Abstract
In contrast to the systemic vasculature, where hypoxia causes vasodilation, pulmonary arteries constrict in response to hypoxia. The mechanisms underlying this unique response have been the subject of investigation for over 50 years, and still remain a topic of great debate. Over the last 20 years, there has emerged a general consensus that both increases in intracellular calcium concentration and changes in reactive oxygen species (ROS) generation play key roles in the pulmonary vascular response to hypoxia. Controversy exists, however, regarding whether ROS increase or decrease during hypoxia, the source of ROS, and the mechanisms by which changes in ROS might impact intracellular calcium, and vice versa. This review will discuss the mechanisms regulating [Ca2+]i and ROS in PASMCs, and the interaction between ROS and Ca2+ signaling during exposure to acute hypoxia.
1. Introduction
A unique aspect of the pulmonary circulation is the pressor response to hypoxia. While the systemic circulation dilates in response to decreased oxygen (O2) concentrations in order to increase blood flow and O2 delivery to tissues, alveolar hypoxia rapidly increases pulmonary vascular resistance beginning within 1–2 minutes after a drop in O2 levels. Vasoconstriction is maintained for the duration of the hypoxic exposure and rapidly reverses with reoxygenation. This mechanism is thought to divert blood flow from regions of the lung where ventilation is poor in an effort to maintain arterial O2 tension. However, complications arise when alveolar hypoxia is global and prolonged, as can occur with residence at high altitude or with many chronic lung diseases, resulting in the development of pulmonary hypertension.
The exact mechanism underlying the generation of hypoxia-induced increases in pulmonary vascular tone is unknown. Over the past 50 years, numerous studies have sought to determine whether the hypoxic response is localized to pulmonary arterial smooth muscle cells (PASMCs), or whether signals from the circulation or neighboring cells, (i.e, the endothelium) are required. It is now generally well accepted that while the endothelium can influence the magnitude of the response, the PASMCs themselves sense and respond to the hypoxic stimulus.
The smooth muscle cells surrounding the pulmonary arteries provide structural integrity for the vascular wall and are vital for precise regulation of vessel tone, pulmonary arterial pressure and pulmonary vascular resistance. The control of PASMC function is complex, and it has become increasing well recognized that increases in intracellular calcium concentration ([Ca2+]i) and changes in reactive oxygen species (ROS) generation play key roles in hypoxic pulmonary vasoconstriction (HPV). This review will focus on the interaction between ROS and Ca2+ signaling in PASMCs during exposure to acute hypoxia.
2. Ca2+ signaling in PASMCs
2.1. Mechanisms regulating [Ca2+]i in PASMCs
[Ca2+]i is one of the most important regulators of pulmonary vascular function. In PASMCs, changes in [Ca2+]i modulate contraction(Buckley et al., 1995; Busse and Mulsch, 1990; Emori et al., 1989; Kohno et al., 1992; Martin and Michaelis, 1990; Whorton et al., 1984), cell proliferation and growth by facilitating progression through the cell cycle(Mogami and Kojima, 1993; Rodman et al., 2005; Shukla et al., 2005) and the activation of genes via regulation of Ca2+-sensitive transcription factors(Altura et al., 2003; Ginty et al., 1991; Hardingham et al., 1998; Pribnow et al., 1992; Rothman et al., 1994). That Ca2+ signaling plays a crucial role in a wide array of cell functions underscores the importance of understanding the mechanisms regulating Ca2+ homeostasis under both physiologic and pathophysiologic conditions.
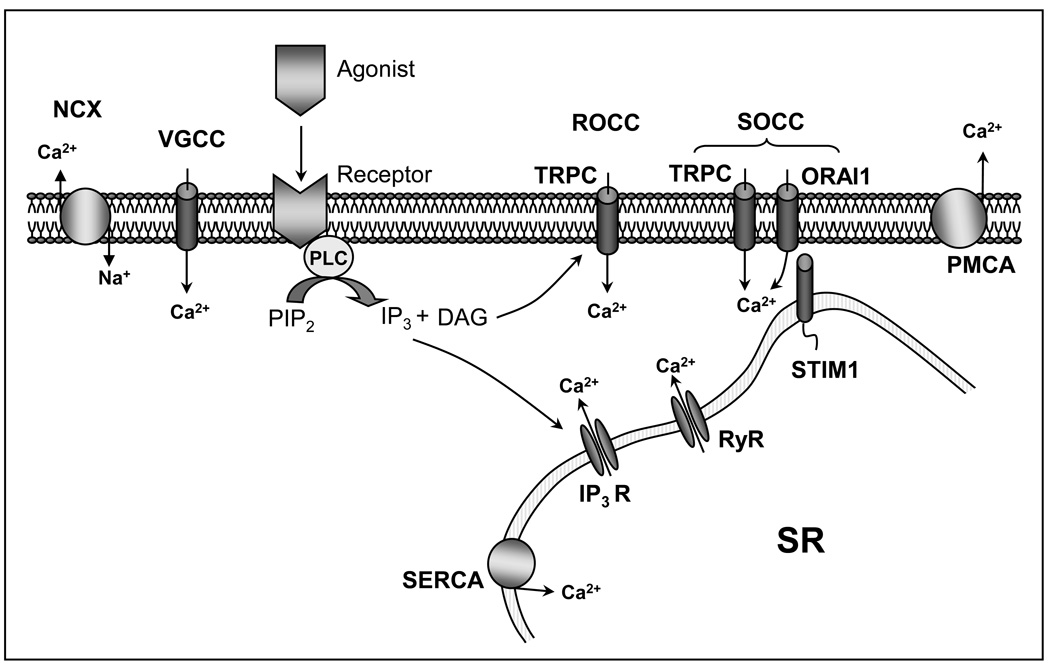
PASMCs express several Ca2+ influx/efflux pathways that may contribute to Ca2+ handling and regulation (Figure 1). Plasmalemmal Ca2+ channels conduct extracellular Ca2+ into the cells, while receptors on the sarcoplasmic/endoplasmic reticulum mediate Ca2+ release from internal stores into the cytoplasm. Mechanisms for removing cytoplasmic Ca2+ from the cell include the plasma membrane Ca2+-ATPase (PMCA) and Na+/Ca2+ exchange (NCX), the activities of which help to maintain low basal [Ca2+]i.
Figure 1.
Schematic illustrating calcium handling pathways in pulmonary arterial smooth muscle cells. PMCA= plasma membrane Ca2+-ATPase; NCX= Na+/Ca2+ exchange
PASMCs are excitable cells, containing both L-type(Amenta et al., 1998; Fan et al., 2002; Golovina et al., 2001; Resnik et al., 2006; Ricci et al., 2000; Wang et al., 2005; Yuan, 1995) and T-Type(Rodman et al., 2005) voltage-gated Ca2+ channels (VGCC). As suggested by their name, VGCC activity is controlled in large part by membrane potential (Em). L-type, or long-lasting, Ca2+ channels exhibit sensitivity to dihydropyridines and are activated by large depolarizations at potentials between −20 and +20 mV. At the normal resting Em, which in PASMCs appears to be controlled primarily by voltage-gated K+ (KV) channels, little activation of these channels occurs, resulting in low basal PASMC [Ca2+]i and tone(Shimoda et al., 2000). However, when Kv channels are inhibited, the consequent depolarization activates VGCCs and increases [Ca2+]i(Yuan, 1995). T-type, or transient, Ca2+ channels have also been identified in PASMCs(Muramatsu et al., 1997; Rodman et al., 2005). T-type channels require small depolarizations in order to activate, are only activated from very negative holding potentials, conduct inward currents at membrane potentials between −70 and 40 mV and typically inactivate rapidly at physiological membrane potentials(Kuga et al., 1990; Mishra and Hermsmeyer, 1994; Wu et al., 2003).
