Abstract
This study examined neural activity with event-related potentials (ERPs) in middle childhood during a computer-simulated ball-toss game, Cyberball. Experiencing fair play initially, children were ultimately excluded by the other players. We focused specifically on “not my turn” events within fair play and rejection events within social exclusion. Dense-array ERPs revealed that rejection events are perceived rapidly. Condition differences (“not my turn” vs. rejection) were evident in a posterior ERP peaking at 420 ms consistent, with a larger P3 effect for rejection events indicating that in middle childhood rejection events are differentiated in < 500 ms. Condition differences were evident for slow-wave activity (500–900 ms) in the medial frontal cortical region and the posterior occipital-parietal region, with rejection events more negative frontally and more positive posteriorly. Distress from the rejection experience was associated with a more negative frontal slow wave and a larger late positive slow wave, but only for rejection events. Source modeling with Geosouce software suggested that slow wave neural activity in cortical regions previously identified in functional imaging studies of ostracism, including subgenual cortex, ventral anterior cingulate cortex and insula was greater for rejection events vs. “not my turn” events.
Keywords: Social exclusion, Social rejection, Middle childhood, Event-related potentials (ERPs), Source modeling
Introduction
The importance of social affiliation in middle childhood can be inferred from how children spend their time with nearly half spent in social activities among peers (Grusec & Lytton, 1988). Interaction with peers in childhood serves as an opportunity for the development of social cognitive and social perceptive abilities. At the same time, peer interactions can be a source of stress as in peer rejection and social exclusion, with some children faring better or worse than others.
Peer rejection and exclusion have a broad scope of effects, directly impacting a child’s academic and social functioning (Buhs & Ladd, 2001; Ladd, Herald-Brown, & Reiser, 2008; Ladd & Troop-Gordon, 2003). As well, peer rejection figures prominently in the emergence and maintenance of mental health concerns including disruptive behavior problems (Dodge et al., 2003), interpersonal difficulties, (Downey, Lebolt, Rincón, & Freitas, 1998), lowered self-esteem and increased levels of internalizing problems such as anxiety and depression (Deater-Deckard, 2001; Ladd, 2006). At the extreme, the experience of social exclusion and rejection has been associated with violence. For instance, in an analysis of 15 school shootings between 1995 and 2001, acute or chronic peer rejection was present in all but two of the cases (Leary, Kowalski, Smith, & Phillips, 2003). While the effects of peer rejection can be diverse, as a process its tends to be a stable and difficult for the child to overcome (Jiang & Cillessen, 2005).
The Neuroscience of Social Exclusion
A growing body of work employs lab-based methods to study peer rejection in middle childhood (Reijntjes, Dekovic, & Telch, 2007; Reijntjes, Dekovic, Vermande, & Telch, 2007; Reijntjes, Stegge, Terwogt, Kamphuis, & Telch, 2006a, 2006b), but few if any studies have done so from a neuroscience perspective. Recently social neuroscientists have begun to examine the neural correlates of social exclusion in adults with a simple interactive game called Cyberball (Williams & Jarvis, 2006). In this game, a participant makes and receives throws from two other cyber players during a “fair play” portion of the game. Then seamlessly, the other players only throw to one another, leaving the participant out of the game. This exclusion experience is mildly distressing to people. Eisenberger et al. (2003) observe that the degree of social distress to exclusion on the Cyberball task is linearly related to fMRI blood-oxygen-level dependent (BOLD) signal in some of the neural circuitry common to physical pain (Eisenberger, Jarcho, Lieberman, & Naliboff, 2006; Eisenberger & Lieberman, 2004; Panksepp, 2003).
Since the seminal work of Eisenberger and colleagues (Eisenberger, Lieberman, & Williams, 2003) a range of paradigms have used neuroimaging to examine the neural substrates of sensitivity to rejection and ostracism. This work has specifically shed light on which brain regions are implicated in an overall social exclusion and experienced distress. In particular, the dorsal anterior cingulate cortex (ACC) (Eisenberger et al., 2003; Kross, Egner, Ochsner, Hirsch, & Downey, 2007), subgenual/ventral ACC (Masten et al., 2009; Somerville, Heatherton, & Kelley, 2006), right venterolateral PFC (Eisenberger et al., 2003; Masten et al., 2009), medial PFC, posterior cingulate (Kross et al., 2007; Masten et al., 2009) and insula (Eisenberger et al., 2003; Kross et al., 2007; Masten et al., 2009) have all been linked to social exclusion distress.
Though fMRI work continues to be important for identifying brain regions responsive to social exclusion, fMRI BOLD signal change occurs over a time course of 2 seconds or more. In turn, most if not all of the neuroimaging studies to date using cyberball have averaged fMRI BOLD signal over an entire exclusion block. Everyday experience would suggest that social interactions involve perception of rapidly unfolding events, occurring in a fraction of a second. As such, a major hurdle for the social neurosciences is to illuminate how we continuously experience and react to the social world as it rapidly unfolds. Social cognitive theorists have written at length about the putative cognitive operations occurring as social information is processed (Crick & Dodge, 1994; Downey & Walker, 1989). Yet, these processes are rarely if ever studied in real-time, leaving as open questions “when?” and “how quickly?” salient social events are registered in awareness. Thus, the issue of timing, central to unpacking the neural and psychological events of social cognition, is well suited to the real-time temporal resolution of event-related brain potentials (ERPs). ERPs can be used to move the discussion from the overall rejection experience, as studied in fMRI, to rejection events and the timing of neural responses within these events.
Using Event Related Potentials to Study Social Exclusion
Among the ERPs identified as markers of cognitive-affective and evaluative processes, two in particular, the feedback related-negativity (fERN) and the late positive potential (LPP) stand out as potentially relevant to a social exclusion context. The fERN is observed when feedback (monetary loss, wrong response) indicates that an outcome is worse than expected (Hajcak, Holroyd, Moser, & Simons, 2005; Holroyd & Coles, 2002), and is thought to engage a feedback monitoring system. The fERN occurs about 250–350 msec post feedback and appears on the scalp at medial frontal regions. Source localization studies, estimating the location of this ERP’s neural generators, consistently point to the anterior cingulate cortex ACC (Gehring & Willoughby, 2002; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003), a region also implicated in social pain during the Cyberball task (Eisenberger et al., 2003). In the Cyberball game, one can think of the ball not being thrown to the participant during exclusion as an expectancy violation that engages the ACC in a way that elicits an ERP deflection for an outcome worse than expected.
The LPP is an ERP component emerging from about 200–300 ms and extending often as far as 1000–2000 ms post stimulus presentation. The LPP is thought to reflect facilitated attention to emotional stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Schupp et al., 2000). ERP studies examining the LPP typically focus on posterior midline cortical regions, finding that the amplitude of this ERP is greater for emotionally arousing versus neutral stimuli (Schupp et al., 2000), is observed in children (Hajcak & Dennis, 2009) as well as adults, and is reduced under conditions of voluntary suppression-reappraisal of negative emotion (Moser, Hajcak, Bukay, & Simons, 2006). Recently, we completed the only study to date employing event-related potentials in a social exclusion paradigm with a sample of young adults (n = 28) (Crowley, Wu, McCarty et al., 2009). Focusing specifically on rejection events, when the ball was kept from the participant during the exclusion portion of the Cyberball game, we observed a left-frontal ERP, similar in form to the LPP in a time window of 580–900 ms post event. Moreover, there was a strong association between neural activity in the left prefrontal-medial frontal cortical region for the exclusion event over this time course and self-reported exclusion distress assessed at the end of the game.
