Abstract
Inheritance of the APOE4 allele is a well established genetic risk factor linked to the development of late onset Alzheimer's disease. As the major lipid transport protein in the central nervous system, apolipoprotein (apo) E plays an important role in the assembly and maintenance of synaptic connections. Our previous work showed that 7 month old human apoE4 targeted replacement (TR) mice displayed significant synaptic deficits in the principal neurons of the lateral amygdala, a region that is critical for memory formation and also one of the primary regions affected in Alzheimer’s disease, compared to apoE3 TR mice. In the current study, we determined how age and varying APOE genotype affect synaptic integrity of amygdala neurons by comparing electrophysiological and morphometric properties in C57BL6, apoE knockout, and human apoE3, E4 and E2/4 TR mice at 1 month and 7 months. The apoE4 TR mice exhibited the lowest level of excitatory synaptic activity and dendritic arbor compared to other cohorts at both ages, and became progressively worse by 7 months. In contrast, the apoE3 TR mice exhibited the highest synaptic activity and dendritic arbor of all cohorts at both ages. C57BL6 mice displayed virtually identical synaptic activity to apoE3 TR mice at 1 month; however this activity decreased by 7 months. ApoE knockout mice exhibited a similar synaptic activity profile with apoE4 TR mice at 7 months. Consistent with previous reports that APOE2 confers protection, the apoE4-dependent deficits in excitatory activity were significantly attenuated in apoE2/4 TR mice at both ages. These findings demonstrate that expression of human apoE4 contributes to functional deficits in the amygdala very early in development and may be responsible for altering neuronal circuitry that eventually leads to cognitive and affective disorders later in life.
Keywords: APOE, targeted, replacement, mice, synaptic, integrity
1.1 Introduction
APOE4 carriers represent one quarter of the population in most developed countries and have the highest risk for contracting late onset Alzheimer’s disease (AD) compared to non-APOE4 carriers (Rubinsztein and Easton, 1999). In contrast, APOE2 carriers represent approximately 15% of the population and theoretically deter Alzheimer’s disease compared to presence of the APOE3 allele (Corder et al., 1994). Humans are the only species to express three isoforms of apolipoprotein (apo) E, each differing by a single amino acid (Weisgraber, 1994). This singular difference has dramatic effects on apoE receptor kinetics (Bohnet et al., 1996) which may explain apoE isoform specific effects on synaptic transmission. Maintaining synaptic integrity is arguably the most important central nervous system (CNS) function needed to avoid AD. We define synaptic integrity as a functional synaptic unit with unimpaired neuronal transmission. The loss of synapses or decline in synaptic function is the strongest correlate of cognitive decline in AD (Terry et al., 1991). Multiple studies in animals show that deficits in synaptic transmission occur prior to the detection of any pathognomonic hallmarks of AD (i.e. plaques and tangles) (Buttini et al., 2002) (Mucke et al., 2000) (Raber et al., 2000). Since cognitive deficits can be detected in APOE4 carriers very early in life (Acevedo et al., 2010) (Reiman et al., 2004) (Snowdon et al., 2000), determining when these deficits begin could provide very useful information for treating the disease. Furthermore, understanding how apoE2 expression confers protection against AD illuminates an entirely new research strategy for development of novel therapeutics.
Numerous groups have used the human apoE TR model to study the role of apoE in AD (Trommer et al., 2005) (Blain et al., 2006) (Osorio et al., 2007) (Yun et al., 2005) (Bales et al., 2009). Previously, we showed that young adult apoE4 TR mice displayed reduced excitatory synaptic transmission, dendritic arborization and spine density in the lateral amygdala compared to apoE3 TR mice, and that these deficits occurred in the absence of any pathological hallmarks such as gliosis, amyloid deposition or neurofibrillary tangles (Wang et al., 2005). In support of this, Dumanis et al. found that spine density was reduced in cortical layers II/III of apoE4 mice (vs. apoE3 mice) that became progressively worse with age (Dumanis et al., 2009). Behavioral studies demonstrate spatial memory deficits in young apoE4 TR mice compared to apoE3 mice (Grootendorst et al., 2005) that also became worse with age (Bour et al., 2008a) lending further support to apoE4 deficits in synaptic integrity. Furthermore, Trommer et al. found that young apoE4 mice exhibit reduced hippocampal long term potentiation (LTP) in the dentate gyrus compared to apoE3 mice (Trommer et al., 2004). In contrast, Korwek et al. found enhanced LTP in the CA1 field of apoE4 mice (Korwek et al., 2009), demonstrating that the effect of apoE4 on neuronal transmission is regionally specific.
The amygdala is a limbic structure that participates significantly in memory formation (Akirav and Richter-Levin, 2002) (Fried et al., 2001) (McGaugh, 2004) and like the hippocampus is one of the earliest structures to undergo neurodegeneration in AD (Hamann et al., 2002) (Cuenod et al., 1993). The amygdala also regulates neural processes that govern affective states, such as depression and anxiety which are very prevalent in AD. For example, Hamann et al. found significant impairment in fear conditioning in AD patients, suggesting a loss of synaptic integrity in the amygdala (Hamann et al., 2002). Behavioral studies in mice have also shown that apoE differentially impacts multiple measures of anxiety (Siegel et al., 2010) (Raber, 2007) (Bour et al., 2008b) (Grootendorst J, 2005).
Herein we examine both the effect of age and APOE genotype on synaptic integrity using the human apoE TR mice. We used apoE2/4 heterozygous mice to measure the effect of apoE2 expression on synaptic integrity since apoE2 homozygous mice develop type III hyperlipidemia (Sullivan et al., 1998), a chronic pro-inflammatory state that can confound interpretation of CNS phenotypes. Analysis of “wild type” C57BL6 (C57) mice provided us an opportunity to make evolutionary comparisons between murine and human apoE while apoE knockout (KO) mice were used to assess the absence of apoE on synaptic integrity in the amygdala. Overall, our data suggests that age and APOE genotype have a significant impact on regulating synaptic transmission and neuronal morphology.
