Abstract
N-truncated/modified forms of amyloid beta (Aß) peptide are found in diffused and dense core plaques in Alzheimer's disease (AD) and Down's syndrome patients as well as animal models of AD, and represent highly desirable therapeutic targets. In the present study we have focused on Ntruncated/modified Aβ peptide bearing amino-terminal pyroglutamate at position 11 (AβN11(pE)). We identified two B-cell epitopes recognized by rabbit anti-AβN11(pE) polyclonal antibodies. Interestingly, rabbit anti-AβN11(pE) polyclonal antibodies bound also to full-length Aβ1-42 and N-truncated/modified AβN3(pE), suggesting that the three peptides may share a common B-cell epitope. Importantly, rabbit anti-AβN11(pE) antibodies bound to naturally occurring Aβ aggregates present in brain samples from AD patients. These results are potentially important for developing novel immunogens for targeting N-truncated/modified Aβ aggregates as well, since the most commonly used immunogens in the majority of vaccine studies have been shown to induce antibodies that recognize the N-terminal immunodominant epitope (EFRH) of the full length Aβ, which is absent in N-amino truncated peptides.
Keywords: N-truncated amyloid beta (Aß) peptide, Alzheimer's disease immunotherapy, immunodominant epitope, B cell epitope
1. INTRODUCTION
The accumulation of fibrillar and oligomeric forms of amyloid-beta (Aβ) peptide in the brain has been hypothesized to play a central role in the neuropathology of Alzheimer's Disease (AD) (Master et al., 1985; Walsh and Selkoe, 2004; Haas and Selkoe, 2007). The main Aβ variants detected in the human brain are Aβ1-40 and Aβ1-42, however a significant proportion of AD brain Aβ consists also of N-terminal truncated/modified species (Mori et al., 1992; Seubert et al., 1992; Saido et al., 1995; Tekirian et al., 1998; Guntert et al., 2006; Wirths et al., 2010). Previous studies have demonstrated that these shortened Aβ forms are significantly more resistant to degradation, aggregate more rapidly in vitro and exhibit similar or, in some cases, increased toxicity in hippocampal neuronal cultures compared to the full-length peptides (Pike et al., 1995; Russo et al., 2002; Schilling et al., 2006; Youssef et al., 2007; D'Arrigo et al., 2009). Also, it has been demonstrated that N-truncated Aβ peptides progressively accumulate in the brain of Familial Alzheimer's disease (FAD) and Down syndrome patients as well as in the brain of sporadic AD patients at the earliest stages of AD even before the appearance of clinical symptoms (Saido et al., 1995; Tekirian et al., 1998; naslund et al., 1994; Kumar-Singh et al., 2000; Huse et al., 2002; Sergeant et al., 2003; Piccini et al., 2005; Vanderstichele et al., 2005; Liu et al., 2006). In addition, the presence of intraneuronal pool of N-truncated Aβ peptides has been shown to correlate with the progression of pathology and neuronal loss in transgenic mice models APP/PS1KI and TBA2 (Casas et al., 2004; Bayer et al., 2008; Wirths et al., 2009). Thus, the N-terminally truncated/modified Aβ peptides represent highly desirable and abundant therapeutic targets.
Most of N-truncated Aβ peptides have been considered to be the degradation products of full-length Aβ, however, the cloning and overexpression in cultured cells of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) led to the conclusion that Aβ11-40/42 may be generated intracellularly directly by BACE1 cleavage of APP (Vassar et al., 1999; Huse et al., 2002; Lee et al., 2003; Liu et al., 2006). This shortened form of Aβ peptide may be further modified by cyclization of the N-terminal glutamate resulting in a peptide bearing amino-terminal pyroglutamate at position 11 (AβN11(pE)). This modification protects the peptide from degradation by most aminopeptidases leading to its accumulation and aggregation.
Anti-Aβ antibodies have been shown to disrupt Aβ aggregates, block aggregation, attenuate toxicity, as well as promote the clearance of the peptide in the central nervous system (CNS). Immunotherapy approaches, both active immunization with Aβ peptide, or passive transfer of anti-Aβ antibodies, have been demonstrated to decrease amyloid deposits and associated neuronal and inflammatory pathologies and reverse Aβ-related cognitive deficits in several amyloid precursor protein transgenic (APP/Tg) mouse models (Schenk et al., 1999; Bard et al., 2000; Wilcock et al., 2004; Brody and Holtzman, 2008; Biscaro et al., 2009; Lemere, 2009), as well as canine and primates models of amyloidosis (Lemere et al., 2004; Head et al., 2008). Interestingly, the majority of the previous studies used mainly Aβ1-40 or Aβ1-42 as an immunogen for active immunization, which induced antibodies specific for amino-terminal part (EFRH epitope) of Aβ. However, most of the N-truncated/modified forms of the Aβ lack this critical B-cell epitope. Thus, novel immunogens directed to generate anti-N-truncated/modified Aβ antibodies should be designed and considered for vaccine preparations for AD.
In the present study we have focused on N-truncated/modified Aβ peptide bearing amino-terminal pyroglutamate at position 11 (AβN11(pE)). We produced anti-AβN11(pE) polyclonal antibodies in rabbits, and identified two B-cell epitopes recognized by these antibodies. Interestingly, rabbit anti-AβN11(pE) polyclonal antibodies bound also to full-length Aβ1-42 and Ntruncated/modified AβN3(pE), suggesting that the three peptides may share a common B-cell epitope. Importantly, we demonstrated that rabbit anti-AβN11(pE) antibodies bound to Aβ deposits present in AD brain and inhibit AβN11(pE)-induced cytotoxicity in IMR-32 differentiated neuroblastoma cells. We believe our results are potentially important for developing novel immunogens targeting N-amino-truncated/modified AβN11(pE) and AβN11(pE) as well as full-length Aβ1-42, three main pathological species of the Aβ peptide present in human brain.