Goals of the current study
In this study we simulated peer rejection using an ERP version of the virtual ball-tossing game Cyberball to identify patterns of neural activity related to peer rejection events and experienced distress among children 8–12 years of age. From a neuroscientific and developmental perspective, we wondered to what extent evidence for engagement of a feedback monitoring system would be evident in middle childhood at the level of the ERP for rejection events. In turn we sought to know whether discrete ERP responses to rejection events, particularly slow wave neural responses, would predict global estimations of how distressing the exclusion experience was for the children, as we have shown in adults. Such evidence would broaden our view of slow wave ERPs to suggest they are related not only to facilitated attention processes and to reappraisal processes in the moment, but also as relevant neural correlates of global emotional experiences.
Children sat for a high-density EEG assessment while they played the Cyberball game. We applied temporal-spatial principal components analysis to identify correlated neural activity for two types of events during the Cyberball game: rejection events, when the ball did not come to the child during a social exclusion experience, and “not my turn events” in which the ball did not come to the child in the course of fair play. First, based on our adult work (Crowley, Wu, McCarty et al., 2009), we hypothesized that the magnitude of a central ERP slow wave would be sensitive to rejection events during social exclusion in the Cyberball game. Second, we hypothesized that the magnitude of the slow wave for rejection events would track the degree of ostracism distress reported by the child. Finally, we used the EEG source modeling software Geosource to examine changes in neural responses across the “not my turn” event and the rejection event for slow wave activity with a focus on cortical regions previously identified in adult and adolescent work to be sensitive to the social exclusion manipulation.
Methods
Participants
Thirty-three children (16 female) 8–12 years of age (mean = 10.76, SD = 1.32) participated for forty dollars compensation. Children’s ethnic backgrounds were as follows: 30 Caucasian, 3 African-American. They played Cyberball while electroencephalogram (EEG) was recorded. Families were recruited via mass mailings with addresses provided by a credit and information agency. The Human Investigation Committee of the Yale University School of Medicine approved this research. The parent of each child provided written parental informed consent and the child gave their written assent.
Procedure
Each participant sat 60 cm before a 17 in. LCD monitor in a dimly lit (60w bulb), sound attenuated room.
The Cyberball Social Exclusion Task
Cyberball is a virtual ball-toss game in which a participant plays with two other players on a computer. Abruptly, the others exclude the participant, only throwing to one another. This exclusionary experience is distressing to participants, as per their self-reports of distress on a Need Threat Scale (described below) (Eisenberger et al., 2003; Williams, 2007).
When the game began, the child's glove was at the bottom center of the screen; the gloves of the other two players, chosen by the computer, were to the left and right of the screen center. Pictures of the other “players” appeared above their names and respective gloves. Participants used their left and right index fingers on a response pad to throw left or right to the other players. The child was led to believe s/he would be playing with two other children over the internet. Then the child was told a picture was taken of them with a camera (focused on them) that the other players would see. The child then overheard one experimenter telling a second experimenter s/he would knock on the door (closed) when the other players were ready to play on the internet. Three to five minutes elapsed before the knock occurred.
Prior to beginning the experiment, the child’s gender and ethnicity were identified. Settings within the game ensured that the other players on the screen were of a similar age, ethnic appearance and gender (drawing on a bank of opponent pictures taken at the Child Study Center for use in research). At the outset of the game, the child saw an actual Google™ webpage, followed by a “Cyberball” web page, followed by a screen with a green status bar. Several other modifications were introduced to make the Cyberball game more engaging to children. The child chose from one of six different ball gloves to be his or her personal glove throughout the game. A female voice narrated instructions on the computer screen. From throw to throw, the ball traveled randomly along different paths (straight line, arc or sine wave); lifelike sound effects occurred as the ball traveled (swoosh) and landed in a glove. After the experiment, the child and parent were debriefed and informed that the other players were not real.
Our ERP version of Cyberball consisted of 155 trials across two blocks, a fair play block (108 trials) and then an exclusion block (47 trials). During the 108-trial fair play block, the cyber-players threw to the participant 36 times. Whether a ball was thrown to the participant during any one trial was pseudorandom and predetermined within a list such that the participant waited for either 0, 1, 2 or 3 throws by the other players before receiving the ball again (frequency 12, 12, 10 and 2, respectively). Cyber-players threw to one another and not to the participant 36 times (“not my turn” events). The participant threw back to the other “players” for the remaining 36 trials. Seamlessly, fair play folded into a 47-trial exclusion block. This block represented 96% exclusion. Of the 47 exclusion trials, the ball only came to the participant three times to maintain attention, once on trial fourteen, twenty-five and thirty-nine. Only 36 exclusion events from this block were used in ERP analyses. Eleven trials were not used. These included the first five trials of the exclusion block, the three throws to the participant during this block, and the three thrown back from the participant to the computer players.
Immediately after the game, children completed the Need Threat Scale (van Beest & Williams, 2006), a reliable and valid 20-item ostracism distress measure (Masten et al., 2009; Sebastian, Viding, Williams, & Blakemore, 2009; van Beest & Williams, 2006) which has been related to fMRI BOLD signal in previous research (Eisenberger et al., 2003). Children responded on the computer while still wearing the EEG cap. A female voice stated each item and the child made his or her response to the item with a mouse. Once it was clear the child understood how to use the mouse, the experimenter left the room while the child completed the need threat assessment. The Need Threat Scale gauges feelings of distress along four dimensions (5 items each): belonging (“I felt rejected”), self-esteem (“I felt liked”), meaningful existence (“I felt invisible.”), control (“I felt powerful”), on a 5-point choice, from “Not at all” to “Extremely”. Cronbach’s alpha internal consistency scores, for each of the four scales, were computed for the data collected in this sample. They were .84, .82, .82, .63 and respectively. A majority of the research on the neural correlates of social exclusion relies on the sum of these four scales as an index of ostracism distress. For this scale, lower scores indicated greater distress.
Electrophysiological Recording and Preprocessing
Using standard procedures, a high-density EEG was recorded from 128 Ag/AgCL electrodes (Electrical Geodesics Incorporated (EGI), Inc.) with Netstation v.4.2 software (EGI, Inc.) and high impedance amplifiers, sampled at 250hz (.1 Hz high pass, 100 Hz, low pass). All electrodes were referenced to Cz for recording. Before beginning, all impedances were at or under 40k ohms. The E-prime v.1.2 (Psychology Software Tools, Inc.) software package controlled the stimulus presentation.
