Abstract
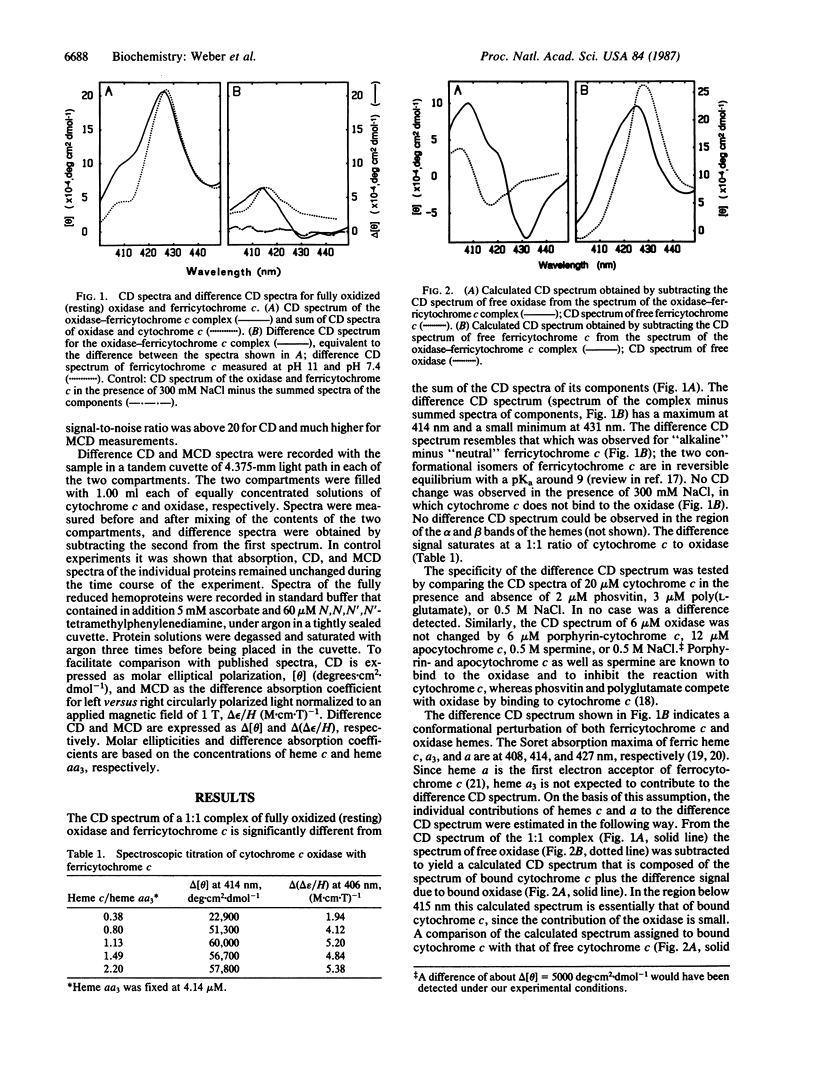
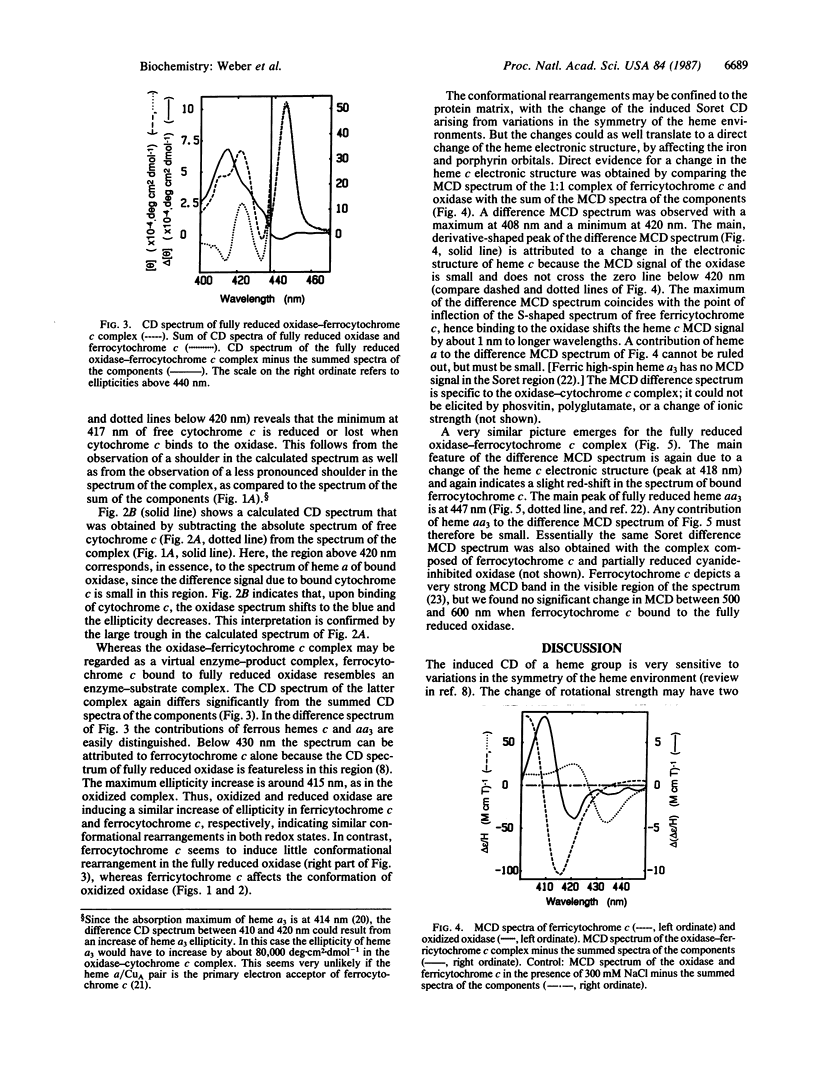
Binding of cytochrome c to cytochrome c oxidase induces a conformational change in both proteins as well as a change of the electronic structure of the heme of cytochrome c, indicating an altered heme c-protein interaction. This follows from the observation that the induced circular dichroism (CD) and magnetic circular dichroism (MCD) spectra of the oxidase-cytochrome c complex in the Soret region differ from the summed spectra of oxidase plus cytochrome c. Spectral changes occur in the complex composed of either the two ferric or the two ferrous hemoproteins. The difference CD and MCD signals saturate at a ratio of 1 heme c per heme aa3. The difference spectra are specific to the cognate complex. The results are interpreted to reflect a direct relationship between the recognition/binding step and the electron-transfer reaction. The conformational rearrangement induced in cytochrome c by cytochrome c oxidase consists of a structural rearrangement of the heme environment and possibly a change of the geometry of the heme iron-methionine-80 sulfur axial bond. This rearrangement may decrease the reorganizational free energy of electron transfer by adjusting the heme c geometry to a state between that of ferri- and ferrocytochrome c.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antalis T. M., Palmer G. Kinetic characterization of the interaction between cytochrome oxidase and cytochrome c. J Biol Chem. 1982 Jun 10;257(11):6194–6206. [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Bosshard H. R. Alkaline isomerization of ferricytochrome c: lysine is not replacing methionine at the sixth co-ordination site of the haem iron. J Mol Biol. 1981 Dec 25;153(4):1125–1149. doi: 10.1016/0022-2836(81)90471-x. [DOI] [PubMed] [Google Scholar]
- Chessa G., Filippi B., Borin G., Moroder L., Palumbo M., Marchiori F. Inhibition of the cytochrome c/cytochrome c oxidase system by cytochrome c derivatives and related fragments. Hoppe Seylers Z Physiol Chem. 1980 Jul;361(7):1077–1091. doi: 10.1515/bchm2.1980.361.2.1077. [DOI] [PubMed] [Google Scholar]
- Chiang Y. L., Kaminsky L. S., King T. E. A complex of cardiac cytochrome c1 and cytochrome c. J Biol Chem. 1976 Jan 10;251(1):29–36. [PubMed] [Google Scholar]
