Abstract
The core transcriptional regulatory circuitries are important for controlling stem cell self-renewal and differentiation. Nuclear receptors provide an ideal model to regulate gene expression in both ligand-dependent and ligand-independent manners. Recent studies of regulatory events by nuclear receptors in neural stem cells, embryonic stem cells, and induced pluripotent stem cells (iPSCs), provided unique insights into mechanisms of stem cell regulation and provided invaluable resources for regenerative medicine. Nuclear receptors have been shown to be key players in stem cell self-renewal, pluripotency, and reprogramming. We summarize recent progress of studies on nuclear receptors in stem cell field as well as the potential therapeutic implications of these nuclear receptors and their cognate ligands. These studies not only uncover molecular mechanisms of stem cell regulation, but also provide unique opportunities for drug discovery.
Keywords: Nuclear receptor TLX (NR2E1), histone modification, microRNAs, Wnt, neural stem cells, embryonic stem cells, induced pluripotent stem cells (iPSCs), drug discovery
1. Introduction
Neural stem cells are tissue-specific multipotent stem cells, which have the capacity to differentiate into all three major neural cell types and have the potential to be used for cell-replacement therapy in the treatment of nerve injury, stroke, and neurodegenerative diseases, such as Parkinson's disease, Huntington's disease, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS).
Human embryonic stem cells are established from early embryos and can be cultured over long periods of time while maintaining pluripotency to differentiate toward diverse cell fate. These properties have led to the expectations that human embryonic stem cells can be used as models to understand disease mechanisms, to screen for effective and safe drugs, and to treat patients with various diseases and injuries, such as neurodegenerative diseases and spinal cord injury [1].
Recently, researches on human induced pluripotent stem cells (iPSCs) have rapidly evolved. Human iPSCs were originally generated through viral transduction of four transcription factors (Oct4, Sox2, Klf4 and c-Myc) into human fibroblasts [2, 3]. In addition to fibroblasts, B lymphocytes, hepatocytes and gastric epithelia cells can also be converted to iPSCs using the same aforementioned transcription factors [4, 5]. The development of iPSCs could circumvent the ethical concerns associated with the use of human embryonic stem cells that are derived from human embryos. Moreover, iPSCs, in combination with gene therapy, have been used to correct the human sickle hemoglobin allele in mice and to correct human Fanconi anemia defect in human cells successfully [6, 7]. Human diseased iPSCs from specific patients will enable insights into disease pathogenesis, offer a platform for drug discovery, and provide genetically identical cells for cell replacement therapy.
Nuclear receptors represent a superfamily of ligand-dependent transcription factors that govern aspects of development, reproduction, and metabolic functions. Included in this family are the classical steroid receptors, adopted orphan receptors, and orphan receptors, the ligand of which are not identified yet. In this review, we will highlight the recent discoveries on nuclear receptor functions in stem cells, with an emphasis on the therapeutic potential of nuclear receptor ligands in neural stem cells and iPSCs.
2. Nuclear receptors in neural stem cells
Neural stem cells possess two features: self-renewal and the capability to differentiate into all three major neural cell types, neurons, astrocytes, and oligodendrocytes. Several nuclear receptors have been shown to be expressed in mouse brains and play critical roles in neurogenesis. These nuclear receptors include TLX (Tailess homolog, NR2E1), LXR (Liver X receptor, NR1H2 and NR1H3), RARβ (Retinoic acid receptor β, NR1B2) , RXRs (Retinoid X Receptor, NR2B1, NR2B2 and NR2B3), GCNF (Germ Cell Nuclear Factor 1, NR6A1), TRs (Thyroid Hormone Receptor, NR1A1 and NR1A2 ), PPARγ (Peroxisome Proliferator Activated Receptor γ, NR1C3), GR (Glucocorticoid Receptor, NR3C1), ER (Estrogen Receptor, NR3A1 and NR3A2), and NURR1 (NUR-Related protein 1, NR4A2), [8-11]. Here we focus on the recent progress from studies on TLX, LXR, and RAR in neural stem cells of the mammalian brain and highlight the potential of targeting these nuclear receptors for regenerative medicine.
2.1 TLX in neural stem cells
The orphan nuclear receptor TLX is expressed exclusively in the mammalian central nervous system [12, 13]. In adult mammalian brains, TLX is highly expressed in the two adult neurogenic areas, the subgranular zone of the hippocampal dentate gyrus and the subventricular zone of lateral ventricles.
TLX has been demonstrated to play a critical role in maintaining neural stem cell self-renewal in both developing and adult brains [12, 14-19]. At embryonic stages, TLX is strictly expressed in neural stem cells of the ventricular zone, with a peak of expression at E13.5 when neurogenesis also peaks. Deletion of the Tlx gene at embryonic stages results in significant thinning of the neocortex. Considerable decrease in the number of neural progenitor cells and reduced proliferative capability of neural progenitors is also evident in the germinal regions of embryonic Tlx-null brains [15]. The embryonic defect in the Tlx-null brain is due to prolonged cell cycles and increased cell cycle exit of embryonic neural progenitors. Transient knockdown of Tlx by in utero electroporation led to a premature cell cycle exit and precocious differentiation of neural stem cells. These findings support a critical role for TLX in controlling cell cycle progression of neural stem cells in the developing brain [15]. TLX has also been shown to be required for regulating the timing of embryonic neurogenesis in the cortex [16].
