Abstract
Social animals, such as primates, must behave appropriately in complex social situations such as dominance interactions. Learning dominance information through trial and error would be dangerous, therefore cognitive mechanisms for rapid learning of dominance information by observation would be adaptive. We used a set of digitally edited artificial social interactions to examine whether rhesus monkeys can learn dominance relationships between unfamiliar conspecifics through observation. Our method allowed random assignment of stimulus monkeys to ranks in an artificial hierarchy, controlling for non-behavioral cues that could indicate dominance. Subject monkeys watched videos depicting one stimulus monkey behaving dominantly toward another, and were rewarded for selecting the dominant individual. Monkeys rapidly learned this discrimination across five behavior types in Experiment 1, and transferred performance to novel videos of new individuals in Experiment 2. Additionally, subjects selected the dominant individual more often than expected by chance in probe videos containing no behavioral dominance information, indicating some retention of the relative dominance status of stimulus monkeys from training. Together, our results suggest that monkeys can learn dominance hierarchies through observation of third-party social interactions.
Keywords: Social cognition, observation, social behavior, hierarchy, computerized testing
Many animals live in complex social groups organized into linear dominance hierarchies (Jackson & Winnegrad, 1988; Chase 1980). It is important for each individual to know their place in the hierarchy and act accordingly; aggressing towards a higher-ranking animal can result in injury or death (Lore & Flannelly, 1977). In many species, physical cues such as size and age do not reliably predict rank (Barchas & Mendoza, 1984; Chase, 1982; Holekamp, Sakai, & Lundrigan, 2007). In these cases, dominance hierarchies must be learned through individual interactions, observed behavior, or other sources of information specific to each social group. Learning the dominance hierarchy through aggressive contests with each group member would be dangerous and time-consuming. In contrast, learning dominance relationships by observation of interactions among individuals could be relatively safe and rapid. Critically, learning by observation can also provide much more detailed information about third-party relationships, information that is essential for predicting the behavior of others in fights and alliances (Cheney & Seyfarth, 1990a). Given these selective pressures for observational social learning, it is of interest to determine whether highly social species, such as rhesus monkeys, learn dominance relationships by observing the social interactions of others (Cheney & Seyfarth, 1990a; Paz-y-Mino, Bond, Kamil, & Balda, 2004; Silk, Alberts, & Altmann, 2003).
Recent research suggests that some animals can learn about dominance relationships through observation and that they use this information appropriately in social interactions. Chickens and pinyon jays that observed a familiar dominant individual lose food to an unfamiliar bird later submitted to that bird during their first meeting (Hogue, Beaugrand, & Lague, 1996; Paz-y-Mino, et al., 2004). Male great tits reacted more vigorously toward playback of unfamiliar males that they heard defeat a highly aggressive individual than to playback of those that they heard lose to a less aggressive individual (Peake, Terry, McGregor, & Dabelsteen, 2002). Male members of the African cichlid fish species A. burtoni that observed fights between pairs of unfamiliar individuals later preferred to spend time near the losing individuals, indicating that they had learned dominance relationships by observation alone (Grosenick, Clement, & Fernald, 2007). Importantly, in this last study, the researchers experimentally controlled which fish would be dominant in each demonstration fight, ensuring that subjects based their choices on observed dominance behaviors rather than on physical features that might co-vary with real-world dominance, such as size or health.
Many primate species are intensely social and live in complex social groups made up of stable linear dominance hierarchies (Cheney & Seyfarth, 1990b; deWaal & Luttrell, 1985; Jackson & Winnegrad, 1988). Evidence from behavioral observations and audio playback experiments indicates that nonhuman primates know about third-party social relationships and use that information to guide their behavior. Monkeys selectively reconcile or aggress towards kin of recent combatants (Aureli, Cozzolino, Cordischi, & Scucchi, 1992; Judge, 1982). Dominant female baboons that hear playbacks of their relatives fighting with another individual selectively displace that individual’s relatives (Cheney & Seyfarth, 1999). Female baboons that hear playback of an infant crying selectively look towards the infant’s mother (Cheney & Seyfarth, 1980). During aggressive encounters, Japanese macaques selectively recruit allies that are dominant to their opponents, and avoid recruiting their opponents’ kin (Schino, Tiddi, & Di Sorrentino, 2006). Monkeys can presumably learn third-party dominance relationships only by observing the social interactions of others; individual first-person experience is not sufficient to learn most others’ relationships (Cheney & Seyfarth, 1999). Whereas you could infer that an individual that dominates you would also dominate all individuals that you dominate, this cannot provide information about relationships between two individuals that are both subordinate or both dominant to you. Given the data demonstrating that monkeys do know third-party relationships, and the apparent logical necessity for observational learning for the acquisition of this information, it is likely that monkeys do learn by social observation. However, because all of the findings that demonstrate monkeys’ knowledge of these relationships come from situations in which social experience could not be controlled, it is difficult to draw firm conclusions about how monkeys learn about these social relationships.
