Abstract
We have identified a gene, rig (rat insulinoma gene), that is activated in chemically induced rat insulinomas but not in normal pancreatic islets or in regenerating islets. In the present study, we have found that the insulinoma gene was activated in a BK virus-induced hamster insulinoma cell line and in a spontaneously occurring human insulinoma. From the hamster and human insulinoma cDNA libraries, rig homologues were isolated, and their nucleotide sequences were determined. In the same manner as the rat gene, both hamster and human homologues contained one open reading frame of 435 nucleotides, differing by 32- and 41-base substitutions, respectively. All the base substitutions were same-sense mutations. Accordingly, the deduced 145-amino acid sequence remained invariant in hamster, human, and rat insulinomas, suggesting that rig has evolved under extraordinarily strong selective constraints. Computerized structure analysis indicated that rig-encoded protein is a possible DNA-binding protein. The antisense oligodeoxyribonucleotide complementary to hamster rig mRNA was synthesized and injected into the hamster insulinoma cells. The antisense rig oligodeoxyribonucleotide inhibited DNA synthesis in the insulinoma cells, whereas the sense rig oligodeoxyribonucleotide or antisense insulin oligodeoxyribonucleotide had no inhibitory effect. These results strongly suggest that the activation of rig is both common and potentially significant in the oncogenic growth of pancreatic B cells of islets of Langerhans.
Full text
PDF



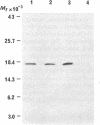
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Sanchez-Pescador R. Sequence of a cDNA encoding Syrian hamster preproinsulin. Diabetes. 1984 Mar;33(3):297–300. doi: 10.2337/diab.33.3.297. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Harlow E., Williamson N. M., Ralston R., Helfman D. M., Adams T. E. Molecular cloning and in vitro expression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985 Jul;5(7):1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Redmond S., Wade-Evans A. Cloning and expression analysis of full length mouse cDNA sequences encoding the transformation associated protein p53. Nucleic Acids Res. 1984 Jul 25;12(14):5609–5626. doi: 10.1093/nar/12.14.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LAZAROW A. Spontaneous recovery from alloxan diabetes in the rat. Diabetes. 1952 Sep-Oct;1(5):363–372. doi: 10.2337/diab.1.5.363. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughon A., Scott M. P. Sequence of a Drosophila segmentation gene: protein structure homology with DNA-binding proteins. Nature. 1984 Jul 5;310(5972):25–31. doi: 10.1038/310025a0. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Skorupa E. S., Kemper B. Single stranded DNA SP6 promoter plasmids for engineering mutant RNAs and proteins: synthesis of a 'stretched' preproparathyroid hormone. Nucleic Acids Res. 1985 Feb 25;13(4):1103–1118. doi: 10.1093/nar/13.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ohsawa K., Hayakawa Y., Nishizawa M., Yamagami T., Yamamoto H., Yanaihara N., Okamoto H. Synergistic stimulation of VIP/PHM-27 gene expression by cyclic AMP and phorbol esters in human neuroblastoma cells. Biochem Biophys Res Commun. 1985 Nov 15;132(3):885–891. doi: 10.1016/0006-291x(85)91890-x. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Regulation of proinsulin synthesis in pancreatic islets and a new aspect to insulin-dependent diabetes. Mol Cell Biochem. 1981 Jun 9;37(1):43–61. doi: 10.1007/BF02355886. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Krovatin W., Jeffrey A., Sauer R. T. The N-terminal arms of lambda repressor wrap around the operator DNA. Nature. 1982 Jul 29;298(5873):441–443. doi: 10.1038/298441a0. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rakieten N., Gordon B. S., Beaty A., Cooney D. A., Davis R. D., Schein P. S. Pancreatic islet cell tumors produced by the combined action of streptozotocin and nicotinamide. Proc Soc Exp Biol Med. 1971 May;137(1):280–283. doi: 10.3181/00379727-137-35561. [DOI] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The protein products of the myc and myb oncogenes and adenovirus E1a are structurally related. Nature. 1983 Dec 22;306(5945):803–806. doi: 10.1038/306803a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Yocum R. R., Doolittle R. F., Lewis M., Pabo C. O. Homology among DNA-binding proteins suggests use of a conserved super-secondary structure. Nature. 1982 Jul 29;298(5873):447–451. doi: 10.1038/298447a0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Kalderon D., Roberts B. L., Colledge W. H., Edge M., Gillett P., Markham A., Paucha E., Richardson W. D. The nuclear location signal. Proc R Soc Lond B Biol Sci. 1985 Oct 22;226(1242):43–58. doi: 10.1098/rspb.1985.0078. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Takasawa S., Yamamoto H., Terazono K., Okamoto H. Novel gene activated in rat insulinomas. Diabetes. 1986 Oct;35(10):1178–1180. doi: 10.2337/diab.35.10.1178. [DOI] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Aizawa T., Furuno A., Muto T. Polyoncogenicity and insulinoma-inducing ability of BK Virus, a human Papovavirus, in Syrian golden hamsters. J Natl Cancer Inst. 1979 Jul;63(1):119–126. [PubMed] [Google Scholar]
- Yamagami T., Miwa A., Takasawa S., Yamamoto H., Okamoto H. Induction of rat pancreatic B-cell tumors by the combined administration of streptozotocin or alloxan and poly(adenosine diphosphate ribose) synthetase inhibitors. Cancer Res. 1985 Apr;45(4):1845–1849. [PubMed] [Google Scholar]
- Yamamoto F., Furusawa M., Furusawa I., Obinata M. The 'pricking' method. A new efficient technique for mechanically introducing foreign DNA into the nuclei of culture cells. Exp Cell Res. 1982 Nov;142(1):79–84. doi: 10.1016/0014-4827(82)90411-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Nose K., Itoh N., Okamoto H. Quantitation of proinsulin mRNA sequences in hamster insulinoma cells in culture by molecular hybridization. Experientia. 1980 Feb 15;36(2):187–188. doi: 10.1007/BF01953722. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Uchigata Y., Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981 Nov 19;294(5838):284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]