Adult Tlx knockout mice have significantly smaller forebrains [20] and severe retinopathies [21-23]. These mice exhibit cortical hypoplasia, limbic system abnormalities, cognitive impairment, and abnormal social behaviors, such as aggressive violence (Chiang and Evans, 1997; Monaghan et al., 1997; Roy et al., 2002; Young et al., 2002). Introduction of human Tlx gene was able to correct the defective phenotypes caused by deletion of the Tlx gene in mice (Abrahams 1995). Sequence analysis revealed that some Tlx mutations are associated with cortical and psychiatric disorders in patients [24, 25].
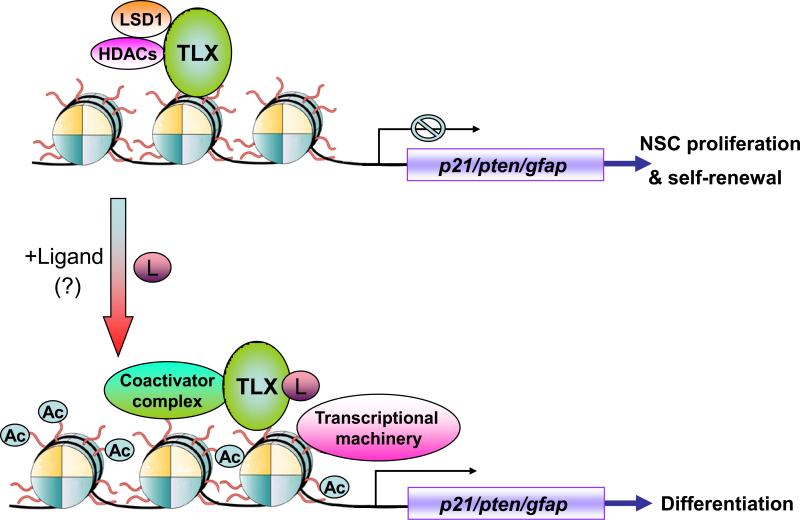
TLX is an essential regulator of neural stem cell maintenance and self-renewal in the adult mammalian brain [14]. While the TLX-expressing cells can proliferate, self-renew and differente into all three major neural cell types in vitro, the Tlx-null cells isolated from adult Tlx-null brains failed to proliferate. Amazingly, reintroducing TLX into Tlx-null cells rescued their ability to proliferate and self-renewal [14]. Recently, the TLX-expressing cells have been identified as type B neural stem cells in the subventricular zone of adult mouse brains [26]. At molecular level, TLX regulates neural stem cell self-renewal by repressing the cyclin-dependent kinase inhibitor p21 and the tumor suppressor pten expression, through epigenetic control [27] (Figure 1). TLX has been shown to interact with histone deacetylase 5 (HDAC5) to regulate p21 and pten gene expression [17]. Both knockdown of HDAC expression or inhibition of HDAC activity led to marked induction of p21 and pten gene expression and reduced neural stem cell proliferation [17]. The HDAC inhibitors valproic acid and trichostatin A have also been shown to reduce the proliferation of neural progenitor cells in the dentate gyrus of adult mouse hippocampus [28].
Figure 1.
Regulation of neural stem cell (NSC) proliferation and differentiation by TLX through epigenetic modulation. TLX recruits histone deacetylases (HDACs) and the lysine-specific histone demethylase 1 (LSD1) to the promoters of its target genes, such as the cyclin-dependent kinase inhibitor, p21, and the tumor suppressor gene, pten, to repress their expression, which in turn maintains neural stem cells in the undifferentiated and self-renewable state. Potential TLX ligands (L) may trigger release of the corepressor complex and lead to the recruitment of a coactivator complex, to activate TLX target genes, which in turn lead to inhibition of cell proliferation and induction of differentiation.
Another epigenetic regulator, the lysine specific demethylase 1 (LSD1), has also been shown to interact with TLX in neural stem cells recently [29]. LSD1 forms a complex with TLX and HDAC5 on the promoter of TLX target genes, p21 and pten, in neural stem cells. As a result, knockdown of LSD1 expression by small RNA interference led to dramatically increased expression of p21 and pten genes. Furthermore, knockdown of LSD1 gene expression in the hippocampus of adult mouse brains, via siRNA expressed by a lentiviral vector, resulted in marked reduction in the proliferation of neural progenitor cells in the subgranular zone of the hippocampus [29]. Treatment with the LSD1 inhibitors, pargyline and tranylcypromine, also caused cell proliferation defect in the hippocampal dentate gyrus of adult mouse brains, suggesting epigenetic regulation of neural stem cells in adult brains [29]. Targeting the interaction between TLX and HDAC/LSD1 may be used to promote neural stem cell differentiation and provide potential avenues for the development of pharmacological tools for the treatment of neurodegenerative diseases. For example, peptides that disrupt TLX-HDAC/LSD1 interactions may trigger neuronal differentiation and serve as drug candidates for the generation of specific neurons.