Controlled laboratory experiments that manipulate exposure to social information are necessary to determine whether monkeys can learn social relationships through observation alone. To date, only one study has used a laboratory approach to this problem. Rhesus monkeys, a species that lives in stable linear dominance hierarchies (deWaal & Luttrell, 1985; Jackson & Winnegrad, 1988), earned food rewards by selecting the dominant individual in short video clips of real social interactions between two unfamiliar individuals (Bovet & Washburn, 2003). Videos included seven different categories of social interactions associated with dominance and were presented to the monkeys in blocks containing a single category of interaction (e.g. threat, displacement, food priority). After learning the discrimination to criterion on one category, two of the three subjects generalized to new videos from that same category as well as to three of the subsequent seven categories. This suggests that monkeys determined dominance by applying a general rule that spanned more than one category of behavior. However, in rhesus monkeys, rank directly impacts physical health; subordinate animals show less efficient hormonal responses to stress challenges and lower immune responses (Gust, 1991; Wilson, Legendre, Pazol, Fisher, & Chikazawa, 2005) and dominant individuals grow faster and reach puberty earlier (Zehr, Van Meter, & Wallen, 2005). Therefore, an alternate explanation for these results is that subjects correctly selected the dominant individual based not on dominance information derived from observed behavior, but on physical differences that co-vary with rank, such as posture, health, or size. Random assignment of stimulus monkeys to dominant status is required to determine whether monkeys use behavior rather than physical characteristics to select dominant animals in these tests.
To determine whether behavioral cues are sufficient to indicate dominance in the absence of possible rank-related physical cues, we used video editing to generate composite videos of artificial dominance interactions that were independent of real-world rank. Together, the set of videos comprised an artificial dominance hierarchy of unfamiliar individuals, with randomly assigned ranks. In this study, we examined whether rhesus monkeys could learn to select dominant individuals in the artificial social interactions. We then tested whether subject monkeys retained information about the ranks of the stimulus monkeys, and whether the learned dominance discrimination transferred to novel stimulus monkeys.
Experiment 1
In Experiment 1, we examined whether rhesus monkeys could learn to select dominant individuals in videos of artificial social interactions between unfamiliar monkeys. Doing so would suggest that they can learn dominance relationships through observation of behavior alone and in the absence of physical features that co-vary with dominance. We then assessed memory for the identity and relative dominance of the stimulus monkeys with identity probe videos depicting individuals seen in training, but without behaviors that indicated dominance. Based on findings from previous experiments and observations, we hypothesized that rhesus monkeys would learn to select the dominant individual in the videos and would transfer performance to identity probe videos containing no dominance information.
Method
Subjects and apparatus
Subjects were six three-year-old male rhesus monkeys (Macaca mulatta) who had been raised by their biological mothers in a large social group until the age of approximately 2.5 years. Monkeys were pair-housed and kept on a 12:12 light:dark cycle with light onset at 7:00 am. Animals received a full ration of food daily and water was available ad libitum.
Testing occurred in the subjects’ home cages. Computerized touch-screen test systems, each consisting of a 15-inch LCD color monitor (3M, St. Paul, MN) running at a resolution of 1024 × 768 pixels, generic stereo speakers, two automated food dispensers (Med Associates Inc., St. Albans, VT), and two food cups below the screen, were attached to the front of each monkey’s cage. Correct responses were rewarded 85% of the time with nutritionally balanced banana flavored pellets (Bio-Serv, Frenchtown, NJ) and the remaining 15% of the time with miniature chocolate candies, intermixed according to a random schedule. One or two test sessions were conducted daily between 10 am and 5 pm, six days per week.
Stimuli
Five adult female rhesus monkeys (aged 9–23 years) that were unfamiliar to the subjects served as stimulus monkeys. Stimulus monkeys were each digitally recorded individually in an outdoor enclosure against the same background. All behaviors occurred on or around a PVC perch in the center of the enclosure. Each monkey was videotaped engaging in the following six elicited behaviors (with two exceptions, described below); 1) walk: walking on the perch from one end to the other; 2) threat: sitting on one end of the perch and threatening toward the other end; 3) submission: sitting on one end of the perch and looking away from the other end; 4) eat: eating food on one end of the perch; 5) jump up: jumping from the floor to the perch; and 6) jump down: jumping from the perch to the floor.