2. MATERIALS AND METHODS
2.1. Materials
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). Synthetic human Aβ1-42, Aβ1-16, Aβ8-42, Aβ17-42, Aβ12-28 and Aβ35-25 as well as Npyroglutamate modified peptides AβN3(pE) and AβN11(pE) were purchased from AnaSpec (San Jose, CA, USA). A monoclonal anti-Aβ antibodieds (4G8, BAM10 and BAM90.1) were from Sigma. HRP-conjugated anti-mouse IgG2b and IgG1 and HRP-conjugated goat anti-rabbit IgG were from Zymed (San Francisco, CA, USA). Super Signal West Dura Extended Duration Substrate kit was from Pierce, Rockford, IL, USA.
2.2. Peptide preparation, WB and dot blot assays
Aβ1-42, AβN3(pE) and AβN11(pE) were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to allow a conversion to the monomer and, after evaporation of solvent, were stored in aliquots at −20°C. Oligomeric Aβ1-42, AβN3(pE) and AβN11(pE) were prepared essentially as described previously by incubation of monomers in DMEM/F12 at 4°C for 24 hrs following overnight incubation at 37°C (Klein, 2002; Solorzano-Vargas et al., 2008). Aβ1-16, Aβ17-42 and Aβ12-28 were dissolved in a water at a concentration of 1 mg/ml. Formation of oligomeric Aβ1-42, AβN3(pE) and AβN11(pE) species was confirmed by the Western Blot. Briefly, after incubation, peptides were separated by electrophoresis on 4-12% polyacrylamide precast NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA, USA) at 100 V for 1 h 45 min and transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA) using a semi dry blot system (Bio-Rad) at 25 V for 50 min. Membranes were blocked in PBS/2% non-fat dry milk/0.2% Triton X-100 overnight at 4°C and incubated overnight at 4°C with primary antibodies: 4G8 (1:2000), BAM10 (1:2000), BAM90.1 (1:2000) or rabbit anti-AβN11(pE) polyclonal IgG. After washing with PBS/0.2% Tween, the membranes were incubated with HRP-conjugated anti-mouse IgG2b or IgG1, 1:2500 or anti-rabbit IgG, for 2 h at RT. Immunoreactive bands were detected by chemiluminescence using SuperSignal West Dura Extended Duration Substrate kit. In competition WB assays, rabbit anti-AβN11(pE) polyclonal IgG were incubated with corresponding phage (109pfu/ml) 1hr at room temperature prior to adding to the membrane. Then the assay was performed as described above. For dot blot assay, peptide monomers prepared as described above, were applied to PVDF membrane and air dried. Rabbit anti-AβN3(pE), anti-AβN11(pE) and anti-Aβ1-42 polyclonal IgG diluted in PBS/2% non-fat dry milk/0.2% Triton X-100 (4μ/ml) were added, and after overnight incubation at 4°C, the immunoreactivity was detected as described above.
2.3. Immunization protocol
All experiments with animals were conducted using protocols approved by our Institutional Animal Care Committee. Three 3-month-old White New Zealand rabbits were immunized subcutaneously (s.c.) at 14 days intervals with 200 μg of human AβN11(pE) oligomers prepared at 37°C as described above. Freund's complete adjuvant was used for primary injection followed by incomplete Freund's adjuvant for three boost injections. Control rabbits were immunized with adjuvant alone or with a non-related peptide. Animals were bled on day 0 and 10 days after the third boost injection. The sera were stored at – 20°C until use.
2.4. Purification of rabbit IgG
IgG were precipitated from rabbit sera with 1.7 M ammonium sulphate and dialyzed against PBS. Then this solution was applied to columns with Protein G-Sepharose (Zymed) and incubated for 1 h at room temperature. Non-bound proteins were removed by washing with PBS, and IgG was eluted with elution buffer (0.2 M glycine, adjusted to pH 2.8 with HCl). After dialysis against water, antibodies were lyophilized and stored at −20°C.
2.5. ELISA for evaluation of anti- AβN11(pE) antibodies
ELISA analysis was carried out using MaxiSorp microtiter plates (Nunc, Roskilde, Denmark) coated overnight with a synthetic peptides at a concentration of 20 μg/ml in carbonate buffer (pH 9.6). After washing with phosphate buffer containing 0.2% Tween-20 (PBS-Tween), plates were blocked with PBS/2% non-fat dry milk for 1 h at 37°C. Plates were washed, then rabbit sera diluted in PBS/2% non-fat dry milk/0.2% Triton X-100 were added and after incubation for 1 h at 37°C, plates were washed with PBS/0.2% Tween. Rabbit anti-human Aβ1-42 polyclonal antibodies (Zymed) as well as rabbit polyclonal anti-AβN3(pE) antibodies obtained in our laboratory (Acero et al., 2009) and mouse anti-Aβ monoclonal antibodies (4G8, BAM10 and BAM90.1) were used as a positive control to confirm the peptide binding to well. HRP-conjugated goat anti-rabbit IgG or anti-mouse IgG1 and IgG2b (Zymed) diluted in PBS/2% non-fat dry milk/0.2% Triton X-100 were added, and plates were incubated for 1 h at 37°C. Plates were washed and 2,2′-azino-bis- (3-ethyl-benzthiazoline-6-sulphonic acid (ABTS) single solution (Zymed) was added. The OD reading at 405 nm was registered using Opsys MR Microplate Reader (DYNEX Technologies, Chantilly, VA, USA). For competition ELISA, sera were preincubated overnight at 4°C with the preparations of synthetic peptides (diluted 1:200, 1:400, 1:600 and 1:800), prior to adding to AβN11(pE)-coated wells. Then the assay was performed as described above.
2.6. Immunohistochemical analysis
Brain tissue
Brain tissue from six AD patients was examined in this study (2–6 hour post-mortem delay). The diagnosis of AD was obtained by the NIANINCDS group criteria Mckhann et al., 1984). Blocks of the temporal cortex were fixed by immersion in a solution of 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, at 4°C for 7 days.