Numerous studies have demonstrated that Ca2+ influx into PASMCs could occur via Ca2+ permeable channels other than VGCCs. In particular, nonselective cation channels (NSCCs) provide an important Ca2+ entry pathway. Based on their proposed modes of activation, these influx pathways were separated into 2 main categories: receptor-operated Ca2+ channels (ROCC), which are activated by ligand binding to membrane receptors, and store-operated Ca2+ channels (SOCCs), which are activated by depletion of intracellular stores(Putney, 1986, 1990). While the activity of VGCCs is primarily driven by depolarization, the activation of NSCCs is relatively voltage-independent. Several labs have demonstrated that Ca2+ influx through NSCCs occurs in PASMCs(Golovina et al., 2001; Snetkov et al., 2003; Wang et al., 2004a), and contributes to PASMC contraction and growth(Golovina et al., 2001; McDaniel et al., 2001; Sweeney et al., 2002; Weigand et al., 2006; Yu et al., 2003); however, these channels do not appear to be active under basal conditions(Lin et al., 2004; Wang et al., 2006; Wang et al., 2004b). The exact molecular identity of the proteins encoding Ca2+-permeable NSCCs remains unclear, although most evidence suggests that these channels may be composed of mammalian homologs of transient receptor potential (TRP) proteins, perhaps in combination with the recently identified ORAI1 and stromal interaction molecule (STIM) proteins(Brough et al., 2001; Cioffi et al., 2003; Lin et al., 2004; Lu et al., 2009; Lu et al., 2008; Ng and Gurney, 2001; Sweeney et al., 2002; Wang et al., 2004a; Wang et al., 2006; Yang et al., 2006; Yu et al., 2003).
There are many sites within PASMCs that can store Ca2+, including mitochondria, lysosomes, and the sarcoplasmic (SR). Of these, the SR is the major contributor in regulating cytosolic [Ca2+](Marin et al., 1999; Pozzan et al., 1994) through release of Ca2+ from channels on the SR membrane or uptake of Ca2+ into the stores via the sarcoplasmic/endoplasmic Ca2+-ATPase (SERCA) pumps(Boittin et al., 1999; Laporte et al., 2004; Wellman and Nelson, 2003). The channels mediating Ca2+ release from intracellular stores can be divided into two categories: ryanodine receptors (RyR), which can be blocked by ryanodine and are activated by caffeine, and inositol triphosphate (IP3) receptors (IP3R), which can be activated by IP3 following agonist stimulation. RyR also contain Ca2+ binding sites, leading to Ca2+-induced Ca2+ release, wgich can be triggered by an increase in cytosolic [Ca2+] due to either IP3-induced release or Ca2+ influx from extracellular sources(Zucchi and Ronca-Testoni, 1997) . Similarly, activation of IP3R by Ca2+ is facilitated by binding of IP3. Three subtypes of RyR have been identified in PASMCs(Yang et al., 2005; Zhang et al., 2003; Zheng et al., 2005), along with three subtypes of IP3 receptors(Zheng et al., 2004). Whether RyRs and IP3Rs access Ca2+ stores that are separate and distinct(Flynn et al., 2001) or are the same pools(Pacaud and Loirand, 1995) remains in debate, and may depend on vascular bed(Janiak et al., 2001) or whether cells are freshly isolated or cultured(Ng et al., 2008).
2.2. Mechanisms that control [Ca2+]i in response to hypoxia
The main site of vasoconstriction in response to hypoxia appears to be the small diameter, resistance arteries, since these vessels exhibit a robust contraction in response to reduced O2 tensions whereas conduit arteries exhibit relaxation or minimal contraction(Dawson et al., 1978; Fishman, 1976; Madden et al., 1985; Nagasaka et al., 1984; Staub, 1985). In PASMCs isolated from resistance arteries, abundant data has been generated in a variety of species demonstrating that acute exposure to hypoxia (minutes to a few hours) is associated with an increase in [Ca2+]i that is largely dependent on Ca2+ entry from the extracellular compartment(Cornfield et al., 1994; Hong et al., 2004; Kang et al., 2002; Salvaterra and Goldman, 1993; Sham et al., 2000; Urena et al., 1996; Vadula et al., 1993; Wang et al., 2005). Consistent with a role for Ca2+ influx, perfusion with Ca2+-free solution reduced HPV to a small transient constriction or abolished it altogether(Dipp et al., 2001; Farrukh and Michael, 1992; Jin et al., 1992; Liu et al., 2001; Robertson et al., 2000; Weigand et al., 2005).
Pharmacologic data indicates that L-type Ca2+ channels contribute significantly to the increase in PASMC [Ca2+]i induced by acute reductions in O2 tension(Bakhramov et al., 1998; Cornfield et al., 1994; Urena et al., 1996; Wang et al., 2005; Yuan, 1995) and to HPV(McMurtry et al., 1976; Redding et al., 1984; Simonneau et al., 1981; Stanbrook et al., 1984; Weigand et al., 2005). However, despite a strong case for VGCC being a main contributor to the Ca2+ influx due to hypoxia, recent studies indicate the presence of VGCC antagonists reduced, but did not abolish, hypoxia-induced increases in [Ca2+]i and Ca2+ entry in PASMCs(Ng et al., 2005; Wang et al., 2005). While experiments examining the effect of specific inhibitors of T-type Ca2+ channels, such as mibefradil, will need to be performed to definitively determine their role in the maintenance of [Ca2+]i during hypoxia, mounting evidence indicates that hypoxia-induced increases in [Ca2+]i can be abolished by antagonists of NSCCs(Ng et al., 2005; Wang et al., 2005; Weissmann et al., 2006). Since release from intracellular stores appears to be a component of the hypoxia-induced increase in [Ca2+]i(Dipp et al., 2001; Jabr et al., 1997; Kang et al., 2002; Zheng et al., 2004; Zheng et al., 2005), a likely scenario is that Ca2+ entry via NSCCs is triggered by store-depletion. Store release in PASMCs in response to hypoxia appears to occur, at least in part, via activation of RyRs(Dipp et al., 2001; Zheng et al., 2005). Blockade of RyRs or depletion of the SR with thapsigargin or cyclopiazonic acid inhibited hypoxia-induced increases in PASMCs [Ca2+]i(Vadula et al., 1993; Zheng et al., 2005) and HPV(Dipp et al., 2001; Du et al., 2005; Leach et al., 1994; Liu et al., 2001; Morio and McMurtry, 2002; Robertson et al., 2000; Zheng et al., 2005). These results indicate that hypoxia induces an initial increase in [Ca2+]i due to Ca2+ release from intracellular stores, with a sustained increase in [Ca2+]i due to Ca2+ influx, likely through both NSCCs and VGCCs. Interestingly, blockade of NSCCs completely prevents the increase in [Ca2+]i induced by hypoxia, whereas blockade of VGCCs only partially inhibited the response(Wang et al., 2005), suggesting the possibility that activation of NSCCs not only leads to Ca2+ influx, but also may provide a depolarizing stimulus that can subsequently contribute to the activation of VGCCs.