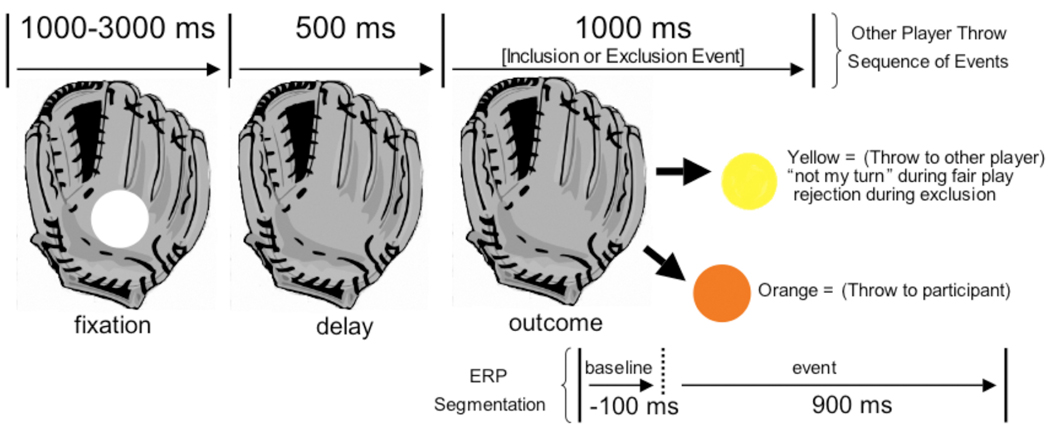
Prior to segmentation, EEG data were low pass filtered at 30 Hz. ERPs were derived only when the ball reappeared after leaving the glove of the cyber-players, but before traveling on the screen (100 ms baseline, 900 ms post stimulus onset, see Figure 1). The EEG for each trial was corrected for blinks and eye movements (Gratton, Coles, & Donchin, 1983). Artifact rejection was used to eliminate ERPs contaminated by movement and eye artifacts. For data to be included in the analyses, a total of no more than 20 channels could be interpolated, per event. The mean number of trials included across the “not my turn” and rejection trial types was comparable t(33) = 1.71, ns (M=30.52, SD = 5.32 vs. M = 29.12, SD = 5.64). The minimum number of trials per average was 16. Averaged data were baseline-corrected by subtracting the average microvolt value across the 100 msec prestimulus interval from the post-stimulus segment. After artifact rejection, the single trial data were re-referenced from the vertex (Cz) to an average reference of all electrodes. The trial by trial data were then averaged separately for each of the 128 electrode sites and separately for the “not my turn” and “rejection” conditions.
Figure 1. Schematic diagram of a Cyber-player’s Glove and Events.
The ball arrives at one of the cyber-players gloves, remains for a fixation period, disappears (delay), and reappears as an outcome event (yellow ball for a “not my turn” event during fair play and a rejection event during exclusion, orange ball indicates a throw to the participant). Ball color and path indicate the type of event. ERPs are segmented on the outcome event.
Analytic Approach
The goals of this study were as follows: first, we examined scalp recorded neural responses for “not my turn” events versus rejection events in a Cyberball social exclusion paradigm in middle childhood to determine if slow wave neural responses differed across the two types of events. Second we related effects of scalp-derived ERP responses to child-reported ostracism distress. Third, we applied GeoSource source estimation software to examine potential neural generators of scalp-derived ERPs based on the existing neuroimaging literature in adolescents and adults. Finally, we examined the relation between putative sources of rejection-related neural activity and child-reported ostracism distress.
Source Analysis
Source estimation was accomplished using GeoSource, Version 1.0.1, electrical source imaging software (Electrical Geodesics, Eugene, OR). Following Luu, Tucker and colleagues, (Luu, Shane, Pratt, & Tucker, 2009; Poulsen, Luu, Crane, Quiring, & Tucker, 2009), neural source estimates of scalp derived ERPs were computed using the distributed linear inverse minimum norm approach with LAURA constraints (Grave de Peralta Menendez, Murray, Michel, Martuzzi, & Gonzalez Andino, 2004). Within GeoSource, the forward model applies a finite difference model (FDM) for accurate computation of the lead field in relation to head tissues. For tissue segmentation, the FDM employs a high-resolution T1-weighted MRI image and whole-head computed tomography (CT) scan. Tissue compartments in the default FDM used here were constructed from the Colin27 MRI average (Holmes et al., 1998). A whole-head CT scan of this same individual, whose Talairach-transformed head closely matches the Montreal Neurological Institute average MRI (MNI305), was also used. Within the FDM, conductivity values were 0.25 S/m (Siemens/ meter) for the brain, 1.8 S/m for the cerebral spinal fluid, 0.018 S/m for the skull, and 0.44 S/m for the scalp (see (Ferree, Eriksen, & Tucker, 2000). Distributed dipole placement relied on the probabilistic map of the MNI305 average brain. Within this probabilistic atlas, cortical gray matter was parceled into 7 mm voxels. Each dipole served as a source location with three orthogonal orientations, resulting in a total of 2,447 source dipole triplets. Resulting estimated source activation voxel intensities and orientations were displayed superimposed on MRI slice views of the Talairach-transformed Colin27 brain. Source regions corresponding to the scalp ERP effects were selected based on previously published effects in the neuroimaging literature on ostracism and social exclusion (Eisenberger et al., 2003, 2007, Kross, et al., 2007, Masten, et al., 2009). We focused specifically on the slow wave neural activity previously identified by Crowley et al. (2009) and relied on a temporal PCA window derived from this dataset for rejection events. From the time periods identified by the temporal PCA, source waveforms within each Brodmann’s area (BA) were generated from the above models. These source waveforms were then analyzed using mean amplitude measures (nanoamperes) within Broadmann areas, averaged over the time course of the slow-wave. Specifically we focused on BA 31 (dorsal posterior cingulate cortex), BA32 (dorsal anterior cingulate cortex), BA23 (ventral posterior cortex), BA25 (subgenual cortex), BA47 (ventrolateral PFC) and insula.
Results
Preliminary Analyses
Prior to examining ERP ostracism relations, we examined sex and age differences in ostracism distress. Females and males did not significantly differ on the Need Threat assessment of ostracism distress (t(31) = .16, ns). Age was unrelated to the Need Threat assessment of ostracism distress (r = .15, ns). We report on relations between age and ERP data below, with no significant relations observed.
