Abstract
Cytokinesis is emerging as a control system defined by interacting biochemical and mechanical modules, which form a system of feedback loops. This integrated system accounts for the regulation and kinetics of cytokinesis furrowing and demonstrates that cytokinesis is a whole-cell process in which the global and equatorial cortices and cytoplasm are active players in the system. Though originally defined in Dictyostelium, features of the control system are recognizable in other organisms, suggesting a universal mechanism for cytokinesis regulation and contractility.
Keywords: Actin, Cell Mechanics, Control System, Cytokinesis, Mechanosensing, Myosin II
Introduction
Cytokinesis, the final step leading to the physical separation of a mother cell into two daughter cells, is often depicted as a linear process regulated by pathways that initially emanate from the mitotic spindle [1–3]. In actuality, because cytokinesis is a process mediated by biochemical interactions as well as the physical parameters of the cell and mechanical inputs, it is regulated by several parallel yet congruent pathways that intersect to form a complex cytokinesis network. This network is an interdependent array of separately regulated circuits and feedback loops that can be broken down into functional modules [4]. Here, we will examine the individual components that work in concert to drive the cytoskeletal remodeling of cytokinesis.
Traditionally, cytokinesis is viewed as occurring through the constriction of the cleavage furrow by a contractile ring composed of anti-parallel actin bundles interdigitated by the force-generating protein, myosin II, whose accumulation at the furrow is presumed to be mitotic spindle mediated [5–7]. The circumferential array of actin and myosin II is found in a number of organisms from Schizosaccharomyces pombe to HeLa cells [6, 8, 9]. However, there are plentiful examples of organisms that do not have a distinctive ring structure, such as adherent mammalian fibroblasts and Dictyostelium [10–12]. In these cell types, actin polymers and myosin II are arranged in a contractile meshwork [11]. These two distinct structural observations imply that the actomyosin organization may be the result, not the cause, of contractility. We suggest that the core principles of cytokinesis – the mechanical and biochemical parameters – are common among organisms and that it is in the regulatory mechanisms where the organismal differences lie. How a cell generates and responds to internal mechanical stress is dependent upon the structure of the cell and force-sensitivity of the protein players involved in cell division. Although the list of proteins involved in cytokinesis is extensive [7, 13], we will focus on the integral players which individually define structural and functional modules: the plasma membrane, actin filaments, myosin II, and actin-crosslinking proteins.
Membrane and Membrane Dynamics
At first glance, cytokinesis is a physical process by which the surface of a cell is severely deformed to promote furrow ingression, bridge formation, and ultimately two new daughter cells. In order for these various characteristic cell shape changes to occur, the plasma membrane must be rapidly remodeled to accommodate cytoplasmic volume conservation, without rupturing under the internal stress associated with those shape changes, and while increasing the final total surface area by ~26% [14–16]. Membrane dynamics are regulated in part by endocytosis and exocytosis – two processes involved in engulfing membrane and depositing lipids respectively to remodel plasma membrane. Genetic mutants of proteins involved in these processes generate cells with cytokinesis defects. Additionally, lipid composition may play a role in membrane and cytoskeleton regulation, since the lipid composition of the furrow region and of the poles are different [17–19]. Whether or not membrane remodeling actively promotes cleavage furrow ingression or passively affects cell shape change by altering surface area to accommodate stress changes currently remains unclear.
The plasma membrane is also an integral component of a physical parameter essential for cell shape change - cortical tension. Cortical tension is defined as the force in the cell cortex and overlying plasma membrane that serves to minimize the surface area (demarcated by the membrane) to volume ratio and it is comprised of all of the mechanical stresses that act at the surface of the cell [20, 21]. Cortical tension affects cleavage furrow ingression dynamics in a multitude of ways: it originally opposes the forces deforming the mother cell, while later acts in the furrow region to aid in pushing out the cytoplasm from the bridge region into the two daughter cells [22]. Concurrently, the cortical tension in the new daughter cells works to withstand and accommodate the cytoplasmic movement from the bridge. How cortical tension affects a dividing cell is dependent not only upon the boundary forces put in place by the plasma membrane, but also on the plasticity of the cytoskeleton.
Actin
The primary structural component of the contractile cytoskeleton is the actin polymer (Fig. 1). These filaments are semi-flexible, meaning that their mechanical characteristics are dictated by two length-scales defined by the polymer contour length (Lc) and the persistence length (Lp) [23, 24]. Lc is the length of the polymer, whereas Lp is the distance between two points on the polymer where those points behave independently of each other. The relationship between Lc and Lp partly describes the mechanical properties of a polymeric network. When Lc>Lp, a polymeric network will stiffen upon the application of large forces. When Lc<Lp, then the network properties are dominated by polymer concentration and crosslinkers [25, 26]. Because the Lp for actin is 10–17 μm and polymer lengths are much shorter (up to 100-fold shorter) than Lp in living cells [8, 11], the properties of the living actin network will be primarily dominated by actin concentration, the lifetime of the actin polymers, and the density, properties and lifetimes of the proteins acting upon and/or crosslinking the actin polymers.