Double immunolabeling
50 μm-thick free-floating sliding microtome brain tissue sections were pretreated with 99% formic acid (Merck, Darmstadt), by immersion for 3 min, at RT and then thoroughly washed several times with TBS (Tris-buffer saline). After blocking with a solution of 0.2% IgG free-albumin (Sigma) in TBS for 20 min at RT, brain slices were incubated overnight at 4°C with anti-AβN11(pE) antibodies (1:200) combined with BAM10 (1:200) diluted in TBS containing 0.2% Triton X-100 (TBS-Tx). Then sections were rinsed several times with TBS-Tx and incubated with a mixture of TRITC-tagged goat-anti-mouse IgG and FITC-tagged goat-anti rabbit IgG (Jackson Immuno. Res. Lab. Inc. West Grove) diluted in TBS.Tx for 1 hour at RT.
To confirm the specificity of anti-AβN11(pE) antibodies in AD brain tissue, a 5μM solution of Aβ35-25, Aβ1-42, AβN3(pE) and AβN11(pE) were preincubated, individually, with anti-AβN11(pE) antibodies at 37°C for 2 hr and immunohistochemistry was performed as previously described.
Confocal microscopy
Sections were mounted in the anti-quenching media Vectashield (Vector Labs. Burlingame) and viewed through a confocal laser scanning microscope (TCP-SP2, Leica, Heidelberg) using a 20x or 100x oil-immersion plan Apochromat objective (NA 1.4). Ten to fifteen consecutive single sections were obtained at 0.8-1.0 μm intervals and sequentially scan for two channels throughout the z-axis of the sample. The resulting stack of images was projected and analyzed onto the two-dimensional plane using a pseudocolor display green (FITC) and red (TRITC). Fluorochromes in double and triple labeled samples were excited at 488nm (for FITC) and 540nm (for TRITC) wavelengths.
2.7. Inhibition of AβN11(pE) toxicity in vitro by selected anti-AβN11(pE) antibodies in IMR-32 cell cultures
Human neuroblastoma IMR-32 cells obtained from the American Type Culture Collection (ATCC, VA, USA) were maintained in DMEM/F12 (1:1) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO) and penicillin-streptomycin (GIBCO) and differentiated for 8-10 days in the presence of 1mM dibutyryl cAMP. To test the inhibitory effect of anti-AβN11(pE) antibodies on the neuronal toxicity induced by AβN11(pE), cells were plated at a density of 1 × 104 cells/ 200 μl per well in 96-well tissue culture plates (Corning, NY, USA). For all assays, AβN11(pE) peptide preparations were mixed to a final concentration of 20 μmol/ml in OPTI-MEM serum free medium containing 200 u/ml penicillin and 200 μg/ml streptomycin. Synthetic Aβ35-25 was used as a negative control. Anti-AβN11(pE) IgGs and IgGs from the rabbit immunizaed with adjuvant only were mixed with AβN11(pE) peptide (1:1 molar ratio) and incubated for 1 hr at room temperature. Before treatment of cells, the old medium was removed and the peptide preparation (with or without antibodies) was added to each well and incubated for 48 hrs. Cell viability was assessed using an XTT cytoxicity assay (sodium 3' {1-[phenylaminocarbonyl]-3-4 tetrazolium}-bis {4-methoxy-6-nitro} benzene sulfonic acid hydrate) (Roche, IN, USA) according to manufacturer's instructions. 50 μl of XTT was added to each plate and incubated for 4 hrs at 37°C and 5% CO2. The OD reading at 500 nm was registered using Opsys MR Microplate Reader (DYNEX Technologies).
2.8. Affinity selection of phages binding to anti- AβN11(pE) antibodies
Selection of phages by biopanning was performed as described previously (Gevorkian et al., 2004). Briefly, a Phage Display Peptide Library from New England Biolabs (Beverly, MA, USA) was used. The displayed 7-mer peptides are expressed at the N-terminus of the minor coat protein (cpIII) of M13 phage. MaxiSorp microtiter plates were coated with goat anti-rabbit IgG (Zymed) at a concentration of 5 μg/ml 1 h at 37°C, washed and blocked with PBS-2%BSA. After washing, polyclonal rabbit anti-AβN11(pE) antibodies diluted 1:200 in PBS-Tween-1% BSA were added and plates were incubated for 1 h at 37°C. Plates were washed, then 1011 plaque-forming units (PFU) from phage library diluted in PBS/BSA 1% were added and plates were incubated overnight at 4°C. Non-specific phages were washed off, and bound phage clones were eluted by triethilamine (0.1 M) and neutralized by Tris-HCl (1M, pH 7.5). Three rounds of biopanning were performed, and 19 individual clones were selected after the third round, amplified and used in direct and competition ELISA to evaluate their binding to anti-AβN11(pE) antibodies. For direct ELISA experiments, MaxiSorp microtiter plates were coated with goat anti-rabbit IgG and blocked as described above. Rabbit anti-AβN11(pE) antibodies diluted 1:100 in PBS-Tween-1% BSA were added and plates were incubated for 1 h at 37°C. After washing, 1010 PFU/ml of each phage clone diluted in PBS-1%BSA were added to plates and incubated overnight at 4°C. After washing, HRPconjugated anti-M13 monoclonal antibody (Invitrogen) was added and incubated for 1 h at 37°C. After washing, ABTS single solution was added. The OD reading at 405 nm was registered using Opsys MR Microplate Reader (DYNEX Technologies). For competition ELISA, plates were coated with synthetic AβN11(pE) preparation and phage clones were incubated overnight with anti-AβN11(pE) antibodies before adding to plates. Then the assay was performed as described above. For competition WB analysis, phage clones (109/ml) were preincubated with rabbit anti-AβN11(pE) antibodies 1h at room temperature and then the assay was performed as described above.
Also, single-stranded DNA was prepared from all positive clones and one negative clone as described previously and used for DNA sequencing [37]. T7 Sequenase version 2.0 Quick Denature Plasmid Sequencing kit (Amersham Pharmacia Biotech, USA) and [α-35S] dATP (Amersham) were used according to the manufacturer's instructions.
2.9. BLAST homology search
A homology between peptide inserts of selected positive clones and known protein sequences was analyzed using BLASTP program (http://blast.ncbi.nlm.nih.gov).
2.10. Statistical analysis
Data were analyzed by ANOVA using SPSS statistical software program (Release 9.0).
