Abstract
The sensitization of hepatocytes to cell death from TNF underlies many forms of hepatic injury including that from toxins. Critical for hepatocyte resistance to TNF toxicity is activation of NF-κB signaling which prevents TNF-induced death by the up regulation of protective proteins. To further define the mechanisms of hepatocyte sensitization to TNF killing, immunoblot analysis comparing livers from mice treated with LPS alone or LPS together with the hepatotoxin galactosamine (GalN) was performed to identify TNF-induced protective proteins blocked by GalN. Levels of CCAAT/enhancer-binding protein β (C/EBPβ) were increased after LPS but not GalN/LPS treatment. In a nontransformed rat hepatocyte cell line, TNF-induced increases in C/EBPβ protein levels were dependent on NF-κB-mediated inhibition of proteasomal degradation. Pharmacological inhibition of c-Jun N-terminal kinase (JNK) did not affect C/EBPβ degradation, indicating that the process was JNK independent. C/EBPβ functioned to prevent cell death as adenoviral C/EBPβ overexpression blocked TNF-induced apoptosis in cells sensitized to TNF toxicity by NF-κB inhibition. C/EBPβ inhibited TNF-induced caspase 8 activation and downstream mitochondrial cytochrome c release and caspase 3 and 7 activation. Studies in primary hepatocytes from c/ebpβ−/− mice confirmed that loss of C/EBPβ increased death from TNF. c/ebpβ−/− mice were also sensitized to liver injury from a nontoxic dose of LPS or TNF. The absence of jnk2 failed to reverse the GalN-induced block in C/EBPβ induction by LPS, again demonstrating that C/EBPβ degradation was JNK independent. Conclusion: C/EBPβ is up regulated by TNF and mediates hepatocyte resistance to TNF toxicity by inhibiting caspase-dependent apoptosis. In the absence of NF-κB signaling, proteasomal degradation of C/EBPβ is increased by a JNK-independent mechanism and promotes death from TNF.
Keywords: apoptosis, c-Jun N-terminal kinase, lipopolysaccharide, liver injury, galactosamine
Tumor necrosis factor α (TNF) is a critical mediator of multiple forms of liver injury including that resulting from toxins,1,2 ischemia/reperfusion,3,4 viral hepatitis5,6 and cholestasis.7,8 Central to TNF's role as a hepatotoxic factor is its ability under certain pathophysiological conditions to induce apoptotic cell death. TNF binding to the type 1 TNF receptor recruits a series of intracellular proteins that ultimately activate initiator caspase 8.9 Caspase 8 activation triggers the sequential release of lysosomal cathepsin B,10 cleavage of the proapoptotic Bcl-2 family member Bid,11 initiation of the mitochondrial death pathway with release of cytochrome c and activation of downstream effector caspases that induce apoptosis.12 Hepatocytes are normally resistant to TNF cytotoxicity through TNF-induced activation of the transcription factor NF-κB.13,14 Loss of NF-κB activity in hepatocytes in culture,14 or in vivo,15 sensitizes the cells to death through this apoptotic pathway.10,13,14 TNF-dependent liver injury from hepatotoxins such as carbon tetrachloride, galactosamine and alcohol may result from a block in protective NF-κB signaling.
A mechanism of NF-κB-mediated resistance to TNF toxicity is down regulation of the mitogen-activated protein kinase c-Jun N-terminal kinase (JNK).16–18 In the absence of NF-κB signaling, TNF-induced JNK activation is converted from a transient to a prolonged response that triggers cell death. Although the central function of JNK is to increase gene transcription through the phosphorylation and activation of the AP-1 component c-Jun, JNK regulates TNF toxicity through non-transcriptional effects on protein degradation. The induction of cell death by JNK overactivation occurs in part from alterations in the half-lives of two proteins that mediate hepatocyte TNF resistance, cFLIP and Mcl-1.19,20 Loss of the transcription factor NF-κB therefore sensitizes hepatocytes to TNF cytotoxicity in part by JNK-dependent effects on protein degradation.
CCAAT/enhancer-binding protein β (C/EBPβ) is a member of a family of transcription factors that regulate a number of critical cellular functions including apoptosis.21 C/EBPβ has three protein forms that in rodents are termed LAP1 (38 kDa), LAP2 (34 kDa) and LIP (20 kDa).21 LAP1 and LAP2 act as transcriptional activators and LIP as a dominant negative. C/EBPβ promotes cell survival in several nonhepatic cell types after DNA damage.22–24 In addition, a novel non-transcriptional function of C/EBPβ as a caspase inhibitor has been described in hepatic stellate cells.22 Whether C/EBPβ performs this function in other cell types is unknown. In hepatocytes C/EBPβ promotes apoptosis from the death receptor Fas.25 C/EBPβ regulation by TNF has been shown to occur in hepatocytes at the level of subcellular localization as TNF induces C/EBPβ translocation to the nucleus where it regulates hepatocyte differentiation and proliferation through effects on gene transcription.26–28 It is unknown whether C/EBPβ functions in hepatocyte death from TNF.
