Abstract
Autism is a neurodevelopmental disorder characterized by abnormal reciprocal social interactions, communication deficits, and repetitive behaviors with restricted interests. BTBR T+tf/J (BTBR) is an inbred mouse strain that displays robust behavioral phenotypes with analogies to all three of the diagnostic symptoms of autism, including low social interactions, reduced vocalizations in social settings, and high levels of repetitive self-grooming. Autism-relevant phenotypes in BTBR offer translational tools to discover neurochemical mechanisms underlying unusual mouse behaviors relevant to symptoms of autism. Because repetitive self-grooming in mice may be a displacement behavior elevated by stressors, we investigated neuroendocrine markers of stress and behavioral reactivity to stressors in BTBR mice, as compared to C57BL/6J, a standard inbred strain with high sociability. Radioimmunoassays replicated previous findings that circulating corticosterone is higher in the BTBR than in B6. Higher basal glucocorticoid receptor mRNA and higher oxytocin peptide levels were detected in the brains of BTBR as compared to B6. No significant differences were detected in corticotrophin releasing factor (CRF) peptide or CRF mRNA. In response to behavioral stressors, BTBR and B6 were generally similar on behavioral tasks including stress-induced hyperthermia, elevated plus-maze, light ↔ dark exploration, tail flick, acoustic startle and prepulse inhibition. BTBR displayed less reactivity than B6 to a noxious thermal stimulus in the hot plate, and less immobility than B6 in both the forced swim and tail suspension depression-related tasks. BTBR, therefore, exhibited less depression-like scores than B6 on two standard tests sensitive to antidepressants, did not differ from B6 on two well-validated anxiety-like behaviors, and did not exhibit unusual stress reactivity to sensory stimuli. Our findings support the interpretation that autism-relevant social deficits, vocalizations, and repetitive behaviors are not the result of abnormal stress reactivity in the BTBR mouse model of autism.
Keywords: autism, mouse models, BTBR
Introduction
Autism is a complex neurodevelopmental disorder affecting approximately 1 in 150 children (Landa, 2008). The etiology of autism is currently unknown but evidence for strikingly high heritability is abundant (Abrahams and Geschwind, 2008, Happe and Ronald, 2008). Linkage and association studies have identified large numbers of de novo and familial candidate genes that may be responsible for susceptibility to autism (Persico and Bourgeron, 2006, Abrahams and Geschwind, 2008, Bourgeron, 2009, Buxbaum, 2009, Lintas and Persico, 2009). Animal models offer opportunities to test genetic hypotheses and evaluate proposed treatments. One strategy utilized with success in mice is the forward genetics approach of identifying inbred strains of mice with phenotypes relevant to the symptoms of a human disease, using multiple well-validated tasks. Forward genetics strain distribution analyses by our group and others identified several inbred strains of mice with low levels of social interaction (Brodkin et al., 2004, Moy et al., 2004, Nadler et al., 2004, Sankoorikal et al., 2006, Bolivar et al., 2007, Crawley, 2007, Moy et al., 2007, Panksepp et al., 2007, Panksepp and Lahvis, 2007, Yang et al., 2007a, Yang et al., 2007b, Fairless et al., 2008, McFarlane et al., 2008, Moy et al., 2008b, Panksepp et al., 2008, Scattoni et al., 2008, Chen et al., 2009, Yang et al., 2009, Roullet et al., 2010, Silverman et al., 2010, Wohr et al., 2010). Of particular interest is BTBR T+tf/J (BTBR), an inbred strain which exhibits lower levels of play soliciting behaviors as juveniles and lacks sociability in the adult social approach task (Bolivar et al., 2007, Moy et al., 2007, Yang et al., 2007a, Yang et al., 2007b, McFarlane et al., 2008), emits fewer ultrasonic vocalizations in various social settings (Scattoni et al., 2008, Scattoni et al., 2009, Roullet et al., 2010, Wohr et al., 2010) and displays high levels of repetitive self-grooming throughout their lifespan (Yang et al., 2007b, McFarlane et al., 2008, Silverman et al., 2010). These behaviors are relevant to all three core symptom domains of autism. Normal scores on measures of general health, motor functions, and sensory abilities including olfaction (Moy et al., 2007, McFarlane et al., 2008, Moy et al., 2008a) support an interpretation of remarkably specific autism-relevant abnormalities in BTBR.
The mechanism underlying the high level of repetitive self-grooming in BTBR is unclear. Rodent self-grooming behavior is an innate behavior elicited in both comforting and stressful situations (van Erp et al., 1994, Moyaho and Valencia, 2002, Kalueff and Tuohimaa, 2004) with ethologically different patterns emerging for each type. A normal grooming pattern includes a cephalo-caudal progression beginning with licking and washing the paws, then the nose and face, head, body, fur, legs, genitals and tail (Berridge and Aldridge, 2000b, a). More frequent bursts of rapid grooming characterize stress-evoked grooming (Kalueff and Tuohimaa, 2004,Kalueff and Tuohimaa, 2005a). BTBR display the normal full sequence of grooming, with high numbers of self-grooming bouts and excessively long durations of self-grooming bouts, often exceeding one minute of continuous self-grooming. Since stress provoking situations in mice are accompanied by heightened grooming behavior, a critical behavioral adaption to stress (Kametani, 1988, Sachs, 1988, Spruijt et al., 1992, van Erp et al., 1994, Kalueff and Tuohimaa, 2005b), and since hyperreactivity to stressors could be the cause of high self-grooming in BTBR, we investigated the possibility that the development and expression of BTBR’s unique autistic-relevant phenotype may be the result of a stress-reactive phenotype, generalized neuroendocrine differences in stress hormones, or the high circulating corticosterone that was previously reported (Benno et al., 2009, Frye and Llaneza, 2010).
The hypothalamic-pituitary-adrenal axis neuroendocrine factors that elicit high levels of self-grooming when administered to rodents include adrenocorticotropic hormone (ACTH) and corticotrophin releasing factor (CRF) (Ferrari, 1958, Gispen et al., 1975, Morley and Levine, 1982, Dunn et al., 1987, Dunn and File, 1987, Sherman and Kalin, 1987, Matsuzaki et al., 1989, Monnikes et al., 1992). Other neuropeptide transmitters also evoke high levels of self-grooming when centrally administered to rodents, including vasopressin, prolactin, substance P, somatostatin, cholecystokinin and oxytocin (Drago et al., 1981, Meisenberg, 1981, Drago et al., 1986, Elliott and Iversen, 1986, Kaltwasser and Crawley, 1987, Van Wimersma Greidanus et al., 1987, Meisenberg, 1988, Pedersen et al., 1988, Stivers et al., 1988, Kaltwasser and Andres, 1989, Van Erp et al., 1993, Amico et al., 2004). To expand the existing neurochemical data in the BTBR, we conducted a baseline comparison of several relevant neuroendocrine factors in BTBR and C57BL6/J (B6), a standard inbred strain with high sociability, low self-grooming, and relative resilience to stressors (Moy et al., 2004, Moy et al., 2007, Yang et al., 2007a, Yang et al., 2007b, McFarlane et al., 2008). Neuroendocrine measures, including corticosterone, CRF, glucocorticoid receptor and oxytocin were chosen based on rodent literature indicating their roles in stress responsivity as well as grooming via specific regional activation in rats and mice (Stenzel-Poore et al., 1994, McCarthy et al., 1996, Dunn and Swiergiel, 1999, Swiergiel and Dunn, 1999, Tronche et al., 1999, Anisman et al., 2001, Ridder et al., 2005, Ring et al., 2006, Roy et al., 2007, Yoshida et al., 2009, Cohen et al., 2010).
To comprehensively characterize basal stress reactivity, we assayed BTBR and B6 mice on two tests for anxiety-like behaviors, four parameters of sensory reactivity, and two depression-relevant tasks. Tasks were chosen both as standard measures of stress-related behaviors in mice, and for relevance to the literature indicating anxiety, high reactivity to stressors, hyperreactivity to sensory stimuli, upset to change, and elevated neurochemical markers of stress in some people with autism (Tordjman et al., 1997, American Psychiatric Association, 2000, Lord et al., 2000, Rogers et al., 2003, Dawson et al., 2004, Rogers and Ozonoff, 2005, Corbett et al., 2006, Lam et al., 2006, Lord and Spence, 2006, Perry et al., 2007, Matson and Shoemaker, 2009, Reaven, 2009, Tordjman et al., 2009, Volkmar et al., 2009, Zwaigenbaum et al., 2009).