1.2 Experimental procedures
Preparation of animals
The TR mice were created by gene targeting as described previously (Sullivan et al., 1997). Briefly, the construction of the TR mice differ from other types of apoE transgenic mice in that human APOE genomic fragments were used to replace the mouse Apoe gene via homologous recombination. All lines of apoE TR mice contain chimeric genes consisting of mouse 5’ regulatory sequences continuous with mouse exon 1 (non-coding) followed by humans exons (and introns) 2–4. Thus, all three lines of apoE TR mice regulate apoE gene expression in the same fashion. The present study uses mice that have been backcrossed to C57B16 mice eight times and therefore are >99.6% C57B16. The animals are genotyped using an allele-specific PCR approach based on Hixson and Vernier (Hixson and Vernier, 1990). All mice are maintained on a normal chow diet (ND) consisting of 4.5% (w/w) fat and 0.2% (w/w) cholesterol (Prolab Isopro, Agway Inc., DeWitt, NY). The animals were handled in accordance with guidelines approved by the Duke and VA Animal Care and Use Committee, which includes minimizing the number of animals used and their suffering. All experiments were performed on age (1 or 7 months) and sex (male) matched animals for each genotype. TR mice were either homozygous for APOE3 (3/3), APOE4 (4/4) or heterozygous for APOE2 and APOE4 (2/4). Age and sex matched C57B16 and apoE KO mice were also used in this study.
Slice preparation
Acute coronal amygdala slices (350 µm thick) from the TR mice were prepared as described previously (Wang et al., 2005) (Klein and Yakel, 2006). Briefly, mice were anaesthetized with 200 mg/kg 2,2,2-tribromoethanol and decapitated. Brains were quickly removed and placed in ice-cold oxygenated artificial cerebral spinal fluid containing (mM): 126 NaCl, 2.5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1 NaH2PO4, 25 NaHCO3 and 10 glucose. Upon removal of the cerebellum, the brain was hemi-sected and the right hemisphere was glued to the Vibratome 1000 Plus (Vibratome, Campden, England) stage and immersed in cooled oxygenated artificial cerebral spinal fluid.
Electrophysiology
All experiments were conducted single-blinded, where the researcher performing the electrophysiological recordings and data analysis were unaware of the APOE genotype. After a 1 hour incubation period single slices were transferred to the recording chamber. Whole-cell patch-clamp recordings were performed in lateral amygdala neurons using patch pipettes with resistances of 3–5 MΩ filled with internal solution (IS) containing (in mM): 140 potassium gluconate, 0.5 CaCl2, 2 MgATP, 2 MgCl2, 5 EGTA, and 10 Hepes (pH 7.2–7.3). For morphological studies, 0.3% biocytin (Sigma) was added to the IS on the day of the experiment. Slices were superfused at room temperature (18–22°C) with artificial cerebral spinal fluid. We clamped cells at −70 mV and recorded spontaneous excitatory post-synaptic currents (sEPSCs) using an Axopatch 200B amplifier, filtered at 1 kHz, and sampled at 10 kHz using pClamp 10.1 software (Axon Instruments). We measured the sEPCSs interval and amplitude using Mini Analysis software (Synaptosoft, Inc. Decatur, GA) and statistical analyses was performed using Origin software (Microcal, Northampton, MA, USA). Averaged data are presented as means ± S.E.M. Statistical significance (P < 0.05) was assessed using a Student's t test. Firing properties and electrical excitability were assessed using whole-cell current-clamp mode. Hyper- and depolarizing step pulses were delivered at 1 s durations ranging from −0.2 to 0.2 nA in 25 pA increments.
Biocytin staining
At the end of the recording session, the patch-pipette was gently retracted from the filled neuron and the brain slice was immediately transferred to a solution containing 4% paraformaldehyde in phosphate buffered saline, pH 7.4 (USB Corp., Cleveland, OH) and stored at 4° C. Slices were incubated for 1–3 days with avidin-biotin-peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Inc., Burmingham, CA) diluted in PBS containing 1% Triton X-100. Slices were then stained with diaminobenzidine (Vector Labs) solution freshly prepared in PBS. The reaction was stopped by rinsing with deionized water and the stained slices were mounted on gelatin subbed slides, air-dried overnight and cover slipped using standard techniques. The morphology of the biocytin-filled neurons was examined using a light microscope (either a Nikon EP3000 or Zeiss Axio ImagerD2), then traced with Neurolucida software (under a 40X objective) and analyzed with Neuroexplorer software (MicroBrightField, Colchester, VT).
1.3 Results
1.3.1 ApoE2 expression attenuates loss of excitatory transmission associated with apoE4 expression in young adult mice
Spontaneous excitatory post-synaptic currents (sEPSCs) were recorded from pyramidal-like neurons in the lateral amygdala from male wild type (C57), apoE KO, apoE3, E4, and E2/4 mice at 7 months of age using whole-cell patch-clamp electrophysiology. At least 2–4 neurons were analyzed from 10–12 animals per genotype. Neurons were classified based on their firing properties in response to positive (depolarizing) current injection (Faber et al., 2001). Both accommodating and regular firing neurons, characteristic of pyramidal neurons, were used for sEPSC analysis (representative image of a pyramidal neuron in Figure 2A). Fast-spiking neurons, which are characteristic of inter-neurons, comprised less than 1% of the total neurons tested and were eliminated from analysis.