Galactosamine/lipopolysaccharide (GalN/LPS)-induced liver injury is a well established experimental model of TNF-dependent hepatic injury.29,30 GalN is a hepatocyte-specific transcriptional inhibitor that at subtoxic doses sensitizes hepatocytes to death from LPS-induced TNF. A feature of this model is that protein changes induced by LPS alone can be contrasted with those from combined GalN/LPS treatment to identify protective proteins whose induction by TNF is blocked by GalN. Employing this approach we have identified C/EBPβ as a factor whose induction by LPS was blocked by the hepatotoxin GalN. In vitro and in vivo studies demonstrate that C/EBPβ blocks TNF-induced apoptosis in hepatocytes at the level of caspase 8 activation. These findings identify C/EBPβ as an NF-κB-regulated anti-apoptotic factor that mediates hepatocyte resistance to TNF toxicity.
Results
GalN Blocks the LPS-induced Increase in C/EBPβ in Mouse Liver
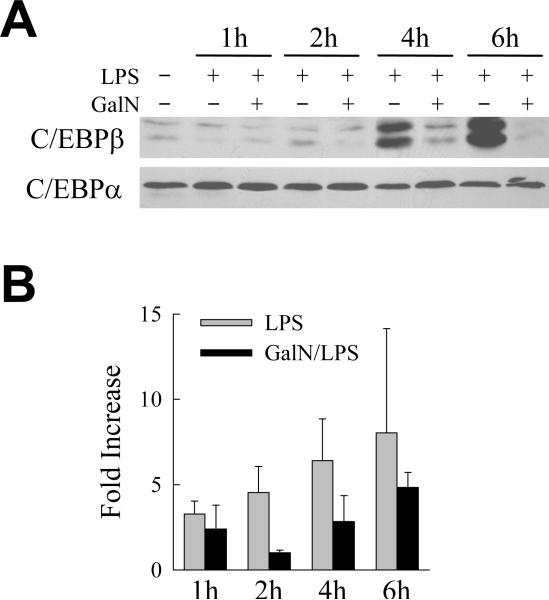
As a strategy to identify novel factors mediating hepatocyte resistance to hepatotoxin-induced, TNF-dependent liver injury, immunoblot analysis was performed on hepatic proteins isolated from LPS- and GalN/LPS-treated mice. An increase in hepatic levels of a specific protein by LPS alone that is blocked by GalN cotreatment identifies that protein as a potential TNF-inducible protective factor. C/EBPβ levels were examined because of the known function of this protein as a caspase inhibitor. LPS administration markedly increased both the LAP1 and LAP2 forms of C/EBPβ protein in mouse liver within 4 h (Fig. 1A). However, cotreatment with GalN blocked this LPS-induced increase in C/EBPβ (Fig. 1A). In contrast, levels of C/EBPα were unaffected by LPS or GalN treatment and served as loading control (Fig. 1A). These findings suggested that a hepatotoxin-mediated inhibition of C/EBPβ induction may sensitize hepatocytes to death from TNF.
Fig. 1.
LPS induction of C/EBPβ protein is blocked by GalN cotreatment. (A) Total hepatic protein was isolated from the livers of an untreated mouse and mice treated for the hours indicated with LPS alone, or LPS together with GalN, and immunoblotted with antibodies against C/EBPβ or C/EBPα. Results are representative of 3 independent experiments. (B) Fold increase in hepatic C/EBPβ mRNA levels as determined by real time PCR at the times indicated after LPS or GalN/LPS treatment. C/EBPβ mRNA levels were normalized to those for TATA-box binding protein (n=3).
GalN inhibits hepatocyte transcription suggesting that this hepatotoxin may block C/EBPβ induction by this mechanism. To determine whether GalN blocked C/EBPβ up regulation at the mRNA level, hepatic levels of C/EBPβ mRNA after LPS or GalN/LPS treatment were determined by real-time PCR. Levels of C/EBPβ mRNA increased 3–8 fold at 1–6 h after treatment with LPS alone (Fig. 1B). GalN did block the increase in C/EBPβ mRNA at 2 h, but at the other time points C/EBPβ mRNA levels increased 2–4 fold despite GalN cotreatment (Fig. 1B). Thus, GalN reduced but did not block completely the LPS-induced increase in C/EBPβ mRNA. These findings are in contrast to the complete absence of any increase in C/EBPβ protein in GalN/LPS-treated mice, suggesting that the lack of C/EBPβ protein induction in these mice was mediated at least in part through changes in protein translation or degradation.
TNF and LPS Induce an NF-κB-dependent Increase in C/EBPβ in RALA Hepatocytes
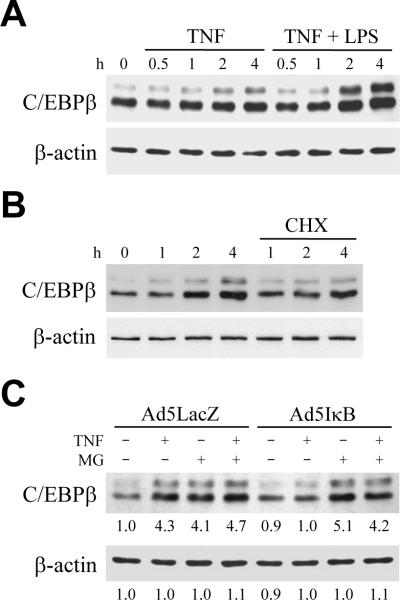
To determine whether TNF and LPS regulate C/EBPβ specifically in hepatocytes, the effects of TNF and LPS on C/EBPβ levels were examined in RALA hepatocytes cultured under nontransformed conditions. TNF treatment increased cellular C/EBPβ protein levels within 2 h (Fig. 2A). The increase in C/EBPβ was further augmented by cotreatment with LPS (Fig. 2A), indicating that TNF and LPS both up regulate C/EBPβ protein content in hepatocytes. The normal up regulation of C/EBPβ by TNF was dependent in part on protein synthesis as the induction was partially blocked by the protein synthesis inhibitor cycloheximide (Fig. 2B).