3. ROS signaling in PASMCs
3.1. Types of ROS and mechanisms of ROS production
It is now recognized that another major component of PASMC signaling is the generation of ROS; O2-derived small molecules, including the O2 radicals superoxide and hydroxyl, and nonradicals that are either oxidizing agents or are easily converted into radicals, such as ozone (O3), singlet O2, and hydrogen peroxide (H2O2). ROS avidly interact with a variety of molecules and have been identified as major contributors to cellular damage, aging and host defense. It has also become clear that ROS play an important role in reversible regulatory processes. For example, H2O2 influences pulmonary vasomotor tone(Jin and Rhoades, 1997; Rhoades et al., 1990) and modifies the function of various proteins including transcription factors, kinases, and phosphatases(Droge, 2002; Franklin et al., 2006; Ichiki et al., 2003; Jin et al., 2000; McCubrey et al., 2006; Rao, 2000; Schmidt et al., 1995).
ROS are produced from a cascade of reactions that starts with the production of superoxide, generated by mitochondrial respiration, xanthine oxidase, uncoupled NO synthase, or via reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase (Nox). Superoxide is highly reactive and rapidly dismutates to H2O2, either spontaneously or catalyzed by superoxide dismutase. Superoxide can also react with nitric oxide to form peroxynitrite, or the iron-catalyzed Fenton reaction leading to the generation of hydroxyl radicals. Of the mechanisms known to produce ROS, evidence suggests that during hypoxia, the mitochondria and Nox appear to be the predominate sources in PASMCs.
3.1.a. Mitochondria
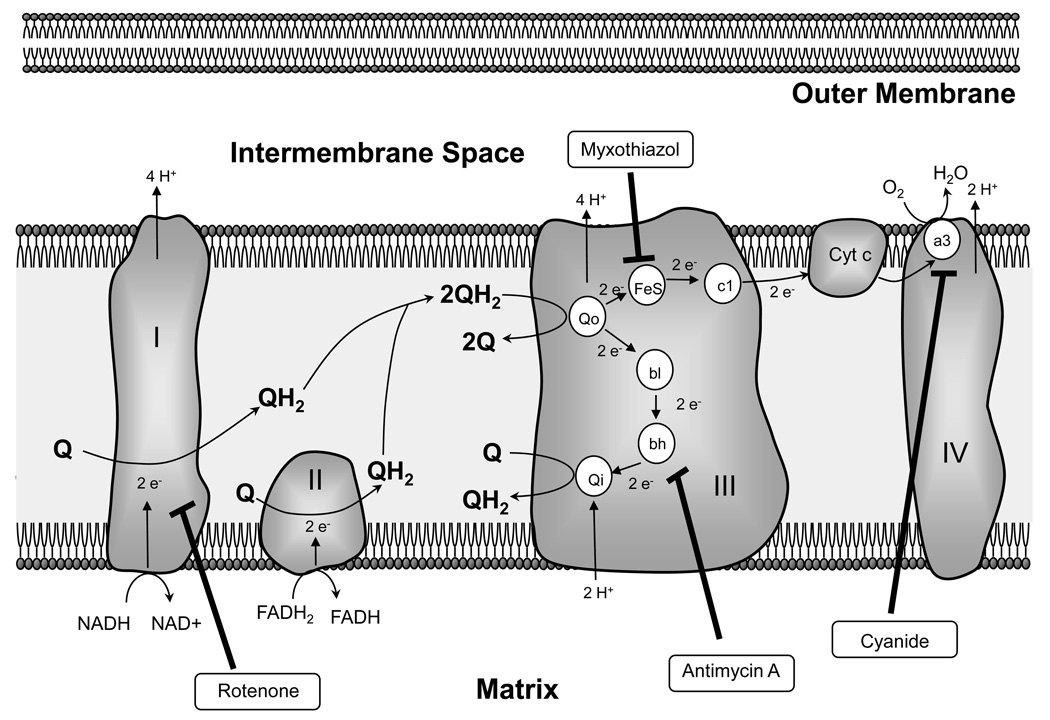
In mitochondria, a series of redox reactions occurs during respiration along which electrons are transferred from a donor molecule (NADH or QH2) to O2, concluding at complex IV (cytochrome oxidase), where molecular O2 is reduced to water (Figure 2). Formation of superoxide occurs upstream of complex IV, primarily by auto-oxidation of flavins in complex I, where superoxide can enter the matrix, and at complex III where superoxide is formed via the Q-cycle. From complex I, two electrons are carried by ubiquinol (QH2) to complex III (coenzyme Q: cytochrome c-oxidoreductase). Another pair of electrons is donated to ubiquinone (Q) at complex II, and also carried by ubiquinol to complex III, where one electron is transferred at the Qo site to the Rieske iron–sulphur protein (Fe-S) and subsequently cytochrome c (Cyt c), while the remaining electron is donated to cytochrome b (bl and bh), followed by transfer to ubiquinone at the Qo site. Cytochrome c then donates the electrons to molecular O2 in complex IV, producing water. Electron leakage can occur at both the Qo and Qi sites of complex III, resulting in production of superoxide that enters either the mitochondrial intermembrane space or the matrix, respectively(Boveris, 1977; Turrens, 2003; Turrens et al., 1985). Once in the matrix, superoxide is converted first to H2O2, by mitochondrial superoxide dismutase, and then to water by glutathione peroxidase (Gpx1). In the inter-membrane space, superoxide can undergo multiple fates; it can be degraded by CuZnSOD, can be scavenged by cytochrome c or can enter the cytosol via voltage-dependent anion channels(Han et al., 2003; Waypa et al., 2001). The relative contribution of complex I and III to superoxide generation appears to be cell and/or tissue specific and dependent on the respiratory status. In PASMCs, rotenone, a complex I inhibitor, and myxothiazol and antimycin A, which prevent production of radicals by inhibiting complex III at the pre- and post-ubisemiquinone sites, respectively, decreased basal ROS production(Archer et al., 1993; Michelakis et al., 2002b; Paky et al., 1993; Waypa et al., 2001), suggesting that constitutive superoxide generation is occurring at both sites.
Figure 2.
Depiction of the mitochondrial electron transport chain.