Experimental Procedures
Mice
Adult mice of the inbred strains BTBR and B6 were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in a conventional mouse vivarium at the National Institute of Mental Health (NIMH), Bethesda, MD, using harem breeding trios. After two weeks with a male, females were separated into individual cages before delivery. Pups were kept with the dam until weaning at postnatal day 21. After weaning, juveniles were housed by sex and strain in standard plastic cages in groups not exceeding four per cage. Mice were housed in a conventional animal facility on a 12h-12h light-dark cycle (lights on from 0700 hr to 1900 hr). Cages were housed in ventilated racks in colony rooms maintained at ~20°C temperature and ~55% humidity. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. Male BTBR and B6 were utilized for all neurochemical assays and behavioral experiments described below. All procedures were conducted in strict compliance with the NIH guidelines for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee.
Neurochemical assays
Naïve mice were individually taken from their home cages to a procedure room 10 feet away, to minimize corticosterone surges from extraneous handling or movement. Three separate cohorts of BTBR and B6 mice were utilized for neurochemical assays: one for corticosterone, one for CRF and oxytocin, and one for glucocorticoid receptor mRNA.
Corticosterone radioimmunoassay
Three behaviorally naïve cohorts of BTBR (N=12) and B6 (N=12) mice were immediately removed from their cages beginning at 1600 hr and were immediately sacrificed by rapid decapitation. Trunk blood was collected in 1.5 ml plastic microcentrifuge tubes. Serum was obtained the following day by centrifugation and was stored frozen. Serum concentrations of corticosterone were determined by radioimmunoassay (MP Biomedicals, Solon, OH). The sensitivity threshold for this commercial assay was 5 ng/ml. Intra- and interassay coefficients of variance were less than 10%.
CRF and oxytocin radioimmunoassay
Frozen brains were cut into 1 mm thick sections containing the hypothalamic paraventricular nucleus (PVN) on a cryostat (Leica, Bannockburn, IL). The sections containing the PVN were placed on microscope slides and the PVN was harvested using a 1 mm micropunch (Stoelting, Kiel, WI). Punches were placed into 150 microliters of 1 N acetic acid and boiled for 20 minutes. Acetic acid extracts were frozen on dry ice and stored at −70°C (Hooi et al., 1989). Subsequently, extracts were thawed and aliquotted into 12mm × 75 mm glass tubes and were dried in a Sorval SpeedVac concentrator. The dried extracts were reconstituted in assay buffer and tissue CRF (BTBR N=10 and B6 N=9) or oxytocin (BTBR N=9 and B6 N=10) concentrations were determined by radioimmunoassay according to the manufacturer’s specifications (Phoenix Pharmaceuticals, Burlingame, CA). The sensitivity of both the CRF and oxytocin assays were 1 pg and the coefficients of variation were less than 10%.
In situ hybridization histochemistry
Brains of BTBR (N=5) and B6 (N=5) mice were rapidly removed, frozen on powdered dry ice and subsequently stored at −70°C. Twelve-micron coronal sections were mounted on SupraFrost Plus slides (Fisher Scientific, Pittsburg, PA) following sectioning on a cryostat. Tissue fixation and hybridization conformed to previously published protocol (Lee et al., 2003, Sharifi et al., 2004, Koenig et al., 2005). Briefly, tissue sections were fixed with 4% paraformaldehyde, acetylated with acetic anhydride (0.25%) in triethanolamine (0.1M, pH 8) and dehydrated through a series of ethanol rinses and delipidated with chloroform. Hybridization buffer containing 1.0X106 cpm of 35S-labeled cRNA probe (synthesized by the riboprobe method) was applied. Antisense cRNA probes were generated using DNA templates for glucocorticoid receptor (GR), obtained from Dr. Roger Miesfeld (Miesfeld et al., 1986), and CRF obtained from Dr. Robert Thompson (Thompson et al., 1987). Slides with the processed tissue sections received twenty-five microliters of hybridization buffer (1.0 × 106 cpm) per section. Labeled sense strand probes served as control for background hybridization. Following hybridization (18 hours at 55°C), slides were rinsed in 4X SSC, incubated with RNase A (20 mg/ml), rinsed under high stringency conditions (0.1X SSC at 68°C), and dehydrated with an ethanol series. The slides were exposed to Kodak BioMax MR film (Eastman Kodak Co., Rochester, NY) for autoradiographic image analysis. Films were developed using GBX developing chemicals (Eastman Kodak Co., Rochester, NY). Autoradiographic images were captured using a Sony CCD video camera interfacing with a Power Macintosh computer via a Quick Time digitizer and Frame Grabber (Data Translation, Marlboro, MA). The data collected from the films were processed using NIH Image 1.67b (Bethesda, MD) to yield relative hybridization values for the regions of interest expressed as relative optical density in arbitrary units (A.U.) and statistical analysis performed.
Behavioral testing
Behavioral experiments were conducted in dedicated behavioral testing rooms during the standard light phase, usually between 1000 and 1500 hr. Mice were brought to a holding room in the hallway of the testing area at least one hour prior to behavioral testing. Separate cohorts of mice were employed for a) stress-induced hyperthermia, b) depression-related tasks, c) elevated plus-maze and light↔dark exploration anxiety-related tasks, d) hot plate and tail flick assessments, e) acoustic startle threshold and prepulse inhibition tasks. A one week period intervened between acoustic startle and prepulse testing. The slightly different fur color markings of B6 (dark brown) and BTBR (dark brown with a light brown ventral patch) prevented fully blind rating in tasks that were scored in real time, such as stress-induced hyperthermia, hot plate and tail flick. However, when scoring was conducted from videotaped sessions, observers were generally unable to identify the strains on the videos in tests such as the elevated plus-maze, forced swim, and tail suspension.
Stress- induced hyperthermia
Group housed mice BTBR (N=12) and B6 (N=10) mice were brought to the testing area in their home cage, one hour before the start of testing. Core basal body temperature was measured at time 1 (T1) with a mouse thermistor probe (Thermalert, Braintree, MA) dipped in olive oil lubricant (STAR fine foods, Fresno, CA) and gently inserted 2 cm into the rectum (T1). Ten minutes after the basal temperature (T1) was obtained, the temperature was measured again by a second insertion of a clean thermistor probe (T2). The increase in body temperature between the first and second measurements represents the hyperthermic stress response, as previously described (Bouwknecht et al., 2000, Papaleo et al., 2008). All tests were performed between 1200 and 1430 hr.
Elevated plus-maze
The elevated plus-maze test was performed as previously described (Holmes et al., 2002a, Holmes et al., 2003c, Bailey et al., 2007) in BTBR (N=12) and B6 (N=12) mice. The apparatus (San Diego Instruments, San Diego, CA) consisted of two open arms (30 × 5 cm2) and two closed arms (30 × 5 × 15 cm3) that extended from a common central platform (5 × 5 cm2). A small raised lip (0.25 cm) around the edges of the open arms helped prevent mice from slipping off. The apparatus was constructed from polypropylene and Plexiglas, with a white floor and clear walls, and elevated to a height of 38 cm above floor level. One hour after bringing the mice to the testing facility, each mouse was placed on the center square facing an open arm and allowed to freely explore the apparatus under a light intensity of ~ 30 lux for 5 minutes. The 5 min session was recorded by a top mounted CCTV camera (Security Cameras Direct, Luling, TX), placed approximately 1 meter from the maze. The maze was cleaned with 70% ethanol and water between subjects, with at least five minutes between cleaning and the start of the next test session, to allow for ethanol evaporation and clearance of ethanol vapor odors. Each 5 minute session was scored by a trained observer using Noldus Observer 8.0 XT software (Noldus Information Technology, Leesburg, VA). Behaviors scored were time spent in the open arms, number of open arm entries, and number of open and closed arm entries combined to yield the sum of total entries (Karlsson et al., 2008). An open or closed arm entry was defined as all four paws within the arm. A center entry was defined as both forepaws in the center. To minimize carryover effects of other behavioral manipulations on the elevated plus-maze task, which is more sensitive to prior experience (Holmes et al., 2003a), the elevated plus-maze was conducted as the first behavioral assay. The second anxiety-related task, light↔dark exploration, was conducted 7 days later.