Fig. 2.
TNF induction of C/EBPβ is mediated by NF-κB-dependent inhibition of proteasomal degradation. (A) RALA hepatocytes were untreated or treated with TNF alone or together with LPS for the indicated number of hours. Total protein was isolated and aliquots immunoblotted with antibodies for C/EBPβ or β-actin. (B) RALA hepatocytes received no pretreatment or were pretreated with cycloheximide (CHX) for 1 h, and then treated with TNF for the number of hours shown. Total protein was immunoblotted for C/EBPβ and β-actin. (C) Following infection with Ad5LacZ or Ad5IκB, cells were left untreated or treated with TNF in the absence or presence of 1 h of MG132 (MG) pretreatment. Total protein was isolated 4 h after TNF administration and immunoblotted with C/EBPβ or β-actin antibodies. Levels of β-actin demonstrated equal loading among protein samples. Immunoblots are representative of 4 independent experiments. Numerical data under the immunoblots represents the relative signal intensity by densitometry scanning of 4 experiments.
TNF-induced activation of the transcription factor NF-κB is a critical protective response for hepatocyte resistance to TNF toxicity.14 To investigate the role of NF-κB in TNF up regulation of C/EBPβ, NF-κB activation was inhibited with the adenovirus Ad5IκB which expresses a mutant IκB that irreversibly binds and inactivates NF-κB.15 The TNF-mediated increase in C/EBPβ was abrogated in Ad5IκB-infected cells, but not in control Ad5LacZ-infected hepatocytes (Fig. 2C), indicating that NF-κB activation mediated the TNF-induced increase in C/EBPβ.
The total block in induction of C/EBPβ protein in GalN/LPS-treated mice despite an increase in C/EBPβ mRNA suggested that NF-κB signaling regulates C/EBPβ in vivo at the level of protein degradation. To test this possibility, cells were treated with TNF in the absence or presence of the proteasomal inhibitor MG132.31 MG132 treatment alone in Ad5LacZ- or Ad5IκB-infected cells increased cellular C/EBPβ protein content to a level equivalent to that in TNF-treated, Ad5LacZ-infected cells (Fig. 2C), demonstrating constitutive regulation of C/EBPβ levels by proteasomal degradation. Cotreatment with MG132 had no effect on C/EBPβ levels in TNF-treated, Ad5LacZ-infected cells (Fig. 2C), indicating that C/EBPβ was not regulated by proteasomal degradation of in these cells. In contrast, MG132 had a marked effect on C/EBPβ levels in cells lacking NF-κB. Inhibition of proteasomal function in Ad5IκB-infected cells increased TNF-induced C/EBPβ content to levels equivalent to those in TNF-treated, Ad5LacZ-infected cells (Fig. 2C). Thus, despite the fact that the TNF-induced increase in C/EBPβ depended in part on protein synthesis (Fig. 2B), the up regulation of C/EBPβ levels by TNF treatment was largely dependent on an NF-κB-dependent inhibition of C/EBPβ protein degradation.
As previous studies have demonstrated that JNK overactivation resulting from a block in NF-κB signaling alters protein degradation,19,20 the possible involvement of JNK in the increased degradation of C/EBPβ with NF-κB inhibition was examined. Pretreatment of cells with the pharmacological JNK inhibitor SP60012532 failed to reverse the block in C/EBPβ up regulation that occurred in the absence of NF-κB signaling (data not shown). These findings in total demonstrate that the up regulation of hepatocyte levels of C/EBPβ in response to TNF is dependent on NF-κB-mediated inhibition of proteasomal degradation by a JNK-independent mechanism.
