Abstract
The low-density lipoprotein receptor-related protein (LRP1) is a multifunctional cell surface receptor that belongs to the LDL receptor (LDLR) gene family and that is widely expressed in several tissues. LRP1 consists of an 85-KDa membrane-bound carboxyl fragment (β chain) and a non-covalently attached 515-KDa (α chain) amino-terminal fragment. Through its extracellular domain, LRP1 binds at least 40 different ligands ranging from lipoprotein and protease inhibitor complex to growth factors and extracellular matrix proteins. LRP-1 has also been shown to interact with scaffolding and signaling proteins via its intracellular domain in a phosphorylation-dependent manner and to function as a co-receptor partnering with other cell surface or integral membrane proteins. LRP-1 is thus implicated in two major physiological processes: endocytosis and regulation of signaling pathways, which are both involved in diverse biological roles including lipid metabolism, cell growth/differentiation processes, degradation of proteases, and tissue invasion. The embryonic lethal phenotype obtained after target disruption of the LRP-1 gene in the mouse highlights the biological importance of this receptor and revealed a critical, but yet undefined role in development. Tissue-specific gene deletion studies also reveal an important contribution of LRP1 in vascular remodeling, foam cell biology, the central nervous system, and in the molecular mechanisms of atherosclerosis.
LRP1 (also known as CD91, or α2macroglobulin receptor, α2MR) is a ubiquitously expressed type 1 transmembrane receptor [1]. The mature form of the receptor is derived from a 600-kDa precursor that is proteolytically processed upon furin cleavage. The processed form of the receptor consists of a carboxyl-terminal β-fragment of 85-kDa, which contains an intracellular and a transmembrane domain. The extracellular portion of the 85 kDa fragment is non-covalently connected to the large amino-terminal 515-kDa α-fragment, which harbors several ligand binding domains that interact with multiple LRP1 ligands (Figure 1) [1, 2].
Figure 1.
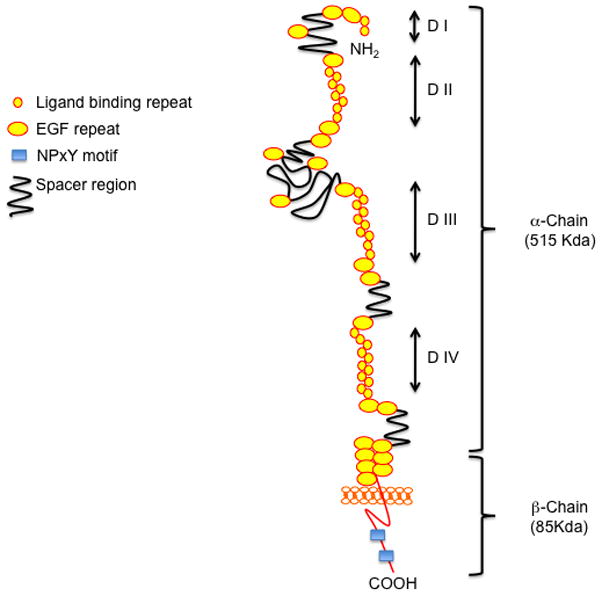
The low-density lipoprotein receptor-related protein (LRP1). LRP1 is a multifunctional receptors that binds a large spectrum of extracellular and intracellular ligands. The extracellular domain consists of ligand-binding-type repeats organized in four binding domains (DI, DII, DIII, DIV) and epidermal precursor homology domains, which contain YWTD repeats and EGF repeats. The cytoplasmic tail of the receptor is containing cytoplasmic NPxY motifs that mediate protein– protein interactions required for endocytosis as well as for the assembly of scaffold proteins related to signal transduction and trafficking such as Dab1, Fe65, JIP1, omp25, and integrin cytoplasmic domain-associated protein 1.
LRP1 is the most multifunctional member of the LDL receptor gene family [3]. It has been implicated in two main biological functions: endocytosis of its numerous ligands and regulation of cell signaling pathways. Through its extracellular domain, LRP1 interacts with at least 40 different ligands ranging from lipoproteins, extracellular matrix glycoproteins, protease/inhibitor complexes, viruses, cytokines and growth factors (Table 1). The large variety of ligands LRP1 recognizes reflects the numerous biological functions this evolutionarily ancient receptor has adopted since its inception in the most primitive metazoans. Its ubiquitous expression, the remarkable structural and sequence conservation among species, the absence of any known functional coding mutations of the LRP1 gene in humans, and the lethality of the conventional knockout in mice reveal that LRP1 is indispensible for cellular physiology.