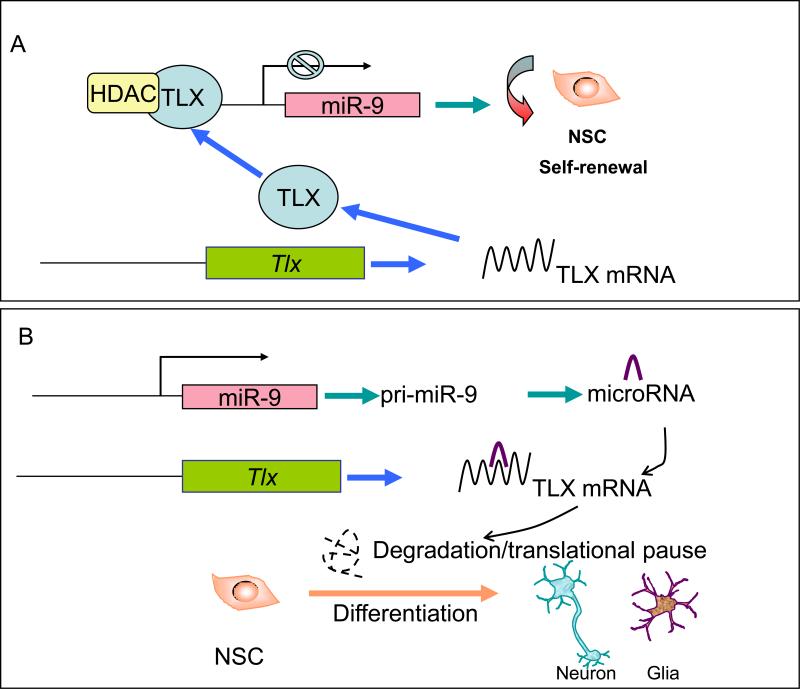
In addition to histone modification, microRNAs, 20-22 nucleotide small RNAs, also play important roles in the regulation of TLX function (Figure 2). MicroRNAs are endogenously expressed small RNAs that negatively regulate downstream target mRNAs, mainly through their 3′ untranslated region (3′ UTR). Two microRNAs, microRNA-9 (miR-9) and lethal-7b (let-7b), have been shown to regulate neural stem cell fate determination by targeting TLX signaling [30, 31]. MiR-9 is one of the microRNAs that are exclusively expressed in the brain. Our recent studies showed that the balance between proliferation and differentiation of neural stem cells can be precisely maintained by miR-9 in a negative feedback loop with TLX. While miR-9 targets the Tlx 3′ UTR to inhibit TLX expression, TLX also binds to the miR-9-1 genomic loci to repress miR-9 precursor transcription. In utero electroporation of miR-9 into the developing mouse brain reduced the expression of TLX protein, decreased the number of proliferative cells in the ventricular zone and induced precocious neuronal differentiation. On the other hand, antisense RNA inhibition of miR-9 expression enhanced neural stem cell proliferation. In this sense, miR-9 antisense RNA could serve as a potential drug to enhance neural stem cell expansion.
Figure 2.
The TLX-microRNA regulatory loop in neural stem cells (NSC). (A) NSC self-renewal is maintained by TLX through its repression of the expression of miR-9 pri-miRNA (pri-miR-9). (B) Under differentiation conditions, TLX protein is decreased, which leads to de-repression of miR-9 expression. miR-9 then binds to TLX mRNA and leads to further inhibition of TLX expression, through either TLX mRNA degradation or TLX translational inhibition. The TLX-miR-9 regulatory loop in turn induces differentiation of neural stem cells into neurons and glia.
In addition to miR-9, let7b is also expressed in mammalian brains and display elevated expression upon neural differentiation. Overexpression of let-7b led to reduced neural stem cell proliferation and increased neural differentiation, through targeting TLX and the cell cycle regulator, cyclin D1 [31]. Studies of microRNA expression profiles in neural stem cells will allow us to identify additional microRNA candidates for neural stem cell regulation.
Signaling by the Wnt family of secreted glycolipoproteins controls embryonic development and adult homeostasis via their downstream effector β-catenin [32, 33]. TLX has been shown to activate Wnt- β-catenin signaling and promote neural stem cell proliferation [34]. Both Wnt7a and a constitutively active β-catenin rescued the proliferation deficiency induced by the treatment of Tlx short interference RNA in adult neural stem cells significantly. Furthermore, introduction of the constitutively active β-catenin into the subventricular zone of adult Tlx-null mice rescued the cell proliferation deficits mediated by deletion of Tlx gene in vivo. These results suggest that TLX acts, at least in part, through the canonical Wnt/β-catenin signaling pathway to control neural stem cell proliferation [34]. This study also presents an interesting possibility that modulators of Wnt signaling may work together with nuclear receptor regulators to enhance the efficacy of neural stem cell-based therapy.
Of particular interest in drug discovery are potential TLX ligand(s), although there is no report for a TLX ligand so far. With the essential role of TLX in neural stem cell maintenance and self-renewal [14], potential TLX ligands, either antagonists or agonists, may serve as excellent drug candidates for neural stem cell-based therapy for neurodegenerative diseases.