3. RESULTS
3.1. Production of anti-AβN11(pE) antibodies and their binding to synthetic Aβ peptides
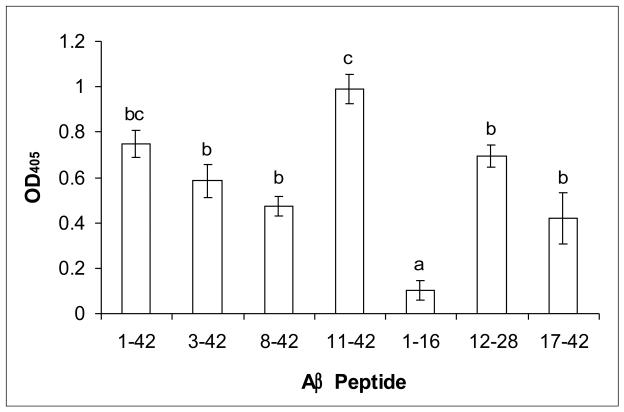
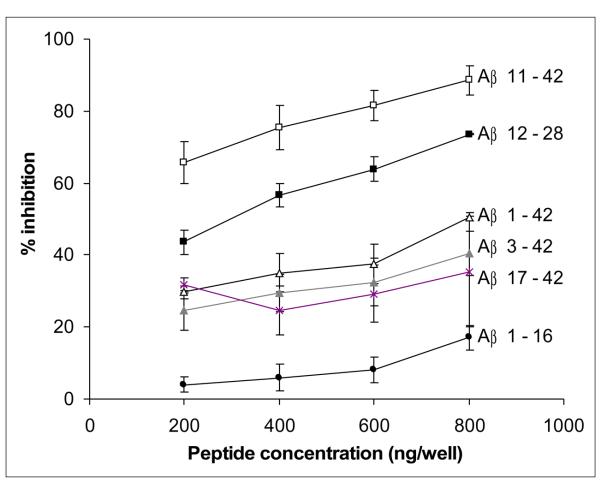
To produce anti-AβN11(pE) antibodies, rabbits were immunized with oligomeric peptide preparation mixed with adjuvant. Animals were bled 10 days after the third boost injection and the production of specific antibodies was tested by ELISA. Anti-AβN11(pE) antibodies were found to bind to AβN11(pE) peptide as well as to AβN3(pE), Aβ1-42, Aβ8-42, Aβ12-28 and Aβ17-42 while no binding to Aβ1-16 was observed (Fig.1). No binding of IgGs from preimmune serum or a serum from a rabbit immunized with the adjuvant alone, to Aβ peptides was detected. The specificity of binding of anti-AβN11(pE) antibodies to Aβ peptides was confirmed by competition ELISA. After preincubation of anti-AβN11(pE) antibodies with AβN11(pE), AβN3(pE), Aβ1-42, Aβ12-28 and Aβ17-42, but not with Aβ1-16, there were inhibition of binding of anti-AβN11(pE) antibodies to oligomeric AβN11(pE) immobilized wells (Fig.2).
Fig.1.
Rabbit anti-AβN11(pE) antibodies are binding in ELISA to AβN11(pE), Aβ1-42, AβN3(pE), Aβ8-42, Aβ12-28 and Aβ17-42 peptides. No recognition of Aβ1-16 was observed. Peptides were prepared as described in Materials and methods and used for covering microtiter plates. Rabbit serum was diluted 1:1000. Optical densities (OD) were registered at 405. Data are means of three independent experiments ±SD. Means denoted with different letters are statistically different (P<0.05). Rabbit preimmune sera as well as sera from rabbits immunized with adjuvant alone showed OD405 < 0.1.
Fig.2.
The specificity of binding of anti-AβN11(pE) antibodies to Aβ peptides was confirmed by competition ELISA. Sera were preincubated overnight at 4°C with the preparations of synthetic AβN3(pE), AβN11(pE), Aβ1-42, Aβ12-28, Aβ17-42 and Aβ1-16 (see Materials and Methods section) prior to adding to AβN11(pE)-coated wells.
3.2. Rabbit anti-AβN11(pE) antibodies bind to Aβ oligomers in Western Blot and Aβ deposits in human brain
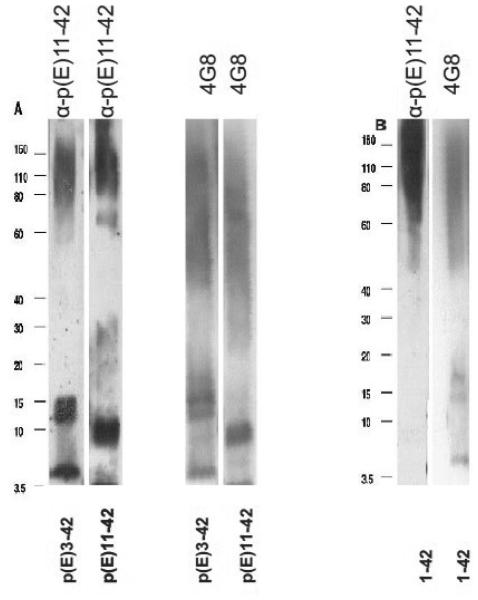
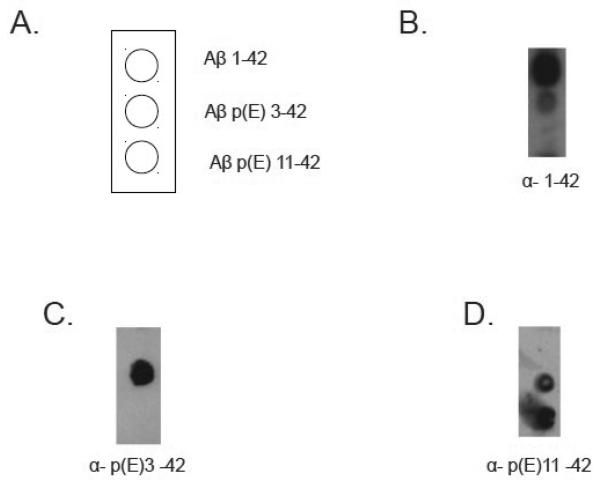
Anti-AβN11(pE) antibodies were tested in Western Blot and found to bind to all forms of AβN11(pE) and AβN3(pE). Interestingly, anti-AβN11(pE) antibodies did not bind to monomeric Aβ1-42, while binding to oligomeric Aβ1-42 was detected (Fig.3B). To further analyze preferential binding of polyclonal anti-AβN11(pE) antibodies we used dot blot assay. HFIP treated synthetic peptides Aβ1-42, AβN3(pE) and AβN11(pE) were diluted in DMSO and applied to PVDF membrane. Anti-AβN11(pE) antibodies bound to AβN3(pE) and AβN11(pE) monomers but failed to stain Aβ1-42 monomers (Fig. 4 D). Anti-Aβ1-42 rabbit IgGs were used as a positive control to stain Aβ1-42 monomers (Fig.4 B).
