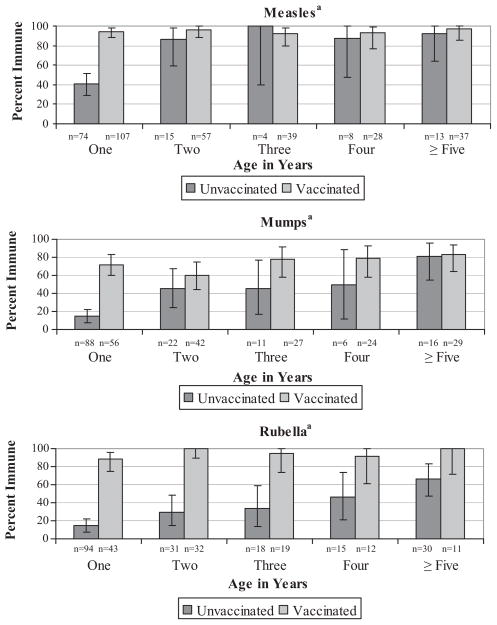
Measles immunity increased with age in children without documentation of a measles-containing vaccine (p < 0.0001) (Fig. 1). In children 5 years or older, 97% with documentation had immunity compared to 92% without documentation. Of the four children with a history of measles, three had serology performed, and all had evidence of protection. Excluding these three children from the analyses did not change the results presented.
Fig. 1.
Proportion of children with protective antibody by age to measles, mumps and rubella in unvaccinated and vaccinated children.
3.2.7. Mumps
Mumps-containing vaccine was documented for 55% of the 321 children with antibody testing done (Appendix 5). Overall 54% of children had protective levels of antibody; of those with no documentation of immunization, 31% had protective levels (Table 2). There was no difference in the proportion protected for those with one dose of vaccine (71%) compared to those with ≥2 doses (91%) (p = 0.29) (Table 3). Mumps immunity increased with age in children without documentation of mumps vaccine (p < 0.0001) (Fig. 1). In children ≥5 years of age, 82% of children with and without documentation of immunization had evidence of immunity. Two children had a history of mumps disease and both were immune. When we excluded these children from the analyses, the results did not change.
3.2.8. Rubella
Rubella-containing vaccine was documented for 38% of the 305 children with rubella antibody testing (Appendix 5). Overall, 54% were immune; of those without rubella vaccine documentation, 30% had protective levels (Table 2). There was no difference in the proportion protected with one (94%) versus two doses of vaccine (100%) (p = 1.0) (Table 2). Immunity increased with increasing age in children without documentation of rubella vaccine (p < 0.0001) (Fig. 1). In children ≥5 years of age, 67% of those without documentation of rubella vaccine were immune compared to 100% of those with rubella immunization. Four children had a history of rubella disease, and two of them had evidence of immunity. Excluding these children from the analyses did not change the results.
3.2.9. Varicella
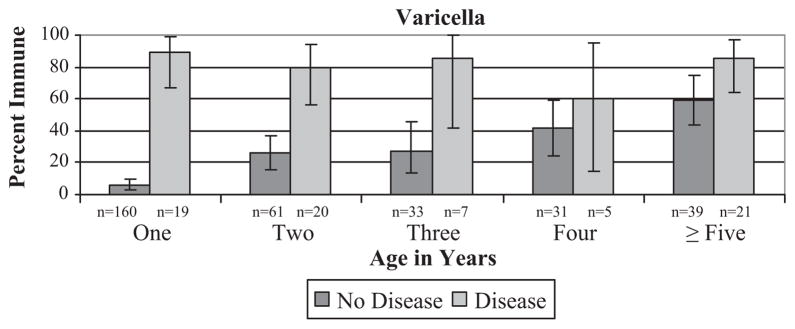
Only 5% of children had documentation of varicella vaccine; 67% of those vaccinated were from Guatemala. The proportion of children with immunity was comparable in those with (38%) and without documentation (33%) (Table 2). A history of varicella disease was significantly associated with immunity (p < 0.0001). In 72 children with a history of varicella disease, 83% had protective levels. Varicella protection increased with age in children without a history of disease (p < 0.0001), but not in children with a history of disease (p = 0.77) (Fig. 2). A history of varicella was 83% sensitive and 78% specific for having protective antibody. While the sensitivity was similar across age groups, there was a significant decline in specificity with increasing age (94% in 12–23 month olds down to 41% in children ≥60 months, (p < 0.0001).