2.2 LXR in neural stem cells
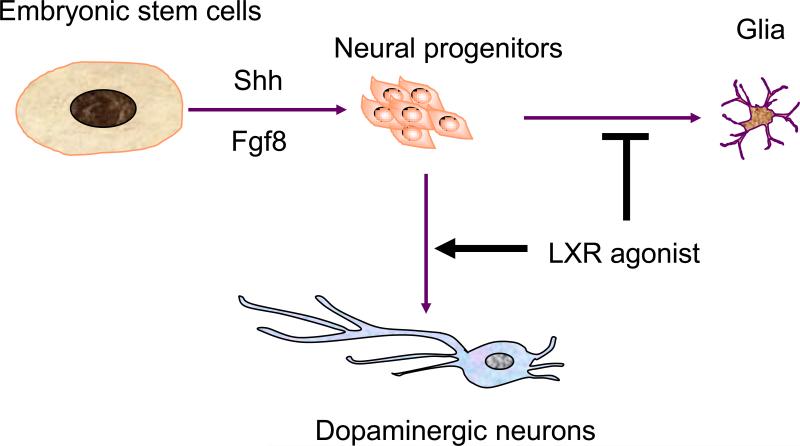
LXRs are ligand-dependent nuclear receptors that are activated by oxidized derivatives of cholesterol (oxysterols) [35]. Upon binding to oxysterols, LXRs form obligate LXR-RXR heterodimers to regulate the transcription of genes controlling cholesterol homeostasis, lipogenesis, and inflammation (Schultz et al., 2000; Zelcer and Tontonoz, 2006). Recently, a novel role of LXR in dopaminergic neuron generation was revealed by characterizing dopaminergic neurogenesis in Lxr-null mice [36]. Genetic ablation of Lxr led to impaired ventral midbrain development that mainly affects the generation of dopaminergic neurons. On the other hand, overexpression of LXRs enhanced dopaminergic neurogenesis, indicating that LXRs are both necessary and sufficient for dopaminergic neurogenesis, and this effect was enhanced by the LXR ligands, oxysterols. Furthermore, in the presence of oxysterols, mouse embryonic stem cells differentiated into dopaminergic neurons more efficiently. Oxysterols also exhibited a selective enhancement of dopaminergic neuronal development in human embryonic stem cells that were treated by sonic hedgehog (SHH) and fibroblast growth factor 8 (FGF8) and in primary ventral midbrain progenitor cultures. These results established a role for the LXR ligands to enhance the generation of midbrain dopaminergic neurons.
One challenge that must be overcome prior to clinical application of stem cell-based therapy is the ability to generate specific and desired cell types. For example, the generation of dopaminergic neurons in large quantity and sufficient purity is an important step for cell-replacement therapy of Parkinson's disease. This study supports potential applications of the LXR ligands in drug discovery and cell replacement therapy for Parkinson's disease (Figure 3). Many nuclear receptors are known to be expressed in embryonic and adult brains, yet their functions are not fully characterized. Defining the role of LXR in neurogenesis may stimulate further studies of other nuclear receptors in neural development and uncovering the potential of their ligands in neurogenesis.
Figure 3.
Implication of LXR ligands in dopaminergic neuron production. Embryonic stem cells can be induced to differentiate into midbrain neural progenitors by the treatment of Shh and Fgf8. These cells can be further induced into dopaminergic neurons by LXR ligands, oxysterols, which promotes dopaminergic neuron induction and inhibits glial differentiation.
2.3 RARs in neural stem cells
There are three RAR genes (RARα, RARβ and RARγ) and three RXR genes (RXRα, RXRβ and RXRγ). Retinoid acid (RA) acts as a cognate ligand for RAR by binding to the RAR-RXR heterodimers. RA has been shown to regulate neural stem cell differentiation, motor neuron axonal outgrowth and neural patterning.
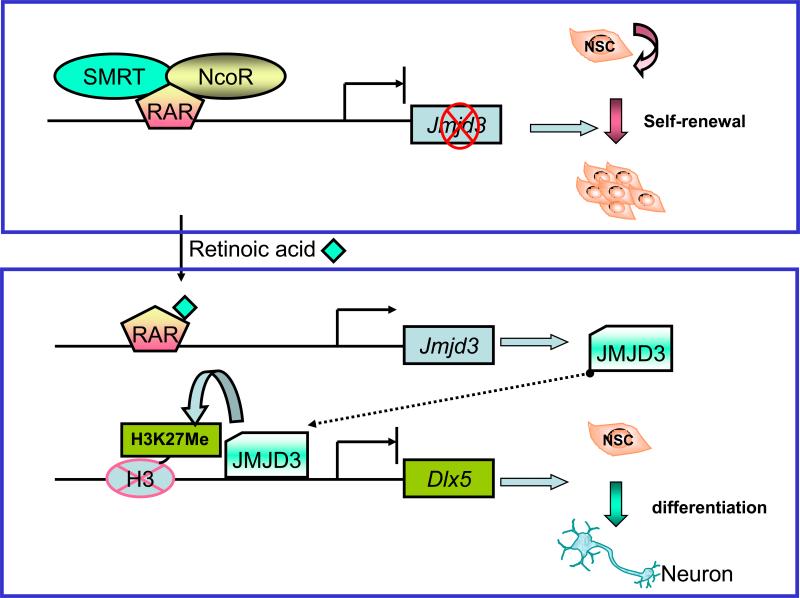
RA induces neuronal differentiation of both neural stem cells and embryonic stem cells by activating genes encoding transcription factors, cell signaling molecules, and structure proteins [37]. It was proposed recently that RA induces neuronal differentiation by releasing the co-repressor SMRT (silencing mediator for retinoid and thyroid receptors) from RAR on the promoter of the histone demethylase JMJD3 (jumonji domain containing 3, histone lysine demethylase). Derepression of JMJD3 expression via the retinoid acid pathway induces neurogenic differentiation [38] (Figure 4). The ability of RA to induce neuronal differentiation can be used to produce specific neural cell types for therapeutic transplantation. The combination of RA and growth factors or neurotrophins has been used to induce various neuronal types from either neural stem cells or embryonic stem cells (table 1). These stem cell-derived neurons may be used to replace damaged or lost neurons in various locations in the brain, including the striatum (for the treatment of Parkinson's or Huntington's disease), the lateral ventricle or the subventricular zone (for the treatment of stroke), the sciatic nerve (for the treatment of peripheral nerve injury), and the cortex ( for the treatment of brain injury) [39].