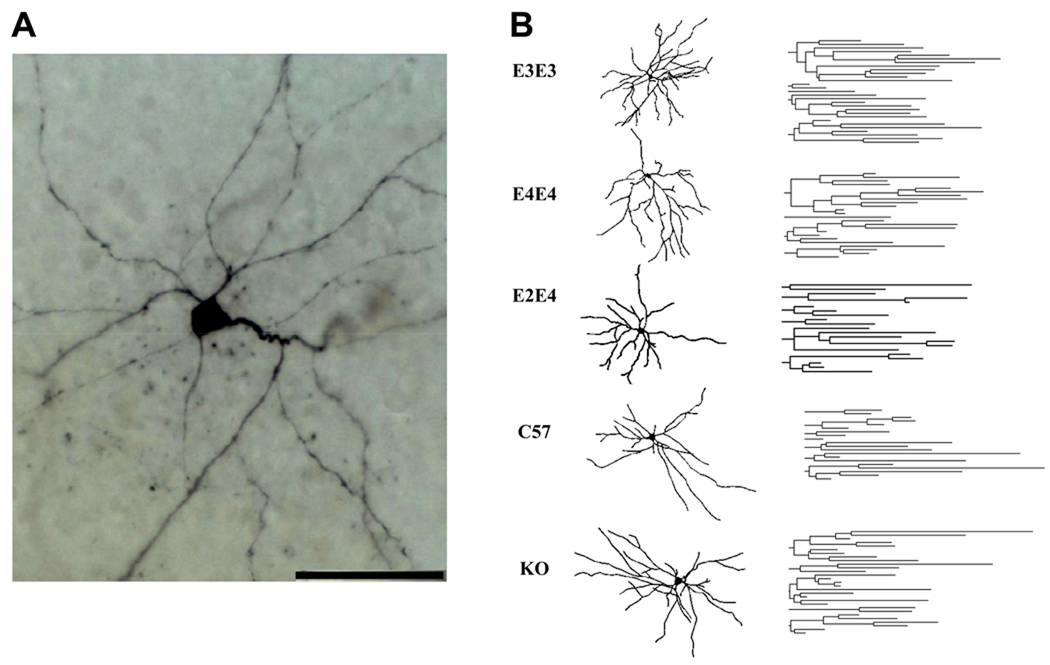
Figure 2.
A. High power image of a representative pyramidal-like lateral amygdala neuron from an apoE3 TR mouse at 7 months of age (scale bar, 50 µm). B. Camera lucida reconstructions (left) and dendrograms (right) corresponding to representative neurons from the indicated cohorts. Quantitative morphological analysis is presented in Table 2.
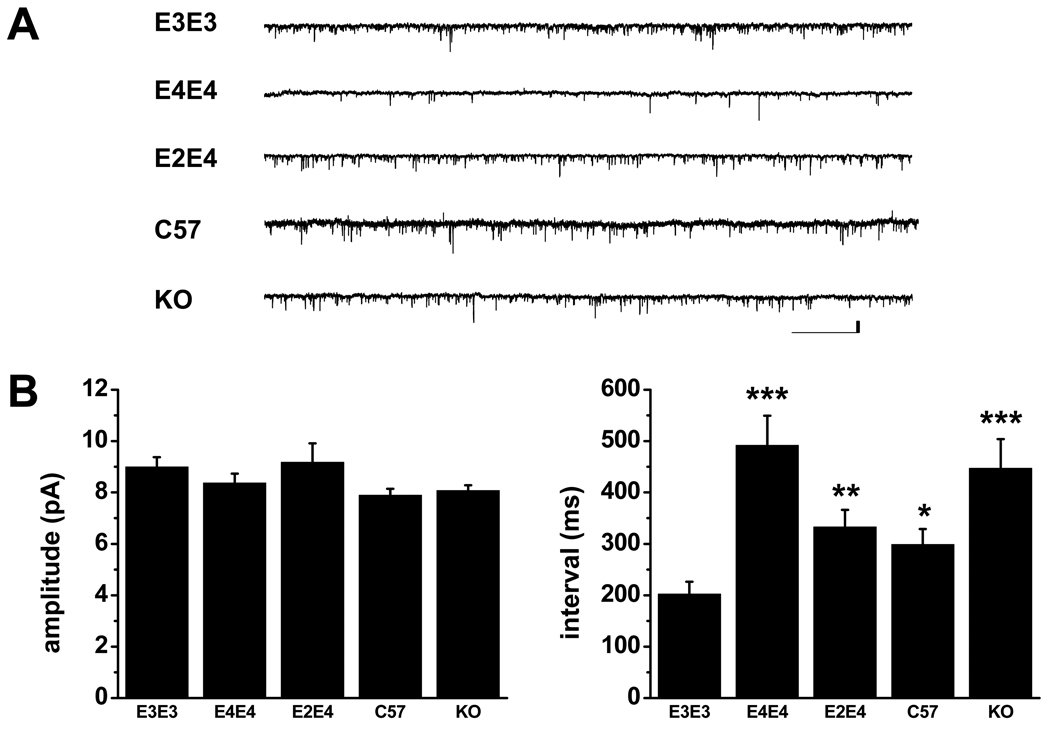
The sEPSC amplitudes and intervals were analyzed from 2 minute continuous recordings. Representative traces from each cohort are illustrated in Figure 1A. A comparison of mean sEPSC amplitudes and intervals are presented in Figure 1B and further summarized in Table 1. The mean amplitudes are similar between each cohort; however, we observed significant differences in the mean interval between each cohort. First, in addition to replicating our previous finding (Wang et al., 2005) where apoE4 mice displayed an increase in the mean sEPSC interval compared to apoE3 mice at 7 months of age, we also observed a similar increase in the interval in apoE KO mice. Secondly, apoE2/4 mice displayed a significant decrease in the mean sEPSC interval compared to apoE4 mice. The sEPSC interval in the C57 mice was most similar to the apoE2/4 mice. Finally, the mean sEPSC interval in the apoE3 mice was significantly less than all other cohorts, suggesting an enhancement in excitatory activity conferred by human apoE3.
