Abstract
TNF-related apoptosis-inducing ligand (TRAIL) is a potential chemotherapeutic agent with high selectivity for malignant cells. Many tumors, however, are resistant to TRAIL cytotoxicity. Although cellular inhibitor of apoptosis 1 and 2 (cIAP-1 and -2) are often over-expressed in cancers, their role in mediating TRAIL resistance remains unclear. Here, we demonstrate that TRAIL-induced apoptosis of liver cancer cells is associated with degradation of cIAP-1 and X-linked IAP (XIAP), whereas cIAP-2 remains unchanged. Lower concentrations of TRAIL causing minimal or no apoptosis do not alter cIAP-1 or XIAP protein levels. Silencing of cIAP-1 expression, but not XIAP or cIAP-2, as well as co-treatment with a second mitochondrial activator of caspases (SMAC) mimetic (which results in rapid depletion of cIAP-1), sensitizes the cells to TRAIL. TRAIL-induced loss of cIAP-1 and XIAP requires caspase activity. In particular, caspase 8 knockdown stabilizes both cIAP-1 and XIAP, while caspase 9 knockdown prevents XIAP, but not cIAP-1 degradation. Cell-free experiments confirmed cIAP-1 is a substrate for caspase 8, with likely multiple cleavage sites. These results suggest that TRAIL-mediated apoptosis proceeds through caspase 8-dependent degradation of cIAP-1. Targeted depletion of cIAP-1 by SMAC mimetics in conjunction with TRAIL may be beneficial for the treatment of human hepatobiliary malignancies.
Keywords: Death receptors, chemoresistance, hepatobiliary cancer
Tumor necrosis factor–related apoptosis-inducing ligand or TRAIL (TNSF10) is a member of the tumor necrosis factor (TNF) superfamily which preferentially induces apoptosis in malignant cells and, therefore, is considered an attractive anti-cancer agent [1]. This ligand initiates signaling cascades by binding to two cognate receptors termed death receptor 4, DR4 (TNSF10A, also referred to as TRAIL receptor 1), and death receptor 5, DR5 (TNSF10B, also referred to as TRAIL receptor 2/KILLER/TRICK-2) [1]. Death receptor oligomerization by TRAIL results in conformational changes within cytoplasmic death domains, facilitating recruitment of FADD and procaspases 8 and 10 to a protein complex termed the death inducing signaling complex (DISC)[2] Caspase 8 activation by induced proximity within this complex can initiate signaling cascades culminating in apoptosis [3]. However, pro-apoptotic signaling by TRAIL can be inhibited by other signaling molecules and cascades, as often observed in cancer cells with primary or acquired resistance to TRAIL [2]. As TRAIL and pro-apoptotic TRAIL agonists enter clinical trials [4], insight into these resistance mechanisms becomes critical in developing strategies to maximize TRAIL efficacy.
Cellular inhibitors of apoptosis 1 and 2 (cIAP-1 and cIAP-2) can inhibit death receptor-mediated apoptosis [5]. These polypeptides belong to the IAP family, a group of intracellular proteins containing one or more zinc-binding baculovirus IAP repeat (BIR) domains. Several IAPs, including cIAP-1, cIAP-2 and X-linked inhibitor of apoptosis (XIAP), also contain a carboxy-terminal RING domain with ubiquitin E3 ligase properties [6]. Although all IAPs can potentially bind to caspases, only XIAP is a direct inhibitor of caspase 9, 3 and 7 [7, 8], whereas cIAP-1 and cIAP-2 are thought to regulate receptor-mediated signaling pathways upstream of mitochondria through their interaction with TNF receptor-associated factor 1 and 2 (TRAF1 and TRAF2) [9]. Mammalian cells contain a natural IAP antagonist, the mitochondrial protein SMAC (second mitochondrial activator of caspases)/DIABLO (direct IAP binding protein with low pI), which is released into the cytosol following mitochondrial membrane permeabilization in response to diverse pro-apoptotic stimuli. SMAC/DIABLO binds to BIR2 and BIR3 domains on IAP proteins inhibiting their function and, thereby, promoting apoptosis [10–12]. As IAPs are frequently up-regulated in tumor cells, small pharmacological compounds that mimic the IAP-binding motif of SMAC/DIABLO (called IAP antagonists or SMAC mimetics) have been developed for cancer therapy. Although initially designed to antagonize XIAP, SMAC mimetics have been shown to bind to cIAP-1 and cIAP-2, and rapidly induce their auto-ubiquitination and proteasomal degradation, resulting in their cellular elimination [13–15]. These drugs strongly enable TNF-α-mediated apoptosis, implicating a substantial role for cIAP-1 and -2 in modulating apoptosis by this death ligand [5, 15, 16]. Although SMAC mimetics have been reported to sensitize cancer cells to TRAIL cytotoxicity, suggesting they may modulate apoptosis by this death ligand as well [17–19], the role of cIAP-1 and/or cIAP-2 in the regulation of TRAIL-mediated apoptosis remains largely unexplored.
The aim of the present study was to investigate a potential role for cIAP-1 and/or cIAP-2 in TRAIL-mediated apoptosis. We chose to use malignant human hepatobiliary cell lines for these studies, because of limited therapeutic options for hepatocellular carcinoma and cholangiocarcinoma [20]. Our results indicate that in a concentration-dependent manner, TRAIL induces apoptosis associated with degradation of cIAP-1 and XIAP, but not cIAP-2. However, only depletion of cIAP-1, but not XIAP, sensitizes tumor cells to TRAIL. TRAIL-induced degradation of cIAP-1 requires caspase 8 activity, and it is, at least in part, due to direct cleavage of cIAP-1 by caspase 8. These findings suggest cIAP-1 modulates the sensitivity to TRAIL, but its inhibitory effect can be overcome by TRAIL concentrations sufficient to cause its degradation by caspase 8.
EXPERIMENTAL PROCEDURES
Cell Lines
The human hepatocellular carcinoma cell lines HuH-7 and Hep3B, and human cholangiocarcinoma cell line Mz-ChA-1 were cultured as previously described by us [21, 22].