Dominance videos
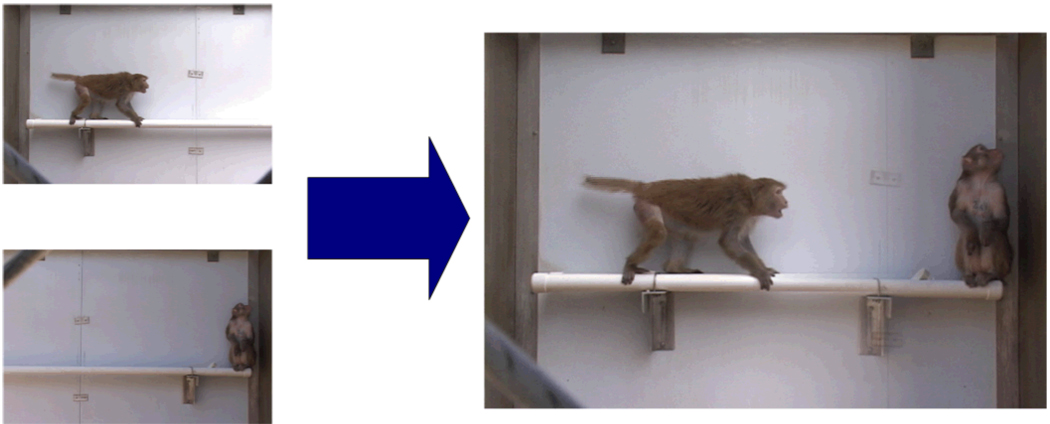
The individual clips were digitally edited into composite videos where two individuals appeared to be interacting (Adobe Premiere Pro 1.5; Figure 1; Supplemental Video). In each interaction, one animal displayed a dominant behavior and the other displayed a subordinate behavior. The dominant animal appeared equally often on the left and right side of the video frame. Five different types of interactions were created using the six individual behaviors; food priority: the dominant individual eats while the subordinate individual averts its gaze; threat-submit: the dominant individual threatens and the subordinate individual averts its gaze; threat-flee: the dominant individual threatens and the subordinate individual jumps off the perch; displace from perch: the dominant individual walks toward the subordinate individual and the subordinate individual jumps off the perch; and displace on perch: the dominant individual jumps from the floor onto the perch near the subordinate individual and the subordinate individual walks away. Table 1 summarizes the behaviors exhibited by the dominant and subordinate member of the pairs for each interaction type.
Figure 1.
Creation of a threat-submit composite video clip containing individuals C and D from the artificial hierarchy. Left panel: still frames from threat video from animal C (top) and submission video from animal D (bottom). Right panel: still frame from the edited composite threat-submit video that was shown to the subjects.
Table 1.
Summary of the dominant and subordinate animals in all the different video interactions presented in training and on identity probe trials.
| Artificial Rank Order Hiearchy A>B>C>D>E |
||||||||
|---|---|---|---|---|---|---|---|---|
| Stimulus Animal | Training Videos | Identity Probes | ||||||
| Dominant animal |
Subordinate animal(s) |
Food priority |
Threat submit |
Threat flee |
Displace from perch |
Displace on perch |
Eat eat | Jump on perch jump on perch |
| A | B,C,D,E | ✓ | ✓ | ✓ | Missing | ✓ | ✓ Missing with E |
Missing |
| B | C,D,E | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ Missing with E |
✓ |
| C | D,E | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ Missing with E |
✓ |
| D | E | ✓ | Missing | Missing | ✓ | ✓ | Missing | ✓ |
| E | None | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Note. Checks indicate videos that were presented for all dominant-subordinate animal pairings in that box. “Missing” indicates videos that were not presented for my dominant-subordinate animal pairing in that box. “N/A” indicates videos that were not produced because they were inconsistent with the hierarchy. The rank order hierarchy that resulted from these interactions appears at the top.
The five stimulus monkeys were randomly assigned to positions in our artificial hierarchy. This random assignment allowed us to control for any physical cues that might identify a dominant or subordinate individual. The artificial hierarchy was created by showing animal A as the dominant individual in all videos in which it appeared, animal B as the dominant individual when paired with all animals except A, and so on through C, D, and E (Figure 1).
One video interaction of each of the five types was created for each pair of animals, with a few exceptions. We were unable to elicit a threat from animal E and unable to get animal A to jump up onto the perch. Consequently, we were unable to construct six of the target composite videos (see Table 1, middle section). The resulting training video set consisted of 44 individual videos, which were mirror flipped across the vertical axis to counterbalance the left-right screen position of the dominant and subordinate animals, resulting in a total of 88 videos. Video clips varied in length from 2 to 10 seconds.
Identity probe videos
Each identity probe video contained two of the five individuals from the artificial hierarchy, as in the training videos. However, identity probe videos contained no dominance information; both stimulus animals engaged in the same behavior (eat or jump up) on opposite sides of the screen. Because the observed behaviors did not indicate dominance, subjects could select the dominant individual only by identifying each individual and remembering their dominance relationship from the training videos. With the available individual clips, we were able to create 12 identity probe videos that were then mirror flipped to counterbalance the left-right screen position of the dominant and subordinate individuals, for a total of 24 videos (Table 1, right section).