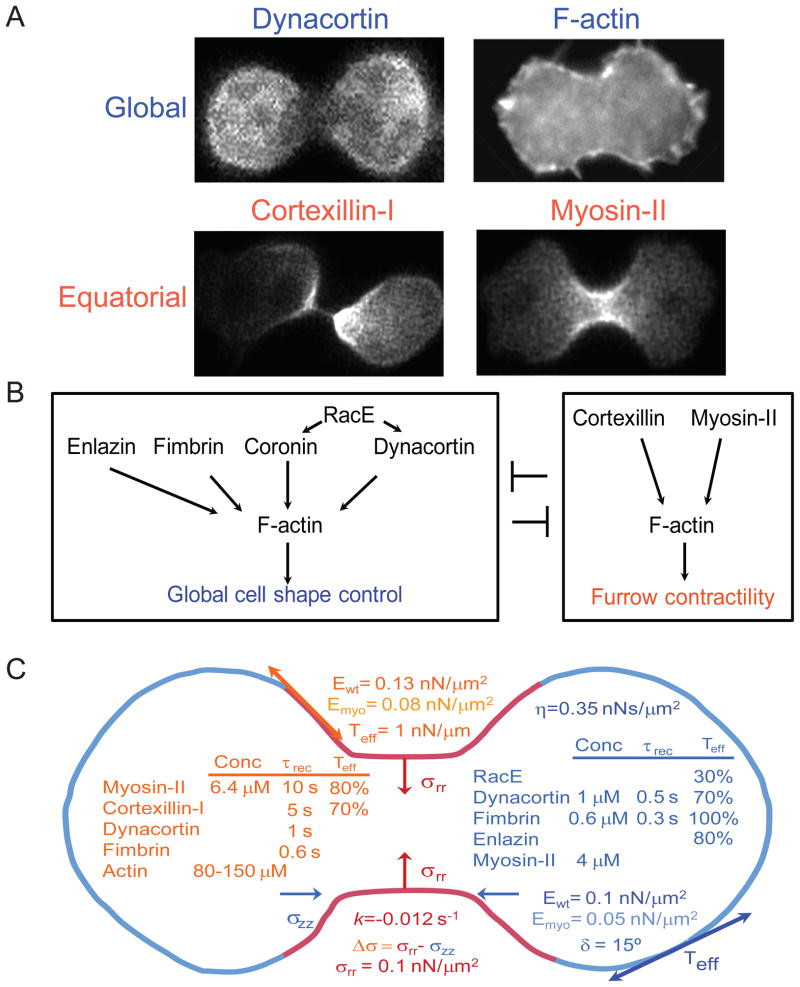
Figure 1. Distinct sets of proteins control equatorial and global cortices.
A. Global proteins are distributed around the cortex but enrich in the polar cortex [11, 40, 41]. Equatorial proteins are similarly distributed around the cortex but enrich in the cleavage furrow cortex [15, 46]. In other words, these two sets of proteins are found in complementary concentration gradients. B. From genetic interactions and cellular distributions, cytokinesis contractility is governed by two modules of proteins, global and equatorial [11, 22, 40, 41, 45]. The global proteins are actin-crosslinking proteins including dynacortin, enlazin (the Dictyostelium ezrin-radixin-moesin-related protein), fimbrin and coronin. These proteins are regulated by RacE. The principle actors in the equatorial module include myosin II and cortexillin. Because of the overlapping complementary nature of the concentration gradients, it is worth noting that global myosin II also contributes to the mechanics of the global cortex. C. Cytokinesis is a mechanical process that is governed by mechanical features defined by the two modules of proteins. Mechanical features include cortical tension (Teff), elasticity (E), and viscosity (η; which is a highly non-linear, force-dependent parameter [79]. The relevant parameter for cytokinesis is listed [22].). The E value varies around the cortex and depends on myosin II (Ewt vs. Emyo) [11]. The mechanical phase angle (δ) indicates the solid-liquid-nature of the cortex, where 0° corresponds to a solid and 90° corresponds to a liquid. The Dictyostelium cortex is viscoelastic and highly solid-like [11, 80, 81]. The wild type cleavage furrow ingression can be described as non-linear with an exponential character. The rate constant k is provided. Along with Laplace pressures generated from surface/cortical tension, the stress differential (Δσ) controls furrow ingression dynamics [22]. Active radial stresses (σrr) are provided. The local concentrations of proteins, their recovery dynamics assessed by FRAP, and their impact on the cortical tension (relative to wild type, which is set at 100%) are provided [11, 40, 80].
Myosin and Myosin Force Generation
The major active force generator of cytokinesis is myosin II (Fig. 1). The functional unit of myosin II is the bipolar thick filament (BTF), comprised of hexameric monomers (M), consisting of two heavy chains, two essential light chains (ECLs), and two regulatory light chains (RCLs) [27]. Myosin II monomers assemble into bipolar thick filaments (BTFs) with most mammalian nonmuscle myosin IIs assembling into BTFs containing 10–30 monomers and in Dictyostelium, into BTFs of up to 70 monomers [28, 29]. Dictyostelium BTF assembly is thought to occur first through a nucleation process in which parallel dimers (D) assemble from two monomers (M), and then two parallel dimers assemble into an anti-parallel tetramer. Subsequent elongation occurs through dimer addition. The assembly of BTFs is regulated by myosin heavy chain kinases (MHCKs), which in Dictyostelium phosphorylate three threonines in the tail of the heavy chain downstream of the assembly domain [30, 31]. Phosphorylation of these sites puts the myosin II monomer in an assembly-incompetent state. Importantly, the phosphomimic (3xAsp) mutant myosin cannot assemble into BTFs and cannot accumulate at the cleavage furrow cortex. Conversely, the unphosphorylatable (3xAla) mutant overassembles into thick filaments, over-accumulates at the cleavage furrow cortex and has severely impaired BTF assembly-disassembly dynamics in the cell cortex [15, 30, 31]. For mammalian nonmuscle myosin II, in addition to heavy chain phosphorylation, RLC phosphorylation helps modulate BTF assembly [32–35]. RLC phosphorylation of assembled myosin II also increases the actin-activated ATPase activity, which is likely due to freeing the motor so that it is more able to bind an actin filament [36–38].
Cell shape dynamics are modulated in large part by the tension produced by myosin II on the actin cytoskeleton. Myosin generates force as it goes through its conformational changes and generates work as it moves relative to the actin filament [27]. The number of myosin II motor heads bound to actin at any one time is dictated by the motor’s duty ratio and the total number of available myosin II heads. The duty ratio is the ratio of time that the motor is strongly bound to the actin filament (the “strongly bound state time”) to the length of the entire ATPase cycle. Of the myosin binding cycle, the force-sensitive step occurs during the conformational changes that precede the ADP-bound, post-stroke conformation. Consequently, resistive tension is generated as the lever arm swings through its power stroke as long as the actin filament is stably anchored by actin linking/crosslinking proteins. This tension results in strain on the lever arm, constraining the lever arm’s swing and ensuring that the motor remains bound in the load-bearing transition (isometric) state for a longer period of time (i.e. increasing the duty ratio of the motor).
Actin Crosslinkers
The final essential components of the cytoskeleton and contractile system that complete the actin-myosin modules are the actin-crosslinking proteins (ACLPs). They are tasked with tethering individual actin polymers to each other and to the plasma membrane, allowing for localized mechanical stress to propagate throughout the network, and pulling in of the plasma membrane during cleavage furrow ingression. The presence of ACLPs on the actin filaments also imparts the network’s deformability characteristics – that is, they dictate how the network responds to internal or external stresses by defining the time-scale of those responses. Additionally, some actin crosslinker binding lifetimes may be load-sensitive due to their inherent molecular flexibility [11, 25, 39].