Fig.3.
Rabbit anti-AβN11(pE) antibodies bound to Aβ oligomers in Western Blot. Oligomers were prepared and analyzed as described in Materials and Methods section. (A) Anti-AβN11(pE) antibodies were used to detect AβN3(pE) and AβN11(pE). A monoclonal anti-Aβ antibody 4G8 was used as a control. (B) Rabbit anti-AβN11(pE) antibodies were used to detect oligomers of Aβ1-42. A monoclonal anti-Aβ antibody (4G8) binding to a central part of Aβ1-42 was used as a control. No binding of anti-AβN11(pE) antibodies to a monomer of Aβ1-42 was observed although a control antibody (4G8) detected it in the same preparation. Migration of the molecular mass standards is indicated.
Fig.4.
Dot blot analysis. Aβ monomers were applied to PVDF membrane as shown in A) and detected with rabbit anti-Aβ1-42 (B), anti-AβN3(pE) (C) and anti-AβN11(pE) (D) antibodies.
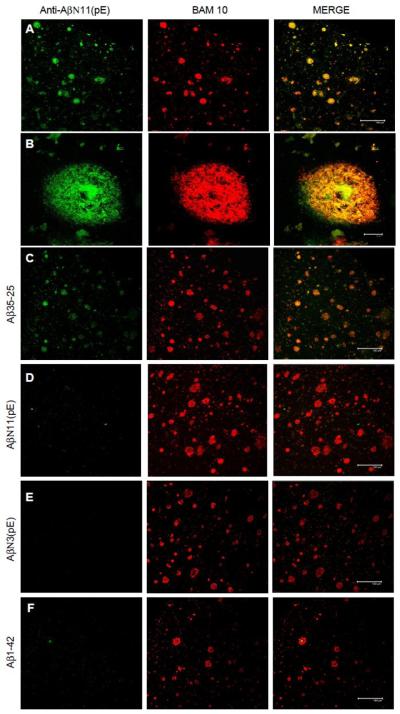
Importantly, anti-AβN11(pE) antibodies recognized amyloid deposits present in human brain from AD patients (Fig.5 A and B), and this binding was inhibited by Aβ1-42, AβN3(pE) and AβN11(pE), but not by Aβ35-25 (Fig.5 C-F).
Fig.5.
Anti-AβN11(pE) antibodies bound to amyloid aggregates in 50 μm-thick brain tissue sections of temporal cortex from AD patients (A,B), and this binding is inhibited by AβN11(pE) (D), AβN3(pE) (E) and Aβ1-42 (F), but not by Aβ35-25 (C). Each panel shows, from the left: the reactivity of anti-AβN11(pE) antibodies (shown in green); the reactivity of a mouse anti-human Aβ1-42 monoclonal antibody (BAM10) (shown in red); the merge between red and green channels. A, C-F: scale bar represents 150 μm; B: scale bar represents 10 μm.
3.3. Inhibition of AβN11(pE) toxicity in vitro by anti-AβN11(pE) antibodies in IMR-32 cell cultures
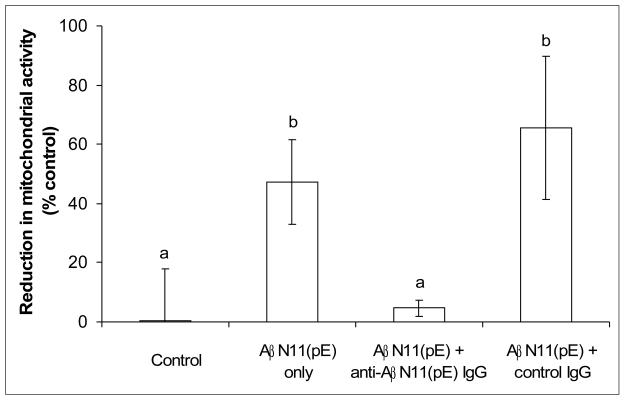
We assessed the ability of anti-AβN11(pE) antibodies to inhibit AβN11(pE)-induced cytotoxicity in IMR-32 differentiated neuroblastoma cells using XTT assay. Overnight preincubation of AβN11(pE) with anti-AβN11(pE) antibodies prior to adding to cells resulted in inhibition of cytotoxicity (Fig.6). Control IgGs from a rabbit immunized with adjuvant only did not show any inhibition activity.
Fig.6.
Anti-AβN11(pE) antibodies inhibit AβN11(pE) induced neurotoxicity in human differentiated neuroblastoma IMR-32 cell cultures. Cell viability was assessed using an XTT toxicity assay. Data presented are means ±SE of three independent experiments.
3.4. Affinity selection of phages binding to anti- AβN11(pE) antibodies
To identify the immunodominant region of AβN11(pE) peptide, the library of random heptapeptides displayed as a fusion to the minor coat protein of M13 phage was screened with rabbit anti-AβN11(pE) antibodies. Three rounds of biopanning were performed and 19 clones were randomly selected from the eluate after the third round. Binding of these clones to anti-AβN11(pE) antibodies was tested in ELISA. DNA sequences of heptapeptides coding inserts of 10 positive and 9 negative phage clones were determined and the deduced amino acid sequences of all positive clones are shown in Table 1. Peptide inserts of the seven positive clones could be grouped into one of two motifs having consensus sequences Q(E,R)HHHQ(E)HL(P) (phage clones C6, C12 and C15) and K(E,G)I(VF)AEA(G,D)L(S,P)F(R,Y) (phage clones C7, C11, C17 and C19). The first motif has a homology with an amino-terminal part of AβN11(pE) (aa 11-15) suggesting that the peptide inserts of these clones are mimotopes of an epitope present at the amino-terminal region of AβN11(pE). The second motif has a homology with a central part of AβN11(pE) (aa 20-24) suggesting a presence of another B cell epitope in this region. The remaining three positive clones (C1, C3, and C10) with no homology with AβN11(pE) could not be grouped into a clear motif.