Reagents
Recombinant human TRAIL (375-TEC) was from R&D Systems (Minneapolis, MN). The pan-caspase inhibitor Q-VD-OPH, and the caspase 8 inhibitor z-IETD-fmk were from Enzyme Systems Products (Solon, OH). The cathepsin B inhibitor CRA 025850 was a kind gift from Dr. Leslie Holsinger from Virobay (Menlo Park, CA). The proteasome inhibitor MG132 was from Calbiochem (La Jolla, CA), The SMAC mimetic JP1584 was from Gemin X (Montreal, Quebec, Canada) in collaboration with Joyant Pharmaceuticals (Dallas, TX). Bafilomycin A1 was from Sigma Aldrich (St. Louis, MO).
Immunoblot analysis and antibodies
Immunoblot analysis of whole-cell lysates was performed as previously described by us [23]. Primary antibodies were: goat polyclonal anti-cIAP-1 (AF8181) and goat polyclonal anti-Bid (AF860) was from R&D Systems; rabbit polyclonal anti-cIAP-2 (NBP1-27972) was from Novus Biologicals (Littleton, CO); mouse monoclonal anti-XIAP (610716) and mouse monoclonal anti-RIP1 (610458) were from BD Transduction Labs (San Jose, CA); rat monoclonal anti-HA tag (clone 3F10; 11-867-243-001) was from Roche Applied Science (Indianapolis, IN); mouse monoclonal anti-caspase 8 (clone 1C12; 9746) was from Cell Signaling Technology (Beverly, MA); goat polyclonal anti-caspase 8 (sc-6136) and goat polyclonal anti-actin (sc-1615) were from Santa Cruz Biochemicals (Santa Cruz, CA). Mouse monoclonal anti-PARP (clone C2-10) was a generous gift of Dr. S.H. Kaufmann (Mayo Clinic, Rochester, MN). All primary antibodies were used at a concentration of 1 μg/ml, except actin (0.25 μg/ml), XIAP and RIP1 (0.5 μg/ml).
Apoptosis Assays
Apoptosis was quantified by assessing the nuclear morphology after staining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma Aldrich) using fluorescence microscopy at excitation and emission wavelengths of 380 and 430 nm, respectively. Caspase-3/7 activity in cell cultures was assessed using the Apo-ONE™ homogeneous caspase-3/7 kit (Promega, Madison, WI) following the supplier’s instructions.
Short Hairpin RNA knockdown of cIAP-1, cIAP-2, XIAP, Caspase 9 or Caspase 8
Short hairpin RNA (shRNA) against cIAP-1, cIAP-2, XIAP, caspase 8 and caspase 9 were from Sigma: MISSION shRNA lentiviral plasmid, target sequence TGGTTAAATCTGCCTTGGAAA [GenBank accession no. NM_001166 for cIAP-1]; target sequence CAGTTCGTACATTTCTTTCAT [GenBank accession no. NM_001165 for cIAP-2]; target sequences GCACTCCAACTTCTAATCAAA and AGCTGTAGATAGATGGCAATA [GenBank accession no. NM_001167 for XIAP]; target sequence GAATCACAGACTTTGGACAAA [GenBank accession no. NM_001228 for caspase 8], and target sequence CAGCTTCCAGATTGACGACAA [GenBank accession no. NM-001229 for caspase 9]. HuH-7 cells were transfected using OptiMEM I (Gibco-Invitrogen, Carlsbad, CA) containing 6 μl/ml Lipofectamine + 6 μl/ml Plus reagent (Invitrogen), and 1 μg/ml plasmid DNA. Forty-eight hours after transfection, fresh complete Dulbecco’s modified Eagle medium (containing 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptavidin) with 1 μg/ml puromycin was added. Surviving clones were separated using cloning rings and individually cultured. Specific protein expression in the clones was assessed by immunoblot analysis.
Real Time-Polymerase Chain Reaction (PCR)
Total RNA was extracted from the cells using the mirVana™ miRNA Isolation Kit (Ambion, Austin, TX) and was reverse-transcribed into complementary DNA with Moloney leukemia virus reverse transcriptase and random primers (Invitrogen). Quantification of the complementary DNA template was performed by real time PCR (LightCycler® 480 II; Roche Applied Science) using SYBR green (Roche) as a fluorophore, as previously described by us [24]. PCR primers were as follows: human cIAP-1: 5′-AGCTAGTCTGGGATCCACCTC-3′ (forward), 5′-GGGGTTAGTCCTCGATGAAG-3′ (reverse); human cIAP-2: 5′-TGGAAGCTACCTCTCAGCCTAC-3′ (forward), 5′-GGAACTTCTCATCAAGGCAGA-3′ (reverse); human XIAP: 5′-TTTGCCTTAGACAGGCCATC-3′ (forward), 5′-TTTCCACCACAACAAAAGCA-3′ (reverse). Primers for 18 S ribosomal RNA, used as internal control, were purchased from Ambion.
Plasmids and Transient Transfection
pEBB-HA-cIAP-1 was a kind gift of Drs. Ezra Burstein (University of Texas Southwestern, Dallas, TX) and Colin Duckett (University of Michigan at Ann Arbor, MI) [25]. pEBB-HA-cIAP-1 was subjected to site-directed mutagenesis to mutate the E3 ligase critical residue His588 (H588→A) to generate pEBB-HA-cIAP-1 H588A using the QuickChange® II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) following the supplier’s instructions. The plasmid was prepared using a DNA miniprep kit (Bio-Rad, Hercules, CA, USA), and subjected to automated sequencing to verify the described mutations and confirm that no additional mutations were present. Cells were transfected using FuGENE® HD transfection reagent (Roche Applied Science) with HD:DNA ratio of 5:2, following the manufacturer’s instructions, and treatments were carried out 48 hours later.