Figure 4.
SMRT (silencing mediator for retinoid and thyroid receptors) regulates the fate specification of neural stem cells through retinoic acid signaling. In neural stem cells (NSCs), retinoic acid receptor (RAR) recruits SMRT and possibly NCoR (nuclear receptor corepressor) to the promoter of Jmjd3, a histone H3 k27 demethylase, to repress its expression. Upon treatment with the RAR ligand retinoic acid, the SMRT corepressor complex is dissociated from Jmjd3 promoter, which results in activation of Jmjd3. The JMJD3 protein in turn demethylates histone H3 lysine 27(H3K27Me) on neuronal genes, such as Dlx5, and initiates neuronal differentiation.
Table 1.
Neuronal types induced by RA and other factors
| Cell types | Inducing factors | Neuronal types |
|---|---|---|
| human embryonic stem cells mouse embryonic neural stem cell |
RA+Shh | Cholinergic dopaminergic |
| Mouse embryonic neural stem cell | RA+CNTF | Dopaminergic |
| human embryonic stem cells | RA+BDNF, RA+TGFα | Dopaminergic |
| Mouse embryonic neural stem cell | RA | Glutaminergic |
| Adult neural stem cell | RA+FBS | mixed |
| Adult neural stem cell | RA+KCl | GABAergic |
| Human olfactory neural cells | RA+Shh | Dopaminergic |
| human embryonic stem cells | Oxysterol | Dopaminergic |
Adapted from [39]. Abbreviations: RA, retinoic acid; Shh, sonic hedgehog; CNTF, ciliary neurotrophic factor; BNDF, brain-derived neurotrophic factor; TGFγ, transforming growth factor γ; FBS, fetal bovine serum.
Neurogenesis is decreased in the adult subventricular zone compared to that in the embryonic brain, which is speculated to be caused by insufficient RA in the adult brain. Indeed, neurogenesis in the subventricular zone of adult brains can be boosted by treatment with agonists of RARα and RARβ [40]. RARβ-null mice displayed complex alterations of dopamine-induced stereotypic motor behaviors, including exaggeration of head bobbing movement and reduction in rearing activity. The loss of RARβ signaling in the mutant mice resulted in reduction of cyclin E2, a key cell cycle controller of transition from G1 to M phase [41]. RARβ signaling thus plays a crucial role in setting up striatal compartments that may be engaged in neural circuits of psychomotor control [41].
The cerebellar and cerebral cortexes of the adult central nervous system express RARβ. Activation of RARβ appears to be critical for neurotrophic and neuritogenic effects of RA. After spinal cord injury in adult mammals, axons do not normally regrow, which generally leads to paralysis. RA can stimulate neurite outgrowth in vitro through activation of RARβ. RA signaling cascade is also activated in injury events, such as sciatic nerve lesions and spinal cord contusion injury [42]. RARβ can be activated in a dose dependent manner by its agonist CD2019 and induce neurite outgrowth via phosphoinositide 3-kinase (PI3K) signaling. In a model of spinal cord injury, CD2019 also induces axonal outgrowth of descending corticospinal fibers and promotes its functional recovery. These data suggest that RARβ agonists may be of therapeutic potential for human spinal cord injuries [43, 44].
In a rat model of ALS, a fatal neurodegenerative disorder caused by extensive damage of motor neurons, changes in the distribution and expression of retinoid receptors has been observed. These changes may be part of a spinal cord protective response to acute injury and to chronic degeneration. In the ALS rat model, loss of RXRβ, and to a lesser extent RARβ/α, was detected in lumbar spinal cord at an early pre-symptomatic phase and throughout the disease progression [45]. Gliosis and motor neuron loss are key pathogenic features in ALS. The selective expression of retinoid receptors in astrocytes and motor neurons may provide further clues to the role of retinoid signaling in neurodegeneration and suggest new treatment strategies based on retinoid-modulating agents [46].
3. Nuclear receptors in pluripotent stem cells
Stem cell-based therapy for neurodegenerative diseases is particularly attractive, given the limited regenerative capacity of the mammalian central nervous system. One of the potential sources of cell-replacement therapy is human embryonic stem cells. However, the use of human embryonic stem cells has been associated with ethical concerns. The recent discovery on reprogramming of somatic cells to embryonic stem cell-like cells, presents an alternative avenue toward cell-based therapy.
The self-renewal of embryonic stem cells is regulated by core transcriptional regulatory circuitry [47]. Oct4, Sox2, and Nanog are central to the transcriptional regulatory hierarchy that specifies embryonic stem cell identity because of their unique expression patterns and their essential roles during early development [47-50]. Recently, Nanog was identified to be a key regulator of the gateway to ground state of pluripotency [49]. Systematic studies of the expression profiles of nuclear receptors in embryonic stem cells revealed the importance of nuclear receptors in the maintenance and differentiation of embryonic stem cells [9]. Several nuclear receptors have been reported to be required for embryonic stem cell maintenance and early differentiation. These receptors include Errβ (Estrogen-related receptor β, NR3B2), LRH-1 (Liver Receptor Homolog-1, NR5A2), DAX-1 (Dosage-sensitive sex reversal, Adrenal hypoplasia critical region, on chromosome X, gene 1, NR0B1), COUP-TFs (Chick Ovalbumin Upstream Promoter-Transcription Factors, NR2F1, NR2F2 and NR2F6 ), GCNF, RARs, RXRs, and TR2 [9, 11, 51-53]. Among these nuclear receptors, Esrrβ, LRH-1, DAX-1, along with RARγ and RXRγ, exhibit unique expression pattern. The expression of these nuclear receptors is abundant in undifferentiated mouse embryonic stem cells, and declined gradually during early differentiation [9].