As a group, ACLPs are extremely versatile. They can organize actin filaments into bundles or networks (meshworks) based on their molecular structures, intermolecular configurations, number of actin binding domains, kinetic properties, in vivo concentrations and ratio to actin concentrations. Some actin crosslinkers containing more than one actin-binding domain (ABD) can monomerically link actin filaments, whereas others form parallel or anti-parallel dimers to crosslink the actin network. This flexibility of configurations and characteristics accounts for the different affinities as well as the different surfaces of the actin filaments to which these ACLPs bind.
Actin crosslinkers can be categorized generally into two spatial networks, based on their localization in a dividing cell and their role in cytokinesis [11, 22, 40–44] (Fig. 1). ACLPs that constitute the equatorial network are found predominantly along the equator of a dividing cell and prevail in an inverse gradient to those ACLPs which comprise the global network and are found predominantly at the cell’s polar cortices. In Dictyostelium, the equatorial network is comprised primarily of myosin II and two isoforms of cortexillin [15, 41–43, 45, 46]. Of the two, the cortexillin I isoform is more dominant in cleavage furrow contractility and mechanosensory responses (discussed below) and can anchor the actin network to the plasma membrane. The major actin crosslinkers in metazoans and S. pombe cells are respectively anillin and the anillin-related proteins Mid1 and Mid2 . These appear to provide the major organizational role of the actin network in these systems [47–53]. In the Dictyostelium system, the global network, constituted of dynacortin, fimbrin, coronin, and enlazin, is regulated by the Rac-family small GTPase, RacE [41, 54–56]. RacE is found to be important in cell mechanics as severe mechanical defects have been observed in RacE mutants, and in RacE null cells, dynacortin and coronin do not accumulate in the cortex [41, 55]. Dynacortin and fimbrin work in conjunction with RacE to slow the rate of cleavage furrow ingression induced by myosin II and cortexillin [22].
This braking phenomenon observed in Dictyostelium with the global actin crosslinkers dynacortin and fimbrin is similarly observed in other systems. In mammalian systems, α-actinin parallels the behavior of dynacortin and fimbrin in that it slows furrow ingression [44]. Upon overexpression of α-actinin, the rate of furrow ingression decreases and actin polymers are stabilized. Conversely, in α-actinin-diminished cells, actin accumulation at the cleavage furrow decreases and the furrow ingression rate increases.
Myosin-ACLP Interactions
While myosin II is one of the primary force-generating proteins in cytokinesis, its mechanical impact is dependent upon two independent factors: (1) the characteristics of the actin crosslinkers and how they counter the mechanical forces generated by the cell, and (2) whether or not the actin network is responding to mechanical stress [11, 41, 57, 58]. All of these components contribute to the cell’s cortical tension and viscoelastic nature, although to different degrees. By defining the viscoelastic nature of the network, these proteins determine how the cortex is remodeled over a variety of time-scales.
Actin crosslinkers exert different effects in wild type cells versus myosin II null (myoII) cells, implying that myosin II affects the kinetics and perhaps structures of these crosslinkers [11]. This relationship further suggests that crosslinkers have the unique ability to respond to force, thereby influencing cell mechanics. Three prime examples of this type of interplay can be discerned through the interactions between myosin II and dynacortin, fimbrin, and cortexillin I (discussed in the Mechanosensing section below) [11, 58].
In the case of dynacortin, the loss of the crosslinker from a wild-type background reduces viscoelasticity by 50% and cortical tension by ~25%. The pulling of myosin II against actin filaments may result in a longer binding time of dynacortin to actin, as dynacortin has a slower off-rate in wild type cells (450 ms τrec), than in myosin II heavy chain (myoII) null cells (290 ms τrec). On the other hand, loss of fimbrin has no effect on cortical tension (measured on the 1-s time-scale), but a discernable effect on the viscoelasticity of the cell (~50%) (for technical reasons, only measured on the 0.1–100 ms time-scale). However, the cortical tension is affected in myoII null strains when fimbrin is depleted. This time-scale-dependency change is likely the result of changing off-rates of fimbrin in the presence and absence of myosin II – in wild type cells, fimbrin’s τrec is 260 ms, which is faster than the 680 ms measured in the myoII mutant. Two possibilities are consistent with this data. In the first, myosin II may pull on the actin polymers, removing fimbrin from the actin network. In the second, without myosin II actively rearranging the filaments, fimbrin may dwell inside larger bundled networks. Both scenarios are supported by in vitro data in which myosin II can pull filaments from fimbrin-crosslinked networks [59].
Mechanosensing Module Activated During Cytokinesis
All cells can mechanosense – the ability to perceive and respond to internally or externally derived mechanical stress. Cells have developed feedback loops by combining force-generating machinery with the ability to sense mechanical stress, a process which likely evolved from the need of simple cells to respond to changing environmental cues. These mechanical feedback loops have been appropriated for a number of complex processes involving a range of length- and time-scales, including, but not limited to hearing, blood pressure regulation, and muscle contraction. During cell division, a mother cell must separate appropriately into two daughter cells under a variety of external environments. Therefore, a mechanical feedback loop must have evolved to respond to these internally derived forces, allowing for crosstalk across the heterogeneous cytoskeletal network to occur. Two observations in Dictyostelium supported this original hypothesis: (1) Dividing cells that exhibit a cellular asymmetry recruit myosin II to correct that asymmetry before proceeding through cytokinesis [57] and (2) dividing cells that are overlaid with an agarose sheet, respond to that compression with a higher accumulation of myosin II at the cleavage furrow [60].