3.1.b. NADPH oxidase
A second important source of ROS is via Nox. Originally described in phagocytes, five Nox isoforms (Nox1-5) have now been identified. Nox produces superoxide or H2O2 via electron transfer from cytosolic NADPH to FAD, and subsequently to molecular O2 to form superoxide, which is then dismutated to H2O2. In vascular smooth muscle, Nox 1, 2, 4 and 5 are expressed and contribute to generation of ROS, although the exact distribution of isoforms varies with vascular bed and species(Fulton, 2009; Lassegue and Griendling, 2010). Nox1 and Nox2, or gp91 phox, are membrane bound and require the assembly of additional membrane associated (p22phox) or cytosolic (Rac 1 and 2, p47phox, and p67phox) subunits for activation(Lambeth et al., 2007) (Table 1), and have been implicated in a variety of cardiovascular diseases(Lassegue and Griendling, 2010). Nox4, also abundant in pulmonary vascular smooth muscle(Ismail et al., 2009; Mittal et al., 2007; Rathore et al., 2008), differs from Nox1 and Nox2 in that it exhibits constitutive activity, does not appear to require additional subunits other than p22phox(Lassegue and Griendling, 2010) and appears to produce primarily H2O2 rather than superoxide(Serrander et al., 2007a), although the mechanism by which this occurs is unclear. In contrast to the other Nox isoforms, Nox5 is not expressed in rodents, does not require p22phox or the cytosolic subunits and is the only isoform that is directly activated by Ca2+ due to four canonical EF-hands in the N-terminus(Jagnandan et al., 2007; Serrander et al., 2007b). In addition, the NADPH-binding domain of Nox5 contains a calmodulin binding site(Tirone and Cox, 2007), and PKC-mediated phosphorylation of Ser/Thr residues in the FAD-binding domain may increase Ca2+-sensitivity(Jagnandan et al., 2007).
Table 1.
Localization, function and activation of NADPH oxidase (Nox) isoforms.
| Isoforms | Tissue expression | Subunits | Activation | Functions | Refs |
|---|---|---|---|---|---|
| Nox1 | colon epithelium, vascular smooth muscle, endothelial cells, kidney | NoxO1 NoxA1 p22phox Rac | PKC, PLD, growth factors | proliferation, migration, apoptosis | (Clempus and Griendling, 2006; Hoidal et al., 2003; Mittal et al., 2007) |
| Nox2 | phagocytes, endothelial cells, pulmonary arteries, heart | p47phox p67phox p22phox Rac | PKC | immunity, HPV | (Clempus and Griendling, 2006; Lassegue and Griendling, 2010; Liu et al., 2006; Mittal et al., 2007) |
| Nox3 | inner ear non vascular | p22phox NoxO1 | constitutive | (Hoidal et al., 2003; Lassegue and Griendling, 2010) | |
| Nox4 | vascular and airway smooth muscle, kidney | p22phox | constitutive, Ang II, TGFβ, hypoxia, | proliferation, migration | (Clempus and Griendling, 2006; Liu et al., 2006; Mittal et al., 2007) |
| Nox5 | spleen, kidney, testes, lung, cancers not found in rodents | p22phox | Ca2+ | contraction, cell growth | (Fulton, 2009) |
3.2. ROS generation in response to hypoxia
Numerous investigators have demonstrated changes in ROS production in PASMCs during hypoxia, suggesting that ROS may be key mediators in HPV; however, controversy exists concerning whether ROS increase or decrease during hypoxia. Since O2 is the major substrate for ROS formation and hyperoxia increased ROS generation(Brueckl et al., 2006; Chandel and Budinger, 2007; Freeman and Crapo, 1981), it was reasonable to hypothesize that, due to substrate limitation, hypoxia would decrease ROS. Indeed, several reports indicated that this was the case, contending that either mitochondrial production of ROS was impaired(Archer and Michelakis, 2002; Archer et al., 1993; Michelakis et al., 2002a), or that there was a decrease in ROS generation by microsomal NADH oxidoreductase(Mohazzab et al., 1995; Mohazzab and Wolin, 1994a, b). Reports of hypoxia-induced decreases in ROS production were countered, however, by numerous labs which consistently showed increased ROS generation in response to hypoxia(Jernigan et al., 2004; Killilea et al., 2000; Leach et al., 2001; Liu et al., 2003; Marshall et al., 1996; Paddenberg et al., 2003; Rathore et al., 2006; Wang et al., 2007; Waypa et al., 2001; Waypa et al., 2006; Waypa and Schumacker, 2002, 2005; Weissmann et al., 2003). The mechanism by which hypoxia might induce an increase in mitochondrial ROS production remains unresolved; however, it has been hypothesized that decreased interaction between O2 and protein or lipids at complex III could prolong the lifetime of ubisemiquinone or that hypoxia might increase the access of O2 to the semiquinone radical at complex III. In both cases, O2 levels cause alterations in the lipid–protein structure such that electron transfer from ubisemiquinone to O2 increases, despite decreased availability of oxygen.
The reasons underlying discrepant reports regarding the effects of hypoxia on ROS remain incompletely understood. Some investigators have attempted to use pharmacological inhibitors of mitochondrial-derived ROS to resolve the controversy, with varying results. Most agree that rotenone blocks the hypoxia-induced changes in ROS, regardless of whether the response was an increase(Rathore et al., 2006; Wang et al., 2007; Waypa et al., 2001; Waypa et al., 2006; Waypa and Schumacker, 2002; Weissmann et al., 2003) or decrease(Archer et al., 1993; Michelakis et al., 2002a). Myxothiazol also blocked the acute hypoxic responses in PASMCs(Archer et al., 1993; Michelakis et al., 2002a; Wang et al., 2007; Waypa et al., 2001; Waypa et al., 2006; Waypa and Schumacker, 2002; Weissmann et al., 2003), although more distal electron transport chain inhibitors, such as antimycin-A, had no effect. These data suggest that the mitochondrial subunits prior to the ubisemiquinone site of complex III act to increase generation of ROS in PASMCs during hypoxia.
In addition to mitochondria, studies have also indicated a role for Nox mediated generation of ROS during hypoxia. Acute hypoxia increased Nox activity and translocation of p47phox to the plasma membrane in pulmonary arteries(Rathore et al., 2008). Consistent with these findings, the putative Nox inhibitors, apocynin and diphenyleneiodonium, blocked the hypoxic increase in ROS in PASMCs(Marshall et al., 1996; Rathore et al., 2008); however, a caveat is that both of these inhibitors can have nonspecific effects, as diphenyleneiodonium is a broad spectrum inhibitor of electron transporters(Bedard and Krause, 2007) and apocynin is able to act as an antioxidant independent of its effects on Nox(Heumuller et al., 2008). Nonetheless, if activation of Nox did occur in these studies, it is likely this was secondary to hypoxic activation of PKC, since inhibition of PKCε blocked the hypoxia-induced Nox activity and increase in ROS. Interestingly, rotenone and myxothiazol both attenuated the hypoxia-induced activation of PKCε activity(Rathore et al., 2006), suggesting a link between mitochondrial and Nox generated ROS.