Cell-free system
Aliquots of 40 ng of human recombinant cIAP-1 (R&D Systems), 500 ng of human recombinant Bid (R&D Systems) or 50 ng of human recombinant PARP (a generous gift of Dr. S.H. Kaufmann) were incubated for 30 min at 37°C in 20 μl of assay buffer (50 mM HEPES [pH 7.2], 50 mM NaCl, 0.1% CHAPS, 10 mM EDTA, 5% glycerol, 10 mM DTT) in the presence or absence of active recombinant human caspase 8 (Biovision Mountain View, CA; 1 unit), or active recombinant human caspase 3 (BioVision; 0.5 unit), with or without Q-VD-OPH (100 μM). One unit of the recombinant caspase 8 or caspase 3 is defined as the enzyme activity that cleaves 1 nmol of the caspase substrate IETD-pNA or DEVD-pNA, respectively, per hour at the indicated conditions. The reaction was stopped by the addition of electrophoresis sample buffer and samples were subsequently subjected to SDS-PAGE and blotted for cIAP-1, Bid or PARP.
Caspase 8 immunoprecipitation
HuH-7 cells (approx. 1.5 × 107 cells/sample) were solubilized in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 10% glycerol, complete protease inhibitor cocktail [Roche]) for 30 minutes on ice. After centrifugation at 13,000 g for 15 minutes, 2 mg-aliquots of the supernatant (2 mg/ml) were pre-cleared using protein A agarose beads for 1 hours at 4°C, then incubated for 2 hr at 4°C with 2 μg of anti-caspase 8 polyclonal antibody. One hundred microliters protein A agarose beads were then added to each sample and incubated under agitation overnight at 4°C. The following day, the beads were washed four times with ice-cold PBS and proteins were solubilized in SDS sample buffer, clarified by centrifugation, subjected to SDS-PAGE, and analyzed by immunoblot.
Statistical analysis
All data represent at least three independent experiments and are expressed as mean ± standard error (SEM) unless otherwise indicated. Differences between groups were compared using an unpaired two-tailed t-test, and p values < 0.05 were considered statistically significant.
RESULTS
Cellular depletion of cIAP-1 enhances the efficiency of TRAIL-mediated apoptosis
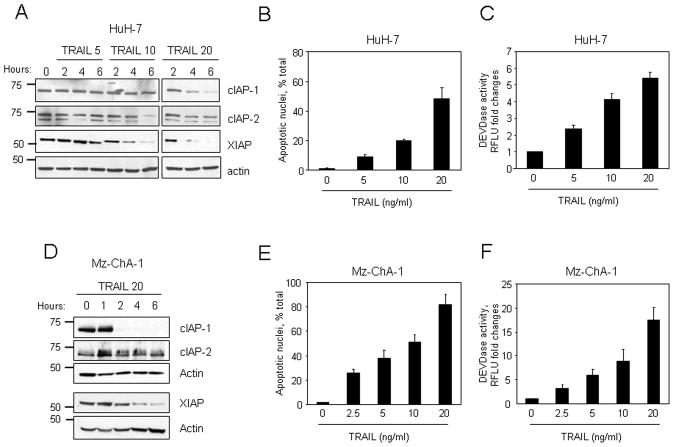
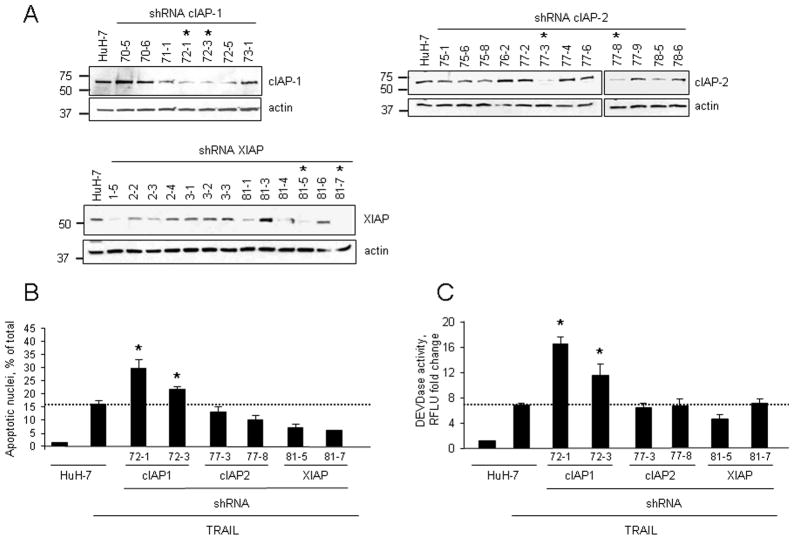
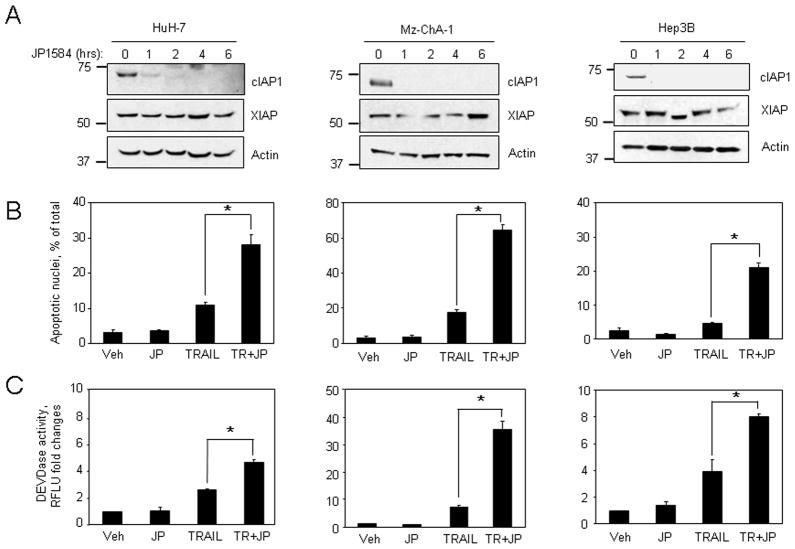
We initially examined cellular levels of cIAP-1, cIAP-2 and XIAP in the hepatocarcinoma cell line HuH-7 during treatment with increasing concentrations of TRAIL (0–20 ng/ml). Low concentrations of TRAIL (≤10 ng/ml) did not affect IAPs protein levels and were associated with modest apoptosis. However, TRAIL concentrations which more efficiently induced apoptosis (20 ng/ml), also resulted in decrease of cIAP-1 and XIAP protein expression (Fig. 1A–C). Similar findings were also observed in the cholangiocarcinoma cell line Mz-ChA-1 (Fig. 1D–F). In contrast, no significant changes in cIAP-2 protein levels were identified in either cell line (Fig. 1A and D). These results suggest cIAP-1 and XIAP depletion may be necessary for efficient TRAIL-induced apoptosis. To test this interpretation of the data, wild-type and HuH-7 clones stably expressing shRNA targeting cIAP-1, cIAP-2, or XIAP were treated with low concentrations (5 ng/ml) of TRAIL for 6 hr. Two clones with successful knockdown of each protein were selected and utilized for these studies (Fig. 2A). Only