TABLE 1.
LRP1 known ligands
| Proteins involved in lipoprotein metabolism |
| Apolipoprotein E-enriched lipoproteins (chylomicron and VLDL remnants), Lipoprotein lipase (LPL), Hepatic lipase, Sphingolipid activator protein |
| Proteases and protease/inhibitor complexes |
| Activated α2-macroglobulin, α2-macroglobulin protease complexes, Pregnancy zone protein-protease complexes, Aprotinin, urokinase plasminogen activator (uPA), pro- uPA, plasminogen activator inhibitor (PAI-1), uPA/PAI-1 complexes, tissue-type plasminogen activator (tPA), tPA/PAI-1 complexes, Thrombin/PA-1, Thrombin/anti- thrombin III, Thrombin/protease nexin-1, Thrombin/heparin cofactor II, Neuroserpin, Neuroserpin/tPA complexes, C1s/C1q inhibitor, Protease/protein C inhibitor, Elastase/α1-anti-trypsin, MMP-9, MMP-13, TSP-2/MMP-2 complexes, Tissue factor pathway inhibitor (TFPI), Factor VIIa/TFPI, Factor VIIIa, Factor IXa, Factor IXa/protease nexin-1, β-amyloid precursor protein |
| Matrix proteins |
| Thrombospondin-1, Thrombospondin-2, Fibronectin |
| Intracellular proteins |
| Receptor associated protein (RAP), Calreticulin, HIV Tat protein |
| Growth factors |
| Platelet-derived growth factor (PDGF), Midkine, Insulin-like growth factor (IGF)- binding protein-3 (IGFBP-3), Connective tissue growth factor (CTGF/CCN2), Transforming growth factor (TGF-β) |
| Others |
| Circumsporozoite protein, Lactoferrin, Ricin A, Saposin, Rhinovirus A peptide (monomer), Gentamicin, Polymycin B, Pseudomonas exotoxin A, Complement C3, Collectins (via calreticulin) |
Besides its role in endocytosis [3, 4], several studies have shown that LRP1 is essential for multiple signaling pathways. These functions have been described in the vascular wall, in neurons, adipose tissue, and numerous other tissues. In the vascular wall, LRP1 plays a major role in controlling vascular smooth muscle cells (vSMCs) proliferation, and protects against atherosclerosis. Mice lacking LRP1 in vSMCs exhibit hyperplasia of the aortic wall, disruption of the elastic lamina, aortic aneurysm formation and greatly enhanced susceptibility to atherosclerotic lesion development [5]. LRP1-deficient mice fed a high cholesterol diet develop massive foam cell formation within the arterial wall, leading to the complete occlusion of the lumen of the aorta and the mesenteric arteries, and thus the death of the animals from progressive large vessel obstruction. The mechanism by which LRP1 protects against the formation of atherosclerotic lesions is mediated through control of at least two distinct signaling pathways in vSMCs by the receptor: the platelet-derived growth factor BB (PDGF-BB) and the transforming growth factor-β (TGFβ) signaling pathways, which both play major roles during atherosclerosis [5–7]. Excessive smooth muscle hyperplasia of the aorta associated with LRP1-deficiency is accompanied by a major increase in the expression of the PDGF receptor β(PDGFRβ), increased PDGFRβ phosphorylation, and increased phosphorylation of Smad2, a downstream component of the TGFβ pathway that mediates the TGFβ transcriptional response. The PDGF-BB pathway has been previously described as a target of the TGFβ signaling pathway [8–10]. Moreover, LRP1 is identical to the TGFβ receptor (V), a member of the TGFβ receptor superfamily that is expressed together with TGFβ receptor I, II and III at the cell surface [11]. Thus, activation of the TGFβ pathway in the absence of LRP1 can further activate the PDGF-BB signaling pathway, by increasing the expression of PDGFRβ in the arterial wall and thereby promoting atherosclerotic lesion formation.