Fig. 2.
Proportion of children with protective antibody to varicella by varicella disease history and age.
4. Discussion
This large comprehensive study with country-specific data examined protective antibody levels to vaccine antigens to determine whether internationally adopted children had serologic evidence of protection against vaccine preventable diseases and to determine if documentation of immunization was associated with protective antibody levels. Table 4 summarizes previously published studies examining immunization verification in internationally adopted children (5–15). The countries evaluated, ages of children studied and laboratory methods used may account for some of the differences seen between studies. Some studies presented data for children with ≥3 doses, without indicating how many additional doses were given [5–7,9,11,12]. In order to make direct comparisons with the existing literature, we have presented our data by the specific number of doses as well as by ≥3 doses of vaccine for diphtheria, tetanus, polio, and HBV and ≥1 dose for measles, mumps, rubella, and varicella.
Table 4.
Review of literature of serologic studies to assess protective antibody levels in internationally adopted children.
| Hostetter [5] | Miller [6] | Schulpen [7] | Viviano [9] | Miller [10] | Cilleruelo [11] | Verla-Tebit [12] | VanSchaik [13] | Staat | |
|---|---|---|---|---|---|---|---|---|---|
| Publication year | 1998 | 2001 | 2001 | 2006 | 2007 | 2008 | 2009 | 2009 | Current |
| Sample size | 26 | 70 | 133 | 70 | 50 | 637 | 465 | 495 | 748 |
| Countries of origin | E. Europe | All | China (98) | E. Europe & | EthiopiaEritrea | All | All | All | All |
| Russia | Other (35) | Russia (69) | |||||||
| China | |||||||||
| Age (months) | 36 (median) | 42.6 (mean) | 21.3 (mean) | 76 (mean) | 47.7 (mean) | 28 (mean) | 19.4 (mean) | 17.8 (median) | 15 (median) |
| Vaccination criteria | ≥3 DTPs | ≥3 DTP | ≥3 DTP | ≥3 DTP | NS | All | ≥3 DTP | NS | ≥3 DTP |
| ≥3 Polio | ≥3 Polio | ≥3 Polio | ≥3 Polio | ≥3 Polio | |||||
| ≥1 MMR | Any HBV | ≥2 HBV | 3 Hib | ||||||
| ≥1 MMR | ≥1 Measles | HBV by dose | |||||||
| ≥1 MMR or V | |||||||||
| Diphtheria | |||||||||
| Test used | NS | ELISA | ToBI | Neut Assay | – | ELISA | ELISA | ToBI | ELISA |
| Protective level | NS | NS | >0.10 IU/mL | >0.01 IU/mL | – | >0.10 IU/mL | ≥0.10 IU/mL | >0.10 IU/mL | >0.10 IU/mL |
| Percent protected | 35% | 88% | 61% (China) | 96% | – | 76% | 95% | 48% | 85% |
| 71% (Other) | |||||||||
| Tetanus | |||||||||
| Test used | NS | ELISA | ToBI | ELISA | NS | ELISA | ELISA | ToBI | ELISA |
| Protective level | NS | NS | >0.10 IU/mL | >0.10 IU/mL | NS | >0.10 IU/mL | ≥0.50 IU/mL | >0.10 IU/mL | >0.10 IU/mL |
| Percent protected | 35% | 61% | 58% China | 91% | 50% | 92% | 87% | 56% | 95% |
| 94% Other | |||||||||
| Polio | |||||||||
| Test used | – | Neut Ab | Neut Ab | Neut Ab | NS | Neut Ab | Neut Ab | Micro-Neut Assay | Neut Ab |
| Protective level | – | NS | >1:4 | ≥1:5 | NS | ≥1:2 | ≥1:40 | >0.10 IU/mL | ≥1:8 |
| Percent protected | |||||||||
| Type 1 | – | 58% | 71% (China) | 83% | – | 89% | 58% | – | 94% |
| Type 2 | – | 65% | 94% (China) | 99% | – | 96% | 82% | – | 96% |
| Type 3 | – | 62% | 79% (China) | 63% | – | 90% | 52% | – | 96% |
| Overall | – | NS | 65% (China) | NS | 50% | 86% | – | 58% | 93% |
| Hepatitis B (HBV) | |||||||||
| Test used | – | – | – | ELISA | Std Methods | ELISA | EIA | EIA | ELISA |
| 1 dose | – | – | – | Any = 69% | – | – | ≥2 = 94% | – | 50% |
| 2 doses | – | – | – | – | – | – | 61% | ||
| 3 doses | – | – | – | – | 76% | – | 77% | ||
| Overall | – | – | – | 12% | – | 76% | 60% | ||
| Hib | – | – | – | – | – | – | – | – | 66% |
| MMRV | |||||||||
| Test used | – | ELISA | – | ELISA | Std Methods | ELISA | ELISA | – | See Methods |
| Measles | – | 90% | – | 62% | 80% | 79% | 81% | – | 95% |
| Mumps | – | 66% | – | 56% | 100% | 30% | – | – | 72% |
| Rubella | – | 79% | – | 86% | 60% | 38% | – | – | 94% |
| Varicella | – | – | – | – | – | – | – | – | 38% |
NS = not stated; MMR = measles, mumps and rubella; V = varicella; ELISA = enzyme linked immunosorbant assay; ToBI = toxin-binding inhibition test; Neut Ab = neutralizaing antibody.