Figure 1.
A. Representative traces of whole-cell patch-clamp recordings illustrating sEPSCs in lateral amygdala neurons from designated cohorts at 7 months of age. Scale bar is 10 pA, 2 ms. B. Comparison of mean ± SEM amplitude (left) and interval (right) of sEPSCs at 7 months of age (C). No significant differences in amplitude occurred. Compared to E3/E3, significant differences in the sEPSC interval were observed in C57 (* p<0.05), E2/E4 (** p<0.005), E4/E4 and KO (*** p<0005). There was also a significant difference (p<0.01) between the E4/E4 and E2/E4 cohorts. See Table 1 for N values.
Table 1.
Summary of mean sEPSC amplitudes and intervals from pyramidal-like neurons in the lateral amygdala.
| 1 month | 7 months | |||||
|---|---|---|---|---|---|---|
| Genotype | # of mice (neurons) |
Amplitude (pA) |
Interval (ms) |
# of mice (neurons) |
Amplitude (pA) |
Interval (ms) |
| E3/E3 | 11 (23) | 8.3 ± 0.3 | 188 ± 24* | 12 (31) | 9.0 ± 0.4 | 202 ± 24 |
| E4/E4 | 12 (28) | 8.2 ± 0.3 | 302 ± 29 | 10 (26) | 8.4 ± 0.4 | 492 ± 58*** |
| E2/E4 | 8 (24) | 9.1 ± 0.5 | 208 ± 26** | 12 (23) | 9.2 ± 0.7 | 333 ± 33** |
| C57 | 6 (20) | 7.4 ± 0.5 | 194 ± 23*** | 11 (26) | 7.9 ± 0.3 | 299 ± 30* |
| KO | 8 (19) | 7.4 ± 0.6 | 254 ± 26 | 12 (30) | 8.1 ± 0.2 | 447 ± 57*** |
At 7 months of age, significant differences in mean ± SEM sEPSC intervals compared to apoE3/E3 are noted (*p<0.05, **p<0.005 and ***p<0.0005. There was also a significant difference between apoE4/E4 and apoE2/E4 (p<0.01) at 7 months. At 1 month of age, significant differences in mean ± SEM sEPSC intervals compared to apoE4/E4: *p<0.005, **p<0.05) and ***p<0.01. Within each cohort, there was a significant difference in mean interval between 1 month and 7 months (p<0.05) for all groups except apoE3.
1.3.2 Neuronal morphological analysis as a function of age and APOE genotype
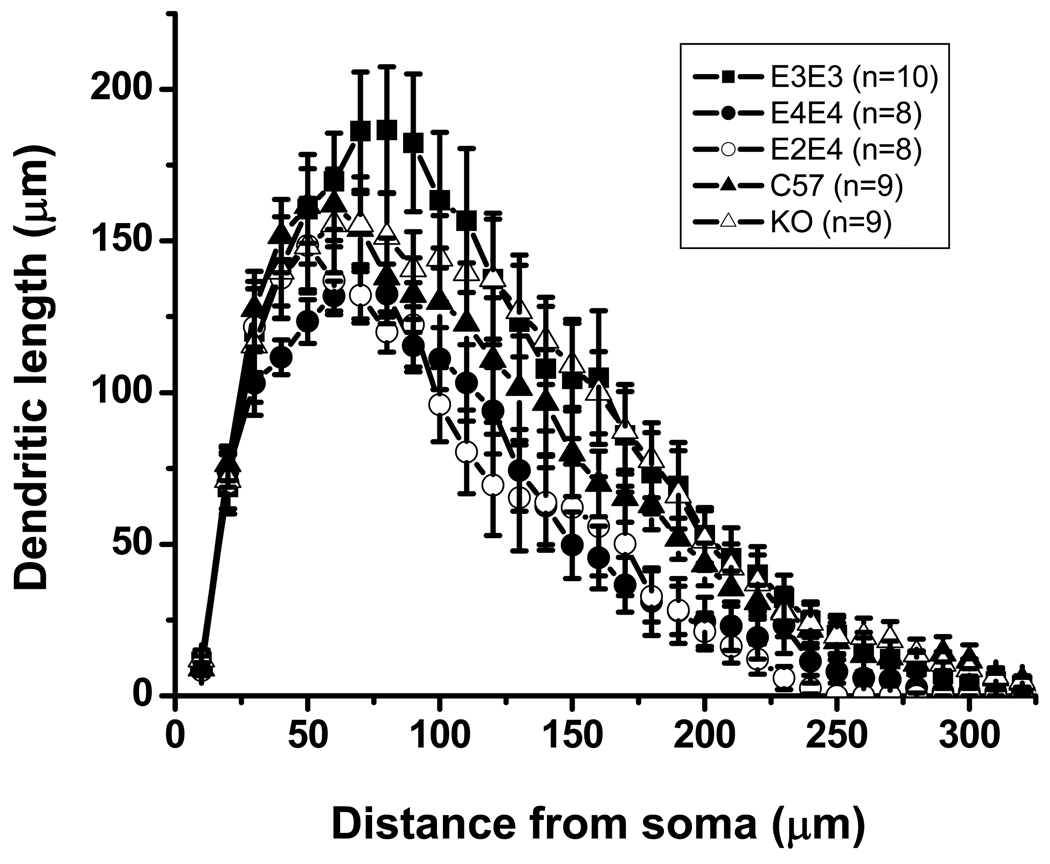
Differences in morphological properties of neurons can provide one explanation for different sEPSC frequencies. Thus, we analyzed biocytin-filled neurons from each cohort (originating from the sEPSC studies) for quantitative differences in morphological properties. The apoE3 neurons showed the most complex branching pattern of all genotypes, whereas the apoE4 and apoE2/4 mice exhibited the least complex pattern (Figure 2B) and were significantly less complex than apoE3. Scholl analysis, which represents the geometrical organization of dendritic arborization, further revealed that apoE4 and E2/4 mice displayed a significant reduction in dendritic length and number of nodes (Table 2) compared to apoE3 mice (Fig. 3). The morphological properties of apoE KO and C57 neurons were not significantly different than apoE3 neurons (Fig. 3, Table 2), although there was a trend toward a decrease in dendritic arbor.