3.1. Errs in pluripotent stem cells
Errβ has been identified as a regulator for the maintenance of embryonic stem cells. Errβ can interact with Oct4 and co-occupy on the Nanog proximal promoter, where it positively regulates Nanog expression [54]. Errβ has also been shown to interact with Nanog and activate Oct4 expression to sustain the self-renewal and pluripotency of embryonic stem cells [10, 47]. Knockdown of Errβ expression using either short hairpin RNA lentivirus or small interfering RNA oligonucleotides induces embryonic stem cell differentiation [55, 56].
Errβ-null mice have severely impaired placental formation, and die at E10.5 post coitum [57], consistent with its role in embryonic stem cells. In addition, the Errβ-null mice displayed abnormal trophoblast proliferation [58], similar to the phenotype observed upon treatment of the Errβ ligand diethylstilbestrol. Binding of diethylstilbestrol to Errβ promoted the dissociation of coactivators from Errβ and consequently inhibited transcriptional activitation of Errβ target genes, many of which are involved in the self-renewal and pluripotency of embryonic stem cells [59]. Interestingly, although Errβ is abundantly expressed in mouse embryonic stem cells, it is undetectable in human embryonic stem cells [9].
Recently, it has been shown that Errs can mediate reprogramming of mouse embryonic fiborblasts to iPSCs along with Oct4 and Sox2. Errβ can replace Klf4 in reprogramming assays in the absence of c-Myc. The Errβ-reprogrammed cells share similar characteristics with human embryonic stem cells.
3.2. LRH-1 in pluripotent stem cells
The nuclear receptor LRH-1 belongs to the nuclear receptor subfamily V. It is expressed in the inner cell mass of the blastocyst and in the embryonic ectoderm at the epiblast stage of embryonic development. LRH-1 co-localizes with stem cell pluripotency regulators, Nanog and Sox2, in embryonic stem cells. LRH-1 binds to the promoter of Oct4 and regulates Oct4 expression in epiblast stage of mouse embryonic development (Gu et al., 2005). LRH-1-null mice is embryonic lethal, displaying a loss of Oct4 expression in epiblasts and die between E6.5 and 7.5 (Gu et al., 2005). Recently, LRH-1 has been shown to enhance reprogramming efficiency with retroviral introduction of the four reprogramming factors (Oct4, Sox2, Klf4 and c-Myc). Furthermore, LRH-1 was able to replace Oct4 in reprogramming, although it failed to replace Sox2 or Klf4 in the reprogramming cocktail [11]. Mutation analysis revealed that the LRH-1 DNA binding domain is critical for the reprogramming function, while the ligand binding domain is dispensable, suggesting that LRH-1 ligands may not facilitate the reprogramming process [11]. SF1 (Steroidogenic Factor 1, NR5A1) is another nuclear receptor that belongs to the same nuclear subfamily V as LRH-1. Similar to LRH-1, SF1 was also able to replace Oct4 in the reprogramming event [11].
3.3. Dax-1, COUP-TF, and GCNF in pluripotent stem cells
DAX-1 is another orphan nuclear receptor that appears to be important in early mouse embryogenesis. DAX-1 was demonstrated to act in part through interacting with Nanog [50]. It was shown that DAX-1 maintains the self-renewal property of embryonic stem cells under the control of STAT3 and Oct4 signaling pathways and inhibits the transcriptional activity of Oct4 in embryonic stem cells [60, 61].
The OCUP-TFs represent the most conserved subfamily of nuclear receptors that play key roles in angiogenesis, neuronal development, cell fate determination, and metabolic homeostasis [62]. Recently, COUP-TFII has been shown to be retinoic acid-responsive [63]. Overexpression of COUP-TFI in embryonic stem cells resulted in high level of Nanog gene expression [53].
Unlike COUP-TFs, the expression of which occurs late in the differentiation process of embryonic stem cells, the expression of GCNF is activated early during differentiation. In mouse embryonic stem cells, GCNF induces differentiation by repressing the pluripotency genes, Oct4 and Nanog [52]. The roles of Dax-1, COUP-TFs, and GCNF in reprogramming of somatic cells in the presence or absence of ligands await further investigation.
3.4. Potential applications of nuclear receptors in diseased iPSCs
A couple of recent studies described the initial characterization of neurological disease models using neuronal cells derived from patient-specific pluripotent stem cells and provided proof-of-concept for the application of these cells as platforms for drug discovery. Recently, iPSCs derived from the ALS patient fibroblasts have been generated and differentiated into motor neurons [64]. Motor neurons have also been derived form human embryonic stem cells and used to identify compounds that support motor neuron growth [65, 66]. Patient-specific iPSCs were also generated from skin fibroblasts of spinal muscular atrophy patients using a reprogramming cocktail (Oct4, Sox2 Nanog, and Lin28) [67]. The iPSCs could be differentiated into spinal motor neurons harboring the SMN1 mutation. Treatment of the motor neurons with valproic acid, a histone deacetylase inhibitor, rescued the defective phenotype of the diseased motor neurons partially. Many nuclear receptors recruit histone deacetylases to regulate gene expression, therefore are potential valproic acid targets. Moreover, many ligands of nuclear receptors are known drugs, with extensive clinical trial data resources. Combining nuclear receptor ligands with the platform of iPSC technology may provide novel avenues for the design, screening, and test of drugs for a variety of human diseases.