Procedure
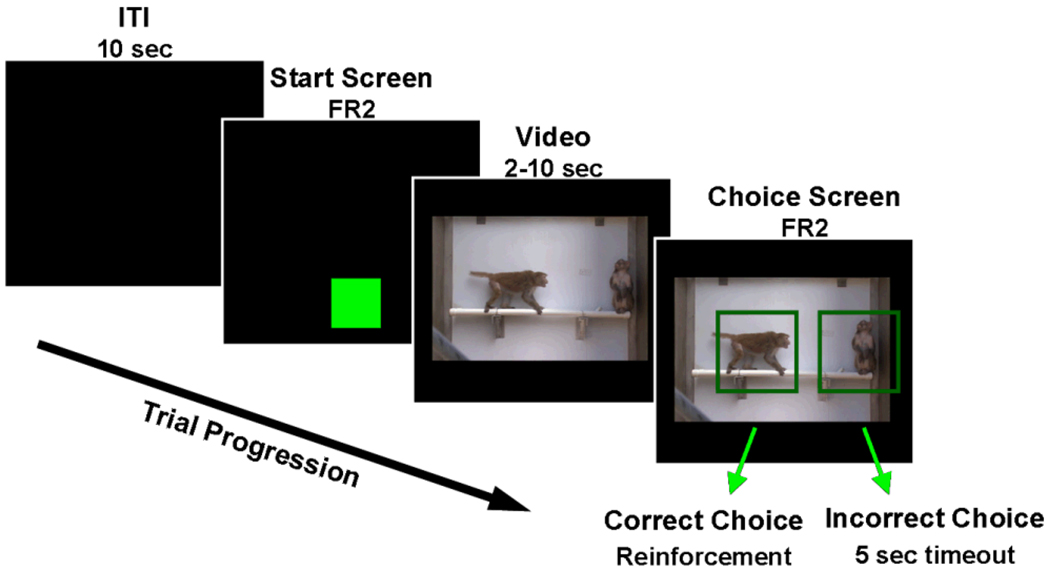
During testing, the monkeys in each pair were separated by an opaque plastic divider with holes that allowed visual, auditory, and tactile contact, but prevented the monkeys from touching the computer screen in the adjacent cage. Computer screens were locked to the front of each monkey’s cage and the door was raised, giving subjects full visual and tactile access to the screen during testing. After a 10-second inter-trial interval (ITI), a green box appeared at the bottom of the screen and remained until the monkey touched it (FR2) to start a trial. A composite video (720 × 480 pixels, 29.97 frames/second) appeared and played in the center of the screen, then froze on the last frame. A square green outline appeared around each stimulus monkey and the subject could select either monkey by touching inside the appropriate outline (FR2). Selection of the monkey showing a dominant behavior resulted in a food reward and auditory secondary reinforcer, while selection of the subordinate monkey resulted in a different auditory stimulus and a five second time out, during which the screen was black (Figure 2).
Figure 2.
Progression of a trial. After an inter-trial interval (ITI), monkeys started a trial by pressing the green start square. A sample video played and froze on the last frame. Two green squares appeared, one around each of the individuals in the video. The monkeys could earn food by selecting the individual that had shown a dominant behavior.
Each training session consisted of 176 trials, organized into two blocks of 88 trials; all 88 videos clips were presented in random order in each block. Monkeys completed a minimum of six training sessions before moving on to identity probe sessions. Each identity probe session consisted of 176 training video trials with 12 identity probe trials interspersed in a pseudorandom fashion (1 identity probe trial was randomly inserted in every block of 13 training trials, excluding the initial 20 warm-up trials of each session), for a total of 188 trials. Monkeys completed eight sessions with identity probes, resulting in four presentations of each of the 24 identity probe videos. To prevent subjects from learning the new discriminations during identity probe sessions, every identity probe trial response was reinforced, whether correct or not.
Data Analysis
Acquisition of the dominance discrimination was analyzed with a repeated-measures ANOVA and a post-hoc, two-tailed, paired t-test between the first and last sessions. We tested the directional hypothesis that accuracy would exceed that expected by chance (50%) using one-tailed, one-sample t-tests with an alpha level of .05. Proportions were arcsine transformed prior to analyses to better approximate normality (Aron & Aron, 1999). Effect sizes for the ANOVA and t-tests are reported as partial eta-squared and Cohen’s d, respectively.
Results and Discussion
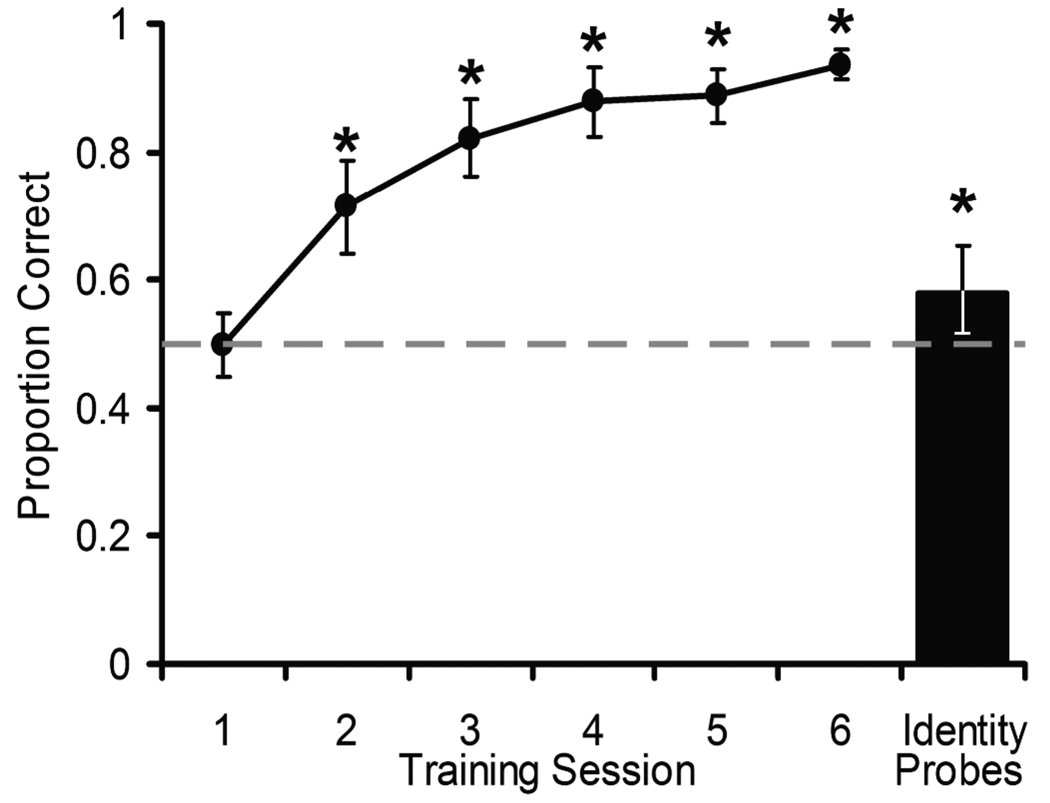
Monkeys acquired the dominance discrimination over the first six training sessions (F(5,25) = 55.52, p < .001, η2 = .917; Figure 3) and a post-hoc test confirmed a significant improvement from the first session to the last session (first: M = .50; last: M = .94; t(5) = 14.45, p < .001, d = 5.90; Figure 3). Acquisition was rapid, as accuracy was significantly above chance by the second training session (M = .71, t(5) = 5.50, p < .001, d = 2.25; Figure 3). Because the monkeys learned so rapidly, they appear to have selected the dominant individual by applying a general rule that spanned behavior types and individual stimulus monkeys. It is unlikely that monkeys solved the dominance discrimination by applying rules specific to a given video or behavior type, as they viewed 44 different videos and their mirror-flipped counterparts that depicted five types of interactions, many of which bore little perceptual or behavioral resemblance to each other. Additionally, because we randomly assigned stimulus monkeys to different ranks in the hierarchy, subjects could not have chosen the dominant individual based solely on cues that co-varied with real-world dominance rank, such as health or size. Dominance was the only variable that remained consistent across all video types; therefore, it is likely that subject monkeys were classifying individuals according to dominance.