C/EBPβ Regulates Hepatocyte Death from TNF
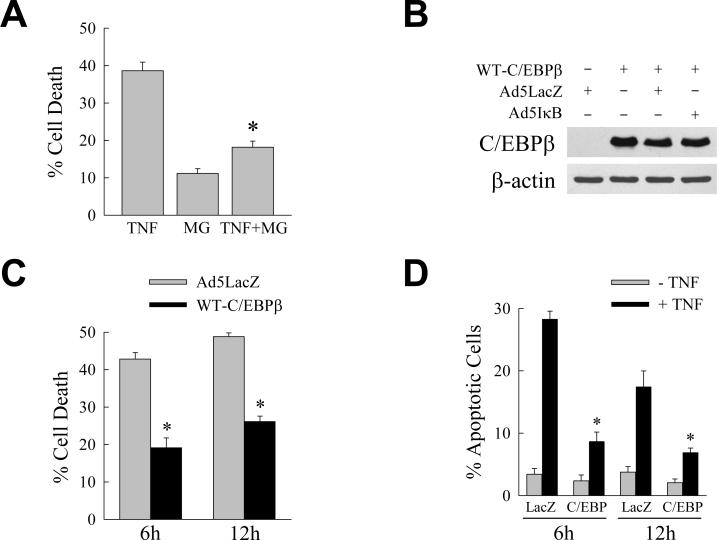
Studies in nonhepatic cells have demonstrated an anti-apoptotic function for C/EBPβ.22–24 The ability of proteasomal inhibition to increase levels of C/EBPβ led us to investigate whether MG132 was able to block hepatocyte death from TNF. Despite mild toxicity from MG132 treatment alone, proteasomal inhibition significantly decreased the amount of cell death in Ad5IκB-infected cells treated with TNF (Fig. 3A). To determine whether C/EBPβ functioned to prevent RALA hepatocyte death from TNF, the effect of C/EBPβ overexpression on TNF-induced apoptosis in RALA hepatocytes with an inhibition of NF-κB activation was assessed. Cells infected with the C/EBPβ-expressing adenovirus WT-C/EBPβ alone, or coinfected with WT-C/EBPβ and either Ad5LacZ or Ad5IκB, expressed increased levels of C/EBPβ as compared to cells infected with Ad5LacZ alone (Fig. 3B). Cells were coinfected with Ad5IκB and either Ad5LacZ as a control or WT-C/EBPβ and treated with TNF. When compared to Ad5IκB/Ad5LacZ-coinfected cells, the amount of cell death after TNF treatment was significantly decreased in Ad5IκB/WT-C/EBPβ-coinfected cells at 6 and 12 h by MTT assay (Fig. 3C). The ability of C/EBPβ expression to block cell death from TNF was confirmed by fluorescence microscopic studies of cells costained with acridine orange/ethidium bromide to quantify the numbers of apoptotic and necrotic cells. As previously established, death from NF-κB inactivation and TNF was predominantly apoptotic and no significant increase occurred in the numbers of necrotic cells (data not shown). The marked increase in apoptotic cells with TNF administration was significantly reduced by adenoviral expression of C/EBPβ (Fig. 3D). Thus, the NF-κB-dependent increase in C/EBPβ in TNF-treated RALA hepatocytes is a mechanism of cellular resistance to TNF-induced apoptosis.
Fig. 3.
C/EBPβ blocks RALA hepatocyte death from TNF. (A) Percentage cell death at 6 h by MTT assay in Ad5IκB-infected cells treated with TNF or MG132 (MG) alone or in combination (*P<0.00001 as compared to cells treated with TNF alone; n=8). (B) Immunoblots of total protein from RALA hepatocytes infected with WT-C/EBPβ, Ad5LacZ and Ad5IκB as indicated and probed for C/EBPβ or β-actin. (C) Percentage cell death by MTT assay at 6 and 12 h after TNF treatment in cells coinfected with Ad5IκB and either Ad5LacZ or WT-C/EBPβ (*P<0.01 as compared to TNF-treated Ad5IκB/Ad5LacZ-coinfected cells; n=3–4). (D) The percentage of apoptotic cells in Ad5IκB/Ad5LacZ (LacZ) and Ad5IκB/WT-C/EBPβ (C/EBP) coinfected cells untreated or TNF treated for 6 or 12 h (*P<0.01 as compared to TNF-treated Ad5IκB/Ad5LacZ-infected cells; n=3–4).
C/EBPβ Inhibits TNF-induced Caspase Activation
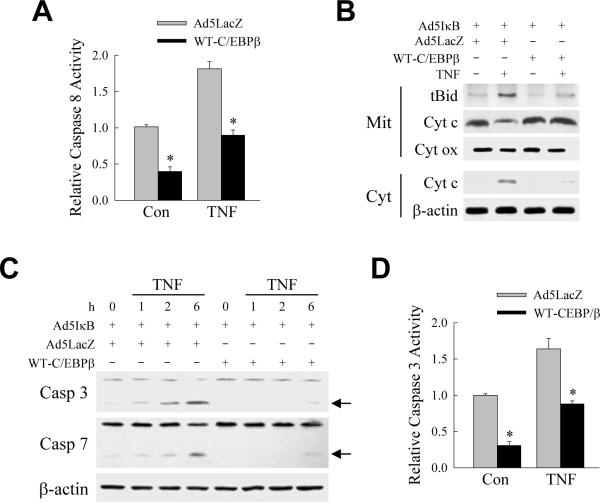
The sensitization of hepatocytes to TNF toxicity by NF-κB inhibition occurs through caspase-dependent apoptosis.17,33 The ability of C/EBPβ to function as a caspase inhibitor suggested that the mechanism of C/EBPβ's inhibition of TNF-induced apoptosis may be through blocking caspase activation. Adenoviral expression of C/EBPβ significantly decreased levels of activity of the initiator caspase 8 in both untreated and TNF-treated cells in which NF-κB was inhibited by Ad5IκB (Fig. 4A). Inhibition of caspase 8 by C/EBPβ prevented TNF-induced activation of the mitochondrial death pathway as WT-C/EBPβ decreased the amount of truncated Bid that translocated to the mitochondria and blocked the cytochrome c release from mitochondria into cytoplasm that occurred in Ad5IκB/Ad5LacZ-coinfected cells (Fig. 4B). In contrast, levels of cytochrome oxidase, a mitochondrial protein not released during apoptosis, were equivalent in Ad5LacZ- and WT-C/EBPβ-infected cells after TNF treatment and indicated equal protein loading (Fig. 4B). As a result of the inhibition of cytochrome c release, downstream effector caspase 3 and 7 activation was blocked in cells over expressing C/EBPβ as detected by decreases in the active, cleaved caspase forms on immunoblots (Fig. 4C). The block in caspase 3 activation was confirmed by measurement of caspase 3 activity which was significantly decreased by C/EBPβ over expression in both untreated and TNF-treated cells (Fig. 4D). These results indicate that C/EBPβ blocks TNF-induced apoptosis by the inhibition of caspase activation.