Light ↔ dark exploration test
The light ↔ dark exploration test was conducted as previously described (Crawley and Goodwin, 1980, Holmes et al., 2003a, Holmes et al., 2003b, Bailey et al., 2007) in BTBR (N=12) and B6 (N=12) mice. The apparatus consisted of a polypropylene cage (44 × 21 × 21 cm3) separated into two compartments by a partition, with a rectangular opening (12 × 5 cm2) at floor level. The larger compartment (28 cm long) was open topped, transparent, and lit using overhead fluorescent ceiling lights (~ 400 lux). The smaller compartment (14 cm long) had black painted sides and was covered at the top with black Plexiglas, creating a closed dark space (~ 5 lux). The subject mouse was individually placed in the light compartment, facing away from the partition, and allowed to freely explore the apparatus for 10 minutes. The apparatus was cleaned with 70% ethanol after each subject. The number of transitions, i.e. entries between the two compartments, and the total time spent in the dark compartment were detected by photocells located within the partition, across the opening between the two chambers. Data from the beam breaks were automatically analyzed by dedicated software (fabricated by Bruce Smith, George Dold, and co-workers, Research Services Branch, NIH, Bethesda, MD). Latency to enter the dark side was scored by a trained observer using a stopwatch.
Forced swim test
The Porsolt forced swim test was conducted as previously described (Porsolt et al., 1977, Lucki, 2001, Holmes et al., 2002b) in BTBR (N=10) and B6 (N=10) mice. Mice were gently placed in a transparent Plexiglas cylinder (20 cm in diameter) filled with tap water (25 ± 2° C). The cylinder was filled to a depth of 12 cm, to prevent mice from using their tails to support themselves in the water. A top mounted CCTV camera (Security Cameras Direct, Luling, TX) was placed approximately 30–50 cm above the cylinder to record the session, for subsequent scoring of time spent immobile. Each cylinder was thoroughly cleaned with soap and water and air dried prior to the next test session. Immobility was defined as the cessation of limb movements except minor movement necessary to keep the mouse afloat. Immobility behavior was sampled every 5 seconds during the last 4 minutes of a 6 minute test session by a highly experienced observer.
Tail suspension test
The tail suspension test was conducted as previously described (Steru et al., 1985, Holmes et al., 2002a, Holmes et al., 2002b, Crowley et al., 2005) in BTBR (N=10) and B6 (N=10) mice. Mice were securely fastened by the distal end of the tail to a flat metallic surface and suspended in a visually isolated area. A CCTV camera (Security Cameras Direct, Luling, TX) was placed approximately 1 meter in front of the metallic surface from which the mice were suspended to record each session, for subsequent scoring of time spent immobile. The metallic area was cleaned with 70% ethanol and water between subjects with at least five minutes between cleaning and the start of the next test session to allow for ethanol evaporation, clearance of ethanol vapor odors and complete dryness. The presence or absence of immobility, defined as the absence of limb movement, was sampled every 5 seconds over a 6 minute test session by a highly trained observer.
Hot plate pain sensitivity
Response to an acute thermal stimulus was measured using the hot plate test as described previously (Blakeman et al., 2003, Wiesenfeld-Hallin et al., 2005, Chadman et al., 2008) in BTBR (N=16) and B6 (N=12) mice. The mouse was placed on a flat, black metal surface (IITC Life Science, Inc., Woodland Hills, CA) maintained at 55 °C and surrounded by a square transparent plexiglass barrier to prevent jumping off. The latency to the first paw lick, jump or vocalization was measured by an observer using a foot pedal-controlled timer. A maximum cut-off time of 30 seconds was used to prevent the risk of tissue damage to the paws.
Tail flick pain assessment
Response to thermal stimulation of the tail was conducted as previously described (Blakeman et al., 2003, Wiesenfeld-Hallin et al., 2005, Chadman et al., 2008) in BTBR (N=12) and B6 (N=12) mice. Mice were gently held in place with the tail lying along the groove of the tail-flick monitor (Columbus Instruments, Columbus, OH). An intense photobeam was directed at the tail. The latency for the mouse to move its tail out of the path of the beam was timed automatically by the apparatus. To prevent any tissue damage there was a maximum cutoff latency of 10 seconds.
Acoustic startle threshold
Acoustic startle was measured in the SR-Lab System (San Diego Instruments, San Diego, CA) as previously described (Paylor and Crawley, 1997, Chadman et al., 2008) in BTBR (N=18) and B6 (N=17) mice. Test sessions began by placing the mouse in the Plexiglas holding cylinder for a 5 minute acclimation period. Over the next 8 minutes, mice were presented with each of six trial types across five discrete blocks of trials for a total of 30 trials. The intertrial interval was 10–20 seconds. One trial type measured the response to no stimulus (baseline movement). The other five trial types measured the response to a startle stimulus alone, consisting of a 40 ms sound burst of 80, 90, 100, 110 or 120 dB. Startle amplitude was measured every 1 millisecond (ms) over a 65 ms period beginning at the onset of the startle stimulus. The seven trial types were presented in pseudorandom order such that each trial type was presented once within a block of seven trials. The maximum startle amplitude over this sampling period was taken as the dependent variable. A background noise level of 70 dB was maintained over the duration of the test session.
Prepulse inhibition of acoustic startle
Prepulse inhibition of acoustic startle was conducted as previously described (Paylor and Crawley, 1997, Dulawa and Geyer, 2000, Holmes et al., 2001, Chadman et al., 2008) in BTBR (N=10) and B6 (N=11) mice. Test sessions began by placing the mouse in the Plexiglas holding cylinder for a 5 minute acclimation period. Over the next 10.5 minutes, mice were presented with each of seven trial types across six discrete blocks of trials for a total of 42 trials. The intertrial interval was 10–20 seconds. One trial type measured the response to no stimulus (baseline movement) and another measured the response to the startle stimulus alone which was a 40 ms 110 dB sound burst. The other five trial types were acoustic prepulse plus acoustic startle stimulus trials. The seven trial types were presented in pseudorandom order such that each trial type was presented once within a block of seven trials. Prepulse tones were 20 ms at 74, 78, 82, 86, and 90 dB, presented 100 ms prior to the 110 dB startle stimulus. Startle amplitude was measured every 1 ms over a 65 ms period beginning at the onset of the startle stimulus. The maximum startle amplitude over this sampling period was taken as the dependent variable. A background noise level of 70 dB was maintained over the duration of the test session.
Statistical analysis
Strain differences between BTBR and B6 were analyzed with a Student’s unpaired t-test, on measures including neuroendocrine factors such as corticosterone, CRF, GR mRNA and oxytocin peptide, and on behavioral outcomes in stress-induced hyperthermia, forced swim, tail suspension, elevated plus-maze, the light↔dark exploration, hot plate and tail flick pain assessments using StatView statistical software (Citewise.com, Acton, MA). Acoustic startle threshold and prepulse inhibition were analyzed with Repeated Measures ANOVA followed by Tukey’s post hoc analysis, where applicable, using StatView statistical software (Citewise.com, Acton, MA). All data were graphed using SigmaPlot version 11.0 (Systat Inc., San Jose, CA).