Studies not included in the table: In Murray [8] of 100 children tested, 70% had antibody to HBV while in 55 tested for varicella, 25% had protective antibody; In Miller [14] four children with written evidence of 3–6 polio vaccines had incompletely protective titers and in Saiman [15] only HBV immunity was assessed: protective antibody following 0, 1, 2 and 3 doses of vaccine was 31%, 24%, 67% and 69%, respectively.
For diphtheria, the Verla-Tebit [12] study found a higher proportion of children (from Russia, China, & Guatemala) with ≥3 doses of vaccine to be protected (95%) compared to 85% in our study. For tetanus, they reported a lower proportion protected (87%) compared to ours (95%) which may be due to the different definitions of protection that were used (>0.50 IU/mL compared to ours >0.10 IU/mL). Similar to our data, two previous studies [11,12] detected an increase in protective antibody with additional number of doses for diphtheria and tetanus.
There were major differences in our polio results compared to other published studies. We found high levels of the proportion protected overall (90%) and by each serotype (93–95%) using a definition of protection of ≥1:8. The overall proportion protected ranged from 50% to 86% in other studies [6,7,9–13]. When we analyzed our data using a titer of ≥1:32 to compare to the Verla-Tebit study [12] which used ≥1:40, we still had higher levels of overall protection (84%) and to serotypes 1–3 (87%, 93%, and 90%, respectively) compared to their study which had 58%, 82% and 52% with protective antibody for serotypes 1–3, respectively. One possible explanation for the lower immunity in other studies is that complement fixation testing may have been inadvertently performed in some studies. This may have occurred without the knowledge of investigators since most commercial laboratories offer both tests.
The results of HBV testing are difficult to compare to other studies since many did not provide dose-specific data. For children with ≥3 doses of vaccine, we found the proportion protected in our study was slightly higher (77%) compared to Saiman (69%) [15]. Without regard to number of doses, our population had lower rates of protection (60%) compared to the children in the Cilleruelo study (76%) that took place during similar time periods as ours [11]. In the Verla-Tebit study, 94% of children with ≥2 doses of vaccine were protected [12]. One explanation may be that many children in that study may have had 3 or more doses of vaccine. While it is not entirely clear why our study and others have reported a lower than expected proportion of children with protective antibody compared to vaccine trials, one explanation may be due to waning immunity as evidenced by the difference we saw in children with longer intervals from the last vaccine dose to testing and boosting seen in those children who received an additional dose and repeat testing. Also in our study, we found no association with lack of protection and receipt of the third dose prior to 6 months of age.
To our knowledge, our study provides the first data on Hib antibody in internationally adopted children. While there is controversy over the serologic value that should be used as a cut-off to define protection, we included this vaccine antigen in our study to better understand whether internationally adopted children had adequate levels of protection. Only 24% of children evaluated had documentation of Hib vaccination. Using the two different proposed definitions of protection (≥1.0 and ≥0.15 IU/mL), 66% and 74% of children with 3 doses had protection, respectively while 20% and 43% without documentation had protection. The reason for a lower than expected level of protection with three doses could not be determined in our study, however studies have shown that children with different ethnic backgrounds, such as Native Americans may have a decreased immune response to Hib vaccine [27]. Given a large proportion of children immunized with Hib were Guatemalan children, this may have played a role in the decreased immune response. In children ≥5 years of age with no history of immunization, 85% had evidence of Hib protection using the ≥0.15 value. Currently, it is not recommended that children ≥5 years of age be vaccinated, since it is assumed most children are immune and there is widespread vaccination in the US [2,3]. However, with recent outbreaks in the US, children without documentation of Hib immunization or immunity may benefit from receiving one dose of Hib vaccine upon arrival to the US [36].