Fig. 4.
C/EBPβ blocks TNF-induced caspase activation. (A) Relative levels of caspase 8 activity in untreated control (Con) and 4 h TNF-treated cells. Cells were coinfected with Ad5IκB and either Ad5LacZ or WT-C/EBPβ as indicated (*P<0.0002 as compared to Ad5IκB/Ad5LacZ-coinfected cells with the same treatment; n=7). (B) Immunoblots of mitochondrial (Mit) or cytosolic (Cyt) proteins from coinfected cells untreated or treated with TNF and probed with antibodies for truncated Bid (tBid), cytochrome c (Cyt c), cytochrome oxidase (Cyt ox) or β-actin. (C) Immunoblots of total protein from cells coinfected with Ad5IκB and Ad5LacZ or WTC/EBPβ as indicated. Cells were untreated or treated with TNF for the indicated number of hours. Proteins were immunoblotted with antibodies for caspase 3 (Casp 3), caspase 7 (Casp 7) or β-actin. Arrows indicate the cleaved forms of caspase 3 and caspase 7. Immunoblots are representative of results from 3 independent experiments. (D) Relative levels of caspase 3 activity in untreated and 6 h TNF-treated cells coinfected with Ad5IκB and either Ad5LacZ or WT-C/EBPβ (*P<0.0002 as compared to Ad5IκB/Ad5LacZ-coinfected cells with the same treatment; n=4).
Absence of C/EBPβ Sensitizes Primary Hepatocytes to Death from TNF
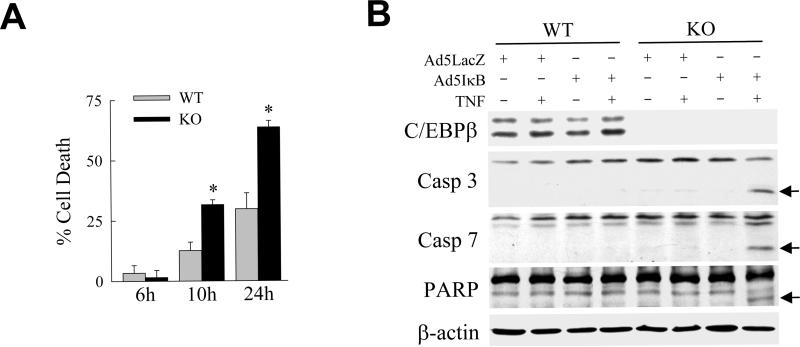
Our findings suggested that the loss of C/EBPβ would result in an increase in hepatocyte sensitivity to TNF. To investigate this possibility, primary hepatocytes were isolated from littermate control wild-type mice and c/ebpβ knockout mice, placed in culture and examined for their sensitivity to TNF-induced death. TNF treatment alone was not sufficient to induce death in either wild-type or C/EBPβ null hepatocytes (data not shown). When the hepatocytes were sensitized to TNF by infection with Ad5IκB, however, cell death at 10 and 24 h in the knockout cells was 2-fold greater than in wild-type cells (Fig. 5A). Knockout cells had greater levels of the cleaved active forms of caspase 3 and 7 that resulted in increased caspase activity as indicated by cleavage of the caspase substrate PARP (Fig. 5B). We have therefore been able to demonstrate with both overexpression and loss-of-function approaches that C/EBPβ mediates hepatocyte resistance to TNF cytotoxicity.
Fig. 5.
C/EBPβ knockout hepatocytes undergo increased cell death from TNF. (A) Percentage cell death by MTT assay at the indicated times after TNF treatment in Ad5IκB-infected wild-type and c/ebpβ knockout hepatocytes (*P<0.00005 as compared to untreated cells; n=12). (B) Total protein from untreated or TNF-treated Ad5IκB-infected primary mouse hepatocytes from wild-type (WT) and c/ebpβ knockout (KO) mice were immunoblotted for C/EBPβ, caspase 3 (Casp 3), caspase 7 (Casp 7), PARP or β-actin. The cleaved forms of caspase 3 and 7 and PARP are indicated by arrows. Data are representative of 3 independent experiments.