Results
Basal hypothalamic pituitary adrenal axis activity in B6 and BTBR mice
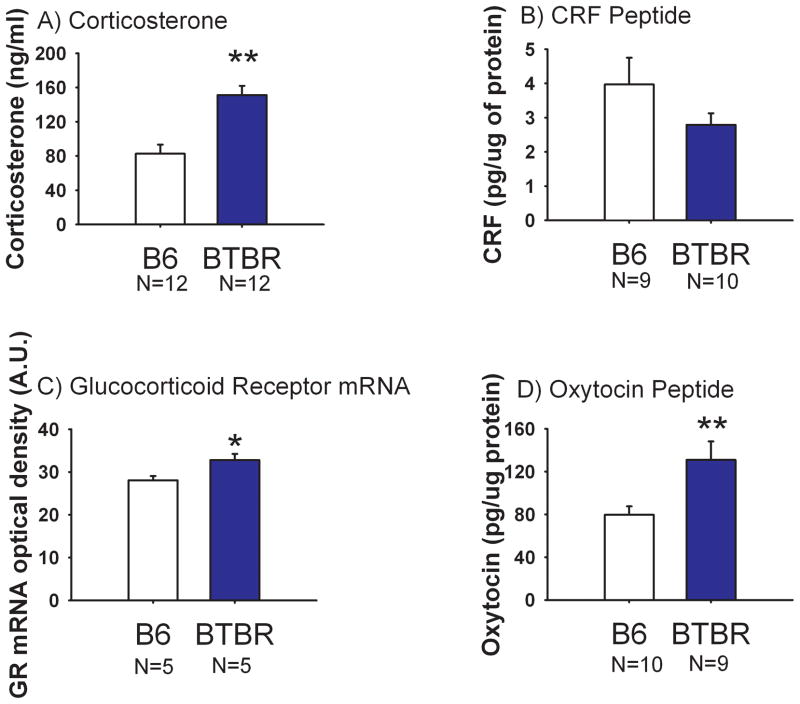
Figure 1 illustrates the quantitative assessment of circulating corticosterone, CRF, GR mRNA and oxytocin peptide for BTBR and B6 mice. Plasma corticosterone from trunk blood was significantly higher in BTBR as compared to B6 (Panel A, t(1, 22) = 4.52, p<0.01). CRF peptide levels in the paraventricular nucleus of the hypothalamus (PVN) did not differ between the two strains (Panel B, t(1, 17) = 1.44, NS). Quantitative in situ hybridization of CRF mRNA in the PVN did not differ between BTBR and B6 (data not shown, t(1, 8) = 1.02, NS). GR mRNA in the CA1 region of the hippocampus was higher in BTBR than in B6 (Panel C, t(1, 8) = 2.70, p<0.05). However, BTBR and B6 did not differ in GR mRNA levels in the CA2 region of the hippocampus, nor in the PVN (data not shown, CA2 t(1, 8) = 0.06, NS, PVN t(1, 8) = 0.33, NS). Oxytocin peptide levels in the PVN were significantly higher in BTBR as compared to B6 (Panel D, t(1, 17) = 2.81, p<0.05).
Figure 1. Comparison of stress-related neuroendocrine factors in BTBR versus B6 mice.
Plasma levels of corticosterone, corticotrophin releasing factor (CRF) and oxytocin were determined by radioimmunoassay. Glucocorticoid receptor (GR) mRNA was determined by in situ hybridization. (A) Plasma corticosterone levels were higher in BTBR than B6, **p<0.01. N=12 BTBR, N=12 B6. (B) CRF detected in the paraventricular nucleus of the hypothalamus (PVN) did not differ between BTBR and B6. N=10 BTBR, N=9 B6. (C) GR mRNA measured by optical density units in the CA1 region of the hippocampus was significantly higher in BTBR than B6, *p<0.05. N=5 BTBR, N=5 B6. (D) Oxytocin peptide levels in the PVN were significantly higher in BTBR than B6, **p<0.01. N=9 BTBR, N=10 B6. Data are shown as ± SEM in all Figures.
Stress-induced hyperthermia
Figure 2 illustrates stress-induced hyperthermia. The mean basal body temperature, T1, was 36.01° ± 0.22° for BTBR and 35.89° ± 0.17° for B6, indicating no strain difference at baseline (t(1, 20) = 0.69, NS). The expected significant increase in body temperature at T2 compared to T1 was detected in both B6 (Panel A, t(1, 18) = 6.84, p<0.05) and BTBR (Panel B, t(1, 22) = 5.321, p<0.05). No strain difference was detected in delta, the change in body temperature (Panel C, t(1, 20) = 0.65, NS).
Figure 2. Similar stress-induced hyperthermia in BTBR and B6.
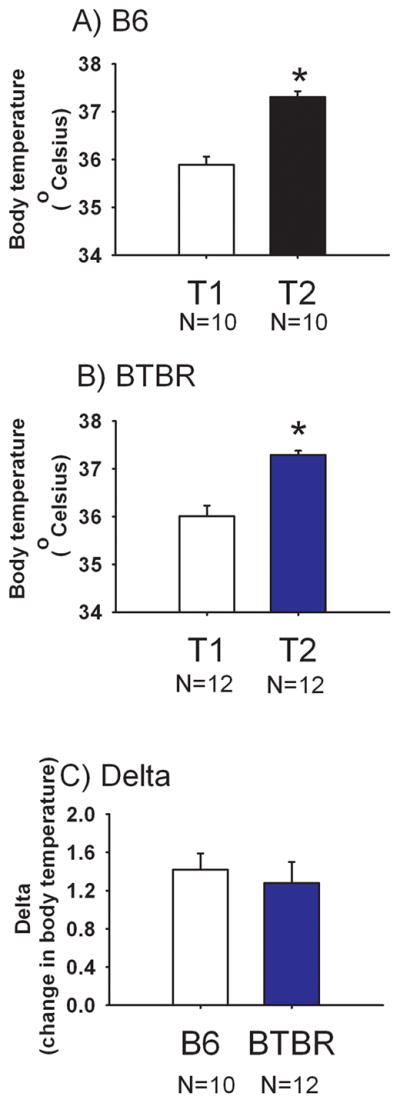
Body temperatures displayed by (A) B6 and (B) BTBR at the time point before (T1) and at the time point 10 minutes after (T2) handling and insertion of the rectal probe, *p<0.05 T2 versus T1. (C) No strain difference was detected in the stress-induced change in body temperature. N=12 BTBR, N=10 B6.
Forced swim and tail suspension
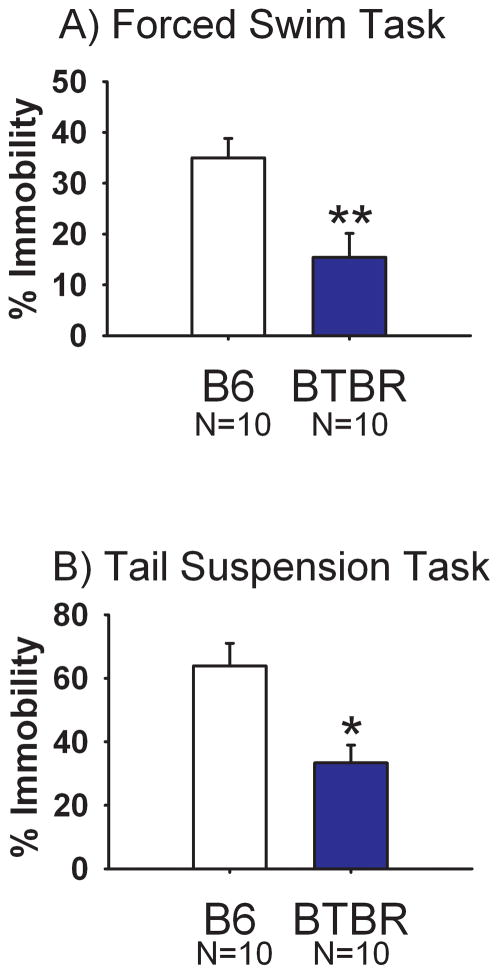
Figure 3 illustrates immobility scores for the forced swim task and the tail suspension test. During forced swim, immobility time was significantly lower in BTBR as compared to B6 (Panel A, t(1, 18) = 3.20, p<0.01). During tail suspension, immobility was similarly lower in BTBR as compared to B6 (Panel B, t(1, 18) = 3.37, p <0.05).
Figure 3. Less depression-relevant immobility in BTBR than B6.
Percent immobile observations were significantly lower in BTBR than in B6 mice on both (A) forced swim and (B) tail suspension. Data are presented as % time immobile over the last 4 minutes of the test session for the forced swim task, and over the entire 6 minute test session for the tail suspension task, *p<0.05 ** p < 0.01. N=10 BTBR, N=10 B6.