clones with shRNA targeting cIAP-1 were sensitized to TRAIL-mediated apoptosis, whereas cIAP-2 or XIAP cellular depletion had no significant effect on apoptosis inhibition (Fig. 2B–C). To further implicate cIAP-1 loss as a mechanism facilitating TRAIL cytotoxicity, HuH-7 cells, Mz-ChA-1 cells, and the TRAIL-resistant Hep3B cells, were treated with non-toxic concentrations of TRAIL in the presence or absence of the SMAC mimetic JP1584. In all cell lines, JP1584 alone induced rapid depletion of cIAP-1, but not XIAP, without evident toxicity (Fig 3A). More importantly, apoptosis was significantly enhanced in cells treated with TRAIL plus JP1584 as compared to cells treated with TRAIL alone (Fig. 3B–C). Collectively, these data suggest that efficient TRAIL-mediated apoptosis may be facilitated by reducing cIAP-1 cellular levels.
Figure 1. Degradation of cIAP-1 and XIAP is associated with TRAIL-mediated apoptosis.
(A) Hepatocellular carcinoma cells HuH-7 were treated with increasing concentrations of TRAIL (0–20 ng/ml). At the indicated time points, cell lysates were obtained and analyzed by immunoblotting for cIAP-1, cIAP-2, and XIAP. Actin was used as protein loading control. After 6 hours of treatment, apoptosis was assessed (B) by fluorescence microscopy after DAPI staining and (C) by measuring caspase 3/7 activation (DEVDase activity; relative fluorescence units - RFLU) with a fluorogenic assay, as described in Experimental Procedures. (D) Cholangiocarcinoma cells Mz-ChA-1 were treated with TRAIL (20 ng/ml). At the indicated time points, cell lysates were obtained and analyzed by immunoblotting for cIAP-1, cIAP-2, and XIAP. Actin was used as protein loading control. (E,F) Mz-ChA-1 were treated with increasing concentrations of TRAIL (0–20 ng/ml) and apoptosis was assessed by (E) fluorescence microscopy and (F) caspase 3/7 activation. Values of RFLU are expressed as fold increase over control value (untreated).
Figure 2. Knock-down of cIAP-1, but not XIAP or cIAP-2, sensitizes to TRAIL-mediated apoptosis.
(A) Clones of HuH-7 cells stably transfected with shRNA against cIAP-1, cIAP-2 or XIAP were assessed by immunoblot analysis to verify efficiency of protein knock-down. Actin was included as a loading control. (B) Selected cIAP-1, cIAP-2 or XIAP shRNA clones were treated with TRAIL (5 ng/ml) for 6 hr. Apoptosis was assessed (B) morphologically and (C) by measuring caspase 3/7 activation. Data are expressed as mean ± SEM compared to TRAIL-treated HuH-7 cells. The asterisk (*) indicates statistical significance of p < 0.05.
Figure 3. SMAC mimetic induces loss of cIAP-1 and enhances sensitivity to TRAIL-mediated apoptosis.
(A) HuH-7, Mz-ChA-1, and Hep3B cells were treated with the SMAC mimetic JP1584 (500 nM). At the indicated time points, cell lysates were obtained and expression of cIAP-1 and XIAP was analyzed by immunoblotting. Actin was used as loading control. (B,C) Cells were treated with medium alone (vehicle - Veh), or human recombinant TRAIL (HuH-7: 5 ng/ml; Mz-ChA-1: 2.5 ng/ml; Hep3B: 20 ng/ml), or JP1584 (500 nM), or a combination (TR+JP) for 6 hr. Apoptosis was quantitated (B) by morphological criteria and (C) by measuring caspase 3/7 activation. The asterisk (*) indicates statistical significance of p < 0.05.