Table 2.
Morphological properties of pyramidal-like neurons in the lateral amygdala from 7 month old ApoE TR mice
| E3/E3 (n=10) |
E2/E4 (n=7) |
E4/E4 (n=8) |
C57 (n=9) |
KO (n=8) |
|
|---|---|---|---|---|---|
| Soma area (µm2) | 110 ± 12 | 102 ± 6 | 114±13 | 140±14 | 132 ± 13 |
| Number of primary dendrites | 4.5 ± 0.3 | 4.7 ± 0.4 | 4.8 ± 0.4 | 4.4 ± 0.4 | 4.6 ± 0.5 |
| Number of nodes Total | 18.0 ± 1.9 | 14.7 ± 0.4* | 12.6 ± 1.5* | 17.4 ± 1.4 | 16.8 ± 1.7 |
| dendritic length (µm) | 2635 ± 322 | 1696 ± 122* | 1697 ± 144* | 2247 ± 158 | 2677 ± 280 |
Significant differences are in comparison to apoE3/E3 (*p<0.05).
Figure 3.
Scholl analysis of averaged lateral amygdala neurons from the indicated ApoE cohort at 7 months of age representing the dendritic length and the number of dendritic intersections crossing each 10 µm radius progressively more distal to the soma. See Table 2 for N values.
1.3.3 Pubescent apoE4 TR mice display reduced excitatory activity
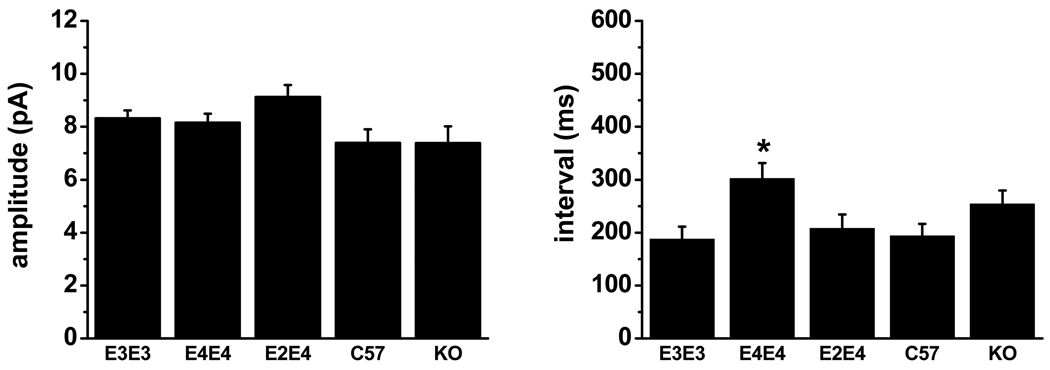
We recorded sEPSCs from pyramidal-like neurons in the lateral amygdala from C57, ApoE KO, apoE3, E4, and E2/4 mice at 1 month of age to determine whether the synaptic integrity phenotype observed at 7 months was present at an earlier stage of development. We observed no significant difference in the mean amplitude between the various cohorts (Figure 4). However, apoE4 TR mice showed an increase in the mean sEPSC interval that was significantly higher than all other cohorts, except the apoE KO mice. Further comparison of each cohort (between 1 and 7 months) showed that apoE3 synaptic activity did not change with age but the mean sEPSC interval significantly increased in all other cohorts, with the largest increase occurring in the apoE4 mice.
Figure 4.
Comparison of mean ± SEM amplitude (left) and interval (right) of sEPSCs at 1 month of age. The mean interval was significantly lower in apoE4 TR mice compared to apoE3 (p<0.005), apoE2/E4 (p<0.05) and C57 (p<0.01). See Table 1 for N values.
We assessed morphological properties in the apoE3, E4 and E2/4 mice at 1 month of age and observed no statistically significant difference in these cohorts of mice (Table 3). Further comparison of each apoE cohort between 1 and 7 months showed that total dendritic length did not change with age in the apoE3 TR mice but significantly decreased in apoE4 and apoE2/4 mice. All three cohorts also showed a significant decrease in soma area between 1 and 7 months.
Table 3.
Morphological properties of pyramidal-like neurons in the lateral amygdala from 1 month old ApoE TR mice
| E3/E3 (n=10) |
E2/E4 (n=7) |
E4/E4 (n=10) |
|
|---|---|---|---|
| Soma area (µm2) | 182 ± 17 | 150 ± 9 | 178 ± 16 |
| Number of primary dendrites | 5.3 ± 0.5 | 5.1 ± 0.3 | 5.6 ± 0.3 |
| Number of nodes | 19.8 ± 1.8 | 21.6 ± 1.7 | 17.9 ± 1.4 |
| Total dendritic length (µm) | 2862 ± 225 | 2799 ± 270 | 2423 ± 230 |
There were no significant differences between the three apoE cohorts; however, apoE4/E4 exhibited a trend toward a decrease in the number of nodes and total dendritic length.