Macrophage lipoprotein receptors can accelerate progression of atherosclerosis by facilitating uptake of atherogenic particles such as the oxidized lipoproteins [12]. In the particular case of LRP1, deletion of the receptor in macrophages has also been shown to increase atherosclerosis in mice [13]. Transplantation of macrophage LRP1−/− bone marrow into lethally irradiated female LDLR−/− recipient mice resulted in a 40% increase in atherosclerosis [13]. Deletion of LRP1 in macrophages however, did not alter plasma lipid levels or plasma lipoprotein profiles, demonstrating no significant contribution of macrophage LRP1-mediated remnant clearance in influencing plasma lipoprotein levels in vivo. LRP1−/− macrophages displayed increased expression of proinflammatory cytokines such as IL-1β, IL-6 and tumor necrosis factor-α expression, and suppression of the pAkt survival pathway [14]. Thus, macrophage LRP1 might protect against atherosclerosis by decreasing inflammation, but also by facilitating efferocytosis, an atheroprotective effect by which apoptotic cells are removed from the lesions by phagocytic cells [13–15].
Since LRP1 in the liver participates in the removal of atherogenic apoE rich lipoproteins from the circulation, its role in that tissue during atherogenesis has also been investigated. Hepatic LRP1 plays a clear protective role in atherogenesis but independent of plasma cholesterol [16]. The mechanism by which hepatic LRP1 affects the development of atherosclerotic lesions is not clear. It might involve clearance of other LRP1 ligands that are related to atherosclerosis such as t-PA or u-PA.
Recently, we have shown that LRP1 is required for normal signaling through a canonical Wnt5a dependent pathway in mouse embryogenic fibroblasts (MEF), and that activation of this pathway prevents intracellular cholesterol accumulation, a prominent and necessary feature of the atherosclerotic lesion formation [17]. LRP1 also regulates LXR-mediated gene transcription and participates in reverse cholesterol transport by controlling cPLA2 activation and ABCA1 expression [18]. LRP1 is further required for lipolysis and for the stimulation of fatty acid synthesis independent of noradrenergic signals, through inhibition of GSK3β and its previously unknown target acetyl-CoA carboxylase (ACC) [17]. LRP1 thus, functions as a physiological integrator of cellular lipid homeostasis with signals that regulate cellular proliferation and vascular wall integrity.
Besides its large contribution to the protection against atherosclerosis, recent work has also shown a role for LRP1 and one of its ligands, tissue plasminogen activator (tPA), in the regulation of vascular tone [19] and the permeability of the blood brain barrier permeability (BBB) [20]. tPA regulates vascular contractility through LRP1 and this is reversed by a physiological tPA inhibitor, plasminogen activator inhibitor 1 (PAI-1). The authors reported that vasoconstriction induced by tPA requires a functional interaction between LRP1 and alpha(v)beta(3) integrin [21]. The mechanism of this interaction and the signaling pathways involved, however, remain unknown. Regulation of BBB permeability is important for neuronal homeostasis and protects the brain against toxins that constantly enter the circulation from the external environment and through the gut. The authors demonstrate that tPA directly induces BBB permeability and that this is blocked by anti-LRP1 antibodies and by the receptor-associated protein (RAP), a chaperone protein that blocks the binding of most of the known LRP1 ligands. These results thus suggest that the tPA-dependent regulation of BBB permeability requires the expression of LRP1 [20].
LRP1 is also playing an important role in the central nervous system (CNS), especially in neurons where it is highly expressed [22, 23] and where it interacts with numerous neuronal proteins such as the postsynaptic density protein 95 (PSD-95) and the N-methyl-D-aspartate (NMDA) receptor [24]. In the brain, glutamate is the main excitatory neurotransmitter, which also plays an important role in neuronal cell death in neurodegenerative diseases [25, 26]. Moreover, LRP1 has been shown to regulate calcium signaling in vitro [27], an important second messenger during glutamate neurotransmission. The active form of α2-macroglobulin (α2M), an LRP1 ligand, inhibits the calcium-dependent NMDA response and the expression of NMDA receptors through a signaling pathway involving LRP1 [28]. Mice lacking LRP1 in neurons exhibit a severe movement disorder, hyperactivity, and premature death [24].