Elevated plus-maze and light↔dark exploration
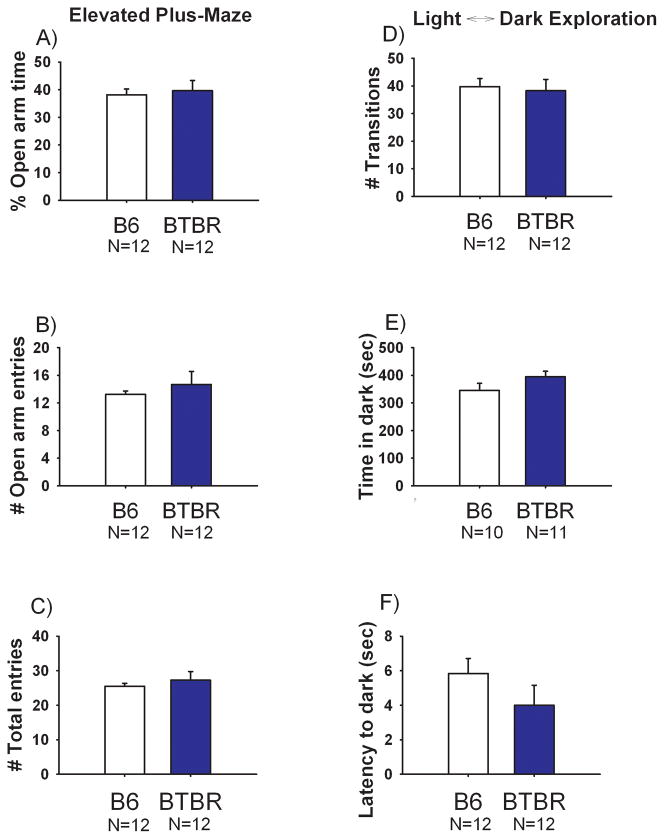
Figure 4 illustrates the lack of an anxiety-like phenotype in BTBR compared to B6 in the elevated plus-maze and light↔dark task. The strains did not differ on percentage of time spent on the open arms of the elevated plus-maze (Panel A, t(1, 22) = 0.34, NS). Entries into the open arms (Panel B, t(1, 22) = 0.73, NS), or total entries (Panel C, t(1, 22) = 0.66, NS). No significant differences were detected between strains in the total number of transitions between the light and dark compartments (Panel D, t(1, 22) = 0.29, NS), cumulative time spent in the dark chamber (Panel E, t(1, 19) = 1.48, NS) or the latency to first entry into the dark chamber (Panel F, t(1, 22) = 1.25, NS).
Figure 4. Similar anxiety-related scores in BTBR and B6.
Elevated plus-maze scores revealed no significant differences in (A) percentage of time spent on the open segments (B) entries into the open arm segments and as an internal locomotion control (C) total entries between BTBR and B6. Light↔dark exploration detected no significant differences in (D) number of transitions between the light and dark sides of the apparatus (E) time spent in the dark chamber and (F) latency to enter the dark portion of the apparatus between BTBR and B6. N=12 BTBR, N=12 B6.
Hot plate and tail flick
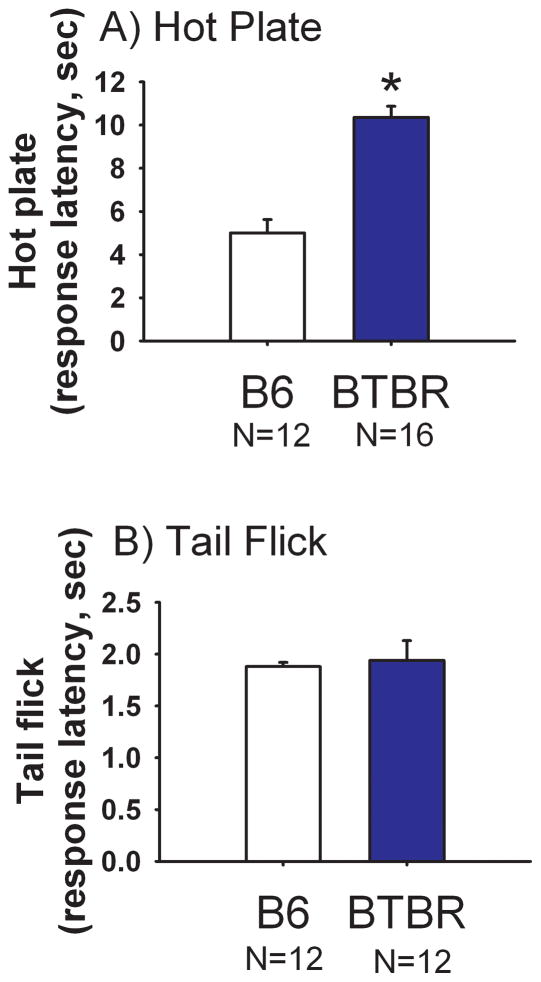
Figure 5 illustrates responses to painful sensory stimuli in BTBR and B6. Higher latencies to react to an aversive stimulus on the hot plate task were detected in BTBR compared to B6 in two independent groups of mice (Cohort 1: Panel A, t(1, 26) = 6.73, p<0.05). A second cohort of mice yielded similar results (data not shown): BTBR latency 11.4 ± 0.68 sec, B6 latency 6.6 ± 0.63 sec (t(1, 24) = 5.14, p<0.0001). No significant effect of strain was observed on tail flick pain sensitivity (Panel B, t(1, 22) = 0.29, NS).
Figure 5. Reduced response on hot plate but not on tail flick in BTBR as compared to B6.
(A) Latency to jump, lick or vocalize was significantly higher in BTBR than B6 on the hot plate test, *p<0.05. N=16 BTBR, N=12 B6. (B) Latency to move the tail away from a hot beam of light did not differ between BTBR and B6. N=12 BTBR, N=12 B6.
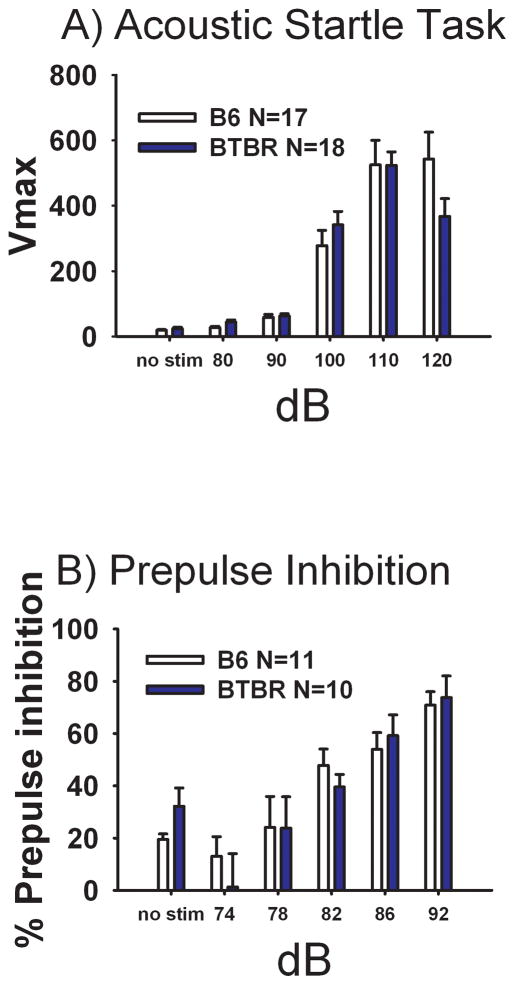
Acoustic startle stimulus and prepulse inhibition
Figure 6 Panel A illustrates startle reactivity over a variable range of decibel levels in both BTBR and B6 mice. Both strains displayed graded startle reactivity as expected (B6: F(5, 16) = 34.58, p<0.001), (BTBR: F(5, 17) = 55.74, p<0.001). No strain difference was detected on startle response at any decibel level (F(1, 33) = 0.15, NS), with similar thresholds apparent. Panel B depicts the expected increase in PPI as the decibels of prepulse increased for both strains (B6: F(1, 10) = 16.38, p<0.001), (BTBR: F(1, 9) = 10.14, p<0.001). No significant strain differences were observed at any level of PPI (F(1, 19) = 0.002, NS).
Figure 6. Similar acoustic startle and sensorimotor gating in BTBR and B6.
No significant strain differences at any decibel level were detected on (A) Acoustic startle responses for BTBR and B6. N=18 BTBR, N=17 B6, (B) Prepulse inhibition of acoustic startle N=10 BTBR, N=11 B6.