Table 1.
Peptide sequences and reactivity of selected positive phage clones with rabbit anti-AβN11(pE) antibodies.
| PHAGE CLONE | SEQUENCE | OD405 nm |
|---|---|---|
| C6 | Q H H H Q H L | 0.95±0.1 |
| C12 | R H H H Q H P | 0.85±0.05 |
| C15 | E H H H E H L | 0.89±0.12 |
| C7 | K I A E A P F | 0.39±0.12 |
| C11 | E V A E G S R | 0.5±0.05 |
| C17 | K I A E A L F | 0.25±0.08 |
| C19 | G F A E D L Y | 0.23±0.11 |
| C1 | F I D P D R M | 0.38±0.04 |
| C3 | S H K D D T M | 0.78±0.11 |
| C10 | D L L M G H P | 0.78±0.17 |
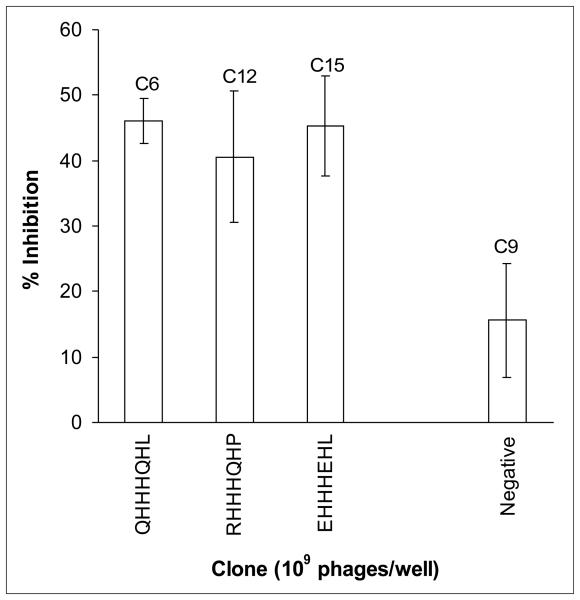
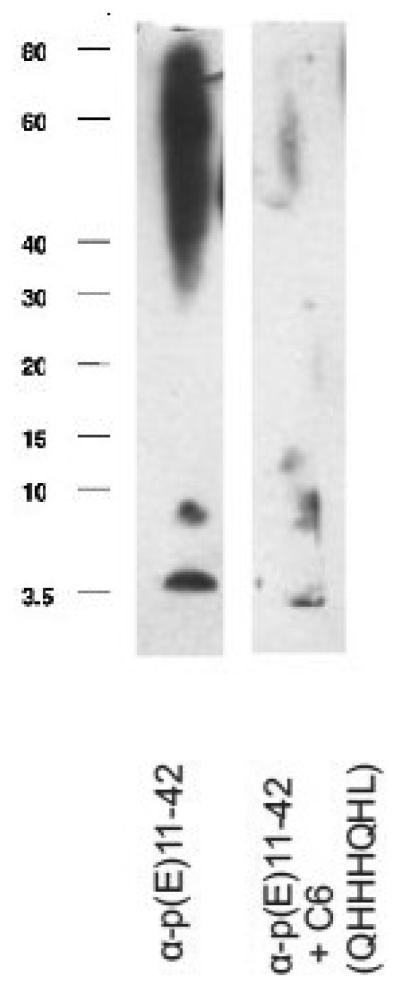
To test the binding specificity of the selected clones, the ability of the clones to inhibit the binding of anti-AβN11(pE) antibodies to AβN11(pE) peptide was studied in a competition ELISA (Fig.7). Three clones bearing a multiple histidine motif (C6, C12 and C15) showed the highest inhibition of binding to AβN11(pE) peptide, while four clones with inserts mimicking the epitope in the central region of AβN11(pE) (phage clones C7, C11, C17 and c19) and the remaining positive clones (C1, C3 and C10) showed a background inhibition similar to a negative phage clone C9 bearing a peptide sequence DVSAIMG. Also, in competition WB analysis phage C6 bearing a multiple histidine motif inhibited the binding of anti-AβN11(pE) antibodies to all forms of the peptide (Fig.8).
Fig.7.
Inhibition of the anti-AβN11(pE) antibody binding to AβN11(pE) by the selected phage clones. Plates were coated with AβN11(pE), and anti-AβN11(pE) antibodies were added after overnight incubation with or without phage clones. Optical densities (OD) were registered at 405. Data are means of three independent experiments ±SD.
Fig.8.
Inhibition of the anti-AβN11(pE) antibody binding to AβN11(pE) by the phage bearing a multiple histidine motif (C6). Phage preparation (109/ml) was incubated with rabbit anti-AβN11(pE) antibodies 1h at room temperature prior to adding to AβN11(pE)-blotted nitrocellulose membrane and the assay was performed as described in Materials and Methods.
4. DISCUSSION
It has been demonstrated that Aβ aggregates present in the brain of sporadic AD patients and in Down syndrome were significantly different and more toxic compared with Aβ present in normal brain, and this was correlated with the predominance of the N-truncated species over full length Aβ1-42 (Saido et al., 1995; Piccini et al., 2005; Vanderstichele et al., 2005; Schilling et al., 2008). Importantly, significant quantities of N-truncated Aβ peptides were post-translationally modified pyroglutamate-containing forms of Aβ (AβN3(pE) and AβN11(pE)). The resistance toward proteolytic degradation by aminopeptidases decreases the rate of their clearance and enhances their accumulation in the brain. In addition, pyroglutamate-containing Aβ species have been shown to have an increased aggregation propensity and proposed to play an important role during the initiation of the disease (He et al., 1999; Schilling et al., 2006). Finally, it has been demonstrated recently that inhibition of glutaminyl cyclase, an enzyme responsible for pyroglutamate formation, reduced plaque load in two different transgenic mouse models of AD accompanied by alleviated plaque-associated inflammation and a significant memory improvement (Schilling et al., 2008). Thus, collectively, these data suggest that anti-Aβ immunotherapeutic strategies should take into account pyroglutamate-containing forms AβN3(pE) and AβN11(pE) and emphasize the need to search for immunogens capable to target N-truncated/modified species, since the great majority of previously reported immunotherapy studies are based on EFRH epitope absent in these peptides.