Sensitization to LPS- and TNF-induced Liver Injury Occurs in the Absence of C/EBPβ
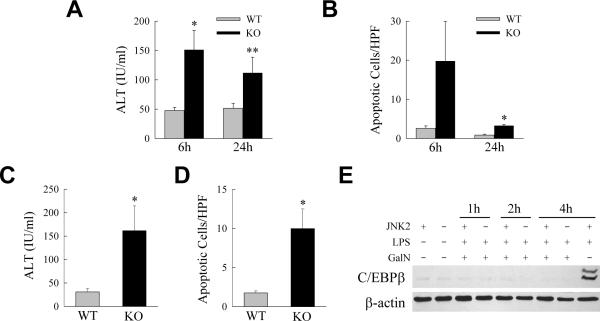
The in vivo function of C/EBPβ in LPS-induced liver injury was determined. The ability of C/EBPβ to block TNF-dependent liver injury in vivo was examined by comparing the degree of liver injury in wild-type and c/ebpβ−/− mice after the administration of a usually nontoxic dose of LPS. Wild-type mice had normal ALT levels after treatment with low dose LPS, but ALT levels were increased in knockout mice (Fig. 6A). Reflective of the predominantly apoptotic nature of TNF-induced hepatocyte death, a much greater increase occurred in the numbers of TUNEL-positive cells in LPS-treated c/ebpβ−/− mice as compared to littermate controls (Fig. 6B). The steady-state numbers of TUNEL-positive cells in the liver were increased 8-fold at 6 h and 4-fold at 24 h in the null mice as compared to control mice. To insure that injury from LPS represented toxicity from TNF, C/EBPβ null mice were examined for sensitivity to injury from TNF. An injection of TNF led to liver injury in knockout but not wild-type mice as demonstrated by increased serum ALT levels (Fig. 6C) and numbers of apoptotic cells (Fig. 6D) at 6 h. C/EBPβ therefore mediates hepatocyte resistance to TNF toxicity in vivo as well as in vitro.
Fig. 6.
C/EBPβ knockout mice are sensitized to liver injury from LPS and TNF. (A) Serum ALT levels in control wild-type (WT) and c/ebpβ knockout (KO) mice 6 and 24 h after LPS injection (*P<0.03, **P<0.001 as compared to wild-type mice; n=10–12). (B) Numbers of apoptotic cells by TUNEL staining in the livers of the same animals (*P<0.0001 as compared to wild-type mice; n=5–7). (C) Serum ALT levels in wild-type mice and c/ebpβ knockout mice 6 h after injection with TNF (*P<0.05 as compared to wild-type mice; n=4). (D) Numbers of apoptotic cells in the livers of the same animals (*P<0.01 as compared to wild-type mice; n=4). (E) Immunoblots of total hepatic protein from untreated and GalN/LPS-treated wild-type and jnk2 null mice for C/EBPβ and β-actin. The last lane contains protein from a wild-type mouse injected with LPS alone to demonstrate normal levels of C/EBPβ induction by LPS. Blots are representative of three experiments.
In the absence of NF-κB signaling, TNF-induced JNK activation is converted from a transient to prolonged response that triggers cell death in part through altered protein degradation of anti-apoptotic proteins. To examine whether the pro-apoptotic effects of JNK during TNF-dependent injury in vivo are mediated via degradation of C/EBPβ, we investigated the effect of loss of jnk2 on C/EBPβ induction after GalN/LPS treatment. Our investigations have previously demonstrated that the jnk2 gene expresses the JNK isoform(s) that promotes liver injury from GalN/LPS.34 Western blots of total liver protein from GalN/LPS-treated wild-type and jnk2 null mice for C/EBPβ revealed that the absence of jnk2 failed to reverse the GalN-induced inhibition of C/EBPβ induction by LPS (Fig. 6E). Mice null for jnk1 are not protected from GalN/LPS toxicity34 and also failed to up regulate C/EBPβ (data not shown). Thus, consistent with the in vitro findings in cells with NF-κB inhibition, C/EBPβ degradation that occurred in vivo during GalN/LPS-induced liver injury was not mediated by JNK.
Discussion
Significant progress has been made in defining the mechanisms by which hepatocytes lose resistance to TNF toxicity and undergo TNF-induced cell death. Critical for resistance to TNF-induced apoptosis is the ability of the hepatocyte to activate the NF-κB, signaling pathway in response to TNF stimulation.13–15 Prominent among the forms of hepatic injury mediated by sensitization to TNF toxicity are those induced by hepatotoxins.1,2 Hepatotoxins invariably impair macromolecular synthesis suggesting that they may sensitize hepatocytes to TNF-dependent injury from a toxin-induced block in the transcriptional or translational induction of protective signals by NF-κB. The identification of the protective protein effectors of NF-κB signaling may therefore increase our understanding of the mechanisms of toxic liver injury and suggest new therapies for its prevention.