- Errede B., Kamen M. D. Comparative kinetic studies of cytochromes c in reactions with mitochondrial cytochrome c oxidase and reductase. Biochemistry. 1978 Mar 21;17(6):1015–1027. doi: 10.1021/bi00599a012. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Angström J. A 1H-NMR longitudinal relaxation study of the interaction between cytochrome c and cytochrome c oxidase. Biochim Biophys Acta. 1983 Feb 17;722(2):291–296. doi: 10.1016/0005-2728(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Georgevich G., Darley-Usmar V. M., Malatesta F., Capaldi R. A. Electron transfer in monomeric forms of beef and shark heart cytochrome c oxidase. Biochemistry. 1983 Mar 15;22(6):1317–1322. doi: 10.1021/bi00275a001. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Kaminsky L. S., Wright R. L., Davison A. J. Effect of alcohols on the rate of autoxidation of ferrocytochrome c. Biochemistry. 1971 Feb 2;10(3):458–462. doi: 10.1021/bi00779a017. [DOI] [PubMed] [Google Scholar]
- Kaminsky L. S., Yong F. C., King T. E. Circular dichroism studies of the perturbations of cytochrome c by alcohols. J Biol Chem. 1972 Mar 10;247(5):1354–1359. [PubMed] [Google Scholar]
- Kornblatt J. A., Luu H. A. The interactions of cytochrome c and porphyrin cytochrome c with cytochrome c oxidase. The resting, reduced and pulsed enzymes. Eur J Biochem. 1986 Sep 1;159(2):407–413. doi: 10.1111/j.1432-1033.1986.tb09883.x. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo S. L., Ellis W. R., Jr, Crutchley R. J., Gray H. B. Long-range electron transfer in heme proteins. Science. 1986 Aug 29;233(4767):948–952. doi: 10.1126/science.3016897. [DOI] [PubMed] [Google Scholar]
- Michel B., Bosshard H. R. Spectroscopic analysis of the interaction between cytochrome c and cytochrome c oxidase. J Biol Chem. 1984 Aug 25;259(16):10085–10091. [PubMed] [Google Scholar]
- Myer Y. P. Conformation of cytochromes. 3. Effect of urea, temperature, extrinsic ligands, and pH variation on the conformation of horse heart ferricytochrome c. Biochemistry. 1968 Feb;7(2):765–776. doi: 10.1021/bi00842a035. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Cytochrome c binding to enzymes and membranes. Biochim Biophys Acta. 1974 Dec 30;346(3-4):261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Borden D., Koppenol W. H., Margoliash E. Electrostatic interactions in cytochrome c. The role of interactions between residues 13 and 90 and residues 79 and 47 in stabilizing the heme crevice structure. J Biol Chem. 1980 Feb 25;255(4):1689–1697. [PubMed] [Google Scholar]