In our study, most children had documentation of measles immunization, and in contrast to other studies, we found that the majority of children with one or two doses of vaccine were protected [6,9–12]. In addition, measles protection increased with age regardless of vaccine history, suggesting that many children had prior infection with wild-type measles or had undocumented immunizations. Thus, the higher protection seen in our study could be due to age differences between the study populations or in other studies, the inclusion of children tested <12 months of age. Even though only a small proportion of children had documentation of mumps or rubella vaccine, many children had evidence of immunity and even in those with documentation, immunity increased with age.
Very few children had documentation of varicella immunization. Even in those who did, few had evidence of immunity. The lack of protective antibody may be due to problems with difficulty in maintaining cold chain vaccine storage. A history of varicella disease was associated with protective antibody, and that protection increased with age.
Some limitations should be considered in interpreting our findings. While our study had a large sample size, there were still small numbers for many countries and regions. In addition, in recent years the demographics of international adoption have changed with more children adopted from Ethiopia and other African countries. It will be important for future studies to assess whether children from countries not included in large numbers in our study have similar findings. We also could not determine whether protective levels of antibody were due to immunization, infection with wild-type disease or boosting from natural disease. Also, for polio, children with protective antibody, regardless of immunization documentation, could have been infected or boosted their immune response with vaccine-type polio virus exposure.
Our study found serology to be a useful tool for verifying a child’s protection against vaccine-preventable diseases. In our population of internationally adopted children, the proportion of children with protective antibody in our study was fairly comparable to expected protective antibody levels after a primary series for tetanus [20] (96% versus 100%) and polio [22] (91% versus 100%) and after one dose of measles [30] (94% versus 95%) and rubella [37] (94% versus >90%) vaccines. Children in our study had slightly lower levels of protection compared to expected levels with a primary series for diphtheria [17] (87% versus 96%), hepatitis B [25] (77% versus 95%) and one dose of mumps [32] (77% versus >95%). Protective antibody levels were much lower than expected for a primary series of Hib vaccine [27] (67% versus >95%) and one dose of varicella vaccine [35] (38% versus >95%). There are several reasons why lower than expected levels of protection were seen in our study. These expected levels of protection result from vaccine trials, under ideal, controlled conditions with a specific interval for testing from the last documented dose of vaccine. Antibody levels in these conditions would be optimal, and it would be expected that in field conditions the antibody levels would be somewhat lower. For varicella, the most likely explanation for the very low levels of protection is due to the inability to keep the vaccine at the appropriate temperature [35].
Administering unnecessary vaccines to internationally adopted children increases the number of visits and may negatively impact the transition for these children [38]. Since screening tests are recommended for all internationally adopted children, serologic testing for vaccine antibodies can be performed at the initial visit to guide immunization decisions for those without a complete immunization record. However, given the high proportion of protective antibody levels in children with documentation of immunizations, a reasonable approach would be to consider birth country vaccine doses to be valid, similar to the recommendations for other immigrants [2–4] and to complete the series as appropriate for the child’s age. If serologic testing is done and protective antibody levels are found for diphtheria, tetanus, polio, Hib or HBV, children should still complete the immunization series for that vaccine according to their age if additional doses of vaccine are recommended. Given the high proportion of protection in children without vaccine documentation, serologic testing to verify immunity should be done to determine which immunizations, if any, are needed. Additional studies to examine the cost-effectiveness of different strategies for immunization decisions in internationally adopted children should be considered while taking into account the medical and psychological affects of administering unnecessary immunizations.
5. Conclusions
In our study, the vast majority of internationally adopted children had serological evidence of protective antibodies to most vaccine antigens. Our country-specific data indicate that in children with immunization documentation, most had protective antibody levels. Therefore neither re-immunization nor serologic testing is needed, the record can be accepted as valid and the immunization series should be completed as appropriate for the child’s age. Our study results provide additional data for evidence-based guidelines for immunization policy makers.
Supplementary Material
Acknowledgments
Salary support to Dr. Stadler during this research was provided by Molecular Epidemiology Child Environmental Health NIEHS training grant 5-T32-ES010957-08, through the Department of Environmental Health, Division of Biostatistics and Epidemiology, University of Cincinnati College of Medicine. Dr. Trehan is partly supported by NIH training grant 5-T32-HD049338-03.