To complement pharmacologic studies, several investigators have used genetic alterations to further explore the role of mitochondrial proteins in the hypoxic ROS response. Overexpression of mitochondrial catalase and Gpx1, which would be expected to augment ROS scavenging, attenuated hypoxia-induced increases in ROS(Wang et al., 2007; Waypa et al., 2006), whereas Gpx1 gene deletion to prevent ROS removal has the opposite effect(Wang et al., 2007). Furthermore, in nonvascular cells, silencing subunits of the mitochondrial electron transport chain reduced hypoxia-induced ROS signaling(Guzy et al., 2005; Mansfield et al., 2005), suggesting that the mitochondria are a primary source for elevating ROS levels. Interestingly, lungs from p47phox knockout mice increased ROS in response to hypoxia, whereas a reduction in ROS was observed in wild-type mice, suggesting that reduced production of extracellular ROS derived from Nox might mask an increase in intracellular mitochondrial-derived ROS under some experimental conditions(Weissmann et al., 2006). It is also possible that within the cell, ROS generation is a localized event and that compartmentalization of either ROS or the indicator could produce differential results. With respect to the indicator, experiments using recently developed ratiometric, redox-sensitive fluorescence resonance energy transfer (FRET) probes indicated that hypoxia-induced an increase in ROS which was inhibited by catalase and antioxidants(Waypa et al., 2006; Waypa et al., 2010). Consistent with the possibility of compartmentalized ROS production, recent reports using indicators targeted to the mitochondrial matrix, intermembrane space and cytoplasm show that ROS levels vary within these compartments, with hypoxia increasing ROS in the cytosol and intermembrane space but decreasing ROS in the mitochondrial matrix(Waypa et al., 2010). These results suggest the possibility that the differences reported regarding the effects of hypoxia on ROS formation in PASMCs could reflect a lack of differentiation between extracellular and intracellular sources of ROS or measurements of intracellular ROS within different sub-compartments of the cell. Overall, however, it would appear that the predominance of evidence argues for increased cytosolic ROS during hypoxia, with a fall in ROS occurring extracellularly and within the mitochondrial matrix.
4. Interplay between ROS and [Ca2+]i
4.1. ROS modulate [Ca2+]i
Substantial evidence has accumulated demonstrating that ROS can influence vascular smooth muscle [Ca2+]i. With respect to hypoxia and PASMCs, in experiments where a ratiometric ROS probe was used in conjunction with a FRET-based ratiometric Ca2+ sensitive probe, the increase ROS and [Ca2+]i appeared to occur virtually simultaneously(Waypa et al., 2006). Moreover, catalase, antioxidants and pharmacological inhibitors of the potential sources of ROS, including rotenone and myxothiazol, reduced or prevented the hypoxia-induced elevation in [Ca2+]i in PASMC(Leach et al., 2001; Rathore et al., 2006; Wang et al., 2007; Waypa et al., 2006). In contrast, antimycin A or cyanide, which inhibits Complex IV, had no effect on or enhanced the [Ca2+]i response to hypoxia(Leach et al., 2001; Rathore et al., 2006; Wang et al., 2007; Waypa et al., 2006; Waypa and Schumacker, 2002). Consistent with these results, HPV was prevented in the presence of rotenone or myxothiazol(Archer et al., 1993; Leach et al., 2001; Rounds and McMurtry, 1981; Waypa et al., 2001; Weissmann et al., 2003), but not by cyanide(Archer et al., 1993; Leach et al., 2001; Waypa et al., 2001; Weissmann et al., 2003).
In the case of studies where ROS was found to decrease during hypoxia, it has been proposed that reduced ROS leads to inhibition of K+ channels and depolarization, with subsequent activation of VGCCs and increased [Ca2+]i(Archer and Michelakis, 2002; Archer et al., 2008; Archer et al., 1993; Michelakis et al., 2002a; Michelakis et al., 2002b; Michelakis et al., 2004; Reeve et al., 1995). On the other hand, ROS have been shown to induce Ca2+ release from ryanodine-sensitive stores(Lin et al., 2007; Pourmahram et al., 2008; Suzuki et al., 1998) either via action on redox-sensitive cysteine thiols in RyRs(Eu et al., 1999) or stimulation of RyRs via generation of the β-NAD+ metabolite, cyclic ADP-ribose(Dipp and Evans, 2001; Evans and Dipp, 2002). Release of SR Ca2+ could then serve to increase Ca2+ entry via store-dependent NSCCs(Wang et al., 2005) and/or activation of VGCCs, secondary to inhibition of K+ channels and depolarization(Post et al., 1995). Elevated H2O2 levels could also initiate an increase in [Ca2+]i through the activation of phospholipase C(Gonzalez-Pacheco et al., 2002), resulting in the production of both IP3 and diacylglycerol. While IP3 generation would lead to Ca2+ release from internal stores, diacylglycerol could serve to activate NSCCs, resulting in Ca2+ influx, as well as activation of VGCCs secondary to depolarization(Weissmann et al., 2006). Superoxide and peroxynitrite have also been shown to inhibit SERCA in systemic vascular smooth muscle(Grover et al., 2003; Suzuki and Ford, 1991), which would serve to elevate cytosolic [Ca2+] by decreasing Ca2+ uptake into the SR; however, whether this occurs in PASMCs has not been examined.
4.2. [Ca2+]i influences ROS generation
While several studies provide evidence that ROS alter [Ca2+]i, as described in the preceding section, several reports also indicate that changes in [Ca2+]i may impact ROS formation. With respect to Nox isoforms, only Nox5 is directly Ca2+-sensitive by virtue of four canonical N-terminal EF-hands(Fulton, 2009). Nox5 also contains a binding site for calmodulin in the NADPH-binding domain and PKC-mediated phosphorylation of Ser/Thr residues in the FAD-binding domain may increase the Ca2+-sensitivity of Nox5(Fulton, 2009). In contrast, [Ca2+]i may control the activity of other Nox isoforms by regulating the cytosolic subunits. For example, under certain circumstances, Nox-dependent generation of ROS requires serine phosphorylation of p47phox, which occurs in response to activation of protein kinase C(Lassegue and Griendling, 2010). Although the specific PKC isozyme(s) responsible for mediating phosphorylation of p47phox has not been identified, it is possible that conventional, or Ca2+-sensitive, PKC isozymes might be involved. On the other hand, recent evidence suggests that Nox activation in PASMCs during hypoxia is mediated by PKCs(Rathore et al., 2008), which is not Ca2+-sensitive; however, in this case ROS production was only measured during brief hypoxic challenge, and it is not clear whether longer duration of hypoxia might lead to Ca2+-dependent activation of Nox and generation of ROS.
5. Conclusions
Over the last two decades, extensive work has been performed aimed at identifying the role of ROS in the pulmonary vascular response to hypoxia. While abundant evidence now indicates that ROS are an essential factor in HPV, there continues to be some debate regarding the direction of the change in ROS with hypoxia, and how ROS might interact with other signaling pathways, namely [Ca2+]i, to produce HPV. The preponderance of evidence appears to indicate that hypoxia induces an increase in ROS formation, likely mitochondrial in origin, which can trigger increases in [Ca2+]i and activation of PKC, perhaps further enhancing ROS production through activation of Nox. It also seems increasingly likely that ROS signaling, like that for Ca2+, is highly compartmentalized within the cell, perhaps within microdomains where mitochondria, the SR and/or plasma membrane channels are in close approximation. The consequence of this type of compartmentalization on PASMC function during acute hypoxia remains to be fully resolved.