To explore whether or not such a feedback system exists in cells, micropipette aspiration (MPA) was used to apply stress to the surface of dividing cells equivalent to that experienced at the cleavage furrow cortex (0.1–1 nN/μm2) [57, 58, 61] (Fig. 2). This MPA-induced external stress leads to a deformation of the cortex over ~10–30 μm2 of surface area, which is on the scale of the deformation that the cleavage cortex imposes during furrow ingression. For comparison, the surface area of the cleavage furrow cortex at the beginning of cell division is ~200 μm2, which reduces to 5–10 μm2 near the end of division. The applied stress leads to the accumulation of myosin II, as well as the actin crosslinking protein cortexillin I, at the cell cortex inside the micropipette tip. The augmentation of myosin II and cortexillin I at the site of deformation is interdependent – neither protein accumulates in the absence of the other. Additionally, the recruitment of myosin and cortexillin to the micropipette tip is independent of the global crosslinking proteins fimbrin, dynacortin, and enlazin, suggesting that myosin II and cortexillin I are unique in delineating a mechanosensory module. In this module, cortexillin I would allow myosin II to generate force and experience tension by anchoring the actin network, locking the motor into its isometric state. Additionally, the full bipolar thick filament assembly dynamics are required as either inhibition of BTF assembly or disassembly kills the mechanosensitive localization of myosin II. Taken together, these data imply that the myosin II-cortexillin I mechanosensory module has three elements required for its cooperative and coordinated response to mechanical stress: (1) mechanosensitive myosin II mechanochemistry, (2) myosin II BTF dynamics, and (3) cortexillin I-mediated actin-filament stabilization.
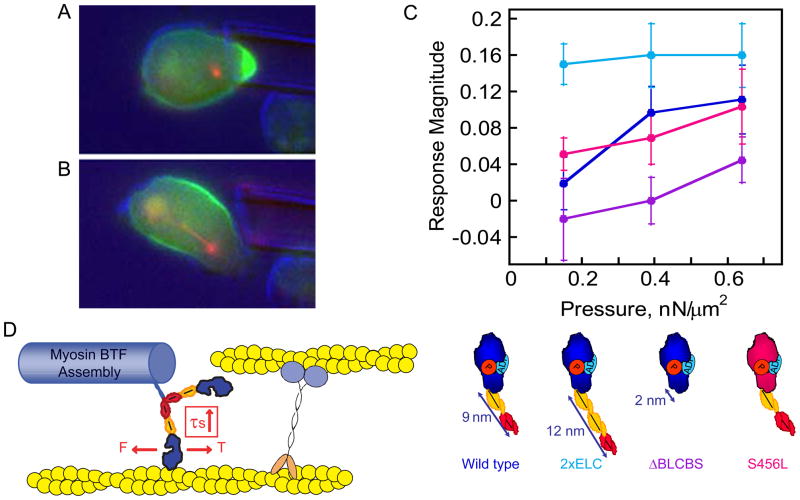
Figure 2. Mechanical stress directs myosin II and cortexillin accumulation.
A. Cortexillin accumulates in response to applied mechanical stress. B. After the cell escapes from the applied pressure, the cell proceeds through cell division. For A and B, green, GFP-cortexillin I; red, RFP-tubulin. C. The myosin II lever arm length sets the pressure-dependency of mechanosensitive localization. This observation strongly implicates the myosin motor and the isometric state as the determinant for this localization. D. The cellular mechanosensor defined by myosin II and cortexillin. The motor domain is the active element with the lever arm tuning the pressure dependency. Cortexillin provides an anchor so that the myosin motor can generate tension, stalling at the isometric state. Mechanosensitive accumulation also depends on the myosin II bipolar thick filament assembly dynamics. Panels modified from references [57, 58].
To determine if myosin II was the active element of the sensor, the pressure-dependencies of the mechanosensory response of wild type myosin II and mutant myosin II proteins with altered lever-arm lengths were measured and compared (Fig. 2B). To this end, three mutants were generated: the 2xELC mutant with an extra essential light chain, 13 nm lever arm and 4 μm/s unloaded actin sliding velocity; the ΔBLCBS mutant with both light chain binding sites deleted, containing a 2 nm lever arm and 0.6 μm/s unloaded actin sliding velocity; and the S456L mutant with a wild type lever arm and ATPase activity but a 10-fold slower unloaded actin filament sliding velocity. For comparison, wild type myosin II has a 9 nm lever arm and a 3 μm/s unloaded actin sliding velocity. The two lever-arm mutants have full actin-activated ATPase activity, imparting fully phosphorylated wild type myosin II characteristics. The longer lever arm mutant 2xELC was more responsive than wild type, while the shorter lever arm mutant ΔBLCBS was less responsive, requiring much higher pressures to generate the mechanosensitive accumulation of myosin II and cortexillin I. The S456L lever arm mutant showed a near wild type mechanosensitive response. These data clearly imply that myosin II’s mechanochemistry is the active element of the myosin II-cortexillin I mechanosensitive module, given that myosin’s Fmax is inversely related to the lever arm length. The implication of this lever arm-length dependency is that as myosin II produces force, it also experiences tension by pulling against an anchored actin filament. Consequently, the motor stalls at the isometric state, which further causes the myosin II motor to dwell tightly bound to the actin filament (Fig. 2C).
Structure-function analysis revealed that the BTF assembly/disassembly dynamics were essential for mechanosensitive localization. Therefore, to determine where the sensitive step in the assembly pathway might exist, kinetic simulations were conducted using measured kinetic or equilibrium constants for each step of the assembly pathway [58]. These simulations revealed two interesting properties. First, BTF sizes will be dispersed exponentially, with the minimal BTF (BTF3 where the BTF contains three dimers) the most frequent size. This distribution occurs if the population of 80% free assembly-incompetent (heavy chain phosphorylated) monomer (M0) is maintained. Second, the major rate-limiting step is the conversion of M0 to the assembly-competent monomer (M). These characteristics suggest that one of three possible assembly mechanisms occurs during the mechanosensitive response. In the first mechanism, assembly is initiated upon the activation of a heavy chain phosphatase. In the second possibility, assembly is driven by the local inactivation of MHCK. In the third scenario, assembly is promoted by cooperative interactions of smaller BTFs with the actin polymer. Here, the mini-BTFs are stabilized by force locking the motor in the isometric state, which leads to the local accumulation of more myosin II monomers, promoting their insertion into the pre-existing BTF.