ERP Analysis
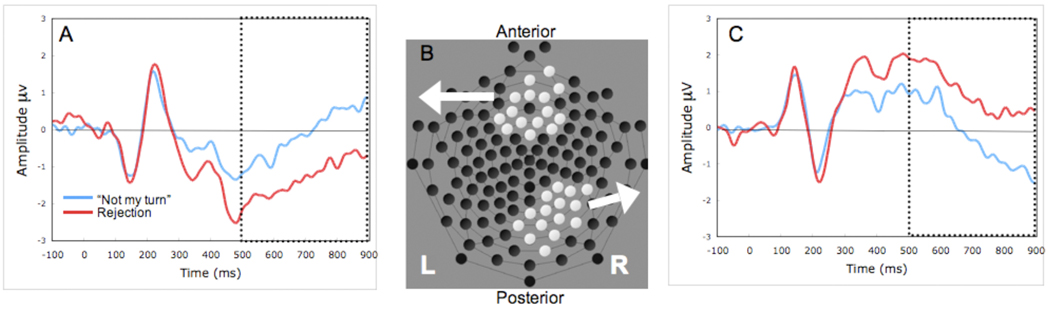
We used temporal principal component analysis (PCA) with a correlation matrix and varimax rotation, conducted on 129 channels of EEG data for two conditions (“not my turn”, rejection). Loadings higher than 0.4 were used to determine the time interval of each factor following Molfese (Molfese, Nunez, Seibert, & Ramanaiah, 1976). The temporal PCA yielded four components accounting for 77.97% of the variance in the ERP signal. Temporal Factor 1 accounted for 50.10%, of the variance and consisted of a slow wave apparent in time interval 500–900 ms (peak time 804 ms). Temporal Factor 2 accounted for 15.39% of the variance and appeared as a 264–656 ms time interval (peak time 420 ms). Temporal Factor 3 accounted for 7.47% and appeared as a 176–300 ms time interval (peak time 224 ms). Temporal Factor 4 accounted for 5.00% of the variance and appeared as a 112–184 ms time interval (peak time 136 ms). Next, a spatial principal component analysis (using the correlation matrix) followed by Varimax rotation was conducted on the spatial dimension of the time interval for each temporal factor. Factor loadings higher than 0.7 or lower than −0.7 were used to determine the effective channels of each spatial factor. Two factors were extracted from the spatial PCA with this cutoff. Factor 1 corresponded to 15 channels in the medial frontal region (see Figure 2, highlighted channels, anterior). Factor 2 corresponded to 15 channels in the occipital-parietal region (see Figure 2, highlighted channels, posterior).
Figure 2. ERP waveforms, and scalp topography for rejection events (red) and “not my turn” events (blue).
(A) Average ERP waveforms at medial frontal electrodes (spatial PCA derived, temporal PCA window 500 to 900 ms, dotted box). (B) 128-electrode geodesic sensor layout, spatial PCA derived electrodes for medial frontal and occipital-parietal spatial factors. (C) Average ERP waveforms at occipital-parietal electrodes (spatial PCA derived, temporal PCA window 500 to 900 ms, dotted box).
Subsequent ERP analyses were based on the separate grand averages of the channels for the two PCA-derived spatial factors (medial frontal, occipital-parietal), for the two conditions (“not my turn”, rejection). A temporal PCA was used to window the data. Grand averages for the ERPs derived from spatial clustering are displayed in Figure 2. We examined differences in neural response (mean voltage) to social exclusion with 2 × 2 repeated-measures ANOVAs for condition ("Not my turn", rejection) × location (medial frontal, occipital-parietal), separately for each of the temporal PCA windows.
The effect for Factor 1 (slow wave, 500–900 ms) was completely accounted for by a condition × location interaction, F(1, 32) = 8.09, p < .01, Partial η2 = .20, Observed Power = .79. There were no significant main effects. Paired-sample t-tests were used to further examine the interaction effect. At the frontal region, the rejection condition (M = − 1.31 µV, SE = .41) had a greater negativity than the "Not my turn" condition (M = −.12 µV, SE = .35), t(32) = 2.94, p < .01; at the occipital-parietal region, the rejection condition (M = .97 µV, SE = .49) had a greater positivity than the "Not my turn" condition (M =−.21 µV, SE = .40), t(32) = −2.44, p < .05.
There were no significant main effects for Factor 2 (frontal negativity, 264–656 ms). Like Factor 1, the effect for Factor 2 was also completely accounted for by a condition × location interaction, F(1, 32) = 4.36, p < .05, Partial η2 = .12, Observed Power = .53, which appeared frontally as a negativity, maximal at 420 ms. Paired sample t-tests were again used to decompose the interaction effect. At the medial frontal region, the condition difference (“not my turn” vs. rejection) was not significant, t(32) = 1.87, p = .07, ns. At the occipital-parietal region, "Not my turn" (M = .29 µV, SE = .49) had less positive amplitude than the rejection condition (M = 1.33 µV, SE = .57), t(32) = −2.14, p < .04.
For factor 4 (112 – 184 ms) there was a main effect of region, F(1, 32) = 23.29, p < .001, Partial η2 = .421, Observed Power = .99. Paired sample t-test showed that ERPs at the medial frontal region (M = −.92 µV, SE = .18) were more negative than the ERPs at the occipital-parietal region (M = 1.15 µV, SE = .26), t(32) = −4.83, p < .001. There were no significant effects for factor 3 (176–300 ms).
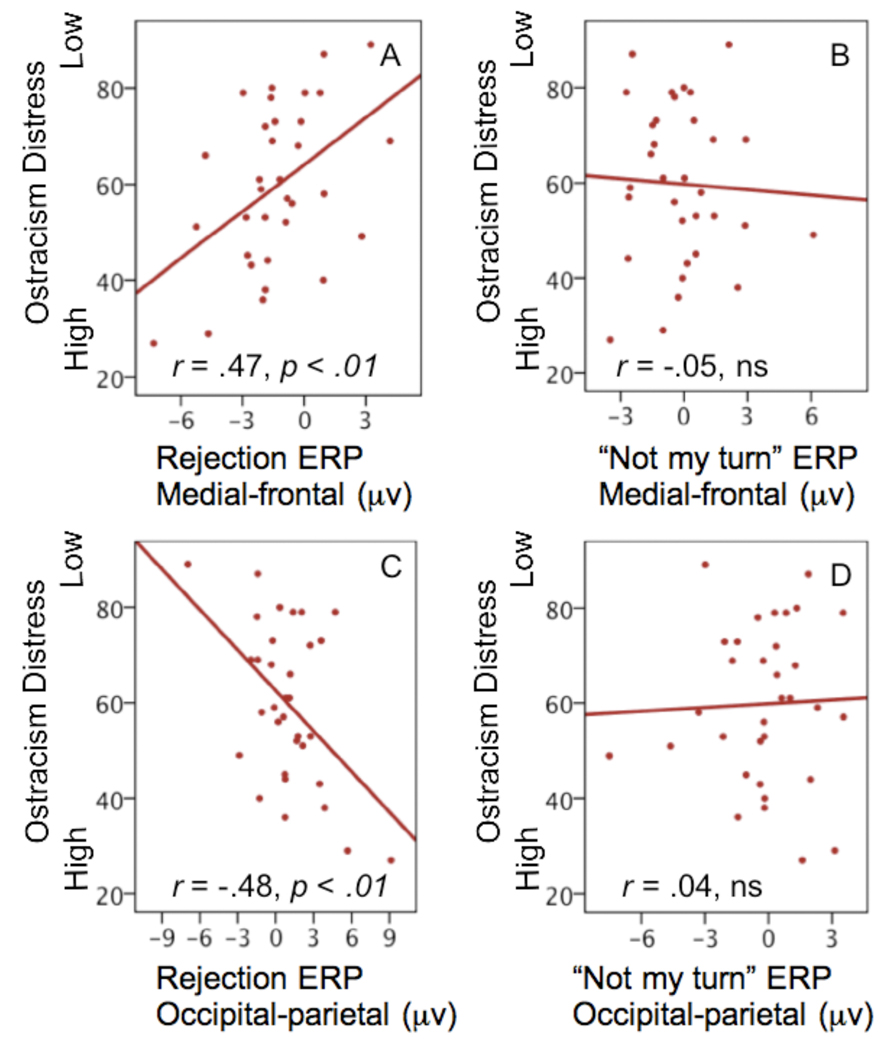
We next used Pearson correlations to examine the relation between self-reported ostracism distress and mean voltage in the rejection and “not my turn” conditions, for each of the four PCA-derived temporal windows, and the medial frontal and occipital-parietal regions. Based on our previous work (Crowley, Wu, McCarty et al., 2009) we hypothesized that the late slow wave would be most strongly associated with ostracism distress for the rejection event. The PCA-derived window for the slow wave is outlined for the medial frontal and occipital parietal ERPs in Figure 2. As predicted, the late slow wave for the rejection event was strongly associated with ostracism distress, (r = .47, p < .01 (medial frontal), r = −.48, p < .01 (occipital-parietal). Applying a Bonferroni correction these two a priori predictions remain significant at p < .025. For descriptive purposes, all correlations, uncorrected, are presented in Table 1. Providing discriminant validity for these relations, “not my turn” events were unrelated to ostracism distress (r=−.05 and r = .04, respectively) for the slow wave window. Scatter plots of these four relations are presented in Figure 3. No other correlations were significantly related to ostracism distress, but the mean amplitude for temporal factor 2 (264 – 656 ms) was related to ostracism distress as a trend, r = .33, p = .06 (medial frontal), r = −.31, p = .07 (occipital parietal).