Discussion
In the current study, we assessed age-dependent electrophysiological and morphological properties of principle neurons within the lateral amygdala in male human apoE TR, apoE KO, and C57 mice. Our results revealed that the human apoE TR mice display differential effects on synaptic integrity compared to each other, as well as to C57 and apoE KO mice. First, expression of apoE4 results in significantly lower dendritic arbor and excitatory transmission compared to non-apoE4 mice by 7 months of age. Second, co-expression of apoE2 with apoE4 attenuates the loss in synaptic activity, but does not alter dendritic length. This result supports the hypothesis that expression of apoE2 has a protective effect on synaptic integrity. Third, the apoE KO mice display reduced excitatory transmission similar to apoE4 mice, while C57 mice are more similar to apoE2/4 mice at 7 months. Fourth, a significant reduction in excitatory activity is present in apoE4 TR mice as early as 1 month of age while the loss of dendritic arbor has not yet begun to manifest. Finally, all cohorts (except apoE3 mice) show significant reductions in excitatory transmission with age (i.e. 1 to 7 months).
Whole cell recordings in brain slices from 1 month old mice revealed significant differences in excitatory transmission (i.e. sEPSC interval) between apoE4 mice and all other cohorts examined (except apoE KO mice). Although the frequency of synaptic transmission was lowest in the apoE4 mice at 1 month, we did not detect any statistically significant difference in neuronal morphology compared to apoE2/4 or apoE3 mice. Dumanis et. al found significant differences in neuronal morphology (i.e. spine number) within cortical layers II/III of 1 month apoE TR mice (Dumanis et al., 2009). This same study, however did not detect any morphological differences in the dentate gyrus at 1 month. Thus, the effect of APOE genotype on neuronal morphology appears to vary from one brain region to another. Comparisons between 1 and 7 month old apoE2/4 and apoE4 mice showed an increase in the sEPSC interval and a decrease in dendritic arbor with no change in the apoE3 mice. We did not extend morphological analysis to the C57 or KO mice as no significant differences were observed in the sEPSC interval at 1 month when compared to apoE3 mice.
The apoE4-associated deficit in synaptic activity in the lateral amygdala is consistent with previous electrophysiological studies performed in the hippocampus of apoE TR mice. For example, LTP in the dentate gyrus was significantly lower in apoE4 mice compared to apoE3 and C57 mice at 2–4 months (Trommer et al., 2004). In contrast, apoE4 mice displayed enhanced LTP in the CA1 field (Korwek et al., 2009). These regional differences are likely due to the complex circuitry of the hippocampus, where the dentate and CA1 comprise distinct circuitries and differing synaptic connections with various brain regions.
The reduction in synaptic activity in apoE KO is similar to two previous reports showing synaptic alterations in the hippocampus (Krugers et al., 1997) (Veinbergs et al., 1999) however differs from two other studies which found no differences in LTP, behavior or neuronal morphology, between apoE KO mice and controls (C57 mice) (Anderson et al., 1998) (Fagan et al., 1998). We did not detect any difference in neuronal morphology in 7 month old apoE KO mice compared to C57 or apoE3 TR mice. It is possible that synaptic deficits that occur in apoE KO mice do not appear until later in life, as Ji et al found that spine density and dendritic length was reduced in apoE KO mice at 12 months (Ji et al., 2003), although this finding is refuted by a study which found no differences between apoE KO and C57 mice at 12 months (Anderson et al., 1998). These contrasting studies highlight the inherent problems associated with using apoE KO mice to study the function of human apoE isoforms, as compensatory changes in other proteins and the extreme hyperlipidemic phenotype of apoE KO mice make it difficult to draw comparisons with human apoE expressing mice. Analysis of C57 mice showed that they were most similar to apoE2/4 and E3 mice at 1 month; however, by 7 months the C57 mice showed a significant reduction in excitatory activity while the apoE3 mice remained unchanged. This indicates that murine apoE functions differently than human apoE3 in the mouse brain with respect to excitatory transmission in the amygdala. This is not surprising given that murine and human apoE are very distant in evolutionary terms (e.g. only 70% amino acid homology between mouse and human apoE) (Weisgraber, 1994).
The two major findings from this study are; one, the age dependent increase in mean sEPSC interval in all cohorts except the apoE3 TR mice, and two, the attenuation of synaptic activity (mean sEPSC interval) by apoE2 in the apoE2/4 mice. These results indicate a significant APOE genotype effect on synaptic transmission in the TR mice. Analogies can be drawn with human cognitive studies which show that both age and APOE genotype have a significant impact on cognitive ability (Riley et al., 2002) (Snowdon et al., 2000). Our results also support the idea that the APOE alleles function in a co-dominant manner, otherwise the phenotype in the apoE2/4 mice would be indistinguishable from the apoE4 mice. Our results also support human gene association studies for relative risk of AD between varying APOE genotypes (Roses et al., 1995). For example, APOE2/4 heterozygotes have a significantly lower risk of developing AD compared to APOE4/4 homozygotes (Saunders et al., 1993) (Corder et al., 1993).
In our earlier study (Wang et al., 2005) we were able to show a correlation between reduced synaptic transmission and reduced dendritic arbor in the apoE4 mice at 7 months. Herein we found 1 month old apoE4 mice displayed reduced synaptic transmission compared to apoE2/4 and apoE3 mice, however we did not detect any differences in neuronal morphology between the three cohorts. Since we were unable to measure spines in the 1 month cohorts it is possible that varying numbers of spines could account for the difference in synaptic activity. We are encouraged by the study in similar cohorts of mice (1 month TR mice) which found varying spine counts, albeit in a different brain region (Dumanis et al., 2009). We plan to use Golgi staining techniques in future studies to aid in determining the number of spines, since we were unable to reliably count spines in any of these cohorts due to technical limitations with imaging the biocytin-filled cells.