Recently, we have demonstrated that the immunodominant region of AβN3(pE) is located at its amino terminus (Acero et al., 2009). In the present study we identified two major B-cell epitopes in AβN11(pE): the first one located in the amino-terminal part and the second one in the central part of the peptide. Peptide inserts of selected positive phage clones are mimicking antigenic properties of these epitopes. Interestingly, two independent web-servers (ABCpred and Epitopia) for predicting B-cell epitopes in an antigen sequence also pointed to these regions of AβN11(pE) (aa 11-18 and aa 19-26) as putative B-cell epitopes (Saha and Raghava, 2006; Rubinstein et al., 2009). Further studies in animal models would be needed to determine if peptides identified in this study may induce specific B-cell response to natural epitopes present in AβN11(pE). Importantly, anti-AβN11(pE) rabbit polyclonal antibodies bound also to Aβ1-42 and AβN3(pE), suggesting that the three peptides may share a common B-cell epitope, and immunization with selected mimotopes may induce cross-reacting antibodies binding to all three major forms of Aβ.
Finally, anti-AβN11(pE) antibodies inhibited AβN11(pE)-induced cytotoxicity in IMR-32 differentiated neuroblastoma cells and recognized naturally occurring amyloid aggregates present in brain samples from AD patients. The latter is important since there may be differences in epitope exposure in synthetic peptide aggregates in vitro and natural amyloid deposits formed in brain. Understanding the antigenic and immunogenic properties of mimotopes selected in this study may help to develop immunogens capable to elicit B-cell response mimicking anti-AβN11(pE) one for targeting different pathological species of Aβ. This will represent a promising immunotherapeutic approach for the disease treatment and/or prevention.
In conclusion, by designing new immunogens capable of inducing antibodies against N-amino-truncated/modified Aβ peptides as well as full-length Aβ, one may target all pathological species of the Aβ peptide present in human brain. This should significantly enhance the efficacy of immunotherapy in the CNS of AD patients, because only approximately 0.1% of the antibody in the blood gains entry into the brain.
ACKNOWLEDGMENTS
This work was supported by grants from DGAPA-UNAM (IN200907 and IN209610-3), PASPA-DGAPA-UNAM, CONACyT ( 58081), Mexico to GG; by UC CONACYT MEXUS grant to DHC and GG, as well as NIH RO-1 grants (NIA AG20241 and NINDS NS50895) to DHC and Alzheimer's Association Grant IIRG 91822 to DHC. R.P. and V.I. are recipients of postgraduate fellowship from CONACyT, Mexico.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acero G, Manoutcharian K, Vasilevko V, Munguia ME, Govezensky T, as G, Luz-madrigal A, Cribbs D, Gevorkian G. Immunodominant epitope and properties of pyroglutamate-modified Ab-specific antibodies produced in rabbits. J. Neuroimmunol. 2009;213:39–46. doi: 10.1016/j.jneuroim.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch K, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid-β peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer's disease. Nature Medicine. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Breyahn H, Duan K, Rettig J, Wirths O. Intraneuronal β-amyloid is a major risk factor – novel evidence from the APP/PS1KI mouse model. Neurodegener. Dis. 2008;5:140–142. doi: 10.1159/000113684. [DOI] [PubMed] [Google Scholar]
- Berg MM, Krafft GA, Klein WL. Rapid impact of β-amyloid on paxillin in a neural cell line. J. Neurosci. Res. 1997;50:979–989. doi: 10.1002/(SICI)1097-4547(19971215)50:6<979::AID-JNR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Biscaro B, Lindvall O, Hock C, Efdahl C, Nitsch RM. Aβ immunotherapy protects morphology and survival of adult-born neurons in doubly transgenic APP/PS1 mice. J. Neurosci. 2009;29:14108–14119. doi: 10.1523/JNEUROSCI.2055-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu. Rev. Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier J-M, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 Neuronal Loss with Intraneuronal and N-Terminal Truncated Aβ42 Accumulation in a Novel Alzheimer Transgenic Model. Am. J. Pathol. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arrigo C, Tabaton M, Perico A. N-terminal truncated pyroglutamyl β amyloid peptide Aβpy3-42 shows faster aggregation kinetics than the full-length Aβ1-42. Biopolymers. 2009;91:861–873. doi: 10.1002/bip.21271. [DOI] [PubMed] [Google Scholar]
- Gevorkian G, Petrushina I, Manoutcharian K, Ghochikyan A, Acero G, Vasilevko V, Cribbs DH, Agadjanyan MG. Mimotopes of conformational epitopes in fibrillar β-amyloid. J. Neuroimmunol. 2004;156:10–20. doi: 10.1016/j.jneuroim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Guntert A, Dobeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143:461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- He W, Barrow CJ. The A beta 3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have geater beta-sheet forming and aggregation propensities in vitro than full-length A beta. Biochemistry. 1999;38:10871–10877. doi: 10.1021/bi990563r. [DOI] [PubMed] [Google Scholar]
- Head E, Pop V, Vasilevko V, Hill. M, Saing T, Sarsoza F, Nistor M, Christie LA, Milton S, Glabe C, Barrett E, Cribbs D. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J. Neurosci. 2008;28:3555–3566. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Liu K, Pijak DS, Carlin D, Lee VM-Y, Doms RW. β-Secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer's disease brain. J. Biol. Chem. 2002;277:16278–16284. doi: 10.1074/jbc.M111141200. [DOI] [PubMed] [Google Scholar]
- Klein WL. Aβ toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, De Jonghe C, Cruts M, Kleinert R, Wang R, Mercken M, De Strooper B, Vanderstichele H, Lofgren A, Vanderhoeven I, Backhovens H, Vanmechelen E, Kroisel PM, Van Broeckhoven C. Nonfibrillar diffuse amyloid deposition due to a gamma(42)-secretase site mutation points to an essential role for N-truncated A beta(42) in Alzheimer's disease. Hum. Mol. Genet. 2000;9:2589–2598. doi: 10.1093/hmg/9.18.2589. [DOI] [PubMed] [Google Scholar]