In the lung, a new important role for LRP1 in the course of the inflammatory response has been reported [29]. The authors described that in the absence of LRP1, the surfactant proteins A and D (SP-A et SP-D) bind to the signal inhibitory regulatory protein α (SIRPα). This activates the tyrosine phosphatase SHP-1, blocks Src family kinases and p38 MAP kinases, and thereby inhibits the inflammatory response. By contrast, when LRP1 is expressed, surfactant proteins SP-A and SP-D interact with foreign organisms, apoptotic cells or cell debris, and the presentation of these organisms to LRP1 in macrophages by calreticulin leads to their phagocytosis and inflammatory response in lungs. Interestingly, it has been proposed that adiponectin promotes the uptake of apoptotic debris by peritoneal macrophages via a calreticulin/LRP1 pathway but not through the previously identified adiponectin receptor [30]. Thus, LRP1 is an important component that regulates the initiation of the innate immune response. This function of LRP1 in macrophages is not only important in lung macrophages, but has also implications in other tissues such as the aortas and in clearance of cells that have undergone apoptosis [31] an important physiologic function during development and tissue homoeostasis [32].
As is the case for numerous receptor and membrane proteins, the extracellular domain of LRP1 can be cleaved by cell surface proteases and subsequently released into the extracellular space or the circulation. A circulating form of LRP1 has been found at nM concentration in human plasma [33, 34]. This cleaved form of LRP1 contains the α chain of about 515 kDa and a fragment of the β chain (85 kDa) of about 55 kDa, demonstrating that the cleavage occurs close to the plasma membrane [33]. Enzymes that can mediate this cleavage have been identified and include the neuronal BACE1 protease [35] and a hepatic metalloproteinase [33]. The LRP1 soluble form is present in the plasma of mammals, but also in the blood of birds, and reptiles. In most case the physiological meaning of the extracellular cleavage is not certain, but since the soluble form can still bind most of the LRP1 ligands and thereby reduce their endocytosis by cellular LRP1, the soluble fragment may serve to quench extracellular ligand interaction with the cell or regulate their intracellular trafficking.
Several of the mechanisms by which LRP1 controls cell signaling pathways remain unresolved. One potential mechanism involves the cleavage of the transmembrane domain of the LRP1 β chain by regulated intramembraneous proteolysis (RIP). The released fragment (LRP1-ICD) of approximately 12 kDa might thus translocate to the nucleus where it can regulate the transcription of target genes [36]. RIP is a process by which the first step of proteolysis involves an extracellular cleavage event, which is then followed by intramembraneous processing and the release of a, usually small, cytoplasmic fragment that may have functions in the cytoplasm or in the nucleus, including transcriptional regulation [37]. Several proteins including the amyloid precursor protein (APP), Notch, a transmembrane protein that regulates cell fate decision of ES cells during development, the tyrosine kinase receptor ErbB-4 [38], the receptor CD44 [39], sterol regulatory element binding proteins SREBPs [40], ATF6 [41], Ire1[42], and cadherin [43] function through a RIP mechanism. In most cases, the intramembranous cleavage is done by the presenilin (PS)/γ-secretase complex. In the case of LRP1, the intracellular domain can also be released upon proteolytic cleavage by the presenilin (PS)/γ-secretase complex [36]. However, the precise cleavage site and thus the complete sequence of the released fragment remain unknown. Recently, one potential target of the LRP1-ICD has been identified [44]. Lipopolysaccharide (LPS) increases the proteolytic processing of the ectodomain of LRP1, which results in the γ-secretase-dependent release of the LRP1 intracellular domain (ICD) from the plasma membrane and its subsequent translocation to the nucleus, where it interacts with and represses the interferon-γ promoter [44]. The LRP1-ICD fragment contains numerous motifs that have been implicated in numerous signaling pathways: Two NPXY motifs, where the distal motif is contiguous with a YXXL motif, and two dileucine motifs. The YXXL motif is presumably the most important one mediating LRP1 endocytosis [45]. However, both NPXY motifs can bind and interact with numerous cytosolic proteins such as, DAB1, FE65, JIP1, PSD-95, ShcA or CED-6/GULP [6, 7, 46–50]. In vitro studies have shown that the LRP1-ICD can colocalize with the histone acetyl transferase Tip60 in the nucleus [51], which in turn can regulate transcription upon APP cleavage [52, 53] suggesting that the LRP1-ICD might be able to regulate the transcriptional activity of the APP-Tip60 complex, and thus have a more general function as a regulator of transcription. In order to dissect the in vivo functions of each motifs located in the LRP-ICD, Roebroek and colleagues [54] introduced mutations into the furin cleavage site and into both NPXY motifs located in the cytoplasmic tail of LRP1. Mutation of the NPXY motif of the cytoplasmic domain or in the furin cleavage site caused distinctive liver phenotypes: respectively, either a late fetal destruction of the organ causing perinatal death or a selective enlargement of von-Kupffer cell lysosomes reminiscent of a mild lysosomal storage without an apparent negative effect on animal survival. A mutation of the most distal NPXY motif within the cytoplasmic tail of LRP1 did not exhibit an overt phenotype [54].