Acknowledgements
Due to space restrictions, it was not possible to quote all of the excellent studies that have been published with respect to the research described in this review; our apologies to those whose studies were not cited. This work was supported by the National Institutes of Health (HL 67191).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altura BM, Kostellow AB, Zhang A, Li W, Morrill GA, Gupta RK, Altura BT. Expression of the nuclear factor-kB and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: possible links to hypertension, atherogenesis, and stroke. Am J Hypertens. 2003;16:701–707. doi: 10.1016/s0895-7061(03)00987-7. [DOI] [PubMed] [Google Scholar]
- 2.Amenta F, Bisetti A, Bronzetti E, Coppola L, Felici L, Ferrante F, Mariotta S, Ricci A. Density and localization of calcium channels of the L-type in human pulmonary artery. Clin Exp Hypertens. 1998;20:389–402. doi: 10.3109/10641969809053220. [DOI] [PubMed] [Google Scholar]
- 3.Archer S, Michelakis E. The mechanism(s) of hypoxic pulmonary vasoconstriction: potassium channels, redox O2 sensors, and controversies. News Physiol Sci. 2002;17:131–137. doi: 10.1152/nips.01388.2002. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 6.Bakhramov A, Evans AM, Kozlowski RZ. Differential effects of hypoxia on the intracellular Ca2+ concentration of myocytes isolated from different regions of the rat pulmonary arterial tree. Exp Physiol. 1998;83:337–347. doi: 10.1113/expphysiol.1998.sp004117. [DOI] [PubMed] [Google Scholar]
- 7.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am J Physiol. 1999;277:C139–C151. doi: 10.1152/ajpcell.1999.277.1.C139. [DOI] [PubMed] [Google Scholar]
- 9.Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- 10.Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. Faseb J. 2001;15:1727–1738. [PubMed] [Google Scholar]
- 11.Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–463. doi: 10.1165/rcmb.2005-0223OC. [DOI] [PubMed] [Google Scholar]
- 12.Buckley BJ, Mirza Z, Whorton AR. Regulation of Ca2+-dependent nitric oxide synthase in bovine aortic endothelial cells. Am J Physiol. 1995;269:C757–C765. doi: 10.1152/ajpcell.1995.269.3.C757. [DOI] [PubMed] [Google Scholar]
- 13.Busse R, Mulsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990;265:133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- 14.Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. 2007;42:165–174. doi: 10.1016/j.freeradbiomed.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Cioffi DL, Wu S, Stevens T. On the endothelial cell ISOC. Cell Calcium. 2003;33:323–336. doi: 10.1016/s0143-4160(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 16.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornfield DN, Stevens T, McMurtry IF, Abman SH, Rodman DM. Acute hypoxia causes membrane depolarization and calcium influx in fetal pulmonary artery smooth muscle cells. Am J Physiol. 1994;266:L469–L475. doi: 10.1152/ajplung.1994.266.4.L469. [DOI] [PubMed] [Google Scholar]
- 18.Dawson CA, Grimm DJ, Linehan JH. Influence of hypoxia on the longitudinal distribution of pulmonary vascular resistance. J Appl Physiol. 1978;44:493–498. doi: 10.1152/jappl.1978.44.4.493. [DOI] [PubMed] [Google Scholar]
- 19.Dipp M, Evans AM. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res. 2001;89:77–83. doi: 10.1161/hh1301.093616. [DOI] [PubMed] [Google Scholar]
- 20.Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L318–L325. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- 21.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 22.Du W, Frazier M, McMahon TJ, Eu JP. Redox activation of intracellular calcium release channels (ryanodine receptors) in the sustained phase of hypoxia-induced pulmonary vasoconstriction. Chest. 2005;128:556S–558S. doi: 10.1378/chest.128.6_suppl.556S. [DOI] [PubMed] [Google Scholar]
- 23.Emori T, Hirata Y, Ohta K, Shichiri M, Marumo F. Secretory mechanism of immunoreactive endothelin in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1989;160:93–100. doi: 10.1016/0006-291x(89)91625-2. [DOI] [PubMed] [Google Scholar]
- 24.Eu JP, Xu L, Stamler JS, Meissner G. Regulation of ryanodine receptors by reactive nitrogen species. Biochem Pharmacol. 1999;57:1079–1084. doi: 10.1016/s0006-2952(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 25.Evans AM, Dipp M. Hypoxic pulmonary vasoconstriction: cyclic adenosine diphosphate-ribose, smooth muscle Ca2+ stores and the endothelium. Respir Physiol Neurobiol. 2002;132:3–15. doi: 10.1016/s1569-9048(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 26.Fan QI, Vanderpool K, Marsh JD. A 27 bp cis-acting sequence is essential for L-type calcium channel α1C subunit expression in vascular smooth muscle cells. Biochim Biophys Acta. 2002;1577:401–411. doi: 10.1016/s0167-4781(02)00441-4. [DOI] [PubMed] [Google Scholar]
- 27.Farrukh IS, Michael JR. Cellular mechanisms that control pulmonary vascular tone during hypoxia and normoxia. Possible role of Ca2+ATPases. Am Rev Respir Dis. 1992;145:1389–1397. doi: 10.1164/ajrccm/145.6.1389. [DOI] [PubMed] [Google Scholar]
- 28.Fishman AP. Hypoxia on the pulmonary circulation. How and where it acts. Circ Res. 1976;38:221–231. doi: 10.1161/01.res.38.4.221. [DOI] [PubMed] [Google Scholar]
- 29.Flynn ER, Bradley KN, Muir TC, McCarron JG. Functionally separate intracellular Ca2+ stores in smooth muscle. J Biol Chem. 2001;276:36411–36418. doi: 10.1074/jbc.M104308200. [DOI] [PubMed] [Google Scholar]
- 30.Franklin RA, Rodriguez-Mora OG, Lahair MM, McCubrey JA. Activation of the calcium/calmodulin-dependent protein kinases as a consequence of oxidative stress. Antioxid Redox Signal. 2006;8:1807–1817. doi: 10.1089/ars.2006.8.1807. [DOI] [PubMed] [Google Scholar]
- 31.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 32.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal. 2009;11:2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginty DD, Glowacka D, Bader DS, Hidaka H, Wagner JA. Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991;266:17454–17458. [PubMed] [Google Scholar]
- 34.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Pacheco FR, Caramelo C, Castilla MA, Deudero JJ, Arias J, Yague S, Jimenez S, Bragado R, Alvarez-Arroyo MV. Mechanism of vascular smooth muscle cells activation by hydrogen peroxide: role of phospholipase C gamma. Nephrol Dial Transplant. 2002;17:392–398. doi: 10.1093/ndt/17.3.392. [DOI] [PubMed] [Google Scholar]
- 36.Grover AK, Kwan CY, Samson SE. Effects of peroxynitrite on sarco/endoplasmic reticulum Ca2+ pump isoforms SERCA2b and SERCA3a. Am J Physiol Cell Physiol. 2003;285:C1537–C1543. doi: 10.1152/ajpcell.00299.2003. [DOI] [PubMed] [Google Scholar]
- 37.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 39.Hardingham GE, Cruzalegui FH, Chawla S, Bading H. Mechanisms controlling gene expression by nuclear calcium signals. Cell Calcium. 1998;23:131–134. doi: 10.1016/s0143-4160(98)90111-7. [DOI] [PubMed] [Google Scholar]
- 40.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 41.Hoidal JR, Brar SS, Sturrock AB, Sanders KA, Dinger B, Fidone S, Kennedy TP. The role of endogenous NADPH oxidases in airway and pulmonary vascular smooth muscle function. Antioxid Redox Signal. 2003;5:751–758. doi: 10.1089/152308603770380052. [DOI] [PubMed] [Google Scholar]
- 42.Hong Z, Weir EK, Nelson DP, Olschewski A. Subacute hypoxia decreases voltage-activated potassium channel expression and function in pulmonary artery myocytes. Am J Respir Cell Mol Biol. 2004;31:337–343. doi: 10.1165/rcmb.2003-0386OC. [DOI] [PubMed] [Google Scholar]