Discussion
The BTBR inbred mouse strain displays behavioral phenotypes with analogies to all three core diagnostic symptoms of autism, replicated in multiple cohorts of mice and across several laboratories (Bolivar et al., 2007, Moy et al., 2007, Yang et al., 2007b, McFarlane et al., 2008, Scattoni et al., 2008, Pobbe et al., 2010). High levels of repetitive self-grooming represent a robust phenotype relevant to repetitive behaviors in autism. Given that a) circulating corticosterone levels are high in BTBR (Benno et al., 2009, Frye and Llaneza, 2010), b) self-grooming in mice often occurs as a displacement behavior in the context of stressful stimuli (van Erp et al., 1994, Kalueff and Tuohimaa, 2004, Kalueff and Tuohimaa, 2005b), and c) irritability, upset to change, and hyperreactivity to sensory stimuli are associated symptoms of autism (Lord et al., 2000, Dawson et al., 2004, Lord and Spence, 2006, Volkmar et al., 2009, Zwaigenbaum et al., 2009), it seemed plausible that the autism-relevant phenotypes of BTBR emerged from an underlying hyper-responsivity to stressful stimuli. The present studies investigated the hypothesis that repetitive self-grooming in BTBR is an outcome of unusually high stress responsivity in this strain of mice. The findings presented herein provide several lines of evidence indicating that BTBR are not hyper-responsive to stressors, as compared to B6, on their baseline behavioral profiles. BTBR displayed lower scores than B6 on two depression-relevant behaviors and on hot plate nociception. Lack of differences between BTBR and B6 were found on two anxiety-related tests and on responses to acoustic startle, sensorimotor gating, tail flick nociception and on stress-induced hyperthermia. As compared to several other inbred strains (Crawley et al., 1997), BTBR appears to fall within the low to moderate range of reactivity to stressful stimuli.
Low levels of depression-related immobility exhibited by BTBR in the forced swim and tail suspension tests are consistent with the interpretation of a lack of depression-like phenotypes. One consideration is that BTBR displays high body weights, and higher levels of initial exploratory locomotor activity in the open field, as compared to B6 (Moy et al., 2007, McFarlane et al., 2008, Scattoni et al., 2008, Silverman et al., 2010), which could conceivably affect performance on the forced swim and/or tail suspension tests. However, immobility in these two tasks is not heavily influenced by body weight (Mico et al., 1986, Lucki et al., 2001, Liu and Gershenfeld, 2003) and differences in baseline immobility do not necessarily correlate nor can be explained by differences in locomotor activity (Logue et al., 1997, Lucki et al., 2001).
In agreement with two previous reports (Benno et al., 2009, Frye and Llaneza, 2010), we detected higher circulating corticosterone in BTBR as compared to a standard social strain, B6. High plasma corticosterone usually reflects high levels of activation of the hypothalamic-pituitary-adrenal (HPA) axis, initiated by hypothalamic CRF activation (Koenig et al., 2005). Paradoxically, levels of CRF peptide and mRNA levels in the PVN of the hypothalamus did not differ between BTBR and B6. Hypothalamic CRF is a potent activator of the pituitary-adrenal system, coordinating the physiological and behavioral response to stress (Bale and Vale, 2004). Affective disorders related to heightened stress sensitivity and dysregulation of stress coping mechanisms often involve dysfunctions of regulatory mechanisms of CRF family members (Stenzel-Poore et al., 1994, Smith et al., 1998, Bale et al., 2000, Bale et al., 2002). While CRF activation generally elevates circulating corticosterone, CRF-induced behavioral changes in stress reactivity may be mediated by direct neurotransmitter actions of CRF on brain pathways independent of HPA activation, thereby not stimulating corticosterone release, and insensitive to dexamethasone suppression (Britton et al., 1986, Stenzel-Poore et al., 1994, Pavcovich and Valentino, 1997). Further, while transgenic mice that chronically overexpress CRF exhibit anxiety-like behaviors on the elevated plus-maze (Stenzel-Poore et al., 1994), CRF deficient mice have minimal impairments in stress responses (Dunn and Swiergiel, 1999). Mice with a null mutation in the CRF1 receptor subtype display low anxiety-related phenotypes relevant to stress reactivity, exhibiting high open arm time in the elevated plus-maze, while CRFR2 null mutants display high anxiety-like phenotypes on the elevated plus-maze, indicating a complex central circuitry through which CRF mediates behavioral responses to stressful stimuli (Smith et al., 1998, Bale et al., 2000). The present data suggest that high peripheral corticosterone in BTBR arises from causes distinct from classical CRF-mediated HPA activation.
The physiological mechanism causing high circulating corticosterone in BTBR remains to be determined. However, it is interesting to note that corticosterone regulates metabolic responses, including fat accumulation, insulin resistance and potentially obesity in mice and humans (Bjorntorp and Rosmond, 2000, Bjorntorp, 2001, Smart et al., 2006, Michailidou et al., 2007, Roberge et al., 2007). Corticosterone binds to its receptors in visceral depots which are lipolytic regions with enriched glucocorticoid receptor expression, to activate lipoprotein lipase and inhibit insulin-induced lipid mobilization. These complex interactions lead to triglyceride accumulation and retention in visceral adipose tissue (Bjorntorp, 1996). BTBR are well-documented for displaying increased body weight, elevated abdominal fat and higher fasting insulin levels compared to B6 (Flowers et al., 2007, Scattoni et al., 2008). Elegant studies of whole body glucose metabolism, basal insulin levels and body composition revealed differences between BTBR and B6 that indicate a mild insulin resistant-like phenotype in BTBR (Flowers et al., 2007, Zhao et al., 2009). Abnormalities in circulating corticosterone in BTBR may therefore be linked to peripheral metabolic dysfunctions rather than central nervous system causes.
Another possibility to consider is that corticosterone’s precursor, progesterone, could conceivably be metabolized differently in BTBR. Brain metabolites of progesterone such as 3α, 5α tetrahydroprogesterone (THP) and dihydroprogesterone (5α-DHP) regulate several neurobiological processes including stress reactivity and depression. 3α,5α-THP is a potent stimulator of GABA receptors and has anxiolytic properties (Steimer et al., 1997). Progesterone may have both partial agonist and/or antagonist properties at glucocorticoid receptors and, in mammals, has been shown to reduce HPA negative feedback (Keller-Wood et al., 1988). Abnormalities in basal concentrations of corticosterone, progesterone and the neurosteroid 3α,5α-THP have been reported in BTBR (Frye et al., 2010).
Elevated oxytocin in the PVN of the hypothalamus, detected in the present experiments, offers another possible explanation for the cause of high repetitive self-grooming in BTBR. Oxytocin induces grooming in rodents when microinjected into the lateral ventricle, ventral tegmentum and nucleus accumbens (Drago et al., 1986, Kaltwasser and Crawley, 1987, Pedersen et al., 1988, Drago et al., 1991, Amico et al., 2004). On the other hand, oxytocin plays a key role in affiliative social, grooming, depressive and anxiety-like behaviors in several rodent species (Carter et al., 1992, Winslow et al., 1993, McCarthy et al., 1996, McCarthy et al., 1997, Bale et al., 2001, Ferguson et al., 2001, Insel and Young, 2001, Winslow and Insel, 2002, Amico et al., 2004, Ring et al., 2006, Lee et al., 2009, Macbeth et al., 2009). Oxytocin null mutant mice display impaired social recognition and poor social memory (Ferguson et al., 2000). Given the robust social deficits in BTBR, one may have predicted reduced oxytocin neurotransmission in this strain. However, it is interesting to note that two independent lines of oxytocin null mutant mice displayed normal social approach (Crawley et al., 2007) on a social task in which BTBR is deficient. Another consideration is that postmortem tissue levels of a peptide neurotransmitter do not necessarily reflect synaptic concentrations of the neuropeptide in vivo. High oxytocin peptide levels in the PVN could be caused by higher synthesis and/or lower release. Similarly, the functional outcome of high oxytocin peptide levels in the PVN depends on the concentration and occupancy of postsynaptic oxytocin receptors. Although oxytocin receptors were not investigated within the present experiments, our preliminary data indicate higher levels of oxytocin receptor mRNA in several brain regions (unpublished data by Macbeth, Silverman, Crawley and Young, NIMH). Potential compensatory activation of the oxytocin receptor by vasopressin, a closely related hypothalamic nonapeptide (Caldwell and Young, 2006), represents a further complication in interpreting the implications of elevated oxytocin in the PVN on self-grooming and social behaviors in BTBR.