In conclusion, LRP1 is a large multifunctional receptor with two main biological functions in multiple ligand endocytosis and in the control and integration of intercellular signaling pathways, which are not required for survival of the cell per se, but essential for the maintenance of basal cellular function and development and survival of the organism. Indicative of its importance, LRP1 is expressed in almost all cells, and there is no known disease-related LRP1 coding mutation that has been described in humans to date. Since it participates in such a large number of physiological activities as a co-receptor and also by interacting with numerous adaptor proteins through its cytoplasmic domain, functional dissection of these mechanisms and identification of further LRP1 partners might open new avenues to the treatment of metabolic diseases such as lipid metabolism and atherosclerosis, but also inflammation, Alzheimer disease and obesity.
Acknowledgments
This work was supported by grants from, centre national pour la recherche scientifique (CNRS), University of Strasbourg, Fondation de France, agence nationale de la recherche (ANR-06-PHYSIO-032-01, and ANR-09-BLAN-0121-01), the National Institutes of Health, the American Health Assistance Foundation, the Perot Family Foundation, the Consortium for Frontotemporal Dementia Research (CFR) and the Wolfgang-Paul Program of the Humboldt Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. Embo J. 1988;7:4119–27. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, et al. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A. 1996;93:8460–4. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–84. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–93. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 5.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–32. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 6.Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–13. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- 7.Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini MM, Loukinov D, et al. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function betwenn LRP and the PDGF. J Biol Chem. 2002;277:15499–506. doi: 10.1074/jbc.M200427200. [DOI] [PubMed] [Google Scholar]
- 8.Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–24. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- 9.Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14:681–5. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Taylor LM, Khachigian LM. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-beta involves transactivation by Smads. J Biol Chem. 2000;275:16709–16. doi: 10.1074/jbc.275.22.16709. [DOI] [PubMed] [Google Scholar]
- 11.Huang SS, Leal SM, Chen C-L, Liu IH, Huang JS. Identification of insulin receptor substrate proteins as key molecules for the TβR-V/LRP-1-mediated growth inhibitory signaling cascade in epithelial and myeloid cells. FASEB J. 2004:04-1872fje. doi: 10.1096/fj.04-1872fje. [DOI] [PubMed] [Google Scholar]
- 12.Linton MF, Babaev VR, Gleaves LA, Fazio S. A direct role for the macrophage low density lipoprotein receptor in atherosclerotic lesion formation. J Biol Chem. 1999;274:19204–10. doi: 10.1074/jbc.274.27.19204. [DOI] [PubMed] [Google Scholar]
- 13.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100:670–7. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 14.Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol. 2010;30:787–95. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–95. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espirito Santo SM, Pires NM, Boesten LS, Gerritsen G, Bovenschen N, van Dijk KW, et al. Hepatic low-density lipoprotein receptor-related protein deficiency in mice increases atherosclerosis independent of plasma cholesterol. Blood. 2004;103:3777–82. doi: 10.1182/blood-2003-11-4051. [DOI] [PubMed] [Google Scholar]
- 17.Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, et al. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem. 2009;284:381–8. doi: 10.1074/jbc.M806538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Choi HY, Li WP, Xu F, Herz J. LRP1 controls cPLA2 phosphorylation, ABCA1 expression and cellular cholesterol export. PLoS One. 2009;4:e6853. doi: 10.1371/journal.pone.0006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, et al. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 20.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–40. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akkawi S, Nassar T, Tarshis M, Cines DB, Higazi AA. LRP and {alpha}vbeta3 mediate tPA activation of smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H1351–9. doi: 10.1152/ajpheart.01042.2005. [DOI] [PubMed] [Google Scholar]
- 22.Bu G, Maksymovitch EA, Geuze H, Schwartz AL. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J Biol Chem. 1994;269:29874–82. [PubMed] [Google Scholar]