Figure 3.
Mean accuracy on the dominance discrimination for the initial six sessions of training and the identity probe sessions. Error bars depict 95% CI. The dashed line represents the level of performance expected by chance. Asterisks denote means that are significantly above chance by a one-tailed, one-sample t-test (p < .05).
Accuracy on identity probes, where dominance information was unavailable, was marginally, but significantly, above chance (M = .58, t(5) = 2.13, p = .044, one-tailed, d = 0.87). Above chance performance on the identity probe trials indicates that the subjects remembered the relative ranks of the stimulus monkeys, although performance on these trials was weak. In Experiment 2, we further assessed subjects’ ability to learn the relative dominance relationships of specific individuals from artificial videos.
Experiment 2
The rapid acquisition of the dominance discrimination across five behaviors and five stimulus monkeys in Experiment 1 suggests that subjects perceived the behaviors of the monkeys in the videos as indicating dominance. According to this account, once subjects learned to select the dominant monkey, they applied this same rule to subsequent discriminations. However, it is possible that subjects instead memorized which stimulus animal was correct for each pair of monkeys, as they were reinforced for their selection of the dominant individual on all training presentations of a given pair. To discriminate between memorization of stimulus-specific responses and classification according to dominance, we presented subjects with transfer probe videos of two novel individuals interacting with members of the training hierarchy. Unlike in Experiment 1, these transfer probe videos played through to the end and then moved on to the next trial without allowing the monkeys to make choices and without any reinforcement. This exposed the subjects to information about the new individuals and their place in the hierarchy without reinforcing a choice of the dominant individual. We then presented identity probes again to examine whether monkeys extracted information about the dominance relationships of the two novel individuals from these videos. Finally, we tested whether the subjects transferred the dominance discrimination learned in Experiment 1 to these new videos containing novel individuals by giving subjects the opportunity to select the dominant individual in the transfer probe videos. Based on the strong training results from Experiment 1, we hypothesized that when first given a choice, subjects would select the dominant individual in transfer probe videos that contained one of the two new individuals.
Method
Subjects and apparatus
The subjects and apparatus were the same as used in Experiment 1.
Stimuli
Videos were created using the same editing techniques as used in Experiment 1. Two novel stimulus animals (X and Y) were inserted into the artificial hierarchy, one just below the top-ranking individual and one just above the bottom-ranking individual, creating a hierarchy of seven individuals (A>X>B>C>D>Y>E). Subjects were shown videos of these new animals interacting with the animals immediately above and below them in rank (animals A and B, and D and E, respectively) and no others. Videos of three of the possible social interaction types were presented for each of the possible pairs, along with their mirror flipped counterparts, for a total of 24 transfer probe videos.
Identity probe videos were similar to those used in Experiment 1 except that one of the two individuals in the video was a newly introduced animal (animal X or Y). Because animals A and E were, respectively, always and never reinforced during training, they likely had strong associative strengths that might obscure detection of any potential knowledge about dominance relationships. Consequently, animals A and E were excluded from these identity probe videos. Videos of all other possible pairings of the new individuals with the middle ranking trained animals (B, C, and D) were presented. In these videos both monkeys were shown eat or jump up, in both normal and mirror flipped orientations, for a total of 24 identity probe videos.