These studies identify for the first time that C/EBPβ is an NF-κB-regulated mediator of hepatocellular resistance to TNF toxicity. C/EBPβ is one member of a family of leucine-zipper transcription factors that regulate cell proliferation, differentiation and metabolism through effects on gene expression. In addition to its role in transcription, Buck et al.,22 have demonstrated a novel non-transcriptional function of C/EBPβ as a caspase inhibitor. In the present studies, C/EBPβ was up regulated by LPS/TNF in vitro and in vivo which suggested that this protein may have an anti-apoptotic function in TNF-induced liver injury. Although TNF has been shown to alter the subcellular localization of C/EBPβ26–28 TNF regulation of C/EBPβ protein levels has not been reported previously in hepatocytes. Consistent with a function for C/EBPβ as a protective factor against TNF-induced cell apoptosis was that C/EBPβ up regulation was NF-κB dependent. Although LPS/TNF increased C/EBPβ mRNA levels and protein synthesis, the primary mechanism by which NF-κB regulated cellular C/EBPβ content was through a decrease in proteasomal degradation of C/EBPβ. Findings from both gain-of-function studies in RALA hepatocytes and loss-of-function studies in primary mouse hepatocytes demonstrated that C/EBPβ mediates hepatocyte resistance to TNF toxicity. The absence of C/EBPβ was insufficient by itself to sensitize mouse hepatocytes to death from TNF, but the significance of this finding is unclear as NF-κB activation occurs in primary hepatocytes in response to the stress of the liver perfusion and cell isolation.35 Other NF-κB-dependent protective factors may have been up regulated by this perfusion-induced NF-κB activation that compensated for the loss of C/EBPβ. Alternatively the null mice may have up regulated other compensatory protective factors that negated the loss of C/EBPβ. Nonetheless, the findings identify C/EBPβ as a new anti-apoptotic protein regulated by NF-κB at the level of protein degradation.
Confirmatory of the in vitro hepatocyte data were findings that C/EBPβ was up regulated and functioned in hepatotoxic liver injury in vivo. Identical to results in RALA hepatocytes, hepatic C/EBPβ protein levels were markedly increased by the TNF inducer LPS. Consistent with the ability of GalN to block the up regulation of NF-κB-induced protective signaling, mice cotreated with GalN and LPS failed to up regulate C/EBPβ. C/EBPβ was protective against TNF cytotoxicity as null mice but not wild-type mice developed liver injury from low-dose LPS or TNF alone. Injury in C/EBPβ null mice was far less than that elicited by the combination of GalN and LPS in wild-type mice. These results suggest that C/EBPβ functions as one of a redundant set of NF-κB-regulated anti-apoptotic factors in the hepatocyte. Alternatively, as with the studies in cultured hepatocytes from these mice, the null mice may have responded to the knockout of C/EBPβ by up regulating other anti-apoptotic factors in compensation for the loss of C/EBPβ that in part masked the true importance of C/EBPβ as an anti-apoptotic factor in vivo.
The mechanism of the anti-apoptotic effect of C/EBPβ was at least in part at the level of initiator caspase 8 activation as C/EBPβ blocked the activation of this caspase and therefore the downstream mitochondrial death pathway and effector caspase cleavage. However, further studies must be performed to delineate the mechanism by which C/EBPβ blocks caspase 8 activation to confirm this possibility. Our finding is consistent with that of Buck et al.,22 who similarly found that C/EBPβ inhibited caspase 8 activation in hepatic stellate cells. This effect in hepatocytes appears to be specific for the TNF death pathway. In contrast to the present finding of an anti-apoptotic function for C/EBPβ in TNF-mediated hepatocyte injury, studies in C/EBPβ null mice demonstrated that C/EBPβ promotes hepatocyte apoptosis from the Fas death receptor.25 Fas-mediated cell death is also caspase 8 mediated yet C/EBPβ promoted this form of apoptosis. The mechanism of the differential effect of C/EBPβ on the TNF and Fas death receptor pathways remains to be determined, but the current study suggests the interesting possibility that TNF, through induction of C/EBPβ, may potentiate Fas toxicity.
A protective mechanism of NF-κB signaling is its inhibition of pro-apoptotic JNK overactivation.16–18 JNK signaling alters the half-lives of proteins that mediate hepatocyte resistance to TNF toxicity. JNK1 has been reported to promote TNF-induced death by mediating degradation of the anti-apoptotic factor cFLIP.19 Conversely, other studies have suggested an anti-apoptotic effect of JNK1 through an increase in the half-life of Mcl-1.20 NF-κB, is therefore known to regulate death from TNF through JNK-dependent effects on protein degradation. Levels of C/EBPβ were similarly regulated through NF-κB,-dependent effects on the rate of C/EBPβ protein degradation. However, this effect was JNK independent as it was not blocked in vitro by pharmacological JNK inhibition. The absence of jnk2 in vivo which prevented GalN/LPS-induced liver injury,34 also failed to restore the LPS-induced increase in C/EBPβ indicating that jnk2 potentiation of liver injury does not occur through degradation of C/EBPβ. The findings in this study therefore demonstrate for the first time a JNK-independent effect of NF-κB on protein degradation that modulates hepatocyte resistance to death from TNF.
The new identification of C/EBPβ as an NF-κB-regulated anti-apoptotic factor in the TNF death pathway adds to the mechanistic complexity of TNF-induced hepatocyte injury. This complexity results in part from the presence of both JNK-dependent and JNK-independent effects of NF-κB on proteasomal degradation. The existence of multiple mechanisms of resistance against the TNF-activated apoptotic death pathway attests to the importance of hepatic resistance to TNF toxicity in maintaining normal liver function.
Supplementary Material
Acknowledgements
We thank Dr. David Brenner for providing the Ad5LacZ and Ad5IκB adenoviruses and Dr. Xiao-Ming Yin for the anti-Bid antibody.
Supported in part by NIH grants DK044234 (MJC) and DK056669 (LEG).