TRAIL induces cIAP-1 degradation by a caspase-dependent mechanism
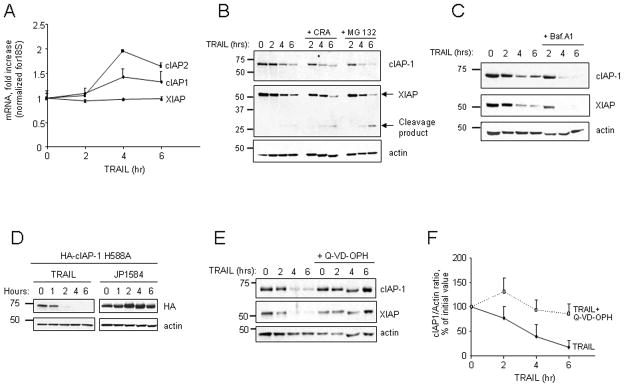
The above studies suggest TRAIL, in a concentration-dependent manner, is capable of down-regulating cIAP-1 levels in order to achieve more efficient apoptosis. Analysis of mRNA expression of IAPs in HuH-7 cells before and after TRAIL stimulation (0–6 hr) revealed that mRNA levels of cIAP-1, cIAP-2 and XIAP were not reduced by TRAIL treatment (Fig. 4A), suggesting that the down-regulation is due to post-transcriptional mechanisms. cIAP-1 has been reported to undergo degradation via trafficking to lysosomes [26], or via a proteosomal-mediated pathway [16, 27]. However, neither disruption of lysosomal function by the vacuolar type H+-ATPase inhibitor bafilomycin A1 nor treatment with the lysosomal cathepsin B inhibitor CRA025850 prevented cellular depletion of cIAP-1 during TRAIL treatment (Fig. 4B–C). The proteasome inhibitor MG132 also failed to stabilize cIAP-1 protein level (Fig. 4B). To ascertain if cIAP-1 auto-ubiquitination mediated by its E3 ubiquitin ligase activity is required for its degradation, cells were transiently transfected with a construct expressing HA-tagged cIAP-1 H588A, in which His588 in the RING domain, a critical residue for the E3 ubiquitin ligase activity of cIAP-1, is mutated to Ala (H588A) [28]. Degradation of HA-cIAP-1 H588A was just as rapid as endogenous cIAP-1 during TRAIL treatment, confirming cIAP-1 degradation is independent of its intrinsic E3 ligase activity (Fig. 4D). Consistent with prior observations [16, 27], the E3 ubiquitin ligase activity was, however, essential for degradation of cIAP-1 after treatment with the SMAC mimetic JP1584 (Fig. 4D). Because caspases play a crucial role in initiation of death receptor-mediated apoptosis, we next tested the possibility that cIAP-1 may be cleaved and degraded by caspases. The broad-spectrum caspase inhibitor Q-VD-OPH did indeed significantly stabilize cIAP-1 protein level during TRAIL treatment, suggesting caspase activity is required for cIAP-1 degradation (Fig. 4E–F). Taken together, these observations suggest that TRAIL-induced cIAP-1 degradation occurs by a caspase-dependent, post-translational process.
Figure 4. TRAIL-mediated degradation of cIAP-1 and XIAP requires caspase activity.
(A) HuH-7 cells were treated with TRAIL (20 ng/ml). At the indicated time points, cIAP-1, cIAP-2 and XIAP mRNA were quantified by real time PCR. Fold increase was determined by normalization to 18S. (B) HuH-7 cells were treated with TRAIL (20 ng/ml) in the presence or absence of the cathepsin B inhibitor CRA 025850 (10 μM; 1 hr pre-incubation), or the proteasome inhibitor MG132 (10 μM; 1 hr pre-incubation), followed by immunoblot analysis for cIAP-1 and XIAP. (C) HuH-7 cells were treated with TRAIL (20 ng/ml) in the presence or absence of the vacuolar type H+-ATPase inhibitor bafilomycin A1 (0.1 μM). Expression of cIAP1 and XIAP was assessed by immunoblot analysis. (D) HuH-7 cells were transiently transfected with a plasmid encoding HA-tagged cIAP-1 with the H588→A mutation in the RING domain, and treated with either TRAIL (20 ng/ml; left panel) or JP1582 (500 nM; right panel); cell lysates obtained at the indicated time points were analyzed by immunoblot for expression of HA-cIAP-1 using an antibody against the HA tag. Actin was used as loading control. (E) HuH-7 cells were treated with TRAIL (20 ng/ml) in the presence or absence of the pan-caspase inhibitor Q-VD-OPH (5 μM; 30 min pre-incubation). Expression of cIAP1 and XIAP was assessed by immunoblot analysis at the indicated time points. Actin was used as loading control. (F) Immunoblots for cIAP-1 from four independent experiments as described in (E) were quantified by densitometry. cIAP-1/Actin ratios were calculated and normalized to time zero, which was arbitrarily set at 100%. Data are expressed as mean ± SEM.
TRAIL-induced degradation of cIAP-1 is caspase 8-dependent
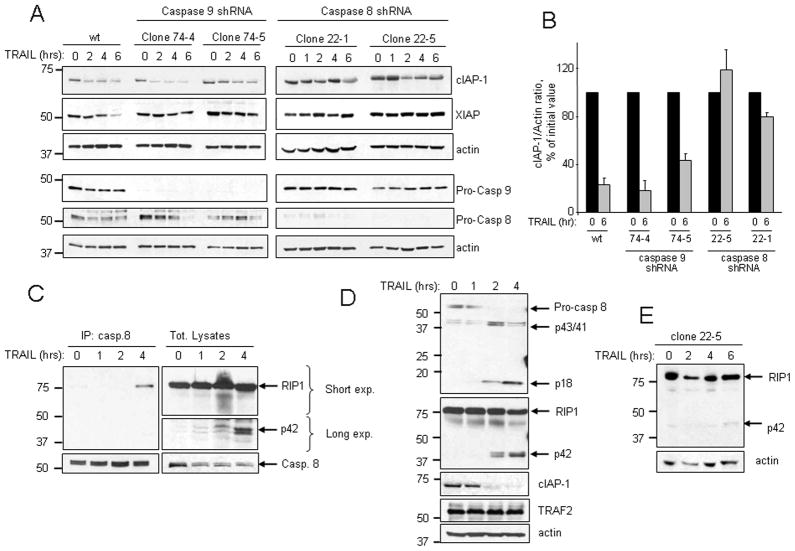
To further define which caspase(s) was involved in cIAP-1 degradation, we initially silenced caspase 8 or 9 in HuH-7 cells by targeted shRNA. Our reasoning was that if caspase 8 participated in cIAP-1 degradation, this was likely a proximal event in TRAIL signaling and important in TRAIL-mediated apoptosis. In contrast, if caspase 9 was necessary for cIAP-1 elimination, it would be more likely that the effector caspases 3, 6, and 7 activated by caspase 9 downstream the mitochondria were responsible for cIAP-1 degradation; in this latter scenario, the caspase-mediated degradation of cIAP-1 would likely be a consequence rather than an active component of TRAIL cytotoxicity. Knockdown of caspase 8 reduced both cIAP-1 and XIAP degradation during TRAIL-treatment, whereas caspase 9 knockdown had no effect on cIAP-1 stability (Fig. 5A–B and 1S). However, caspase 9 knockdown prevented XIAP depletion, suggesting caspase 9 activity is required for XIAP cleavage (Fig. 5A); these observations are consistent with previous findings describing cleavage of XIAP by effector caspases during death receptor-mediated apoptosis [29].