4. Perspective remarks
There are substantial differences between human and mouse expression profiles of nuclear receptors in embryonic stem cells [9]. This discrepancy may reflect the different developmental stages of these two embryonic stem cells. Mouse embryonic stem cells are equivalent to embryonic stage of inner cell mass in blastocysts while human embryonic stem cells are close to epiblast, a later stage of blastocysts [68]. Many nuclear receptors are expressed in embryonic stem cells, although the functional relevance of some of these receptors in embryonic stem cells remains elusive. Considering the expression profiles of these nuclear receptors in embryonic stem cells and their importance as transcriptional sensors for many signaling pathways, further study on nuclear receptors in the maintenance, self-renewal, and pluripotency of embryonic stem cells will provide insights into how to generate iPSCs with high efficiency and facilitate the application of iPSCs in stem cell-based therapy.
Furthermore, since genetic manipulation of iPSCs poses safety concern due to tumorigenic potential of transgenes, small molecules, such as nuclear receptor ligands, are emerging as attractive candidates for reprogramming to replace the genetic reprogramming factors [8, 9, 11, 69]. Nuclear receptors promise to be important players in stem cell-based disease modeling, drug discovery, and cell replacement therapies.
Acknowledgments
We apologize for studies that are not cited due to space limitations. We thank Drs. Ming-Fei Lang, Wendong Li and Mr. Donald Jhung, Jr. for their critical reading and discussion. G.S. is a Herbert Horvitz fellow. This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke grant R01 NS059546 and RC1 NS068370 (to Y.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 5.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 7.Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, Castella M, Rio P, Sleep E, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong Y, Mangelsdorf DJ. Nuclear receptor regulation of stemness and stem cell differentiation. Exp Mol Med. 2009;41:525–537. doi: 10.3858/emm.2009.41.8.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie CQ, Jeong Y, Fu M, Bookout AL, Garcia-Barrio MT, Sun T, Kim BH, Xie Y, Root S, Zhang J, et al. Expression profiling of nuclear receptors in human and mouse embryonic stem cells. Mol Endocrinol. 2009;23:724–733. doi: 10.1210/me.2008-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng B, Jiang J, Kraus P, Ng JH, Heng JC, Chan YS, Yaw LP, Zhang W, Loh YH, Han J, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol. 2009;11:197–203. doi: 10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- 11.Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T, et al. The Nuclear Receptor Nr5a2 Can Replace Oct4 in the Reprogramming of Murine Somatic Cells to Pluripotent Cells. Cell Stem Cell. 2010 doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Monaghan AP, Grau E, Bock D, Schutz G. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121:839–853. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 13.Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Sun G, Yang S, Qu Q, Nakashima K, Shi Y. Nuclear receptor TLX regulates cell cycle progression in neural stem cells of the developing brain. Mol Endocrinol. 2008;22:56–64. doi: 10.1210/me.2007-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy K, Kuznicki K, Wu Q, Sun Z, Bock D, Schutz G, Vranich N, Monaghan AP. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24:8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci U S A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Land PW, Monaghan AP. Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb Cortex. 2003;13:921–931. doi: 10.1093/cercor/13.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 20.Monaghan AP, Bock D, Gass P, Schwager A, Wolfer DP, Lipp HP, Schutz G. Defective limbic system in mice lacking the tailless gene. Nature. 1997;390:515–517. doi: 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- 21.Miyawaki T, Uemura A, Dezawa M, Yu RT, Ide C, Nishikawa S, Honda Y, Tanabe Y, Tanabe T. Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J Neurosci. 2004;24:8124–8134. doi: 10.1523/JNEUROSCI.2235-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci U S A. 2000;97:2621–2625. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang CL, Zou Y, Yu RT, Gage FH, Evans RM. Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev. 2006;20:1308–1320. doi: 10.1101/gad.1413606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar RA, Leach S, Bonaguro R, Chen J, Yokom DW, Abrahams BS, Seaver L, Schwartz CE, Dobyns W, Brooks-Wilson A, et al. Mutation and evolutionary analyses identify NR2E1-candidate-regulatory mutations in humans with severe cortical malformations. Genes Brain Behav. 2007;6:503–516. doi: 10.1111/j.1601-183X.2006.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar RA, McGhee KA, Leach S, Bonaguro R, Maclean A, Aguirre-Hernandez R, Abrahams BS, Coccaro EF, Hodgins S, Turecki G, et al. Initial association of NR2E1 with bipolar disorder and identification of candidate mutations in bipolar disorder, schizophrenia, and aggression through resequencing. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:880–889. doi: 10.1002/ajmg.b.30696. [DOI] [PubMed] [Google Scholar]
- 26.Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schutz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun G, Zhao C, Shi Y. Shi Y, Clegg D, editors. Epigenetic control of neural stem cell self-renewal and specification. Stem Cell Research and Therapeutics. 2008.