We thank Jareen Meinzen-Derr, PhD and Paul Succop, PhD for their assistance with the study. We thank Dee Daniels, Linda Jamison, Rachel Akers, Jen Andriga, Marina Bischoff, Tyler Browning, Vanessa Florian, Kristen Frommier, Emilie Grube, Rotimi Okunade, and Elizabeth Roberts for their assistance with this study, and Dr. David Bernstein for his review of the manuscript. We want to thank all the wonderful children and their families who participated in this study whose participation will help improve the health of internationally adopted children in the future.
Abbreviations
- AAP
American Academy of Pediatrics
- ACIP
Advisory Committee on Immunization Practices
- CDC
Centers for Disease Control and Prevention
- DTP
diphtheria, tetanus and whole-cell pertussis
- DTaP
diphtheria, tetanus and acellular pertussis
- Hib
Haemophilus influenzae type b
- PRP
polyribosylribitol phosphate
- HBV
hepatitis B virus
- ELISA
enzyme-linked immunosorbent assay
- OR
odds ratio
- CI
95% confidence interval
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2010.09.069.
Footnotes
Portions of this work were presented at the Pediatric Academic Societies’ Annual Meeting on May 17, 2005, in Washington, DC.
Financial disclosures: None.
References
- 1.US Department of State. [accessed 17.09.10];Immigrant visas issued to orphans coming to US. http://adoption.state.gov/news/totalchart.html.
- 2.American Academy of Pediatrics. Medical evaluation of internationally adopted children for infectious diseases. In: Pickering LK, Baker CJ, Long SS, McMillan JA, editors. Red book: 2009 report of the Committee on Infectious Diseases. 28. Elk Grove Village, IL: American Academy of Pediatrics; 2009. pp. 177–84. [Google Scholar]
- 3.Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2006;51(RR-2):33–5. [PubMed] [Google Scholar]
- 4.Pickering LK, Baker CJ, Freed GL, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Diseases Society of America. CID. 2009;49:817–40. doi: 10.1086/605430. [DOI] [PubMed] [Google Scholar]
- 5.Hostetter MK, Johnson DE. Immunization status of adoptees from China, Russia, and Eastern Europe. Pediat Res: Prog Issue APS-SPR. 1998;43(4 Suppl 2):147. [Google Scholar]
- 6.Miller LC, Comfort K, Kely N. Immunization status of internationally adopted children. Pediatrics. 2001;108(4):1050–1. [PubMed] [Google Scholar]
- 7.Schulpen TWJ, van Seventer AHJ, Rumke HC, van Loon AM. Immunisation status of children adopted from China. Lancet. 2002;358:2131–2. doi: 10.1016/s0140-6736(01)07188-4. [DOI] [PubMed] [Google Scholar]
- 8.Murray TS, Groth ME, Weitzman C, Cappello M. Epidemiology and management of infectious diseases in international adoptees. Clin Microbiol Rev. 2005;18(3):510–20. doi: 10.1128/CMR.18.3.510-520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viviano E, Cataldo F, Accomando S, Firenze A, Valenti RM, Romano N. Immunization status of internationally adopted children in Italy. Vaccine. 2006;24(19):4138–43. doi: 10.1016/j.vaccine.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Miller LC, Tseng B, Tirella LG, Chan W, Feig E. Health of children adopted from Ethiopia. Matern Child Health J. 2008;12(5):599–605. doi: 10.1007/s10995-007-0274-4. [DOI] [PubMed] [Google Scholar]
- 11.Cilleruelo MJ, de Ory F, Ruiz-Contreras J, Gonzalez-Gonzalez R, Mellado MJ, Garcia-Hortelano M, et al. Internationally adopted children: what vaccines should they receive? Vaccine. 2008;26(46):5784–90. doi: 10.1016/j.vaccine.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Verla-Tebit E, Zhu X, Holsinger E, Mandalakas AM. Predictive value of immunization records and risk factors for immunization failure in internationally adopted children. Arch Pediatr Adolesc Med. 2009;163(5):473–9. doi: 10.1001/archpediatrics.2009.26. [DOI] [PubMed] [Google Scholar]
- 13.van Schaik R, Wolfs TF, Geelen SP. Improved general health of international adoptees, but immunization status still insufficient. Eur J Pediatr. 2009;168(9):1101–6. doi: 10.1007/s00431-008-0895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller LC. Internationally adopted children-immigration status. Pediatrics. 1999;103(5):1078. doi: 10.1542/peds.103.5.1078. [DOI] [PubMed] [Google Scholar]
- 15.Saiman L, Aronson J, Zhou J, et al. Prevalence of infectious diseases among internationally adopted children. Pediatrics. 2001;108:608–12. doi: 10.1542/peds.108.3.608. [DOI] [PubMed] [Google Scholar]