We were able to measure other characteristics of neuronal morphology, however, and confirmed our original finding in the 7 month cohort which showed significant differences between apoE3 and E4 mice. Analysis of the apoE2/4 mice, however, showed no difference in dendritic length compared to apoE4 mice, which again could be explained by varying spine number. In the current study we elected not to use apoE2 homozygous mice in order to avoid any potential confounding effects of hyperlipidemia on synaptic integrity (Sullivan et al., 1998). Future analysis of functional and morphological outcomes using hemizygous apoE TR mice (i.e. E2/E0 and E4/E0) could provide insight into the individual contribution of each isoform; however the apoE2/0 mice would be expected to develop type III hyperlipidemia, a similar confounding variable present in apoE2 and apoE KO homozygous mice.
The mechanism responsible for the reduction in synaptic transmission associated with apoE4 is currently unknown, but several possibilities may account for this effect. The lateral amygdala receives excitatory/glutamatergic afferents originating from the cortex and thalamus (McDonald et al., 2002) and age-dependent pruning of dendrites and spines in cortical and thalamic afferents may contribute to the loss of excitatory activity. Since loss of synaptic integrity within the cortical limbic system is thought to begin very early in AD, potential disruption of these connections in the apoE4 TR mice could explain the reduction in synaptic transmission. While inhibitory inputs can also impact the degree of excitatory activity, our previous report showed no differences in the amplitude or frequency of spontaneous inhibitory synaptic currents (sIPSCs) between apoE3 and E4 (Wang et al., 2005). It is plausible that the reduction in sEPSCs observed in apoE4 mice is due to the concomitant decrease in dendritic length of the post-synaptic cell. However, this does not explain our result seen in apoE2/E4 mice.
We had previously suggested that one explanation for the observed apoE4 deficit (at 7 months) was due to a deficiency in lipids required for neuronal remodeling (Wang et al., 2005). The idea that apoE might regulate synaptic transmission via the distribution of lipids in neuronal membranes was first proposed by Mahley et al. (Mahley, 1988) and Poirier et al. (Poirier, 1994). It is now known that synaptic plasticity depends on glial derived cholesterol (Goritz et al., 2002) which is delivered by apoE-containing HDL particles, and that phospholipids are required for maintaining synaptic plasticity (Koudinov and Koudinova, 2001) (Hering et al., 2003) (Bourre et al., 1989) . Therefore, apoE4 (relative to apoE3 and E2) may be deficient in delivery of essential lipids for maintaining synaptic plasticity.
The observed apoE4 deficit in synaptic integrity could also be due to insufficient levels of functional apoE4 protein which would result in a rate-limiting supply of lipids required for synaptic plasticity. We and others recently showed that protein levels are lowest in the hippocampus and cortex of 3–4 month old male apoE4 mice compared to apoE3 and E2 mice (Sullivan et al., 2009) (Riddell et al., 2008). Another group found a trend towards lower apoE protein expression in the amygdala of female apoE4 TR mice (compared to apoE3 and apoE2 mice) however, no analysis of male TR mice was performed (Siegel et al., 2010). Future studies are needed to determine the effects of gender and age on apoE protein levels.
Human APOE4 carriers display signs of cognitive impairment by the second decade of life (Bourre et al., 1989, Murthy et al., 2002)[41] (Ganguli et al., 2000) (Scarmeas et al., 2005) and on into middle age (Blair et al., 2005) (Wishart et al., 2006). Amygdalar volumes are also significantly reduced in probable AD patients based on MRI morphometry (Cuenod et al., 1993) (Basso et al., 2006). Determining when cognitive impairment begins in APOE4 carriers may reveal insight into the etiology of late onset AD and improve strategies for intervention. Here we provide the first electrophysiology studies in the human equivalent of “pre-adolescents” (i.e. pubescent 1 month old TR mice) which reveal an age and APOE genotype effect on synaptic transmission in the amygdala. Ongoing and future studies in our lab aim to investigate the long-term impact of apoE isoforms on synaptic integrity in aged animals.
Abbreviations
- AD
Alzheimer’s disease
- apo
apolipoprotein (refers to the protein or isoform)
- APOE
designation for human apolipoprotein E gene
- CNS
central nervous system
- C57
murine wild type strain
- sEPSC
spontaneous excitatory post-synaptic currents
- KO
knock out
- LDLR
low density lipoprotein receptor
- LTP
long term potentiation
- TR
targeted replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo SF, Piper BJ, Craytor MJ, Benice TS, Raber J. Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatr Res. 2010;67:293–299. doi: 10.1203/PDR.0b013e3181cb8e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. Journal of Neuroscience. 2002;22:9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Barnes JC, Bliss TV, Cain DP, Cambon K, Davies HA, Errington ML, Fellows LA, Gray RA, Hoh T, Stewart M, Large CH, Higgins GA. Behavioural, physiological and morphological analysis of a line of apolipoprotein E knockout mouse. Neuroscience. 1998;85:93–110. doi: 10.1016/s0306-4522(97)00598-8. [DOI] [PubMed] [Google Scholar]
- Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso M, Gelernter J, Yang J, MacAvoy MG, Varma P, Bronen RA, van Dyck CH. Apolipoprotein E epsilon4 is associated with atrophy of the amygdala in Alzheimer's disease. Neurobiol Aging. 2006;27:1416–1424. doi: 10.1016/j.neurobiolaging.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Blain JF, Sullivan PM, Poirier J. A deficit in astroglial organization causes the impaired reactive sprouting in human apolipoprotein E4 targeted replacement mice. Neurobiol Dis. 2006;21:505–514. doi: 10.1016/j.nbd.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E. APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64:268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- Bohnet K, Pillot T, Visvikis S, Sabolovic N, Siest G. Apolipoprote in (apo) E genotype and apoE concentration determine binding of normal very low density lipoproteins to HepG2 cell surface receptors. Journal of Lipid Research. 1996;37:1316–1324. [PubMed] [Google Scholar]
- Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behavioural brain research. 2008a doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008b;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. Journal of Neuroscience. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature Genetics. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cuenod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Syrota A, Boller F. Amygdala atrophy in Alzheimer's disease. An in vivo magnetic resonance imaging study. Arch Neurol. 1993;50:941–945. doi: 10.1001/archneur.1993.00540090046009. [DOI] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. Journal of neurophysiology. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Murphy BA, Patel SN, Kilbridge JF, Mobley WC, Bu G, Holtzman DM. Evidence for normal aging of the septo-hippocampal cholinergic system in apoE (−/−) mice but impaired clearance of axonal degeneration products following injury. Experimental Neurology. 1998;151:314–325. doi: 10.1006/exnr.1998.6818. [DOI] [PubMed] [Google Scholar]
- Fried I, Wilson CL, Morrow JW, Cameron KA, Behnke ED, Ackerson LC, Maidment NT. Increased dopamine release in the human amygdala during performance of cognitive tasks. Nature Neuroscience. 2001;4:201–206. doi: 10.1038/84041. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Chandra V, Kamboh MI, Johnston JM, Dodge HH, Thelma BK, Juyal RC, Pandav R, Belle SH, DeKosky ST. Apolipoprotein E polymorphism and Alzheimer disease: The Indo-US Cross-National Dementia Study. Arch Neurol. 2000;57:824–830. doi: 10.1001/archneur.57.6.824. [DOI] [PubMed] [Google Scholar]
- Goritz C, Mauch DH, Nagler K, Pfrieger FW. Role of glia-derived cholesterol in synaptogenesis: new revelations in the synapse-glia affair. J Physiol Paris. 2002;96(3–4):257–263. doi: 10.1016/s0928-4257(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Grootendorst JBA, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behavioral Brain Research. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behavioural brain research. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer's disease. Neuropsychologia. 2002;40:1187–1195. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. Journal of Neuroscience. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of Lipid Research. 1990;31:545–548. [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer's disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. J Physiol. 2006;576:865–872. doi: 10.1113/jphysiol.2006.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korwek KM, Trotter JH, Ladu MJ, Sullivan PM, Weeber EJ. ApoE isoform-dependent changes in hippocampal synaptic function. Molecular neurodegeneration. 2009;4:21. doi: 10.1186/1750-1326-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB Journal. 2001;15:1858–1860. doi: 10.1096/fj.00-0815fje. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Mulder M, Korf J, Havekes L, de Kloet ER, Joels M. Altered synaptic plasticity in hippocampal CA1 area of apolipoprotein E deficient mice. Neuroreport. 1997;8:2505–2510. doi: 10.1097/00001756-199707280-00018. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. Journal of Neuroscience. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Hamilton J, Greiner RS, Moriguchi T, Salem N, Jr, Kim HY. Differential effects of n-3 fatty acid deficiency on phospholipid molecular species composition in the rat hippocampus. Journal of Lipid Research. 2002;43:611–617. [PubMed] [Google Scholar]
- Osorio C, Sullivan PM, He DN, Mace BE, Ervin JF, Strittmatter WJ, Alzate O. Mortalin is regulated by APOE in hippocampus of AD patients and by human APOE in TR mice. Neurobiol Aging. 2007;28:1853–1862. doi: 10.1016/j.neurobiolaging.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends in Neurosciences. 1994;17:525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Raber J. Role of apolipoprotein E in anxiety. Neural Plast. 2007;2007:91236. doi: 10.1155/2007/91236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Annals of Neurology. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- Roses AD, Saunders AM, Alberts MA, Strittmatter WJ, Schmechel D, Gorder E, Pericak-Vance MA. Apolipoprotein E E4 allele and risk of dementia. Jama. 1995;273:374–375. author reply 375–376. [PubMed] [Google Scholar]
- Rubinsztein DC, Easton DF. Apolipoprotein E genetic variation and Alzheimer's disease. a meta-analysis. Dementia & Geriatric Cognitive Disorders. 1999;10:199–209. doi: 10.1159/000017120. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, Stern Y. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Markesbery WR. Linguistic ability in early life and the neuropathology of Alzheimer's disease and cerebrovascular disease. Findings from the Nun Study. Annals of the New York Academy of Sciences. 2000;903:34–38. doi: 10.1111/j.1749-6632.2000.tb06347.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Han B, Liu F, Mace BE, Ervin JF, Wu S, Koger D, Paul S, Bales KR. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. Journal of Biological Chemistry. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. Journal of Clinical Investigation. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Annals of Neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Stine WB, Manelli A, Sullivan P, Pasternak JF, LaDu MJ. ApoE isoform-specific effects on LTP: blockade by oligomeric amyloid-beta1–42. Neurobiol Dis. 2005;18:75–82. doi: 10.1016/j.nbd.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Ye GL, Sotak M, Sullivan PM, Pasternak JF, LaDu MJ. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15:2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- Veinbergs I, Mante M, Jung MW, Van Uden E, Masliah E. Synaptotagmin and synaptic transmission alterations in apolipoprotein E-deficient mice. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:519–531. doi: 10.1016/s0278-5846(99)00013-5. [DOI] [PubMed] [Google Scholar]
- Wang C, Wilson WA, Moore SD, Mace BE, Maeda N, Schmechel DE, Sullivan PM. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. 1994:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, Roth RM, Mamourian AC, Tsongalis GJ, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67:1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- Yun SH, Park KA, Sullivan P, Pasternak JF, Ladu MJ, Trommer BL. Blockade of nicotinic acetylcholine receptors suppresses hippocampal long-term potentiation in wild-type but not ApoE4 targeted replacement mice. J Neurosci Res. 2005;82:771–777. doi: 10.1002/jnr.20684. [DOI] [PubMed] [Google Scholar]