Figure 5. TRAIL-mediated degradation of cIAP-1 is caspase-8 dependent.
(A) Parental HuH-7 cells or clones of HuH-7 cells stably transfected with shRNA against caspase 9 or caspase 8 were treated with TRAIL (20 ng/ml). At the indicated time points, cell lysates were obtained and analyzed by immunoblotting for cIAP-1 and XIAP; effectiveness of knockdown was verified by caspase 8 and caspase 9 immunoblot. (B) Immunoblots for cIAP-1 from independent experiments (n=3 for wild-type and 22-1; n=4 for 22-5; n=2 for 74-4 and 74-5) as described in (A) were quantified by densitometry. cIAP-1/Actin ratios were calculated at 6 hr and normalized to time zero, which was arbitrarily set at 100%. Data are expressed as mean ± SEM. (C) HuH-7 cells were treated with TRAIL (20 ng/ml) and co-immunoprecipitation of RIP1 and caspase 8 was performed at the indicated time points as described in Experimental Procedures. (D) HuH-7 cells were treated with TRAIL (20 ng/ml); total cell lysates were obtained at the indicated time points and analyzed by immunoblotting for caspase 8, RIP1, cIAP-1 and TRAF2. Actin was used as loading control. (E) Caspase 8 shRNA HuH-7 cells (clone 22-5) were treated with TRAIL (20 ng/ml); total cell lysates were obtained at the indicated time points and analyzed by immunoblotting for RIP1. Actin was used as loading control.
Previous studies demonstrated that cIAP-1 and cIAP-2 are responsible for Lys-63 polyubiquitination of RIP1 in cancer cells, which, in turn, results in activation of NF-κB-mediated survival signals [14, 30]. When RIP1 ubiquitination is blocked, i.e., by treatment with a SMAC mimetic, RIP1 associates with caspase 8, and is subsequently cleaved by caspase 8 itself, switching from a pro-survival to a pro-apoptotic molecule, promoting further caspase 8 activation [14, 31]. Therefore, TRAIL-mediated degradation of cIAP-1 should result in RIP1 deubiquitination, association to caspase 8 and subsequent RIP1 cleavage. Indeed, TRAIL treatment was associated with formation of a caspase 8:RIP1 complex, as demonstrated by coimmunoprecipitation of endogenous caspase 8 and RIP1 (Fig. 5C), and generation of RIP1 fragments consistent with cleavage by caspase 8 (Fig. 5C and D) [32]. TRAIL-induced cleavage of RIP1 was significantly reduced in cells with caspase 8 knockdown, confirming that caspase 8 is required for RIP1 cleavage (Fig. 5E and 1S). TRAF2, which also functions as E3 ligase for cIAP-1, was not altered by TRAIL treatment (Fig. 5D). Importantly, the kinetic of caspase 8 activation coincided with that of cIAP-1 cleavage and RIP1 cleavage (Fig. 5D), supporting the hypothesis that cIAP-1 degradation is a proximal event in TRAIL signaling.
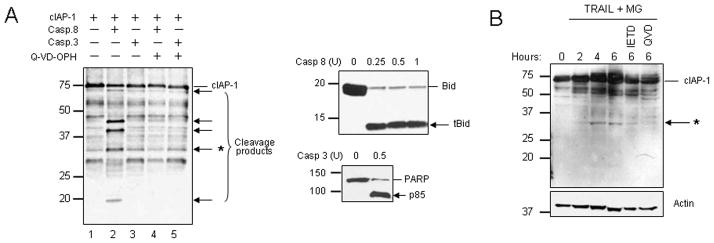
To ascertain if cIAP-1 is a direct substrate of caspase 8, recombinant human cIAP-1 was incubated with recombinant active caspase 8 in a cell-free system, and then subjected to SDS PAGE and immunoblot analysis. The concentration of caspase 8 used in this experiment was able to cleave 95% of the well-established caspase 8 substrate Bid in the same experimental conditions (Fig. 6A – right panel). cIAP-1 was cleaved by caspase 8, generating at least five novel fragments indicative of multiple cleavage sites for caspase 8 within cIAP-1 (Fig. 6A, lane 2). Formation of the fragments was inhibited in the presence of the pan-caspase inhibitor Q-VD-OPH (Fig. 6A, lane 4). Since cIAP-1 has been previously reported to be cleaved by caspase 3 into a 52 kDa and a 35 kDa fragment during apoptosis [33], recombinant cIAP-1 was also incubated with recombinant active caspase 3 to compare the cleavage patterns from the two caspases. Surprisingly, we were not able to reproduce the previous finding, as in our hands, caspase 3 did not cleave cIAP-1 in vitro (Fig. 6A, lane 3) at concentrations which effectively cleave the known caspase 3 substrate PARP (Fig. 6A – right panel).
Figure 6. cIAP-1 is cleaved by caspase-8 in cell-free system and in vivo.
(A) Recombinant human cIAP-1 was incubated with human recombinant active caspase 8 (1 U) or human recombinant active caspase 3 (0.5 unit), in the presence or absence of Q-VD-OPH (100 μM) for 30 minutes at 37°C and analyzed by immunoblot. Newly generated fragments are indicated by the arrows (left panel). As controls, recombinant human Bid or PARP were incubated with human recombinant active caspase 8 (0.25-1 U) or human recombinant active caspase 3 (0.5 unit), respectively, for 30 minutes at 37°C and analyzed by immunoblot (right panel). (B) HuH-7 cells were incubated with TRAIL (20 ng/ml) and MG132 (10 μM; 1 hr pre-incubation), in the presence or absence of Q-VD-OPH (5 μM) or z-IETD-fmk (10 μM). Cell lysates were analyzed by immunoblot for cIAP-1 and actin (loading control). The asterisk (*) indicates a fragment of approximately 32 kDa generated both in vivo and in the cell-free system.