Taken together with previous reports in which BTBR displayed low anxiety-like scores on the elevated plus-maze (Moy et al., 2007, Yang et al., 2009), elevated zero maze (McFarlane et al., 2008), and light↔dark exploration task (Yang et al., 2009), and high exploratory behavior in an empty novel open field (McFarlane et al., 2008, Yang et al., 2009, Silverman et al., 2010), our present findings of comparatively low depression-relevant behaviors, low pain sensitivity, and scores in the normal range for responses to several aversive sensory stimuli, suggest that the baseline profile of behavioral reactivity of BTBR is consistent with an interpretation of low to moderate responsivity on mildly stressful behavioral tasks. The present data, therefore, argue against hyperreactivity to stress as a primary cause of the high repetitive self-grooming in BTBR. Similarly, the low to normal reactivity components of the BTBR phenotype are not analogous to the subset of individuals with autism who display high levels of irritability and hyperreactivity to sensory stimuli, but may be relevant to individuals with autism who display low affect and hyposensitivity to painful stimuli. The complex central circuitry for neuropeptides and neuroendocrine factors mediating stress responses will require further extensive experiments to fully understand the neurochemical mechanisms underlying repetitive behaviors in BTBR mice.
Acknowledgments
Supported by the National Institute of Mental Health Intramural Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington D.C: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Amico JA, Vollmer RR, Karam JR, Lee PR, Li X, Koenig JI, McCarthy MM. Centrally administered oxytocin elicits exaggerated grooming in oxytocin null mice. Pharmacol Biochem Behav. 2004;78:333–339. doi: 10.1016/j.pbb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci. 2001;115:443–454. [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Super-stereotypy I: enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse. 2000a;37:194–204. doi: 10.1002/1098-2396(20000901)37:3<194::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Super-stereotypy II: enhancement of a complex movement sequence by intraventricular dopamine D1 agonists. Synapse. 2000b;37:205–215. doi: 10.1002/1098-2396(20000901)37:3<205::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20:291–302. [PubMed] [Google Scholar]
- Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Blakeman KH, Hao JX, Xu XJ, Jacoby AS, Shine J, Crawley JN, Iismaa T, Wiesenfeld-Hallin Z. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience. 2003;117:221–227. doi: 10.1016/s0306-4522(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RA, Olivier B. Stress-induced hyperthermia in mice: effects of flesinoxan on heart rate and body temperature. Eur J Pharmacol. 2000;400:59–66. doi: 10.1016/s0014-2999(00)00387-3. [DOI] [PubMed] [Google Scholar]
- Britton DR, Varela M, Garcia A, Rosenthal M. Dexamethasone suppresses pituitary-adrenal but not behavioral effects of centrally administered CRF. Life Sci. 1986;38:211–216. doi: 10.1016/0024-3205(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD. Multiple rare variants in the etiology of autism spectrum disorders. Dialogues Clin Neurosci. 2009;11:35–43. doi: 10.31887/DCNS.2009.11.1/jdbuxbaum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Young WSI. Oxytocin and vasopressin: genetics and behavioral implications. In: Lim R, editor. Neuroactive Proteins and Peptides. New York, NY: Springer; 2006. pp. 573–607. [Google Scholar]
- Carter CS, Williams JR, Witt DM, Insel TR. Oxytocin and social bonding. Ann N Y Acad Sci. 1992;652:204–211. doi: 10.1111/j.1749-6632.1992.tb34356.x. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates behavioral response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31:59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral Phenotyping of Transgenic and Knockout Mice. Hoboken, NJ: John Wiley & Sons, Inc; 2007. What's Wrong With My Mouse? [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Drago F, Bohus B, Canonico PL, Scapagnini U. Prolactin induces grooming in the rat: possible involvement of nigrostriatal dopaminergic system. Pharmacol Biochem Behav. 1981;15:61–63. doi: 10.1016/0091-3057(81)90339-7. [DOI] [PubMed] [Google Scholar]
- Drago F, Pedersen CA, Caldwell JD, Prange AJ., Jr Oxytocin potently enhances novelty-induced grooming behavior in the rat. Brain Res. 1986;368:287–295. doi: 10.1016/0006-8993(86)90573-1. [DOI] [PubMed] [Google Scholar]
- Drago F, Sarnyai Z, D'Agata V. The inhibition of oxytocin-induced grooming by a specific receptor antagonist. Physiol Behav. 1991;50:533–536. doi: 10.1016/0031-9384(91)90541-u. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW, Lai YI, Yachabach TL. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8:841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Behav. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Behavioral responses to stress are intact in CRF-deficient mice. Brain Res. 1999;845:14–20. doi: 10.1016/s0006-8993(99)01912-5. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Iversen SD. Behavioural effects of tachykinins and related peptides. Brain Res. 1986;381:68–76. doi: 10.1016/0006-8993(86)90691-8. [DOI] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–217. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferrari W. Behavioural changes in animals after intracisternal injection with adrenocorticotrophic hormone and melanocyte-stimulating hormone. Nature. 1958;181:925–926. doi: 10.1038/181925a0. [DOI] [PubMed] [Google Scholar]
- Flowers JB, Oler AT, Nadler ST, Choi Y, Schueler KL, Yandell BS, Kendziorski CM, Attie AD. Abdominal obesity in BTBR male mice is associated with peripheral but not hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2007;292:E936–945. doi: 10.1152/ajpendo.00370.2006. [DOI] [PubMed] [Google Scholar]
- Frye CA, Llaneza DC. Corticosteroid and Neurosteroid Dysregulation in an Animal Model of Autism, BTBR Mice. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen WH, Wiegant VM, Greven HM, de Wied D. The induction of excessive grooming in the rat by intraventricular application of peptides derived from ACTH: structure-activity studies. Life Sci. 1975;17:645–652. doi: 10.1016/0024-3205(75)90103-4. [DOI] [PubMed] [Google Scholar]
- Happe F, Ronald A. The 'fractionable autism triad': a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18:287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology. 2003a;28:1031–1044. doi: 10.1038/sj.npp.1300164. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003b;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci. 2002a;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003c;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002b;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Hooi SC, Richardson GS, McDonald JK, Allen JM, Martin JB, Koenig JI. Neuropeptide Y (NPY) and vasopressin (AVP) in the hypothalamo-neurohypophysial axis of salt-loaded or Brattleboro rats. Brain Res. 1989;486:214–220. doi: 10.1016/0006-8993(89)90507-6. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Kaltwasser MT, Andres U. The interaction of central oxytocin and cholecystokinin regulates grooming behavior in the rat. Brain Res. 1989;496:380–384. doi: 10.1016/0006-8993(89)91093-7. [DOI] [PubMed] [Google Scholar]
- Kaltwasser MT, Crawley JN. Oxytocin and cholecystokinin induce grooming behavior in the ventral tegmentum of the rat. Brain Res. 1987;426:1–7. doi: 10.1016/0006-8993(87)90418-5. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res Brain Res Protoc. 2004;13:151–158. doi: 10.1016/j.brainresprot.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI) Behav Brain Res. 2005a;160:1–10. doi: 10.1016/j.bbr.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods. 2005b;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kametani H. Analysis of age-related changes in stress-induced grooming in the rat. Differential behavioral profile of adaptation to stress. Ann N Y Acad Sci. 1988;525:101–113. doi: 10.1111/j.1749-6632.1988.tb38599.x. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 2008;195:547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, Silbiger J, Wood CE. Progesterone attenuates the inhibition of adrenocorticotropin responses by cortisol in nonpregnant ewes. Endocrinology. 1988;123:647–651. doi: 10.1210/endo-123-1-647. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nat Clin Pract Neurol. 2008;4:138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Brain Res Mol Brain Res. 2003;115:55–62. doi: 10.1016/s0169-328x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Lintas C, Persico AM. Autistic phenotypes and genetic testing: state-of-the-art for the clinical geneticist. J Med Genet. 2009;46:1–8. doi: 10.1136/jmg.2008.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. An exploratory factor analysis of the Tail Suspension Test in 12 inbred strains of mice and an F2 intercross. Brain Res Bull. 2003;60:223–231. doi: 10.1016/s0361-9230(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Spence S. Autism spectrum disorders: phenotype and diagnosis. In: Moldin S, Rubenstein J, editors. Understanding autism: from basic neuroscience to treatment. New York, NY: Taylor & Francis; 2006. pp. 1–23. [Google Scholar]