The final element of the mechanosensory module is the interaction between cortexillin I and myosin II, leading to their stabilization on the actin polymer network. This interaction likely occurs through cooperative binding to the actin filament. Myosin II motors alone cooperatively bind to actin filaments and this cooperativity appears to depend on the isometric state of the myosin motor, the heterologous proteins associated with the actin polymer, or the structure of the actin filament itself [62–64]. In a complex living system, all three modes of cooperative interactions between myosin II motors may contribute to mechanosensitive localization. The isometric state-dependent mode is strongly suggested by the lever-arm length dependency of the mechanosensory response. For myosin II to be stabilized in the isometric state, the actin filament must be anchored so that when the motor pulls on the filament, tension can be generated and experienced by the motor. To determine if cortexillin I has such characteristics as a stable actin anchor under the influence of force, cortexillin I-actin-binding lifetimes were measured using single molecule analyses [58]. Over a force range of −2 to 2 pN, cortexillin I has an actin-binding life-time of 550 ms, significantly longer than myosin II in either the unloaded or loaded strongly bound state. Thus, cortexillin I remains bound to actin filaments long enough for myosin to generate tension on the filament.
Regulation of the Mechanosensory Response by RacE
The wild-type mechanosensory response during cell division is more vigorous than during interphase [57, 58]. This is possibly due to the greater deformability of dividing cells as compared to interphase cells [11] and/or changes in the localization patterns of global actin crosslinkers [40, 41]. Although wild-type interphase cells do not readily show the mechanosensitive responses except under very high pressure-regimes, RacE null mutants show a strong mechanosensitive localization of both myosin II and cortexillin I during interphase. These data suggest that the mechanosensitive response, while operating throughout the cell cycle, is dampened during interphase by the RacE pathway. RacE, which operates upstream of the actin crosslinkers (e.g. dynacortin) in the global network, also affects the cortical tension of the cell – RacE mutants have a cortical stiffness that is 30% of wild type cells and a varied distribution of ACLPs [41, 55]. Consequently, RacE activity may be modulated throughout the cell cycle, constraining the mechanosensory pathway to cytokinesis by altering the cortical mechanics of the dividing cell.
Biochemical-Mechanical Feedback Loops: Integrating the Modules
It is clear now that cytokinesis depends on a network of pathways, some of which are defined by their biochemical nature, such as the kinases that regulate myosin II assembly and activation [31, 65], and others which are defined by their mechanical nature, such as the changes in cortical tension due to the interplay between myosin II and actin crosslinking proteins [11]. These modules intersect so that the dividing cell system assumes the form of a classic control system (Fig. 3).
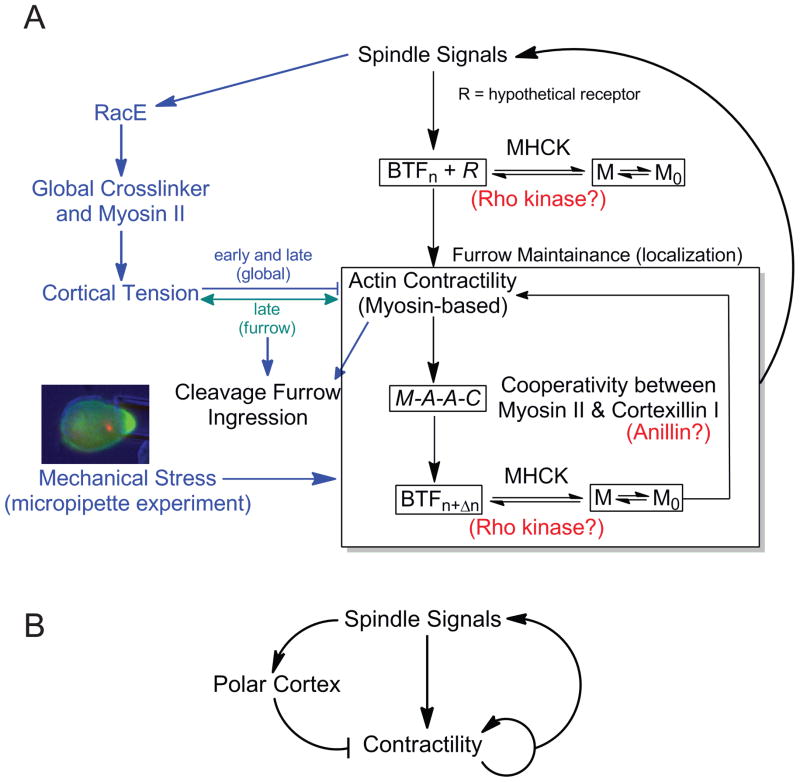
Figure 3. Cytokinesis is a control system characterized by biochemical-mechanical feedback loops.
A. The cartoon depicts the cytokinesis control system. M0 and M refer to assembly-incompetent and assembly-competent myosin II, respectively, which is modulated by MHCK. BTFn refers to bipolar thick filaments assembled with n dimers. M-A-A-C refers to the communication between myosin (M) and cortexillin (C) through actin (A). In metazoans, Rho kinase may function at the step of modulating myosin assembly, and anillin may function analogously to cortexillin. The hypothetical myosin II cortical receptor (R) is yet to be determined. B. This cartoon is a simplified version of the control system in panel A, illustrating the basic structure. From control theory [82], this system has the structure of an incoherent negative feedback system with an extra mechanical stress-activated positive feedback loop acting on the contractility module.
In this control system view, spindle signals are thought to modulate equatorial and global mechanics. The global system feeds through RacE to global crosslinking proteins and myosin II. This establishes the cortical tension of the cell, which acts first to resist, then assist cleavage furrow ingression [11, 22, 41]. Conversely, the spindle signals that direct contractile proteins to the cleavage furrow cortex originate, in part, from central spindle microtubules, which communicate via kinesin-6 proteins – kif12 in Dictyostelium [66] and MKLP1 in metazoans [7, 67] – among other microtubule-based motors. These signals may ultimately lead to the accumulation of myosin II and bipolar thick filament assembly, through the activation of such contractility regulators as INCENP and Aurora kinase (in Dictyostelium and metazoans) and/or Rho-pathway regulators, MgcRacGAP and ECT2 (in metazoans) [68–70]. However, in metazoans, the Rho signal through Rho kinase is not essential for myosin II localization once the regulatory light chain phosphorylation has been provided [33, 52, 68, 71, 72]. Because mechanical stresses can also direct myosin II (and cortexillin I) to sites of high mechanical stress, mechanosensitive localization may provide an independent pathway for accumulating myosin II locally at the equatorial cortex. As the cell is actively elongating, stresses along the equator may help direct myosin II there, providing a redundant mechanism for myosin II localization.