Trial Types
Three trial types were used. Exposure trials exposed subjects to the new monkeys and their place in the hierarchy without explicit food reinforcement. Identity probes assessed retention of dominance information about the new monkeys in the absence of dominance behaviors. Transfer probes assessed transfer of the dominance discrimination to new monkeys engaging in known dominance behaviors.
Exposure Trials
On exposure trials, a transfer probe video showing a dominance interaction between a new monkey and a training monkey played but did not freeze on the final frame and did not present the subject with a choice. Instead, the program proceeded directly to the ITI for the next trial. If subjects were able to learn the dominance relations between individuals just from watching the exposure trial videos without being reinforced for choosing the dominant animal, then they should select the dominant individual on subsequent identity probe trials.
Identity Probe Trials
To test whether subjects learned the dominance relations of the new animals without explicit reinforcement, we presented subjects with identity probe trials, like those presented in Experiment 1. Each video depicted two monkeys, one of which was a newly added monkey, engaged in the same behavior (eat or jump up) and therefore did not contain explicit dominance information. To prevent subjects from learning this discrimination from the identity probe trials, all choices were nonreinforced.
Transfer Probe Trials
To test whether subjects’ selection of the dominant individual in Experiment 1 would transfer to new individuals and videos, we allowed subjects to choose a monkey in the transfer probe videos. These trials were conducted as in Experiment 1, except that the videos displayed were the 24 new transfer probe videos seen in the exposure trials. All choices were nonreinforced.
Procedure
One trial type was presented per session, intermixed with trials of training videos used in Experiment 1. Each session consisted of 200 trials, 176 training trials from Experiment 1 and 24 new trials interspersed pseudorandomly throughout (after 20 warm up trials, 1 new video trial was randomly inserted in every block of 6 training trials until the new videos were exhausted). Five sessions of exposure trials were followed by two sessions of identity probe trials. This pattern was repeated 3 times, until the subjects had been exposed to the transfer videos 15 times each. Then, a single session of transfer probe trials was presented – this was the first time subjects could select a dominant individual from the exposure videos containing the novel stimulus monkeys engaged in dominance interactions. Finally, two additional sessions of identity probes were presented to assess retention of any dominance information gained from making a choice on the transfer probes.
In summary, in this Experiment subject monkeys watched videos of two new stimulus monkeys interacting with the already familiar stimulus monkeys. Four types of trials were presented in sequence: 1) exposure trials, in which one of the two monkeys was shown being dominant to the other, but subjects could not select a monkey at the end of the video; 2) identity probe trials, in which the two monkeys were shown acting with no behavioral indication of dominance, but subjects did select a monkey at the end of the video; 3) transfer probe trials, in which videos seen earlier in exposure trials were repeated, but subjects did select a monkey at the end of the video this time; and 4) a final presentation of the identity probe trials, just as explained above.
Data Analysis
Analyses were conducted as in Experiment 1.
Results and Discussion
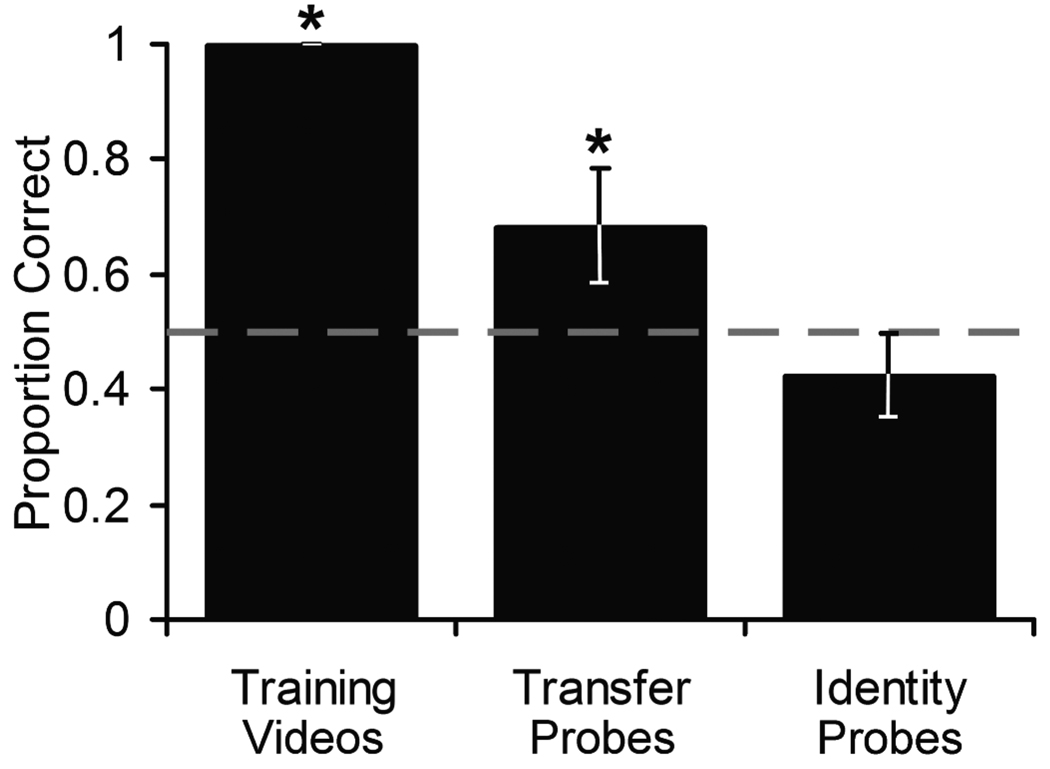
For the single session of choice trials, accuracy with the videos trained in Experiment 1 containing known stimulus monkeys remained significantly above chance (M = .99, t(5) = 32.89, p < .001, d = 13.43; Figure 4). Accuracy on transfer probe trials containing videos of the newly introduced stimulus monkeys was also significantly above chance the first time the monkeys were allowed to choose (M = .68, t(5) = 3.02, p = .015, d = 1.23; Figure 4). This successful transfer to new individuals indicates that subjects based their choices on the observed dominance behaviors and not rote memorization of individual videos or stimulus animals.