Abbreviations
- C/EBPβ
CCAAT/enhancer-binding protein β
- GalN
galactosamine
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PARP
poly(ADP-ribose) polymerase
- TNF
tumor necrosis factor α
Footnotes
Materials and Methods This section is in the Supporting Materials, which is available online.
References
- 1.Czaja MJ, Xu J, Alt E. Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology. 1995;108:1849–1854. doi: 10.1016/0016-5085(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 2.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of tumor necrosis factor α in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 3.Teoh N, Field J, Sutton J, Farrell G. Dual role of tumor necrosis factor-α in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-α gene knockout mice. Hepatology. 2004;39:412–421. doi: 10.1002/hep.20035. [DOI] [PubMed] [Google Scholar]
- 4.Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology. 2001;33:902–914. doi: 10.1053/jhep.2001.23073. [DOI] [PubMed] [Google Scholar]
- 5.Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor α. Proc Natl Acad Sci U S A. 1997;94:8744–8749. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallinowski B, Haseroth K, Marinos G, Hanck C, Stremmel W, Theilmann L, et al. Induction of tumour necrosis factor (TNF) receptor type p55 and p75 in patients with chronic hepatitis C virus (HCV) infection. Clin Exp Immunol. 1998;111:269–277. doi: 10.1046/j.1365-2249.1998.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–G190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 8.Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–626. doi: 10.1053/jhep.2001.22702. [DOI] [PubMed] [Google Scholar]
- 9.Schattenberg JM, Czaja MJ. TNF and TNF receptors. In: Dufour JF, Clavien P-A, editors. Signaling Pathways in Liver Diseases. 2nd ed. Springer-Verlag; Berlin: 2010. pp. 161–179. [Google Scholar]
- 10.Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ. Cathepsin B knockout mice are resistant to tumor necrosis factor-α-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am J Pathol. 2001;159:2045–2054. doi: 10.1016/s0002-9440(10)63056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161–167. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-α-induced liver injury. J Biol Chem. 2001;276:27432–27440. doi: 10.1074/jbc.M102465200. [DOI] [PubMed] [Google Scholar]
- 13.Bradham CA, Qian T, Streetz K, Trautwein C, Brenner DA, Lemasters JJ. The mitochondrial permeability transition is required for tumor necrosis factor α-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998;18:6353–6364. doi: 10.1128/mcb.18.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, et al. NF-κB inactivation converts a hepatocyte cell line TNF-α response from proliferation to apoptosis. Am J Physiol. 1998;275:C1058–C1066. doi: 10.1152/ajpcell.1998.275.4.C1058. [DOI] [PubMed] [Google Scholar]
- 15.Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, et al. NFκB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, et al. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Lo CR, Czaja MJ. NF-κB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- 18.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, et al. Inhibition of JNK activation through NF-κB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 19.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIPL turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Kodama Y, Taura K, Miura K, Schnabl B, Osawa Y, Brenner DA. Antiapoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 21.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buck M, Poli V, Hunter T, Chojkier M. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8:807–816. doi: 10.1016/s1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 23.Ewing SJ, Zhu S, Zhu F, House JS, Smart RC. C/EBPβ represses p53 to promote cell survival downstream of DNA damage independent of oncogenic Ras and p19(Arf) Cell Death Differ. 2008;15:1734–1744. doi: 10.1038/cdd.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon K, Zhu S, Ewing SJ, Smart RC. Decreased survival of C/EBP β-deficient keratinocytes is due to aberrant regulation of p53 levels and function. Oncogene. 2007;26:360–367. doi: 10.1038/sj.onc.1209797. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee D, Kaestner KH, Kovalovich KK, Greenbaum LE. Fas-induced apoptosis in mouse hepatocytes is dependent on C/EBPβ. Hepatology. 2001;33:1166–1172. doi: 10.1053/jhep.2001.24032. [DOI] [PubMed] [Google Scholar]
- 26.Diehl AM, Yang SQ, Yin M, Lin HZ, Nelson S, Bagby G. Tumor necrosis factor-α modulates CCAAT/enhancer binding proteins-DNA binding activities and promotes hepatocyte-specific gene expression during liver regeneration. Hepatology. 1995;22:252–261. doi: 10.1016/0270-9139(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 27.Trautwein C, Rakemann T, Malek NP, Plumpe J, Tiegs G, Manns MP. Concanavalin A-induced liver injury triggers hepatocyte proliferation. J Clin Invest. 1998;101:1960–1969. doi: 10.1172/JCI504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin M, Yang SQ, Lin HZ, Lane MD, Chatterjee S, Diehl AM. Tumor necrosis factor α promotes nuclear localization of cytokine-inducible CCAAT/enhancer binding protein isoforms in hepatocytes. J Biol Chem. 1996;271:17974–17978. doi: 10.1074/jbc.271.30.17974. [DOI] [PubMed] [Google Scholar]
- 29.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, et al. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-α and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1202–R1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- 31.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 32.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFα- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. TNF-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Gao X, Fukumoto S, Tademoto S, Sato K, Hirai K. Differential expression and regulation of chemokines JE, KC, and IP-10 gene in primary cultured murine hepatocytes. J Cell Physiol. 1999;181:361–370. doi: 10.1002/(SICI)1097-4652(199911)181:2<361::AID-JCP18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.