One of the missing pieces in fitting together the different cytokinesis modules is the identification of a cortical receptor/anchor (hypothetical receptor R) for the myosin II bipolar thick filament (Fig. 3). Its existence is supported by several lines of evidence. First, in the presence of actin polymer disruptors, myosin II still associates with the cleavage furrow [73]. Second, headless myosin II heavy chains can localize to the cleavage furrow in Dictyostelium and mammalian cells, provided the cleavage furrow has started to ingress [33, 74]. The hypothetical receptor R may not require mature BTFs for binding, but rather may be able to bind smaller nucleating BTFs, thus allowing free myosin II monomers to interact with the stabilized bound BTF nucleus. In higher metazoans, anillin has been suggested to provide a cortical anchor, but this idea has been challenged by structure-function studies in C. elegans [48, 75, 76]. It is possible that if anillin is one of the anchoring complexes its role is not essential due to mechanosensory-mediated localization. It is likely however that the cortical anchor remains to be identified.
This whole contractile system feeds back on the spindle signals. Precedence exists for cooperative interactions between the contractile zone and the central spindle in Drosophila and mammalian cells [77, 78]. We have found that some spindle-signal-associated proteins accumulate at the cortex in a mechanical stress-dependent, microtubule-independent manner, though they are not required for the mechanosensitive localization of myosin II and cortexillin I, which form the mechanosensor. These observations point towards a feedback loop from contractility to the spindle signals, rounding out the control system.
Cylinder-Thinning Model
All of the biochemical and mechanical modules work together to affect five characteristics of furrow ingression dynamics: cortical tension, radial stresses, compressive stresses, cytoplasmic viscoelasticity and cortical viscoelasticity [22] (Fig. 1). To incorporate the contributions of these parameters, the simple analytical Cylinder-Thinning model was proposed. This model accounts for the cytoplasmic fluid flow from a well-defined furrow bridge into the daughter cells. The Cylinder-Thinning model asserts that Laplace-like pressures originating from the cortical tension generated at the cleavage furrow and active radial stresses (σrr) from myosin II are responsible for the outward flow of the cytoplasm from the furrow. Laplace-like pressures are derived from the surface/cortical tension and the membrane/cortex curvature. Variations in local curvature and/or cortical tension can lead to pressure variations across the cell. The actin crosslinking proteins in the global/polar network, coupled with the viscoelasticity of the cytoplasm and the Laplace-like pressures inherent in the daughter cells, work in concert to produce compressive stresses (σzz) that resist the push from the cleavage furrow. The balance of these properties then controls how fast the furrow ingresses and how the ingression dynamics change over time (and consequently how cell shape changes over time). Significantly, this analysis revealed that all genotypes of cells – from wild type to a broad array of mutants – divide according to their own characteristic trajectories [11, 22, 40]. Furthermore, this analysis revealed that cytokinesis contractility cannot be explained by events at the cleavage furrow cortex/contractile ring alone. Rather, the mechanical and dynamical features of the entire cortex and cytoplasm must be understood in order to explain cytokinesis contractility.
Conclusions
Cytokinesis involves the interplay between mechanical and biochemical pathways in what will likely prove to be a universal mechanistic network. The basic premise for this system is that furrow ingression is the result of mechanical stress which is radiated throughout the cytoskeletal network by an intricate cross-talk between a number of key elements and the cytoskeleton, whose widespread structural integration with itself and the plasma membrane allow for rapid mechanical and chemical signal transduction. The significant overlap of these modules across organisms suggests that fundamental differences in cytokinesis contractile mechanisms are likely due to differences in subtle details of regulation rather than in the network of mechanical and biochemical modules that drive cytokinesis.
Acknowledgments
Our research is supported by the NIH (GM066817 and GM086704) and the American Cancer Society (RSG CCG-114122).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glotzer M. Animal Cell Cytokinesis. Annu Rev Cell Dev Biol. 2001;17:351–86. doi: 10.1146/annurev.cellbio.17.1.351. [DOI] [PubMed] [Google Scholar]
- 2.Dechant R, Glotzer M. Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev Cell. 2003;4:333–44. doi: 10.1016/s1534-5807(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 3.Neujahr R, Albrecht R, Köhler J, Matzner M, Schwartz J-M, Westphal M, et al. Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J Cell Sci. 1998;111:1227–40. doi: 10.1242/jcs.111.9.1227. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DN, Kee Y-S, Luo T, Surcel A. 7.5 Biophysics of Cell Division: Understanding how dividing cells change shape. In: Wirtz D, Egelman EH, editors. Comprehensive Biophysics. Elsevier; 2010. In press. [Google Scholar]
- 5.De Lozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–91. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder TE. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci USA. 1973;70:1688–92. doi: 10.1073/pnas.70.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–9. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 8.Kamasaki T, Osumi M, Mabuchi I. Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J Cell Biol. 2007;178:765–71. doi: 10.1083/jcb.200612018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maupin P, Pollard TD. Arrangement of actin filaments and myosin-like filaments in the contractile ring and of actin-like filaments in the mitotic spindle of dividing HeLa cells. J Ultrastruct Res. 1986;94:92–103. doi: 10.1016/0889-1605(86)90055-8. [DOI] [PubMed] [Google Scholar]
- 10.Fishkind DJ, Wang Y-L. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J Cell Biol. 1993;123:837–48. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, Girard KD, et al. Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr Biol. 2008;18:471–80. doi: 10.1016/j.cub.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBiasio RL, LaRocca GM, Post PL, Taylor DL. Myosin II transport, organization, and phosphorylation: evidence for cortical flow/solation-contraction coupling during cytokinesis and cell locomotion. Mol Biol Cell. 1996;7:1259–82. doi: 10.1091/mbc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–66. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 14.Hategan A, Law R, Kahn S, Discher DE. Adhesively-tensed cell membranes: lysis kinetics and atomic force microscopy probing. Biophys J. 2003;85:2746–59. doi: 10.1016/s0006-3495(03)74697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson DN, Cavet G, Warrick HM, Spudich JA. Quantitation of the distribution and flux of myosin-II during cytokinesis. BMC Cell Biology. 2002;3:4. doi: 10.1186/1471-2121-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauthier NC, Rossier OM, Mathur A, Hone JC, Sheetz MP. Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol Biol Cell. 2009;20:3261–72. doi: 10.1091/mbc.E09-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janetopoulos C, Borleis J, Vazquez F, Iijima M, Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev Cell. 2005;8:467–77. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Echard A. Membrane traffic and polarization of lipid domains during cytokinesis. Biochem Soc Trans. 2008;36(Pt. 3):395–9. doi: 10.1042/BST0360395. [DOI] [PubMed] [Google Scholar]