- Lucki I. A prescription to resist proscriptions for murine models of depression. Psychopharmacology (Berl) 2001;153:395–398. doi: 10.1007/s002130000561. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009;8:558–567. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009;30:1107–1114. doi: 10.1016/j.ridd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Matsuzaki I, Takamatsu Y, Moroji T. The effects of intracerebroventricularly injected corticotropin-releasing factor (CRF) on the central nervous system: behavioural and biochemical studies. Neuropeptides. 1989;13:147–155. doi: 10.1016/0143-4179(89)90085-1. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Schwartz-Giblin S, Wang SM. Does estrogen facilitate social behavior by reducing anxiety? Ann N Y Acad Sci. 1997;807:541–542. doi: 10.1111/j.1749-6632.1997.tb51962.x. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Meisenberg G. Short-term behavioral effects of posterior pituitary peptides in mice. Peptides. 1981;2:1–8. doi: 10.1016/s0196-9781(81)80003-4. [DOI] [PubMed] [Google Scholar]
- Meisenberg G. Vasopressin-induced grooming and scratching behavior in mice. Ann N Y Acad Sci. 1988;525:257–269. doi: 10.1111/j.1749-6632.1988.tb38611.x. [DOI] [PubMed] [Google Scholar]
- Michailidou Z, Coll AP, Kenyon CJ, Morton NM, O'Rahilly S, Seckl JR, Chapman KE. Peripheral mechanisms contributing to the glucocorticoid hypersensitivity in proopiomelanocortin null mice treated with corticosterone. J Endocrinol. 2007;194:161–170. doi: 10.1677/JOE-07-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mico JA, Thierry AM, Chermat R, Steru L, Simon P. Effect of body weight on the tail suspension test in mice. IRCS Medical Science. 1986;14:274. [Google Scholar]
- Miesfeld R, Rusconi S, Godowski PJ, Maler BA, Okret S, Wikstrom AC, Gustafsson JA, Yamamoto KR. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986;46:389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Heymann-Monnikes I, Tache Y. CRF in the paraventricular nucleus of the hypothalamus induces dose-related behavioral profile in rats. Brain Res. 1992;574:70–76. doi: 10.1016/0006-8993(92)90801-f. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. Corticotrophin releasing factor, grooming and ingestive behavior. Life Sci. 1982;31:1459–1464. doi: 10.1016/0024-3205(82)90007-8. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008a;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008b;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyaho A, Valencia J. Grooming and yawning trace adjustment to unfamiliar environments in laboratory Sprague-Dawley rats (Rattus norvegicus) J Comp Psychol. 2002;116:263–269. doi: 10.1037/0735-7036.116.3.263. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Wong JC, Kennedy BC, Lahvis GP. Differential entrainment of a social rhythm in adolescent mice. Behav Brain Res. 2008;195:239–245. doi: 10.1016/j.bbr.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavcovich LA, Valentino RJ. Regulation of a putative neurotransmitter effect of corticotropin-releasing factor: effects of adrenalectomy. J Neurosci. 1997;17:401–408. doi: 10.1523/JNEUROSCI.17-01-00401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Drago F, Noonan LR, Peterson G, Hood LE, Prange AJ., Jr Grooming behavioral effects of oxytocin. Pharmacology, ontogeny, and comparisons with other nonapeptides. Ann N Y Acad Sci. 1988;525:245–256. doi: 10.1111/j.1749-6632.1988.tb38610.x. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Reaven JA. Children with high-functioning autism spectrum disorders and co-occurring anxiety symptoms: implications for assessment and treatment. J Spec Pediatr Nurs. 2009;14:192–199. doi: 10.1111/j.1744-6155.2009.00197.x. [DOI] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, Zink M, Hortnagl H, Flor H, Henn FA, Schutz G, Gass P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Roberge C, Carpentier AC, Langlois MF, Baillargeon JP, Ardilouze JL, Maheux P, Gallo-Payet N. Adrenocortical dysregulation as a major player in insulin resistance and onset of obesity. Am J Physiol Endocrinol Metab. 2007;293:E1465–1478. doi: 10.1152/ajpendo.00516.2007. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wohr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent marking, is modulated by social experience. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.06.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy V, Merali Z, Poulter MO, Anisman H. Anxiety responses, plasma corticosterone and central monoamine variations elicited by stressors in reactive and nonreactive mice and their reciprocal F1 hybrids. Behav Brain Res. 2007;185:49–58. doi: 10.1016/j.bbr.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Sachs BD. The development of grooming and its expression in adult animals. Ann N Y Acad Sci. 1988;525:1–17. doi: 10.1111/j.1749-6632.1988.tb38591.x. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi JL, Brady DL, Koenig JI. Estrogen modulates RGS9 expression in the nucleus accumbens. Neuroreport. 2004;15:2433–2436. doi: 10.1097/00001756-200410250-00026. [DOI] [PubMed] [Google Scholar]
- Sherman JE, Kalin NH. The effects of ICV-CRH on novelty-induced behavior. Pharmacol Biochem Behav. 1987;26:699–703. doi: 10.1016/0091-3057(87)90599-5. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Steimer T, Driscoll P, Schulz PE. Brain metabolism of progesterone, coping behaviour and emotional reactivity in male rats from two psychogenetically selected lines. J Neuroendocrinol. 1997;9:169–175. doi: 10.1046/j.1365-2826.1997.t01-1-00571.x. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stivers JA, Kaltwasser MT, Hill PS, Hruby VJ, Crawley JN. Ventral tegmental oxytocin induces grooming. Peptides. 1988;9 (Suppl 1):223–231. doi: 10.1016/0196-9781(88)90248-3. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. CRF-deficient mice respond like wild-type mice to hypophagic stimuli. Pharmacol Biochem Behav. 1999;64:59–64. doi: 10.1016/s0091-3057(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Seasholtz AF, Herbert E. Rat corticotropin-releasing hormone gene: sequence and tissue-specific expression. Mol Endocrinol. 1987;1:363–370. doi: 10.1210/mend-1-5-363. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, Brailly-Tabard S, Perez-Diaz F, Graignic R, Carlier M, Schmit G, Rolland AC, Bonnot O, Trabado S, Roubertoux P, Bronsard G. Pain reactivity and plasma beta-endorphin in children and adolescents with autistic disorder. PLoS One. 2009;4:e5289. doi: 10.1371/journal.pone.0005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, Hertzig ME, Snow ME, Hall LM, Thompson SM, Ferrari P, Cohen DJ. Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry. 1997;38:705–715. doi: 10.1111/j.1469-7610.1997.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Van Erp AM, Kruk MR, De Kloet ER. Induction of grooming in resting rats by intracerebroventricular oxytocin but not by adrenocorticotropic hormone-(1–24) and alpha-melanocyte-stimulating hormone. Eur J Pharmacol. 1993;232:217–221. doi: 10.1016/0014-2999(93)90776-e. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behav Brain Res. 1994;65:47–55. doi: 10.1016/0166-4328(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Van Wimersma Greidanus TB, Maigret C, Krechting B. Excessive grooming induced by somatostatin or its analog SMS 201–995. Eur J Pharmacol. 1987;144:277–285. doi: 10.1016/0014-2999(87)90380-3. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, State M, Klin A. Autism and autism spectrum disorders: diagnostic issues for the coming decade. J Child Psychol Psychiatry. 2009;50:108–115. doi: 10.1111/j.1469-7610.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Crawley JN, Hokfelt T. Galanin and spinal nociceptive mechanisms: recent results from transgenic and knock-out models. Neuropeptides. 2005;39:207–210. doi: 10.1016/j.npep.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Shapiro L, Carter CS, Insel TR. Oxytocin and complex social behavior: species comparisons. Psychopharmacol Bull. 1993;29:409–414. [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2010 doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social Approach Behaviors are Similar on Conventional Versus Reverse Lighting Cycles, and in Replications Across Cohorts, in BTBR T+ tf/J, C57BL/6J, and Vasopressin Receptor 1B Mutant Mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao E, Keller MP, Rabaglia ME, Oler AT, Stapleton DS, Schueler KL, Neto EC, Moon JY, Wang P, Wang IM, Lum PY, Ivanovska I, Cleary M, Greenawalt D, Tsang J, Choi YJ, Kleinhanz R, Shang J, Zhou YP, Howard AD, Zhang BB, Kendziorski C, Thornberry NA, Yandell BS, Schadt EE, Attie AD. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm Genome. 2009;20:476–485. doi: 10.1007/s00335-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, Chawarska K, Constantino J, Dawson G, Dobkins K, Fein D, Iverson J, Klin A, Landa R, Messinger D, Ozonoff S, Sigman M, Stone W, Tager-Flusberg H, Yirmiya N. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics. 2009;123:1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]