- 43.Ichiki T, Tokunou T, Fukuyama K, Iino N, Masuda S, Takeshita A. Cyclic AMP response element-binding protein mediates reactive oxygen species-induced c-fos expression. Hypertension. 2003;42:177–183. doi: 10.1161/01.HYP.0000079791.26014.04. [DOI] [PubMed] [Google Scholar]
- 44.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L489–L499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol. 1997;122:21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 47.Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C22–C33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- 48.Jernigan NL, Resta TC, Walker BR. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L947–L955. doi: 10.1152/ajplung.00215.2003. [DOI] [PubMed] [Google Scholar]
- 49.Jin N, Hatton ND, Harrington MA, Xia X, Larsen SH, Rhoades RA. H2O2-induced egr-1, fra-1, and c-jun gene expression is mediated by tyrosine kinase in aortic smooth muscle cells. Free Radic Biol Med. 2000;29:736–746. doi: 10.1016/s0891-5849(00)00376-2. [DOI] [PubMed] [Google Scholar]
- 50.Jin N, Packer CS, Rhoades RA. Pulmonary arterial hypoxic contraction: signal transduction. Am J Physiol. 1992;263:L73–L78. doi: 10.1152/ajplung.1992.263.1.L73. [DOI] [PubMed] [Google Scholar]
- 51.Jin N, Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol. 1997;272:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- 52.Kang TM, Park MK, Uhm DY. Characterization of hypoxia-induced [Ca2+]i rise in rabbit pulmonary arterial smooth muscle cells. Life Sci. 2002;70:2321–2333. doi: 10.1016/s0024-3205(02)01497-2. [DOI] [PubMed] [Google Scholar]
- 53.Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L408–L412. doi: 10.1152/ajplung.2000.279.2.L408. [DOI] [PubMed] [Google Scholar]
- 54.Kohno M, Yokokawa K, Horio T, Yasunari K, Murakawa K, Ikeda M, Takeda T. Release mechanism of endothelin-1 and big endothelin-1 after stimulation with thrombin in cultured porcine endothelial cells. J Vasc Res. 1992;29:56–63. doi: 10.1159/000158933. [DOI] [PubMed] [Google Scholar]
- 55.Kuga T, Sadoshima J, Tomoike H, Kanaide H, Akaike N, Nakamura M. Actions of Ca2+ antagonists on two types of Ca2+ channels in rat aorta smooth muscle cells in primary culture. Circ Res. 1990;67:469–480. doi: 10.1161/01.res.67.2.469. [DOI] [PubMed] [Google Scholar]
- 56.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56:439–513. doi: 10.1124/pr.56.4.1. [DOI] [PubMed] [Google Scholar]
- 58.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leach RM, Robertson TP, Twort CH, Ward JP. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am J Physiol. 1994;266:L223–L231. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- 61.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 62.Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1598–L1608. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- 63.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 64.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 65.Liu Q, Sham JS, Shimoda LA, Sylvester JT. Hypoxic constriction of porcine distal pulmonary arteries: endothelium and endothelin dependence. Am J Physiol Lung Cell Mol Physiol. 2001;280:L856–L865. doi: 10.1152/ajplung.2001.280.5.L856. [DOI] [PubMed] [Google Scholar]
- 66.Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L17–L25. doi: 10.1152/ajplung.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L104–L113. doi: 10.1152/ajplung.00058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madden JA, Dawson CA, Harder DR. Hypoxia-induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol. 1985;59:113–118. doi: 10.1152/jappl.1985.59.1.113. [DOI] [PubMed] [Google Scholar]
- 69.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marin J, Encabo A, Briones A, Garcia-Cohen EC, Alonso MJ. Mechanisms involved in the cellular calcium homeostasis in vascular smooth muscle: calcium pumps. Life Sci. 1999;64:279–303. doi: 10.1016/s0024-3205(98)00393-2. [DOI] [PubMed] [Google Scholar]
- 71.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 72.Martin TW, Michaelis KC. Ca2+-dependent synthesis of prostaglandin I2 and mobilization of arachidonic acid from phospholipids in cultured endothelial cells permeabilized with saponin. Biochim Biophys Acta. 1990;1054:159–168. doi: 10.1016/0167-4889(90)90237-8. [DOI] [PubMed] [Google Scholar]
- 73.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 74.McDaniel SS, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin LJ, Yuan JX. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L870–L880. doi: 10.1152/ajplung.2001.280.5.L870. [DOI] [PubMed] [Google Scholar]
- 75.McMurtry IF, Davidson AB, Reeves JT, Grover RF. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res. 1976;38:99–104. doi: 10.1161/01.res.38.2.99. [DOI] [PubMed] [Google Scholar]
- 76.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002a;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 77.Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002b;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 78.Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37:1119–1136. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Mishra SK, Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ Res. 1994;75:144–148. doi: 10.1161/01.res.75.1.144. [DOI] [PubMed] [Google Scholar]
- 80.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 81.Mogami H, Kojima I. Stimulation of calcium entry is prerequisite for DNA synthesis induced by platelet-derived growth factor in vascular smooth muscle cells. Biochem Biophys Res Commun. 1993;196:650–658. doi: 10.1006/bbrc.1993.2299. [DOI] [PubMed] [Google Scholar]
- 82.Mohazzab KM, Fayngersh RP, Kaminski PM, Wolin MS. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial PO2-elicited responses. Am J Physiol. 1995;269:L637–L644. doi: 10.1152/ajplung.1995.269.5.L637. [DOI] [PubMed] [Google Scholar]
- 83.Mohazzab KM, Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery PO2 sensor. Am J Physiol. 1994a;267:L823–L831. doi: 10.1152/ajplung.1994.267.6.L823. [DOI] [PubMed] [Google Scholar]
- 84.Mohazzab KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol. 1994b;267:L815–L822. doi: 10.1152/ajplung.1994.267.6.L815. [DOI] [PubMed] [Google Scholar]
- 85.Morio Y, McMurtry IF. Ca2+ release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92:527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- 86.Muramatsu M, Tyler RC, Rodman DM, McMurtry IF. Possible role of T-type Ca2+ channels in L-NNA vasoconstriction of hypertensive rat lungs. Am J Physiol. 1997;272:H2616–H2621. doi: 10.1152/ajpheart.1997.272.6.H2616. [DOI] [PubMed] [Google Scholar]
- 87.Nagasaka Y, Bhattacharya J, Nanjo S, Gropper MA, Staub NC. Micropuncture measurement of lung microvascular pressure profile during hypoxia in cats. Circ Res. 1984;54:90–95. doi: 10.1161/01.res.54.1.90. [DOI] [PubMed] [Google Scholar]
- 88.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- 89.Ng LC, Kyle BD, Lennox AR, Shen XM, Hatton WJ, Hume JR. Cell culture alters Ca2+ entry pathways activated by store-depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2008;294:C313–C323. doi: 10.1152/ajpcell.00258.2007. [DOI] [PubMed] [Google Scholar]