- 19.Emoto K, Inadome H, Kanaho Y, Narumiya S, Umeda M. Local Change in Phospholipid Composition at the Cleavage Furrow Is Essential for Completion of Cytokinesis. J Biol Chem. 2005;280:37901–7. doi: 10.1074/jbc.M504282200. [DOI] [PubMed] [Google Scholar]
- 20.Derganc J, Božic B, Svetina S, Žekš B. Stability analysis of micropipette aspiration of neutrophils. Biophys J. 2000;79:153–62. doi: 10.1016/S0006-3495(00)76280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56:151–60. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc Natl Acad Sci USA. 2005;102:7186–91. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKintosh FC, Kas J, Janmey PA. Elasticity of semiflexible biopolymer networks. Phys Rev Lett. 1995;75:4425–8. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 24.Luo T, Robinson DN. The role of the actin cytoskeleton in mechanosensation. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues: Mechanotransduction. New York: Springer-Verlag; 2010. [Google Scholar]
- 25.Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz D. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci USA. 2006;103:1762–7. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–5. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 27.Spudich JA. The myosin swinging cross-bridge model. Nat Rev Mol Cell Biol. 2001;2:387–92. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- 28.Niederman R, Pollard TD. Human platelet myosin. II. In vitro assembly and structure of myosin filaments. J Cell Biol. 1975;67:72–92. doi: 10.1083/jcb.67.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahajan RK, Pardee JD. Assembly mechanism of Dictyostelium myosin II: Regulation by K+, Mg2+, and actin filaments. Biochemistry. 1996;35:15504–14. doi: 10.1021/bi9618981. [DOI] [PubMed] [Google Scholar]
- 30.Egelhoff TT, Lee RJ, Spudich JA. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993;75:363–71. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- 31.Yumura S, Yoshida M, Betapudi V, Licate LS, Iwadate Y, Nagasaki A, et al. Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium. Mol Biol Cell. 2005;16:4256–66. doi: 10.1091/mbc.E05-03-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breckenridge MT, Dulyaninova NG, Egelhoff TT. Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells. Mol Biol Cell. 2009;20:338–47. doi: 10.1091/mbc.E08-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beach JR, Egelhoff TT. Myosin II recruitment during cytokinesis independent of centralspindlin-mediated phosphorylation. J Biol Chem. 2009;284:27377–83. doi: 10.1074/jbc.M109.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludowyke RI, Elgundi Z, Kranenburg T, Stehn JR, Schmitz-Peiffer C, Hughes WE, et al. Phosphorylation of nonmuscle myosin heavy chain IIA on Ser1917 is mediated by protein kinase C beta II and coincides with the onset of stimulated degranulation of RBL-2H3 mast cells. J Immunol. 2006;177:1492–9. doi: 10.4049/jimmunol.177.3.1492. [DOI] [PubMed] [Google Scholar]
- 35.Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryanzanov AG, et al. TRPM7 Regulates Myosin IIA Filament Stability and Protein Localization by Heavy Chain Phosphorylation. J Mol Biol. 2008;378:790–803. doi: 10.1016/j.jmb.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrow BD, Chen P, Chisholm RL. Expression of a myosin regulatory light chain phosphorylation site mutant complements the cytokinesis and developmental defects of Dictyostelium RMLC null cells. J Cell Biol. 1994;127:1945–55. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uyeda TQ, Abramson PD, Spudich JA. The neck region of the myosin motor domain acts as a lever arm to generate movement. Proc Natl Acad Sci USA. 1996;93:4459–64. doi: 10.1073/pnas.93.9.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walcott S, Fagnant PM, Trybus KM, Warshaw DM. Smooth muscle heavy meromyosin phosphorylated on one of its two heads supports force and motion. J Biol Chem. 2009;284:18244–51. doi: 10.1074/jbc.M109.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasza K, Nakamura F, Hu S, Kollmannsberger P, Bonakdar N, Fabry B, et al. Filamin A Is essential for active cell stiffening but not passive stiffening under external force. Biophys J. 2009;96:4326–35. doi: 10.1016/j.bpj.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Octtaviani E, Effler JC, Robinson DN. Enlazin, a natural fusion of two classes of canonical cytoskeletal proteins, contributes to cytokinesis dynamics. Mol Biol Cell. 2006;17:5275–86. doi: 10.1091/mbc.E06-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson DN, Spudich JA. Dynacortin, a genetic link between equatorial contractility and global shape control discovered by library complementation of a Dictyostelium discoideum cytokinesis mutant. J Cell Biol. 2000;150:823–38. doi: 10.1083/jcb.150.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stock A, Steinmetz MO, Janmey PA, Aebi U, Gerisch G, Kammerer RA, et al. Domain analysis of cortexillin I: actin-bundling, PIP2-binding and the rescue of cytokinesis. EMBO J. 1999;18:5274–84. doi: 10.1093/emboj/18.19.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber I, Gerisch G, Heizer C, Murphy J, Badelt K, Stock A, et al. Cytokinesis mediated through the recruitment of cortexillins into the cleavage furrow. EMBO J. 1999;18:586–94. doi: 10.1093/emboj/18.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukhina S, Wang YL, Murata-Hori M. alpha-Actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev Cell. 2007;13:554–65. doi: 10.1016/j.devcel.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber I, Neujahr R, Du A, Köhler J, Faix J, Gerisch G. Two-step positioning of a cleavage furrow by cortexillin and myosin II. Curr Biol. 2000;10:501–6. doi: 10.1016/s0960-9822(00)00452-8. [DOI] [PubMed] [Google Scholar]