- Proudfoot A. E., Wallace C. J., Harris D. E., Offord R. E. A new non-covalent complex of semisynthetically modified tryptic fragments of cytochrome c. Biochem J. 1986 Oct 15;239(2):333–337. doi: 10.1042/bj2390333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. The cytochrome c oxidase binding site on cytochrome c. Differential chemical modification of lysine residues in free and oxidase-bound cytochrome c. J Biol Chem. 1978 Sep 10;253(17):6045–6053. [PubMed] [Google Scholar]
- Satterlee J. D., Moench S. J., Erman J. E. A proton NMR study of the non-covalent complex of horse cytochrome c and yeast cytochrome-c peroxidase and its comparison with other interacting protein complexes. Biochim Biophys Acta. 1987 Mar 18;912(1):87–97. doi: 10.1016/0167-4838(87)90251-2. [DOI] [PubMed] [Google Scholar]
- Senn H., Wüthrich K. Amino acid sequence, haem-iron co-ordination geometry and functional properties of mitochondrial and bacterial c-type cytochromes. Q Rev Biophys. 1985 May;18(2):111–134. doi: 10.1017/s0033583500005151. [DOI] [PubMed] [Google Scholar]
- Smith H. T., Staudenmayer N., Millett F. Use of specific lysine modifications to locate the reaction site of cytochrome c with cytochrome oxidase. Biochemistry. 1977 Nov 15;16(23):4971–4974. doi: 10.1021/bi00642a005. [DOI] [PubMed] [Google Scholar]
- Speck S. H., Dye D., Margoliash E. Single catalytic site model for the oxidation of ferrocytochrome c by mitochondrial cytochrome c oxidase. Proc Natl Acad Sci U S A. 1984 Jan;81(2):347–351. doi: 10.1073/pnas.81.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Tollin G., Meyer T. E., Cusanovich M. A. Elucidation of the factors which determine reaction-rate constants and biological specificity for electron-transfer proteins. Biochim Biophys Acta. 1986 Nov 4;853(1):29–41. doi: 10.1016/0304-4173(86)90003-0. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M., Erecińska M. Cytochrome c interaction with membranes. Absorption and emission spectra and binding characteristics of iron-free cytochrome c. Eur J Biochem. 1975 Dec 1;60(1):199–207. doi: 10.1111/j.1432-1033.1975.tb20992.x. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- Vickery L., Nozawa T., Sauer K. Magnetic circular dichroism studies of low-spin cytochromes. Temperature dependence and effects of axial coordination on the spectra of cytochrome c and cytochrome b5. J Am Chem Soc. 1976 Jan 21;98(2):351–357. doi: 10.1021/ja00418a006. [DOI] [PubMed] [Google Scholar]
- Wallace C. J., Proudfoot A. E. On the relationship between oxidation-reduction potential and biological activity in cytochrome c analogues. Results from four novel two-fragment complexes. Biochem J. 1987 Aug 1;245(3):773–779. doi: 10.1042/bj2450773. [DOI] [PMC free article] [PubMed] [Google Scholar]