Figure 4.
Mean accuracy during the one session with transfer probes and all sessions of identity probes combined. Training videos show performance on the 176 trained dominance discrimination videos (used in Experiment 1) during the transfer probe session. Transfer probes show performance on the 24 transfer probe trials during the transfer probe session. Identity probes show performance on all sessions of identity probes combined. Error bars depict 95% CI. The dashed line represents the level of performance expected by chance. Asterisks denote means that are statistically above chance (p < .05).
Accuracy in the four sessions containing identity probe trials did not differ among sessions (repeated-measures ANOVA: F(2,15) = 0.15, p = .928) and data from these session were collapsed for further analyses. On these trials, which contained one of the two newly presented stimulus monkeys and no dominance information, subjects did not select the dominant individual significantly above chance (M = .42, t(5) = −2.04, p = .951, one-tailed; Figure 4). It is possible that the difference in performance on identity probes between Experiments 1 and 2 was due to the differences in reinforcement of the probes (all reinforced and non-reinforced, respectively). However, the monkeys’ poor performance on identity probes in Experiment 2 both before and after choice trials suggests that the relatively brief exposure to these new monkeys may have been insufficient to produce robust learning and retention of information about the observed dominance relationships.
General Discussion
Rhesus monkeys selected the dominant individual in videos of unfamiliar monkeys in artificial social interactions and transferred this judgment to novel videos containing new individuals. These results support the hypotheses that rhesus monkeys can determine third-party dominance relationships among unfamiliar individuals by observation of behavior alone, and bolsters related findings from a study using videos of natural social interactions (Bovet & Washburn, 2003).
Despite rapid learning of the dominance discrimination and performance well above 90%, subjects did not perform well on identity probe trials. These trials required monkeys to remember which individual was dominant in training trials. To do this, subjects must first recognize the individuals shown. In a natural social group, monkeys would have years of experience with group members, making it easier to identify, discriminate, and remember which individuals engaged in third-party interactions. In the current study, subjects were given limited experience with stimulus monkeys they did not know, making it likely that subjects had trouble recognizing individuals in the videos. Inability to discriminate one stimulus monkey from another would explain the poor performance on identity probes in both Experiments 1 and 2. This explanation is supported by a comparison of identity probe performance in the two experiments. We obtained non-significant results with the less familiar transfer monkeys, but significant results with the more familiar training monkeys. Therefore, it is likely that subjects required more exposure or explicit training than was given here to reliably identify unfamiliar monkeys in these types of videos. If monkeys were trained to discriminate individuals before being trained to discriminate dominance, they might be better able to retain information about specific dominance relationships.
Our findings that monkeys can select dominant individuals across different types of artificial social interactions and transfer performance to videos containing new individuals supports the results from observations, field experiments, and laboratory experiments that suggest the monkeys can gain information about others’ social relationships through observation (Bovet & Washburn, 2003; Cheney & Seyfarth, 1999; Judge, 1991). To our knowledge, this is the first study in nonhuman primates to use experimentally controlled social interactions to assess perceived dominance. These findings also support similar studies in non-primate species that used controlled social interactions and found that animals can extract dominance information from observations (Grosenick, et al., 2007; Hogue, et al., 1996; Paz-y-Mino, et al., 2004). Together, these findings suggests that the ability to extract dominance information by observing third-party interactions may be widespread among highly social species.
In social groups with complex dominance relationships, the ability to appropriately express aggression or submission and to maintain stable relationships with other group members is critical to the acquisition of social status, lifetime reproductive success, and survival (Fairbanks & McGuire, 1984; Silk, et al., 2003). Many social behaviors require navigation of a complex series of relationships, coalitions, friendships, and kin networks and do not result from stereotyped responses to eliciting cues (Cheney & Seyfarth, 1990a). Without knowledge of others’ social relations, which can best be gained through observation, animals would not be able to manage the complex social landscapes they live in (Kummer, 1971).
Because social knowledge is vital to adaptive behavior in many species, including humans, more controlled investigations aimed at establishing how specific social information is acquired, processed, and used are called for. The current study lays the groundwork for future laboratory studies aimed at understanding how primates acquire and use social information. We have demonstrated the feasibility of using videos of artificial social interactions to assess social knowledge in primates. The ability to experimentally manipulate social variables of interest, such as dominance, is critical for understanding primates’ social knowledge. Ultimately, a full understanding of social cognition will require the combined use of both naturalistic studies and tightly-controlled laboratory experiments.