Table 1.
Correlations Between Ostracism Distress and Mean Voltages for Temporal-Spatial Factors
| Condition | Temporal Factor |
Spatial Factor 1 Frontal-central |
Spatial Factor 2 Occipital-parietal |
|---|---|---|---|
| "Not my turn" | temporal factor 1 500 – 900 ms |
r = −.05 p = .80 |
r = .04 p = .84 |
| temporal factor 2 264 – 656 ms |
r = −.25 p = .15 |
r = .22 p = .23 |
|
| temporal factor 3 176 – 300 ms |
r = −.09 p = .63 |
r = .15 p = .40 |
|
| temporal factor 4 112 – 184 ms |
r = −.09 p = .62 |
r = .15 p = .40 |
|
| Rejection | temporal factor 1 500 – 900 ms |
r = .47** p < .01 |
r = −.48** p < .01 |
| temporal factor 2 264 – 656 ms |
r = .33 p = .06 |
r = −.320 p = .07 |
|
| temporal factor 3 176 – 300 ms |
r = .12 p = .51 |
r = −.25 p = .16 |
|
| temporal factor 4 112 – 184 ms |
r = .13 p = .47 |
r = −.13 p = .47 |
Figure 3. Scatter plot for ostracism distress scores (Y-axis) and mean slow wave data for rejection events and “not my turn” events (X-axes).
(A) Scatter plot of mean slow wave data for rejection ERP and ostracism distress at the medial frontal region. (B) Scatter plot of mean slow wave data for “not my turn” ERP and ostracism distress at the medial frontal region. (C) Scatter plot of mean slow wave data for rejection ERP and ostracism distress at the occipital-parietal region. (D) Scatter plot of mean slow wave data for “not my turn” ERP and ostracism distress at the occipital-parietal region.
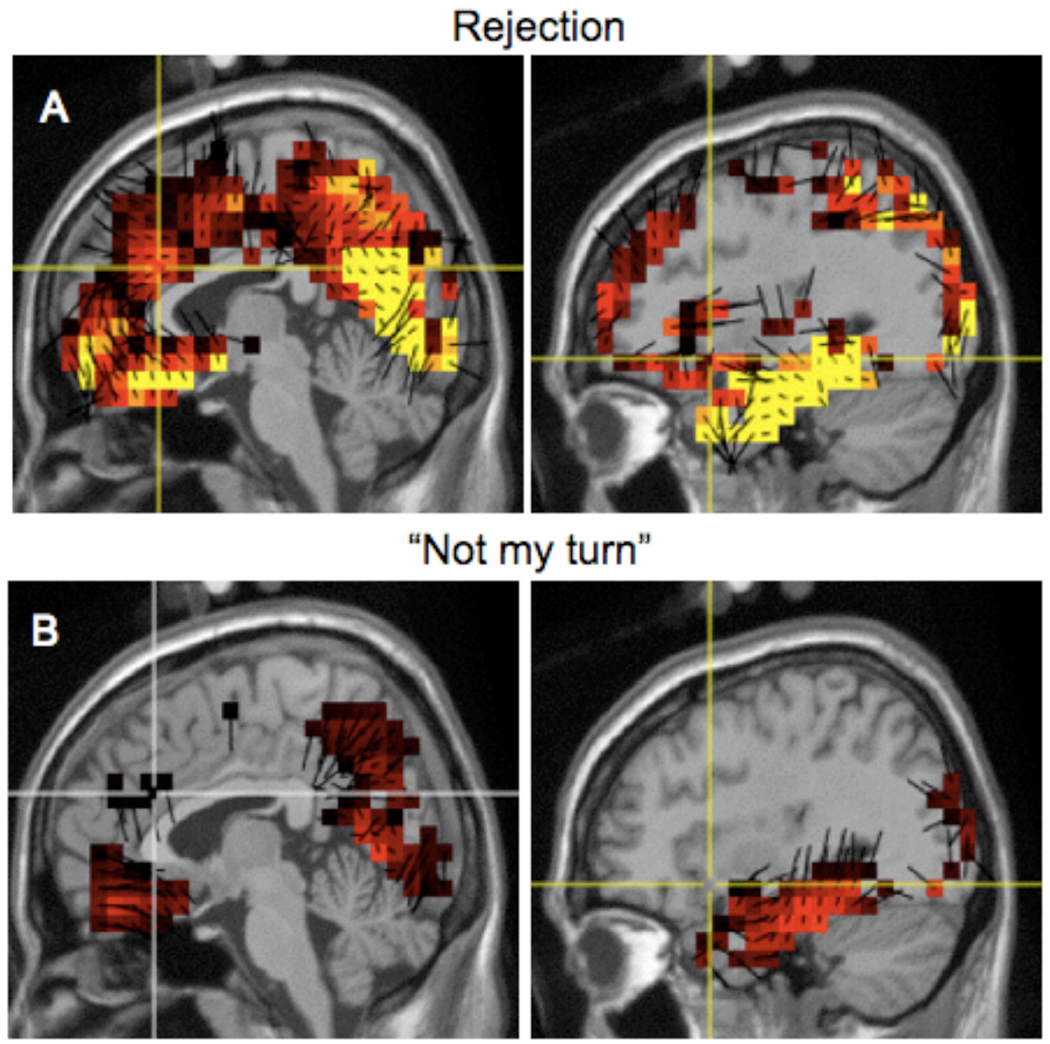
Source analysis located generators of PCA-derived slow wave neural activity (whole head analysis), associated rejection and “not my turn” events. The source distribution for rejection and “not my turn” events are presented in Figure 4. For a priori Brodmann areas previously identified to respond to social exclusion (23, 25, 31, 32, 47, Insula) and current source densities, we conducted separate mixed-models ANOVAs for each brain region of interest, in a 2 (hemisphere: left vs. right) × 2 (condition: reject vs. “not my turn”) design. The restricted maximum likelihood (REML) estimator was used in combination with an unstructured covariance matrix, which has the advantage of estimating the full covariance matrix from the data. Analyses were conducted in the SAS System version 9.2, using PROC MIXED. No hemisphere effects were observed, thus only condition effects are reported here in Table 2. Significant condition effects were found for Brodmann area 23 (ventral posterior cingulate), area 25 (subgenual cortex), and insular cortex. Exploratory analyses using a compound symmetric covariance matrix (covariance structure used by many ANOVA programs) suggested differences in Brodmann area 31 (posterior cingulate cortex) and area 32 (anterior cingulate cortex).