As cIAP-1 fragments were normally not detectable in samples from cells treated with TRAIL, we reasoned that they may be subjected to proteasomal degradation in vivo. Indeed, when HuH-7 cells were treated with TRAIL in the presence of the proteasome inhinbitor MG132, several fragments generated in a time-dependent manner after TRAIL-treatment were identified, the predominant of which (32 kDa in size) appears to match a fragment obtained in the cell-free system (Fig. 6A and 6B - asterisk). More importantly, addition of Q-VD-OPH or the caspase 8 inhibitor z-IETD-fmk prevented the formation of the fragment (Fig. 6B). These results suggest caspase 8 directly participates to cIAP-1 degradation during TRAIL cytotoxicity. Taken together, our data indicate that TRAIL induces caspase 8-dependent loss of IAPs, which results in RIP1 binding to caspase-8, cleavage of RIP1 by caspase 8, and amplification of the apoptotic cascade.
DISCUSSION
The results of this study provide new insights regarding the mechanism of TRAIL cytotoxicity in liver cancer cells, in particular, the role of IAPs in mediating resistance to TRAIL-induced apoptosis. The principal findings indicate that (i) TRAIL-mediated apoptosis is associated with degradation of cIAP-1 and XIAP; (ii) genetic or pharmacological depletion of cIAP-1, but not XIAP or cIAP-2, sensitizes to TRAIL-induced apoptosis; (iii) TRAIL-induced cIAP-1 degradation requires caspase 8 activity. Each of these results is discussed in greater detail below.
Although overexpression of IAP proteins inhibits cell death by various stimuli [34], the precise mechanisms regulating their anti-apoptotic activity remain largely unknown. Direct caspase inhibition has only been established for XIAP, whereas cIAP-1 and cIAP-2 are weak caspase inhibitors despite their ability to bind caspases [35]. Recent studies have implicated cIAP-1 and cIAP-2 in TNF-R1-mediated signaling pathways [5, 15, 16, 36]. In particular, cIAP-1 and cIAP-2 have been shown to ubiquitinate and activate RIP1, promoting cancer cell survival by sustained activation of RIP1-mediated pro-survival signaling pathways [14]. SMAC mimetic compounds cause cIAP-1 and cIAP-2 degradation, resulting in production of TNF-α via activation of NF-κB, generating a TNF-α autocrine loop which results in enhanced TNF-α/TNF-R1-mediated apoptosis [15, 16]. However, the involvement of cellular IAPs in regulation of TRAIL-induced apoptosis is relatively unexplored. Our data in liver cancer cells imply that TRAIL concentrations able to induce apoptosis cause degradation of both cIAP-1 and XIAP proteins, suggesting that cellular removal of cIAP-1 and XIAP may facilitate TRAIL-initiated apoptosis. Subsequent knock-down experiments focused our studies on cIAP-1, as only depletion of cIAP-1 increased cell sensitivity to TRAIL apoptosis, while cells with reduced XIAP expression were indistinguishable from the wild-type cells. Our findings may appear to be at variance with previous observations that inhibition of XIAP sensitizes pancreatic carcinoma cells to TRAIL-mediated apoptosis in vivo and in vitro, suggesting XIAP plays the most critical role in regulating TRAIL signaling [37, 38]. This apparent discrepancy could be explained by differences in the cell lines examined, in particular their relative expression of XIAP and cIAP-1. Indeed, cIAP-1 has been found to be over-expressed in hepatocellular carcinoma due to genetic amplification [39], while high levels of XIAP have been described in pancreatic carcinoma [40, 41]. In our current study, treatment with a SMAC mimetic induced rapid and complete degradation of cIAP-1, but not XIAP, and greatly increased cell sensitivity to TRAIL killing. We are cognizant that degradation of XIAP is not required for inhibition by SMAC mimetics, in contrast to cIAP-1 and cIAP-2 [15, 16]. Thus, while the data employing the SMAC mimetic leave open a possible role for XIAP, shRNA-mediated knockdown experiments implicate cIAP-1 as the predominant IAP in these cells.
In addition to the auto-ubiquitination and proteasomal degradation evoked by the SMAC mimetics, degradation of cIAP-1 can be mediated by other pathways. Recent studies have demonstrated that cIAP-1 is targeted for degradation during CD30 signaling via a mechanism that requires TRAF2 E3 ubiquitin ligase activity, but not cIAP-1 E3 ligase activity and its auto-ubiquitination [42]. Moreover, degradation of the cIAP-1:TRAF2 complex occurs via a lysosomal pathway following stimulation of the TNF superfamily receptor FN14 by its ligand TWEAK [26]. Our data indicate that during TRAIL-induced apoptosis, neither of these mechanisms contributes to cIAP-1 degradation. Specifically, our results demonstrated that cIAP-1 depletion is mediated by caspase 8, although we cannot rule out that other caspases activated downstream of caspase 8 may also be involved in cIAP-1 degradation via a feedback loop. Indeed, previous reports suggest that cIAP-1 can be cleaved by caspase 3 and, possibly, by other downstream caspases [33], although we were not able to reproduce these findings in a cell-free system. Moreover, activation of caspase 9 is necessary to mediate the activation of downstream caspases after mitochondrial permeabilization. In the current study, caspase 9 knockdown did not prevent loss of cIAP-1, supporting the hypothesis that cIAP-1 degradation is a proximal event in TRAIL signaling. Finally, caspase 8 directly cleaved cIAP-1 in a cell-free system, indicating that cIAP-1 is a substrate for caspase 8. Caspase 8 cleavage generates several cIAP-1 fragments, suggesting that multiple cleavage sites are likely present on cIAP-1. At least one of these fragments, the most abundant, was also identified in protein lysates from cells treated with pro-apoptotic concentrations of TRAIL. The cleavage products were only detectable in the presence of a proteasome inhibitor, indicating that the cIAP-1 fragments are likely degraded via the ubiquitin/proteasome system in vivo. Mapping of caspase 8 cleavage sites is complicated by the large number of potential cleavage sites on cIAP-1. A computer-based analysis of the protein sequence revealed 31 putative caspase cleavage sites are present on cIAP-1 [43]. Identifying which of these sites are caspase recognition sites in vivo is beyond the scope of this study and will require detailed analysis.