- Lee EB, Skovronsky DM, Abtahian F, Doms RW, Lee VM-Y. Secretion and intracellular generation of truncated Aβ in β-site amyloid-β precursor protein-cleaving enzyme expressing human neurons. J. Biol. Chem. 2003;278:4458–4466. doi: 10.1074/jbc.M210105200. [DOI] [PubMed] [Google Scholar]
- Lemere CA. Developing novel immunogens for a safe and effective Alzheimer's disease vaccine. Prog. Brain Res. 2009;175:83–92. doi: 10.1016/S0079-6123(09)17506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer's disease Aβ vaccine reduces central nervous system Aβ levels in a non-human primate, the Caribbean vervet. Am. J. Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Solano I, Mann D, Lemere C, Mercken M, Trojanowski JQ, Lee VM-Y. Characterization of Aβ11-40/42 peptide deposition in Alzheimer's disease and young Down's syndrome brains: implication of N-terminally truncated Aβ species in the pathogenesis of Alzheimer's disease. Acta neuropathol. 2006;112:163–174. doi: 10.1007/s00401-006-0077-5. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreyther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J. Biol. Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- Naslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, Silberring J, Gandy SE, Winblad B, Greengard P, Nordstedt C, Terenius L. Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA. 1994;91:8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Russo C, Gliozzi A, Relini A, Vitali A, Borghi R, Giliberto L, Armirotti A, D'Arrigo, Bachi A, Cattaneo A, Canale C, Torrassa S, Saido TC, Markesbery W, Gambetti P, Tabaton M. β-Amyloid Is Different in Normal Aging and in Alzheimer Disease. J. Biol. Chem. 2005;280:34186–34192. doi: 10.1074/jbc.M501694200. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Overman MJ, Cotman CW. Amino-terminal Deletions Enhance Aggregation of beta-Amyloid Peptides in Vitro. J. Biol. Chem. 1995;270:23895–23898. doi: 10.1074/jbc.270.41.23895. [DOI] [PubMed] [Google Scholar]
- Rubinstein ND, Mayrose I, Martz E, Pupko T. Epitopia: a web-server for predicting B-cell epitope. BMC Bioinformatics. 2009;10:287. doi: 10.1186/1471-2105-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo C, Violani E, Salis S, Venezia V, Dolcini V, Damonte G, Benatti U, D'Arrigo C, Patrone E, carlo P, Schettini G. Pyroglutamate-modified amyloid -peptides - A N3(pE) - strongly affect cultured neuron and astrocyte survival. J. Neurochem. 2002;82:1480–1489. doi: 10.1046/j.1471-4159.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- Saha S, Raghava GPS. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins. 2006;65:40–48. doi: 10.1002/prot.21078. [DOI] [PubMed] [Google Scholar]
- Saido TC, Iwatsubo T, Mann DMA, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schilling S, Lauber T, Schaupp M, Manhart S, Scheel E, Bohm G, Demuth H-U. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochemistry. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Sch lenzig D, Landler C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer's disease-like pathology. Nat. Medicine. 2008;14:1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- Sergeant N, Bombois S, Ghestem A, Drobecq H, Kostanjevecki V, Missiaen C, Wattez A, David J-P, Vanmechelen E, Sergheraert C, Delacourte A. Truncated beta-amyloid peptide species in pre-clinical Alzheimer's disease as new targets for the vaccination approach. J. Neurochem. 2003;85:1581–1591. doi: 10.1046/j.1471-4159.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schiossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberburg I, Schenk D. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Solórzano-Vargas RS, Vasilevko V, Acero G, Ugen KE, Martinez R, Govezensky T, Vazquez-Ramirez R, Kubli-Garfias C, Cribbs DH, Manoutcharian K, Gevorkian G. Epitope mapping and neuroprotective properties of a human single Chain Fv antibody that binds an internal epitope of amyloid-beta 1-42. Mol. Immunol. 2008;45:881–886. doi: 10.1016/j.molimm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Tekirian TL, Markesbery WR, Russel MJ, Wekstein DR, Patel E, Geddes JW. N-terminal heterogeneity of parenchymal and cerebrovascular Abeta deposits. J. Neuropathol. Exp. Neurol. 1998;57:76–94. doi: 10.1097/00005072-199801000-00009. T.C. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, De Meyer G, Andreasen N, Kostanjevecki V, Wallin A, Olsson A, Blennow K, Vanmechelen E. Amino-truncated β-amyloid 42 peptides in cerebrospinal fluid and prediction of progression of mild cognitive impairment. Clin. Chem. 2005;51:1650–1660. doi: 10.1373/clinchem.2005.051201. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secterase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman MJ, Gordon MN, Morgan D. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J. Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Breyhan H, Cynis H, Schilling S, Demuth H-U, bayer TA. Intraneuronal pyroglutamate-Abeta 3-42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;118:487–496. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Bethge T, Marcello A, Harmeier A, Jawhar S, Lucassen PJ, Milthaup G. Pyroglutamate Abeta pathology in APP/PS1KI mice, sporadic and familial Alzheimer's disease cases. J. Neural. Transm. 2010;117:85–96. doi: 10.1007/s00702-009-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef I, Florent-Bechard S, malaplate-Armand C, Koziel V, Bihain B, Olivier J-L, Leininger-Muller B, Kriem B, Oster T, Pillot T. N-truncated amyloid-β oligomers induce learning impairment and neuronal apoptosis. Neurobiol. Aging. 2007;29:1319–1333. doi: 10.1016/j.neurobiolaging.2007.03.005. [DOI] [PubMed] [Google Scholar]