Figure 4. Geosource source analysis.
(A) Source distribution for the mean slow wave (500–900 ms) during rejection (medial sagittal view, left panel, lateral sagittal view, right panel). (B) Source distribution for the mean slow wave (500–900 ms) during “Not my turn” (medial sagittal view, left panel, lateral sagittal view, right panel). The brain maps are oriented from the ACC (left panels) and the right insula (right panels).
Table 2.
Source Analysis Results Comparing Rejection and “Not my turn” Events for A Priori Regions of Interest
| Region | F-value | p | Result |
|---|---|---|---|
| BA-23 ventral posterior cingulate | F (1, 32) = 5.84 | p < 0.05 | condition effect |
| BA-25 subgenual cortex | F (1, 32) = 4.56 | p < 0.05 | condition effect |
| BA-31 posterior cingulate | F (1, 32) = 1.52 | p = 0.23 | no condition effect |
| BA-32 anterior cingulate | F (1, 32) = 1.71 | p = 0.20 | no condition effect |
| insula | F (1, 32) = 5.98 | p < 0.05 | condition effect |
| BA-47 venterolateral PFC | F (1, 32) = 2.45 | p = 0.12 | no condition effect |
| Exploratory using Compound Symmetric covariance matrix | |||
| BA-31 posterior cingulate | F (1, 32) = 4.30 | p < 0.05 | condition effect |
| BA-32 anterior cingulate | F (1, 32) = 4.62 | p < 0.05 | condition effect |
Discussion
This study was the first to use event related potentials to examine social exclusion in middle childhood. We employed high-density EEG and a task that explicitly isolated the event-related aspects of Cyberball to allow for event-related potential assessment. We examined ERP differences for neural events that were identical in terms of their spatial orientation on the screen, but differed in context, specifically comparing “not my turn” events in which the ball did not come to the child in the context of ongoing fair play to rejection events in which the ball did not come to the child in the context of social exclusion. A temporal PCA revealed four time windows of correlated neural activity for the events. A spatial PCA isolated the scalp regions most consistently associated with the neural response to these two types of events. Two cortical regions were identified, one in the medial frontal region, comprised of 15 electrodes, and a second in the occipital-parietal region (left) also comprised of 15 electrodes.
As predicted, a slow wave ERP component beginning at 500 ms and continuing on to the end of the event segmentation (900 ms), was significantly more negative in the medial frontal region for the rejection event versus the “not my turn” event, and showed the opposite pattern for the posterior scalp region examined. In turn, the magnitude of rejection ERP slow wave response strongly tracked the child’s later estimation of how distressing the exclusion experience was overall. A second, earlier posterior ERP component (factor 2), beginning at 264 ms through 656 ms was more positive for the rejection event than for the “not my turn” event. This ERP resembled the P3 (300) component, thought to reflect attentional salience. Thus, we can say that in middle childhood, neural detection of rejection was accomplished between 264 and 656 ms, whereas neural response tracked the child’s global estimation of how distressing the experience was beginning at 500 ms through the 900 ms.
Rejection Events and Slow Wave ERPs
Frontal negative slow waves have been observed in anticipation of various arousing stimuli, including aversive noise (Crowley, Wu, Bailey, & Mayes, 2009; Regan & Howard, 1995) and shocks (Baas, Kenemans, Bocker, & Verbaten, 2002), thought to reflect engagement of evaluative processes. Perhaps in this study, the rejection event itself reflects the evaluation and anticipation by the child that the ball will again be thrown to another person. We observed a late positive slow wave resembling an LPP for rejection in the occipital-parietal region which was again greater in magnitude as compared to the “not my turn” event. Most work on the LPP has focused on central posterior cortical regions, but with static picture paradigms. In that work, unpleasant pictures produce a more positive LPP, which has been interpreted as an emotion-facilitated attention effect (Schupp et al., 2000). Data across the frontal and posterior slow wave components suggest cyberball rejection events engage both evaluation and attention processes. They also suggest an electrical current dipole given the flip in relative polarity for the two types of neural events across regions.
While drawing on past literature on the LPP to inform this current work, have refrained from directly labeling the late slow wave to rejection events as an LPP for three reasons. First, rejection represents a different context from that which has been used to elicit the LPP. Second, rejection events produced an accompanying frontal slow wave. Third, it is likely that rejection events have different neural generators than those that drive the LPP previously reported on.
The results also revealed that the social exclusion induced a slow wave neural response, beginning at about 500 ms after a rejection event, that tracked individual differences in the overall level of distress reported by the child. We observed a strong positive relation between ostracism distress in the frontal ERP slow wave for the rejection ERP such that higher distress was associated with a more negative slow wave in the medial frontal region. The magnitude and direction of this effect converges with our adult work using a Cyberball task with ERPs, though not in terms of exact scalp topography. Providing discriminant validity for the rejection-ostracism effect, neural activity for the “not my turn” event was unrelated to children’s reports of ostracism distress. This makes sense because in ongoing fair play, children were not excluded. Thus, their neural responses would not be expected to track their self-reported distress on the Need Threat Scale.
Rejection Event Salience and Feedback
The ERP corresponding to factor 2 (264–656 ms, peak 420 ms) was significantly more positive for rejection events compared to “not my turn” events in the posterior scalp region resembling a P3. This is surprising since the P3 is most strongly elicited for occurrence of infrequent, task-relevant events (Donchin & Coles, 1988) and compared to the rejection events, the “not my turn” events were more infrequent within the context of fair-play. However P3 effects can occur when the stimuli are particularly salient. For instance among opiate addicts, equiprobable visually presented drug cues elicit a P3 response comparable to that for infrequent stimuli (Lubman, Allen, Peters, & Deakin, 2007). With adaptive value of social bonds for the survival of mammalian species, particularly in humans, perhaps there exists a bias for social rejection events to be particularly “salient”.