- 28.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun G, Alzayada K, Stewart R, Ye P, Yang S, Wendong L, Shi Y. The Histone Demethylase LSD1 Regulate Neural Stem Cell Proliferation. Mol Cell Biol. 2010 doi: 10.1128/MCB.01116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao C, Sun G, Li S, Lang M-F, Yang S, Li W, Shi Y. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010. [DOI] [PMC free article] [PubMed]
- 32.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 34.Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12:31–40. doi: 10.1038/ncb2001. sup pp 31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 36.Sacchetti P, Sousa KM, Hall AC, Liste I, Steffensen KR, Theofilopoulos S, Parish CL, Hazenberg C, Richter LA, Hovatta O, et al. Liver X receptors and oxysterols promote ventral midbrain neurogenesis in vivo and in human embryonic stem cells. Cell Stem Cell. 2009;5:409–419. doi: 10.1016/j.stem.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Maden M. Role and distribution of retinoic acid during CNS development. Int Rev Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- 38.Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, Hermanson O, Rosenfeld MG. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 39.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 40.Haskell GT, LaMantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25:7636–7647. doi: 10.1523/JNEUROSCI.0485-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao WL, Tsai HC, Wang HF, Chang J, Lu KM, Wu HL, Lee YC, Tsai TF, Takahashi H, Wagner M, et al. Modular patterning of structure and function of the striatum by retinoid receptor signaling. Proc Natl Acad Sci U S A. 2008;105:6765–6770. doi: 10.1073/pnas.0802109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mey J. New therapeutic target for CNS injury? The role of retinoic acid signaling after nerve lesions. J Neurobiol. 2006;66:757–779. doi: 10.1002/neu.20238. [DOI] [PubMed] [Google Scholar]
- 43.Agudo M, Yip P, Davies M, Bradbury E, Doherty P, McMahon S, Maden M, Corcoran JP. A retinoic acid receptor beta agonist (CD2019) overcomes inhibition of axonal outgrowth via phosphoinositide 3-kinase signalling in the injured adult spinal cord. Neurobiol Dis. 2010;37:147–155. doi: 10.1016/j.nbd.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yip PK, Wong LF, Pattinson D, Battaglia A, Grist J, Bradbury EJ, Maden M, McMahon SB, Mazarakis ND. Lentiviral vector expressing retinoic acid receptor beta2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord. Hum Mol Genet. 2006;15:3107–3118. doi: 10.1093/hmg/ddl251. [DOI] [PubMed] [Google Scholar]
- 45.van Neerven S, Kampmann E, Mey J. RAR/RXR and PPAR/RXR signaling in neurological and psychiatric diseases. Prog Neurobiol. 2008;85:433–451. doi: 10.1016/j.pneurobio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Jokic N, Ling YY, Ward RE, Michael-Titus AT, Priestley JV, Malaspina A. Retinoid receptors in chronic degeneration of the spinal cord: observations in a rat model of amyotrophic lateral sclerosis. J Neurochem. 2007;103:1821–1833. doi: 10.1111/j.1471-4159.2007.04893.x. [DOI] [PubMed] [Google Scholar]
- 47.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 49.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 51.Gupta P, Ho PC, Huq MM, Ha SG, Park SW, Khan AA, Tsai NP, Wei LN. Retinoic acid-stimulated sequential phosphorylation, PML recruitment, and SUMOylation of nuclear receptor TR2 to suppress Oct4 expression. Proc Natl Acad Sci U S A. 2008;105:11424–11429. doi: 10.1073/pnas.0710561105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullen EM, Gu P, Cooney AJ. Nuclear Receptors in Regulation of Mouse ES Cell Pluripotency and Differentiation. PPAR Res. 2007;2007:61563. doi: 10.1155/2007/61563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuang Y, Gudas LJ. Overexpression of COUP-TF1 in murine embryonic stem cells reduces retinoic acid-associated growth arrest and increases extraembryonic endoderm gene expression. Differentiation. 2008;76:760–771. doi: 10.1111/j.1432-0436.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 54.van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol. 2008;28:5986–5995. doi: 10.1128/MCB.00301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 56.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 57.Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 58.Mitsunaga K, Araki K, Mizusaki H, Morohashi K, Haruna K, Nakagata N, Giguere V, Yamamura K, Abe K. Loss of PGC-specific expression of the orphan nuclear receptor ERR-beta results in reduction of germ cell number in mouse embryos. Mech Dev. 2004;121:237–246. doi: 10.1016/j.mod.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguere V. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev. 2001;15:833–838. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun C, Nakatake Y, Akagi T, Ura H, Matsuda T, Nishiyama A, Koide H, Ko MS, Niwa H, Yokota T. Dax1 binds to Oct3/4 and inhibits its transcriptional activity in embryonic stem cells. Mol Cell Biol. 2009;29:4574–4583. doi: 10.1128/MCB.01863-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun C, Nakatake Y, Ura H, Akagi T, Niwa H, Koide H, Yokota T. Stem cell-specific expression of Dax1 is conferred by STAT3 and Oct3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2008;372:91–96. doi: 10.1016/j.bbrc.2008.04.154. [DOI] [PubMed] [Google Scholar]
- 62.Warnecke M, Oster H, Revelli JP, Alvarez-Bolado G, Eichele G. Abnormal development of the locus coeruleus in Ear2(Nr2f6)-deficient mice impairs the functionality of the forebrain clock and affects nociception. Genes Dev. 2005;19:614–625. doi: 10.1101/gad.317905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 65.Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 67.Ebert AD, Yu J, Rose FF, Jr., Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]