- 16.Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J. 2001;20(1):63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Diphtheria. 11. Washington, DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 18.Global Programme for Vaccines and Immunizations. The immunological basis for immunization series. Module 2: Diphtheria. Geneva, Switzerland: World Health Organization; 1993. [accessed 17.09.10]. Expanded programme on immunization. Document WHO/EPI/GEN/93:12. Available from: http://www.who.int/vaccines-documents/DoxTrng/h4tibi.htm. [Google Scholar]
- 19.Moen RC, Oemichen SL, Kiggens AJ, Hong R. ELISA detection of specific functional antibodies in human serum to Escherichia coli, tetanus toxoid, and diphtheria-tetanus toxoids: normal values for IgG, IgA, and IgM. Diagn Immunol. 1986;4:17–23. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Tetanus. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 21.Global Programme for Vaccines and Immunizations. The immunological basis for immunization series. Module 3: Tetanus Update 2006. Geneva, Switzerland: World Health Organization; 2006. [accessed 17.09.10]. Expanded programme on immunization. Available from: http://www.who.int/vaccines-documents/DoxTrng/h4tibi.htm. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Polio. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 23.Global Programme for Vaccines and Immunizations. The immunological basis for immunization series. Module 6: Poliomyelititis. Geneva, Switzerland: World Health Organization; 1993. [accessed 17.09.10]. Expanded programme on immunization. Document WHO/EPI/GEN/93:16. Available from: http://www.who.int/vaccines-documents/DoxTrng/h4tibi.htm. [Google Scholar]
- 24.Modlin JF. Polioviruses. In: Mandell GL, et al., editors. Principles and practice of infectious diseases. 6. New York, NY: Churchill Livingston; 2005. pp. 2141–7. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, Hepatitis B, editors. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 26.Abbott Anti-HBs Kit, package insert 11-2009. Abbott Diagnostics, Inc; [Google Scholar]
- 27.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Haemophilus influenzae type b. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 28.Granoff DM. Assessing efficacy of Haemophilus influenzae type b combination vaccines. CID. 2001;33(Suppl 4):S278–287. doi: 10.1086/322563. [DOI] [PubMed] [Google Scholar]
- 29.Madore DV, Anderson B, Baxter D, et al. Interlaboratory study evaluating quantitation of antibodies to Haemophilus influenzae type b polysaccharide by enzyme-linked Immunosorbent assay. Clin Diagn Lab Immunol. 1999;3:84–8. doi: 10.1128/cdli.3.1.84-88.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Measles. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 31.Leland DS. Measles and mumps. In: Detrick B, et al., editors. Manual of molecular and clinical laboratory immunology. 7. Washington, DC: ASM Press; 2006. pp. 707–11. [Google Scholar]
- 32.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Mumps. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 33.Helfand RF, Keyserling HL, Williams I, et al. Comparative detection of measles and rubella IgM and IgG derived from filter paper blood and serum samples. J Med Virol. 2001;65:751–7. doi: 10.1002/jmv.2100. [DOI] [PubMed] [Google Scholar]
- 34.Zeus VZV AB ELISA Kit, package insert 8-11-00. Raritan, NJ: Zeus Scientific, Inc; 1996. [Google Scholar]
- 35.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Varicella. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 36.Centers for Disease Control and Prevention. Invasive Haemophilus influenzae type B disease in five young children – Minnesota, 2008. MMWR. 2009;58(3):58–60. [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: the pink book. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Rubella. 11. Washington DC: Public Health Foundation; 2009. [accessed 17.09.10]. Available from: www.cdc.gov/vaccines/pubs/pinkbook/default.htm. [Google Scholar]
- 38.Meyerhoff AS, Weniger BG, Jacobs RJ. Economic value to parents of reducing the pain and emotional distress of childhood vaccine injections. Pediatr Infect Dis J. 2001;20:S57–62. doi: 10.1097/00006454-200111001-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.