In the frontal region for the same time window that captured the P3, we observed an ERP negativity (frontal negativity), which resembled an fERN in terms of spatial location (medial frontal region) and valence for rejection events (statistically a trend), though later temporally than is typically reported (420 vs. 250 ms). Given the role of the medial frontal region and the ACC in feedback monitoring and the nature of the task, with the subject receiving feedback about their inclusion or lack thereof, we expected to observe evidence of a feedback negativity. Indeed, other related work with the error-related negativity (ERN), also localized to the ACC (Holroyd, Dien, & Coles, 1998), has shown that social pressure, such as being evaluated by another person, can amplify the ERP response (Hajcak, Moser, Yeung, & Simons, 2005). The negative deflection we observed for social feedback in Cyberball was delayed compared to a typical fERN, perhaps because of the complexity of the feedback delivered which depends on context (rejection and position on the screen) versus typical fERN studies use a symbol to directly indicate an outcome. As well, the fact that this ERP deflection comes out only as a trend may reflect that expectations for getting the ball likely shift over time during rejection. Initially we would expect larger fERN responses, but after repeated exclusions the fERN could be diminished due to decreased expectations of getting the ball. Lastly, we did observe associations between neural activity in the 264–656 ms window and ostracism distress for both frontal and posterior voltages in this time window though they appeared as statistical trends.
Developmental Considerations
The region of frontal scalp activation reported here diverged from our adult ERP work with this paradigm, suggesting a developmental difference between middle childhood and adulthood (Crowley et al., 2009). In particular, the scalp topography was strongly left-lateralized in adults, covering the left midline and left-frontal region. Greater late positive slow wave neural activity in the anterior left frontal-medial frontal region for rejection events was more pronounced for those who experienced less distress. This effect resembled a finding in adults by Kross and colleagues (Kross et al., 2007) who observed greater left inferior frontal effects with fMRI among low rejection sensitive individuals. Because coping processes of emotion regulation have been associated with lateral and medial prefrontal activation (Ochsner et al., 2004; Phan et al., 2005) we reasoned that these neural processes might reflect the mitigation of the social exclusion experience. We speculate that the difference we are seeing in child and adult scalp topography for rejection events may reflect maturational changes in the frontal lobes, with adults having a greater capacity to recruit a broader frontal network to cope with rejection and exclusion. At the same time, we note a degree of developmental continuity across our adult study (Crowley, Wu, McCarty et al., 2009) and this report on social exclusion in middle childhood, both in terms of the timing of a slow wave neural response associated with rejection events (580–900 ms in adults vs. 500–900 ms, present study) and in terms of the magnitude of the ostracism distress-slow wave association (for the frontal region, r = .62 in adults vs. r = .47, present study). This continuity is to be expected since by middle childhood, children easily perceive psychological distance from a social group in terms of sociometric status and popularity (Asher & Coie, 1990).
Source Modeling Social Exclusion Events
A particular strength of this study was its implementation of source modeling through Geosource software. We relied on past neuroimaging work to guide our selection of regions of interest (Eisenberger et al., 2003, 2007, Kross, et al., 2007, Masten, et al., 2009). Findings generally converge on the neuroimaging work where we observed changes across the “not my turn” events contrasted with rejection events for the subgenual cortex, ventral posterior cortex and insula. A less conservative statistical approach suggested a similar pattern of difference for the anterior cingulate cortex and posterior cingulate cortex, two regions which neighbor the subgenual cortex and ventral posterior cortex. The pattern of differences observed here are consistent with the pattern of scalp potentials we observed. Worthy of note, there are no middle childhood neuroimaging studies of ostracism at the time of this report. Thus, we may have overlooked some regional sources relevant to middle childhood.
Contrasting ERP and fMRI Work with Cyberball
There are several important distinctions between our ERP social exclusion paradigm and the recent fMRI studies that used Cyberball, which are worth bearing in mind. First, our Cyberball paradigm departs in terms of length of task, from that used in the neuroimaging work to date. Notwithstanding modifications to make the task more engaging to children, our fair play phase includes 2/3 more trials (108 vs. 60 in Masten et al., 2009) and took about 9 minutes vs. 2 minutes. It is unclear whether this would lead to more intense or less intense feelings of distress later on. Our exclusion portion of the task included 47 trials and lasted 7 minutes versus the version used by Matsen et al. (2009), which had 30 trials and lasted 1 minute. Second, we relied on partial exclusion rather than full exclusion. This could also mitigate the exclusion experience to some degree, but is a necessary modification in an ERP assessment to maintain attention during the exclusion portion of the task. Third, we are examining actual neural activity with an ERP versus inferring neural activity based on hemodynamic response. Both are reliable and valid techniques, but they need not always converge. Fourth, the previous fMRI studies of social exclusion relied on block designs, examining the overall inclusion experience contrasted with the overall exclusion experience. Here we focused just on rejection events or “not my turn” events. It is likely that there are perceptual and regulatory processes at work over the later time course. This report isolates the initial neural reaction to rejection events and their relations with distress.
Study Limitations
This study comes qualified with several limitations. First, we report on a relatively small sample here. While a 5-year age span such as we have examined would be trivial in most typical adult populations, the changes occurring from 8–12 years reflect a dramatic shift in cognitive and perspective taking abilities as well as a transition to greater independence and to middle school. Thus, a more comprehensive examination of age-related changes in neural response to social exclusion would require greater numbers within each age group. Second, the experiment did not have the typical counterbalanced control or intermixed trial types in that “not my turn” events always preceded rejection rejection events, occurring in separate blocks. This was done because of the need to create a realistic situation in which subjects felt excluded. If exclusion preceded inclusion, subjects might have unnecessarily carried this expectation into the “not my turn” trials. Because ostracism distress, as assessed by the Need Threat Scale, tracks neural responding for rejection events (convergent validity), but is uncorrelated with neural responding for “not my turn” events (discriminant validity), we believe we have provided strong evidence for our design choice. Third, while the Need Threat Scale is a reliable and valid instrument and has been primary outcome instrument for research with Cyberball paradigms, it is vulnerable to the same types of inference problems as other self reports, including social desirability, capacity to reflect on internal states and that the assessment occurs “post-exclusion”. Other corroborating indices such as the startle response might be used to provide independent evidence of affective state as has been done in rejection sensitivity research (Downey, Mougios, Ayduk, London, & Shoda, 2004). Fourth, and related to the idea of individual differences in response to exclusion, we examined the neural correlates of distress here, but other constructs such as active coping and reappraisal might provide additional insight into the neural response to rejection events. Lastly, we drew on an EEG assessment to study only time domain effects with ERPs. Neural oscillatory activity, remains a rich, but largely unexplored area for the social neurosciences and a more comprehensive picture of neural response to ostracism and rejection could make use of the frequency domain (Peterson, Gravens, & Harmon-Jones, in press).