In conclusion, our data have highlighted a novel signaling pathway during TRAIL-induced apoptosis mediated by caspase 8-dependent cIAP-1 degradation. Loss of cIAP-1 causes deubiquitination of RIP1, allowing its association with caspase 8 and promoting cell death. These results emphasize the crucial role for cIAP-1 in regulating TRAIL-resistance, and suggest that strategies targeting cIAP-1 expression may be beneficial to restore TRAIL-sensitivity in liver cancer cells.
Supplementary Material
Parental HuH-7 cells or HuH-7 cells stably transfected with shRNA against caspase 8 (clone 22-5) were treated with TRAIL (20 ng/ml) in two independent experiments. At the indicated time points, cell lysates were obtained and analyzed by immunoblotting for cIAP-1 and RIP1.
Acknowledgments
The superb secretarial assistance of Erin Nystun-Bungum is highly appreciated. We thank Dr. Scott H. Kaufmann for providing reagents and for helpful discussion and suggestions.
This work was supported by NIH grant R01 DK63947 (to GJG), the Optical Microscopy Core of P30 DK84567, and the Mayo Foundation.
ABBREVIATIONS
- TRAIL
TNF-related apoptosis-inducing ligand
- IAP
inhibitor of apoptosis protein
- cIAP-1
cellular inhibitor of apoptosis 1
- cIAP-2
cellular inhibitor of apoptosis 2
- XIAP
X-linked inhibitor of apoptosis
- PARP
poly (ADP-ribose) polymerase
- SMAC
second mitochondrial activator of caspases
- FADD
Fas-associated protein with death domain
- TNF-α
tumor necrosis factor alpha
- TNFR1
TNF receptor 1
- TRAF2
TNFR-associated factor 2
- NF-κB
nuclear factor kappa B
- E3
ubiquitin-protein isopeptide ligase
- RIP1
receptor interacting protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 2.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- 5.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 7.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 9.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 10.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 11.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 12.Creagh EM, Murphy BM, Duriez PJ, Duckett CS, Martin SJ. Smac/Diablo antagonizes ubiquitin ligase activity of inhibitor of apoptosis proteins. J Biol Chem. 2004;279:26906–26914. doi: 10.1074/jbc.M313859200. [DOI] [PubMed] [Google Scholar]
- 13.Galban S, Hwang C, Rumble JM, Oetjen KA, Wright CW, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Duckett CS. Cytoprotective effects of IAPs revealed by a small molecule antagonist. Biochem J. 2009;417:765–771. doi: 10.1042/BJ20081677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 18.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y, Liu M, Tang W, Li Y, Lian J, Lawrence TS, Xu L. A Smac-mimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB. BMC Cancer. 2009;9:392. doi: 10.1186/1471-2407-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberts SR, Gores GJ, Kim GP, Roberts LR, Kendrick ML, Rosen CB, Chari ST, Martenson JA. Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin Proc. 2007;82:628–637. doi: 10.4065/82.5.628. [DOI] [PubMed] [Google Scholar]
- 21.Guicciardi ME, Bronk SF, Werneburg NW, Gores GJ. cFLIPL prevents TRAIL-induced apoptosis of hepatocellular carcinoma cells by inhibiting the lysosomal pathway of apoptosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1337–1346. doi: 10.1152/ajpgi.00497.2006. [DOI] [PubMed] [Google Scholar]
- 22.Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J Biol Chem. 2007;282:28960–28970. doi: 10.1074/jbc.M705671200. [DOI] [PubMed] [Google Scholar]
- 23.Guicciardi ME, Bronk SF, Werneburg NW, Yin XM, Gores GJ. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129:269–284. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 25.Csomos RA, Brady GF, Duckett CS. Enhanced Cytoprotective Effects of the Inhibitor of Apoptosis Protein Cellular IAP1 through Stabilization with TRAF2. J Biol Chem. 2009;284:20531–20539. doi: 10.1074/jbc.M109.029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P, McKinlay M, Benetatos CA, Condon SM, Chunduru SK, Yeoh G, Brink R, Vaux DL, Silke J. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–1090. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- 29.Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. Embo J. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clem RJ, Sheu TT, Richter BW, He WW, Thornberry NA, Duckett CS, Hardwick JM. c-IAP1 is cleaved by caspases to produce a proapoptotic C-terminal fragment. J Biol Chem. 2001;276:7602–7608. doi: 10.1074/jbc.M010259200. [DOI] [PubMed] [Google Scholar]
- 34.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 35.Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 36.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, Gschwend JE, Simmet T, Debatin KM, Fulda S. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res. 2008;68:7956–7965. doi: 10.1158/0008-5472.CAN-08-1296. [DOI] [PubMed] [Google Scholar]
- 38.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, Bhanot U, Hasel C, Moller P, Gschwend JE, Simmet T, Debatin KM, Fulda S. Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009;69:2425–2434. doi: 10.1158/0008-5472.CAN-08-2436. [DOI] [PubMed] [Google Scholar]
- 39.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrikhande SV, Kleeff J, Kayed H, Keleg S, Reiser C, Giese T, Buchler MW, Esposito I, Friess H. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer Res. 2006;26:3265–3273. [PubMed] [Google Scholar]
- 41.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. [PubMed] [Google Scholar]
- 42.Csomos RA, Wright CW, Galban S, Oetjen KA, Duckett CS. Two distinct signalling cascades target the NF-kappaB regulatory factor c-IAP1 for degradation. Biochem J. 2009;420:83–91. doi: 10.1042/BJ20082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wee LJ, Tan TW, Ranganathan S. SVM-based prediction of caspase substrate cleavage sites. BMC Bioinformatics. 2006;7(Suppl 5):S14. doi: 10.1186/1471-2105-7-S5-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parental HuH-7 cells or HuH-7 cells stably transfected with shRNA against caspase 8 (clone 22-5) were treated with TRAIL (20 ng/ml) in two independent experiments. At the indicated time points, cell lysates were obtained and analyzed by immunoblotting for cIAP-1 and RIP1.