- 46.Faix J, Weber I, Mintert U, Köhler J, Lottspeich F, Marriott G. Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 2001;20:3705–15. doi: 10.1093/emboj/20.14.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–78. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Straight AF, Field CM, Mitchison TJ. Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol Biol Cell. 2005;16:193–201. doi: 10.1091/mbc.E04-08-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berlin A, Paoletti A, Chang F. Mid2p stabilizes septin rings during cytokinesis in fission yeast. J Cell Biol. 2003;160:1083–92. doi: 10.1083/jcb.200212016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tasto JJ, Morrell JL, Gould KL. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J Cell Biol. 2003;160:1093–103. doi: 10.1083/jcb.200211126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coffman VC, Nile AH, Lee I-J, Liu H, Wu J-Q. Roles of formin nodes and myosin motor activity in Mid1p-dependent contractile-ring assembly during fission yeast cytokinesis. Mol Biol Cell. 2009;20:5195–20. doi: 10.1091/mbc.E09-05-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y, Yan H, Balasubramanian MK. Assembly of normal actomyosin rings in the absence of mid1p and cortical nodes in fission yeast. J Cell Biol. 2008;183:979–88. doi: 10.1083/jcb.200806151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paoletti A, Chang F. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol Biol Cell. 2000;11:2757–73. doi: 10.1091/mbc.11.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larochelle DA, Vithalani KK, DeLozanne A. Role of Dictyostelium racE in cytokinesis: Mutational analysis and localization studies by the use of green fluorescent protein. Mol Biol Cell. 1997;8:935–44. doi: 10.1091/mbc.8.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerald N, Dai J, Ting-Beall HP, DeLozanne A. A role for Dictyostelium RacE in cortical tension and cleavage furrow progression. J Cell Biol. 1998;141:483–92. doi: 10.1083/jcb.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larochelle DA, Vithalani KK, DeLozanne A. A novel member of the rho family of small GTP-binding proteins is specifically required for cytokinesis. J Cell Biol. 1996;133:1321–9. doi: 10.1083/jcb.133.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Effler JC, Kee Y-S, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-specific mechanosensing and contractile protein redistribution control cell shape. Curr Biol. 2006;16:1962–7. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, et al. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr Biol. 2009;19:1421–8. doi: 10.1016/j.cub.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prassler J, Stocker S, Marriott G, Heidecker M, Kellermann J, Gerisch G. Interaction of a Dictyostelium member of the plastin/fimbrin family with actin filaments and actin-myosin complexes. Mol Biol Cell. 1997;8:83–95. doi: 10.1091/mbc.8.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uyeda TQP, Kitayama C, Yumura S. Myosin II-Independent cytokinesis in Dictyostelium: its mechanism and implications. Cell Struct Funct. 2000;25:1–10. doi: 10.1247/csf.25.1. [DOI] [PubMed] [Google Scholar]
- 61.Effler JC, Iglesias PA, Robinson DN. A mechanosensory system controls cell shape during mitosis. Cell Cycle. 2007;6:30–5. doi: 10.4161/cc.6.1.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greene LE, Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980;77:2616–20. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orlova A, Egelman EH. Cooperative rigor binding of myosin to actin is a function of F-actin structure. J Mol Biol. 1997;265:469–74. doi: 10.1006/jmbi.1996.0761. [DOI] [PubMed] [Google Scholar]
- 64.Tokuraku K, Kurogi R, Toya R, Uyeda TQP. Novel mode of cooperative binding between myosin and Mg2+-actin filaments in the presence of low concentrations of ATP. J Mol Biol. 2009;386:149–62. doi: 10.1016/j.jmb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–64. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 66.Lakshmikanth G, Warrick HM, Spudich JA. A mitotic kinesin-like protein required for normal karyokinesis, myosin localization to the furrow, and cytokinesis in Dictyostelium. Proc Natl Acad Sci USA. 2004;101:16519–24. doi: 10.1073/pnas.0407304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuriyama R, Gustus C, Terada Y, Uetake Y, Matuliene J. CHO1, a mammalian kinesin-like protein, interacts with F-actin and is involved in the terminal phase of cytokinesis. J Cell Biol. 2002;156:783–90. doi: 10.1083/jcb.200109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Q, Lakshmikanth GS, Spudich JA, DeLozanne A. The localization of inner centromeric protein (INCENP) at the cleavage furrow is dependent on Kif12 and involves interactions of the N terminus of INCENP with the actin cytoskeleton. Mol Biol Cell. 2007;18:3366–74. doi: 10.1091/mbc.E06-10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Chen Q, Kaller M, Nellen W, Graf R, DeLozanne A. Dictyostelium Aurora kinase has properties of both Aurora A and Aurora B kinases. Euk Cell. 2008;7:894–905. doi: 10.1128/EC.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–82. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dean SO, Spudich JA. Rho kinase's role in myosin recruitment to the equatorial cortex of mitotic Drosophila S2 cells is for myosin regulatory light chain phosphorylation. PLoS ONE. 2006;1:e131. doi: 10.1371/journal.pone.0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uehara R, Goshima G, Mabuchi I, Vale RD, Spudich JA, Griffis ER. Determinants of Myosin II Cortical Localization during Cytokinesis. Curr Biol. doi: 10.1016/j.cub.2010.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dean SO, Rogers SL, Stuurman N, Vale RD, Spudich JA. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc Natl Acad Sci USA. 2005;102:13473–8. doi: 10.1073/pnas.0506810102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zang J-H, Spudich JA. Myosin II localization during cytokinesis occurs by a mechanism that does not require its motor domain. Proc Natl Acad Sci USA. 1998;95:13652–7. doi: 10.1073/pnas.95.23.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol. 2008;18:30–6. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 76.Maddox AS, Lewellyn L, Desai A, Oegema K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev Cell. 2007;12:827–35. doi: 10.1016/j.devcel.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 77.Giansanti MG, Bonaccorsi S, Williams B, Williams EV, Santolamazza C, Goldberg ML, et al. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu CK, Coughlin M, Field CM, Mitchison TJ. Cell polarization during monopolar cytokinesis. J Cell Biol. 2008;181:195–202. doi: 10.1083/jcb.200711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feneberg W, Westphal M, Sackmann E. Dictyostelium cells' cytoplasm as an active viscoplastic body. Eur Biophys J. 2001;30:284–94. doi: 10.1007/s002490100135. [DOI] [PubMed] [Google Scholar]
- 80.Girard KD, Chaney C, Delannoy M, Kuo SC, Robinson DN. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 2004;23:1536–46. doi: 10.1038/sj.emboj.7600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Girard KD, Kuo SC, Robinson DN. Dictyostelium myosin-II mechanochemistry promotes active behavior of the cortex on long time-scales. Proc Natl Acad Sci USA. 2006;103:2103–8. doi: 10.1073/pnas.0508819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Novák B, Tyson JJ. Design principles of biochemical oscillators. Nature Rev Mol Cell Biol. 2008;9:981–91. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]