Conclusion
In summary, this is the first study to examine event-related neural response to social exclusion in middle childhood. The excellent temporal resolution of the ERP allowed us to move research on ostracism and social exclusion to focus specifically on neural activity for rejection events. Our findings suggest that rapidly occurring neural responses to social exclusion events are linked to individual differences in ostracism-related distress in middle childhood. In particular, greater magnitude of frontal late negative potential and greater magnitude of a posterior late positive potential were both associated with greater distress. We observed a pattern of distress-ERP relation in middle childhood similar to our adult work, suggesting some developmental continuity. However the pattern of frontal scalp activity differed from our past work in adults, with neural response in children more localized along the midline. Source modeling suggested that cortical regions sensitive to social exclusion in recent fMRI work (subgenual cortex, ventral anterior cingulate, insula) are also differentially active at the level of the ERP slow wave. Here we focused only on middle childhood. Future work should include adolescent and adult samples for a direct examination of developmental trends.
Acknowledgements
This research was supported by the Bial Foundation (MJC), a NARSAD Young Investigator Award (MJC); NIDA grants RO1-DA-06025 (LCM), DA-017863 (LCM) and KO5 (LCM), and a grant from the Gustavus and Louise Pfeiffer Research Foundation (LCM). This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The authors thank David Reiss and Max Greger-Moser for their thoughtful comments on this manuscript.
References
- Asher SR, Coie JD, editors. Peer rejection in childhood. New York, NY: Cambridge University Press; 1990. [Google Scholar]
- Baas JM, Kenemans JL, Bocker KB, Verbaten MN. Threat-induced cortical processing and startle potentiation. Neuroreport. 2002;13:133–137. doi: 10.1097/00001756-200201210-00031. [DOI] [PubMed] [Google Scholar]
- Crick NR, Dodge KA. A review and reformulation of social information-processing mechanisms in children's social adjustment. Psychological Bulletin. 1994;115:74–101. [Google Scholar]
- Crowley MJ, Wu J, Bailey CA, Mayes LC. Bringing in the negative reinforcements: the avoidance feedback-related negativity. Neuroreport. 2009;20:1513–1517. doi: 10.1097/WNR.0b013e32832ff2f5. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, McCarty ER, David DH, Bailey CA, Mayes LC. Exclusion and micro-rejection: event-related potential response predicts mitigated distress. Neuroreport. 2009;20:1518–1522. doi: 10.1097/WNR.0b013e328330377a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Annotation: Recent research examining the role of peer relationships in the development of psychopathology. Journal of Child Psychology and Psychiatry. 2001;42:565–579. [PubMed] [Google Scholar]
- Dodge KA, Lansford JE, Burks VS, Bates JE, Pettit GS, Fontaine R, et al. Peer rejection and social information-processing factors in the development of aggressive behavior problems in children. Child Development. 2003;74:374–393. doi: 10.1111/1467-8624.7402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:355–372. [Google Scholar]
- Downey G, Lebolt A, Rincón C, Freitas AL. Rejection sensitivity and children's interpersonal difficulties. Child Development. 1998;69:1074–1091. [PubMed] [Google Scholar]
- Downey G, Mougios V, Ayduk O, London BE, Shoda Y. Rejection sensitivity and the defensive motivational system: insights from the startle response to rejection cues. Psychological Science. 2004;15:668–673. doi: 10.1111/j.0956-7976.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Downey G, Walker E. Social cognition and adjustment in children at risk for psychopathology. Developmental Psychology. 1989;25:835–845. [Google Scholar]
- Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126:132–138. doi: 10.1016/j.pain.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Eriksen KJ, Tucker DM. Regional head tissue conductivity estimation for improved EEG analysis. Ieee Transactions on Biomedical Engineering. 2000;47:1584–1592. doi: 10.1109/10.887939. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Medial prefrontal cortex and rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R, Murray MM, Michel CM, Martuzzi R, Gonzalez Andino SL. Electrical neuroimaging based on biophysical constraints. Neuroimage. 2004;21:527–539. doi: 10.1016/j.neuroimage.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Grusec JE, Lytton H. Social development: History, theory, and research. New York, NY: Springer-Verlag Publishing; 1988. [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neuroscience Letters. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Jiang XL, Cillessen AHN. Stability of continuous measures of sociometric status: A meta-analysis. Developmental Review. 2005;25:1–25. [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal Cognitive Neuroscience. 2007;19:945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Ladd GW. Peer rejection, aggressive or withdrawn behavior, and psychological maladjustment from ages 5 to 12: an examination of four predictive models. Child Development. 2006;77:822–846. doi: 10.1111/j.1467-8624.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Leary MR, Kowalski RM, Smith L, Phillips S. Teasing, Rejection, and Violence: Case Studies of the School Shootings. Aggressive Behavior. 2003;29:202–214. [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence of the motivational salience of drug cues in opiate addiction. Psychological Medicine. 2007;37:1203–1209. doi: 10.1017/S0033291707009932. [DOI] [PubMed] [Google Scholar]
- Luu P, Shane M, Pratt NL, Tucker DM. Corticolimbic mechanisms in the control of trial and error learning. Brain Research. 2009;1247:100–113. doi: 10.1016/j.brainres.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese DL, Nunez V, Seibert SM, Ramanaiah NV. Cerebral asymmetry: changes in factors affecting its development. Annals of the New York Academy of Sciences. 1976;280:821–833. doi: 10.1111/j.1749-6632.1976.tb25545.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroscience. Feeling the pain of social loss. Science. 2003;302:237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Peterson CK, Gravens LC, Harmon-Jones E. Asymmetric frontal cortical activity and negative affective responses to ostracism. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsq027. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Poulsen C, Luu P, Crane SM, Quiring J, Tucker DM. Frontolimbic activity and cognitive bias in major depression. Journal of Abnormal Psychology. 2009;118:494–506. doi: 10.1037/a0015920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan M, Howard R. Fear conditioning, preparedness, and the contingent negative variation. Psychophysiology. 1995;32:208–214. doi: 10.1111/j.1469-8986.1995.tb02950.x. [DOI] [PubMed] [Google Scholar]
- Reijntjes A, Dekovic M, Telch MJ. Support for the predictive validity of the SASC-R: linkages with reactions to an in vivo peer evaluation manipulation. Journal of Anxiety Disorders. 2007;21:903–917. doi: 10.1016/j.janxdis.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Reijntjes A, Dekovic M, Vermande M, Telch MJ. Children's feedback preferences in response to an experimentally manipulated peer evaluation outcome: the role of depressive symptoms. Journal of Abnormal Child Psychology. 2007;35:497–507. doi: 10.1007/s10802-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes A, Stegge H, Terwogt MM, Kamphuis JH, Telch MJ. Children's coping with in vivo peer rejection: an experimental investigation. Journal of Abnormal Child Psychology. 2006a;34:877–889. doi: 10.1007/s10802-006-9061-8. [DOI] [PubMed] [Google Scholar]
- Reijntjes A, Stegge H, Terwogt MM, Kamphuis JH, Telch MJ. Emotion regulation and its effects on mood improvement in response to an in vivo peer rejection challenge. Emotion. 2006b;6:543–552. doi: 10.1037/1528-3542.6.4.543. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2009;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods. 2006;38:174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]