Acknowledgement
This work was supported by a grant from the James S. McDonnell Foundation, by Yerkes Center base grant RR-00165 awarded by the Animal Resources Program of the National Institutes of Health, and by the Center for Behavioral Neuroscience under the STC Program of the National Science Foundation under Agreement IBN-9876754. The work of I.A. was supported by the Japan Society for the Promotion of Science. We thank Jane J. Na for help with preparing stimuli and testing subjects and Juliet Ward and Jeff Fisher for help filming monkeys.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/com
Contributor Information
Regina Paxton, Department of Psychology, Emory University and Yerkes National Primate Research Center.
Benjamin M. Basile, Department of Psychology, Emory University and Yerkes National Primate Research Center
Ikuma Adachi, Yerkes National Primate Research Center.
Wendy A. Suzuki, Center for Neural Science, New York University
Mark E. Wilson, Division of Psychobiology, Yerkes National Primate Research Center
Robert R. Hampton, Department of Psychology, Emory University and Yerkes National Primate Research Center
References
- Aron A, Aron E. Statistics in Psychology. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Aureli F, Cozzolino R, Cordischi C, Scucchi S. Kin-oriented redirection among Japanese macaques: An expression of a revenge system? Animal Behaviour. 1992;44:283–291. [Google Scholar]
- Barchas PR, Mendoza SP. Emergent hierarchical relationships in rhesus macaques: An application of Chase's model. In: Barchas PR, editor. Social hierarchies. Westport: Greenwood Press; 1984. pp. 81–95. [Google Scholar]
- Bovet D, Washburn DA. Rhesus macaques (Macaca mulatta) categorize unknown conspecifics according to their dominance relations. Journal of Comparative Psychology. 2003;117:400–405. doi: 10.1037/0735-7036.117.4.400. [DOI] [PubMed] [Google Scholar]
- Chase ID. Social process and hierarchy formation in small groups: A comparative perspective. American Sociological Review. 1980;45:905–924. [Google Scholar]
- Chase ID. Dynamics of hierarchy formation: The sequential development of dominance relationships. Behaviour. 1982;80:218–240. [Google Scholar]
- Cheney DL, Seyfarth RM. Vocal recognition in free-ranging vervet monkeys. Animal Behaviour. 1980;28:362–367. [Google Scholar]
- Cheney DL, Seyfarth RM. The representation of social relations by monkeys. Cognition. 1990a;37:167–196. doi: 10.1016/0010-0277(90)90022-c. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. How monkeys see the world. Chicago: University of Chicago Press; 1990b. [Google Scholar]
- Cheney DL, Seyfarth RM. Recognition of other individuals' social relationships by female baboons. Animal Behaviour. 1999;58:67–75. doi: 10.1006/anbe.1999.1131. [DOI] [PubMed] [Google Scholar]
- deWaal FBM, Luttrell LM. The formal hierarchy of rhesus macaques: An investigation of the bared-teeth display. American Journal of Primatology. 1985;9:73–85. doi: 10.1002/ajp.1350090202. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, McGuire MT. Determinants of Fecundity and Reproductive Success in Captive Vervet Monkeys. American Journal of Primatology. 1984;7:27–38. doi: 10.1002/ajp.1350070106. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmedansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behavior and Immunity. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Hogue M-E, Beaugrand JP, Lague PC. Coherent use of information by hens observing their former dominant defeating or being defeated by a stranger. Behavioural Processes. 1996;38:241–252. doi: 10.1016/s0376-6357(96)00035-6. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Sakai ST, Lundrigan BL. The spotted hyena (Crocuta crocuta) as a model system for study of the evolution of intelligence. Journal of Mammalology. 2007;88:545–554. [Google Scholar]
- Jackson WM, Winnegrad RL. Linearity in dominance hierarchies : A second look at the Individual Attributes Model. Animal Behaviour. 1988;36:1237–1240. [Google Scholar]
- Judge PG. Redirection of aggression based on kinship in a captive group of pigtail macaques. International Journal of Primatology. 1982;3:301. [Google Scholar]
- Judge PG. Dyadic and triadic reconciliation in pigtail macaques (Macaca nemestrina) American Journal of Primatology. 1991;23:225–237. doi: 10.1002/ajp.1350230403. [DOI] [PubMed] [Google Scholar]
- Kummer H. Primate societies: Group techniques of ecological adaptation. Arlington Heights, IL: AHM Publishing Corporation; 1971. [Google Scholar]
- Lore R, Flannelly K. Rat societies. Scientific American. 1977;236:106–116. doi: 10.1038/scientificamerican0577-106. [DOI] [PubMed] [Google Scholar]
- Paz-y-Mino G, Bond A, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Peake TM, Terry AMR, McGregor PK, Dabelsteen T. Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269(1503):1925–1929. doi: 10.1098/rspb.2002.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Tiddi B, Di Sorrentino EP. Simultaneous classification by rank and kinship in Japanese macaques. Animal Behaviour. 2006;71:1069–1074. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): Role of prenatal androgens, social rank, and adolescent body weight. Biology of Reproduction. 2005;72:1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]