- 23.Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J Biol Chem. 1994;269:18521–8. [PubMed] [Google Scholar]
- 24.May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–83. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olney JW, Wozniak DF, Farber NB. Excitotoxic neurodegeneration in Alzheimer disease. New hypothesis and new therapeutic strategies. Arch Neurol. 1997;54:1234–40. doi: 10.1001/archneur.1997.00550220042012. [DOI] [PubMed] [Google Scholar]
- 26.Olney JW, Wozniak DF, Farber NB. Glumate receptor dysfunction and Alzheimer’s disease. Restor Neurol Neurosci. 1998;13:75–83. [PubMed] [Google Scholar]
- 27.Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2000;97:11551–6. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu Z, Strickland DK, Hyman BT, Rebeck GW. alpha 2-Macroglobulin exposure reduces calcium responses to N-methyl-D-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J Biol Chem. 2002;277:14458–66. doi: 10.1074/jbc.M112066200. [DOI] [PubMed] [Google Scholar]
- 29.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 30.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–86. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel M, Morrow J, Maxfield FR, Strickland DK, Greenberg S, Tabas I. The cytoplasmic domain of the low density lipoprotein (LDL) receptor-related protein, but not that of the LDL receptor, triggers phagocytosis. J Biol Chem. 2003;278:44799–807. doi: 10.1074/jbc.M308982200. [DOI] [PubMed] [Google Scholar]
- 32.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Quinn KA, Pye VJ, Dai YP, Chesterman CN, Owensby DA. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP) Exp Cell Res. 1999;251:433–41. doi: 10.1006/excr.1999.4590. [DOI] [PubMed] [Google Scholar]
- 34.Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA. Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997;272:23946–51. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- 35.von Arnim CA, Kinoshita A, Peltan ID, Tangredi MM, Herl L, Lee BM, et al. The low density lipoprotein receptor-related protein (LRP) is a novel beta-secretase (BACE1) substrate. J Biol Chem. 2005;280:17777–85. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- 36.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–43. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 37.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–8. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 38.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155:755–62. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–40. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 41.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–99. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niwa M, Sidrauski C, Kaufman RJ, Walter P. A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell. 1999;99:691–702. doi: 10.1016/s0092-8674(00)81667-0. [DOI] [PubMed] [Google Scholar]
- 43.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–56. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1:ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–94. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 46.Barnes H, Larsen B, Tyers M, van Der Geer P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J Biol Chem. 2001;276:19119–25. doi: 10.1074/jbc.M011437200. [DOI] [PubMed] [Google Scholar]
- 47.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, et al. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–24. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 48.Su HP, Nakada-Tsukui K, Tosello-Trampont AC, Li Y, Bu G, Henson PM, et al. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP) J Biol Chem. 2002;277:11772–9. doi: 10.1074/jbc.M109336200. [DOI] [PubMed] [Google Scholar]
- 49.Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–60. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 50.Chang Y, Tesco G, Jeong WJ, Lindsley L, Eckman EA, Eckman CB, et al. Generation of the beta-amyloid peptide and the amyloid precursor protein C-terminal fragment gamma are potentiated by FE65L1. J Biol Chem. 2003;278:51100–7. doi: 10.1074/jbc.M309561200. [DOI] [PubMed] [Google Scholar]
- 51.Kinoshita A, Shah T, Tangredi MM, Strickland DK, Hyman BT. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J Biol Chem. 2003;278:41182–8. doi: 10.1074/jbc.M306403200. [DOI] [PubMed] [Google Scholar]
- 52.Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 53.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–20. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 54.Roebroek AJ, Reekmans S, Lauwers A, Feyaerts N, Smeijers L, Hartmann D. Mutant Lrp1 knock-in mice generated by recombinase-mediated cassette exchange reveal differential importance of the NPXY motifs in the intracellular domain of LRP1 for normal fetal development. Mol Cell Biol. 2006;26:605–16. doi: 10.1128/MCB.26.2.605-616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