- 90.Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563:409–419. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pacaud P, Loirand G. Release of Ca2+ by noradrenaline and ATP from the same Ca2+ store sensitive to both InsP3 and Ca2+ in rat portal vein myocytes. J Physiol. 1995;484(Pt 3):549–555. doi: 10.1113/jphysiol.1995.sp020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N, Braun-Dullaeus RC, Kummer W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2003;284:L710–L719. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- 93.Paky A, Michael JR, Burke-Wolin TM, Wolin MS, Gurtner GH. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. J Appl Physiol. 1993;74:2868–2874. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- 94.Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- 95.Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med. 2008;45:1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 96.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 97.Pribnow D, Muldoon LL, Fajardo M, Theodor L, Chen LY, Magun BE. Endothelin induces transcription of fos/jun family genes: a prominent role for calcium ion. Mol Endocrinol. 1992;6:1003–1012. doi: 10.1210/mend.6.7.1508217. [DOI] [PubMed] [Google Scholar]
- 98.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 99.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 100.Rao GN. Oxidant stress stimulates phosphorylation of eIF4E without an effect on global protein synthesis in smooth muscle cells. Lack of evidence for a role of H202 in angiotensin II-induced hypertrophy. J Biol Chem. 2000;275:16993–16999. doi: 10.1074/jbc.275.22.16993. [DOI] [PubMed] [Google Scholar]
- 101.Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCepsilon signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2006;351:784–790. doi: 10.1016/j.bbrc.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCε signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Redding GJ, Tuck R, Escourrou P. Nifedipine attenuates acute hypoxic pulmonary vasoconstriction in awake piglets. Am Rev Respir Dis. 1984;129:785–789. doi: 10.1164/arrd.1984.129.5.785. [DOI] [PubMed] [Google Scholar]
- 104.Reeve HL, Weir EK, Nelson DP, Peterson DA, Archer SL. Opposing effects of oxidants and antioxidants on K+ channel activity and tone in rat vascular tissue. Exp Physiol. 1995;80:825–834. doi: 10.1113/expphysiol.1995.sp003890. [DOI] [PubMed] [Google Scholar]
- 105.Resnik E, Herron J, Keck M, Sukovich D, Linden B, Cornfield DN. Chronic intrauterine pulmonary hypertension selectively modifies pulmonary artery smooth muscle cell gene expression. Am J Physiol Lung Cell Mol Physiol. 2006;290:L426–L433. doi: 10.1152/ajplung.00281.2005. [DOI] [PubMed] [Google Scholar]
- 106.Rhoades RA, Packer CS, Roepke DA, Jin N, Meiss RA. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol. 1990;68:1581–1589. doi: 10.1139/y90-241. [DOI] [PubMed] [Google Scholar]
- 107.Ricci A, Bronzetti E, El-Assouad D, Felici L, Greco S, Mariotta S, Sabbatini M, Amenta F. Influence of age on L-type Ca2+ channels in the pulmonary artery and vein of spontaneously hypertensive rats. Mech Ageing Dev. 2000;120:33–44. doi: 10.1016/s0047-6374(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 108.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525(Pt 3):669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res. 2005;96:864–872. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- 110.Rothman A, Wolner B, Button D, Taylor P. Immediate-early gene expression in response to hypertrophic and proliferative stimuli in pulmonary arterial smooth muscle cells. J Biol Chem. 1994;269:6399–6404. [PubMed] [Google Scholar]
- 111.Rounds S, McMurtry IF. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res. 1981;48:393–400. doi: 10.1161/01.res.48.3.393. [DOI] [PubMed] [Google Scholar]
- 112.Salvaterra CG, Goldman WF. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol. 1993;264:L323–L328. doi: 10.1152/ajplung.1993.264.3.L323. [DOI] [PubMed] [Google Scholar]
- 113.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kB. Chem Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 114.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007a;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007b;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 116.Sham JS, Crenshaw BR, Jr, Deng LH, Shimoda LA, Sylvester JT. Effects of hypoxia in porcine pulmonary arterial myocytes: roles of KV channel and endothelin-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L262–L272. doi: 10.1152/ajplung.2000.279.2.L262. [DOI] [PubMed] [Google Scholar]
- 117.Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol. 2000;279:L884–L894. doi: 10.1152/ajplung.2000.279.5.L884. [DOI] [PubMed] [Google Scholar]
- 118.Shukla N, Rowe D, Hinton J, Angelini GD, Jeremy JY. Calcium and the replication of human vascular smooth muscle cells: studies on the activation and translocation of extracellular signal regulated kinase (ERK) and cyclin D1 expression. Eur J Pharmacol. 2005;509:21–30. doi: 10.1016/j.ejphar.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 119.Simonneau G, Escourrou P, Duroux P, Lockhart A. Inhibition of hypoxic pulmonary vasoconstriction by nifedipine. N Engl J Med. 1981;304:1582–1585. doi: 10.1056/NEJM198106253042606. [DOI] [PubMed] [Google Scholar]
- 120.Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol. 2003;140:97–106. doi: 10.1038/sj.bjp.0705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stanbrook HS, Morris KG, McMurtry IF. Prevention and reversal of hypoxic pulmonary hypertension by calcium antagonists. Am Rev Respir Dis. 1984;130:81–85. doi: 10.1164/arrd.1984.130.1.81. [DOI] [PubMed] [Google Scholar]
- 122.Staub NC. Site of hypoxic pulmonary vasoconstriction. Chest. 1985;88:240S–245S. doi: 10.1378/chest.88.4_supplement.240s. [DOI] [PubMed] [Google Scholar]
- 123.Suzuki YJ, Cleemann L, Abernethy DR, Morad M. Glutathione is a cofactor for H2O2-mediated stimulation of Ca2+-induced Ca2+ release in cardiac myocytes. Free Radic Biol Med. 1998;24:318–325. doi: 10.1016/s0891-5849(97)00227-x. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki YJ, Ford GD. Inhibition of Ca2+-ATPase of vascular smooth muscle sarcoplasmic reticulum by reactive oxygen intermediates. Am J Physiol. 1991;261:H568–H574. doi: 10.1152/ajpheart.1991.261.2.H568. [DOI] [PubMed] [Google Scholar]
- 125.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 126.Tirone F, Cox JA. NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett. 2007;581:1202–1208. doi: 10.1016/j.febslet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 127.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 129.Urena J, Franco-Obregon A, Lopez-Barneo J. Contrasting effects of hypoxia on cytosolic Ca2+ spikes in conduit and resistance myocytes of the rabbit pulmonary artery. J Physiol. 1996;496(Pt 1):103–109. doi: 10.1113/jphysiol.1996.sp021668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vadula MS, Kleinman JG, Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol. 1993;265:L591–L597. doi: 10.1152/ajplung.1993.265.6.L591. [DOI] [PubMed] [Google Scholar]
- 131.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004a;286:L848–L858. doi: 10.1152/ajplung.00319.2003. [DOI] [PubMed] [Google Scholar]
- 132.Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1059–L1069. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- 133.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia-inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 134.Wang J, Weigand L, Sylvester J, Shimoda L. Enhanced capacitative Ca2+ entry contributes to elevated resting Ca2+ and tension in pulmonary arterial smooth muscle from rats exposed to chronic hypoxia. Amer Rev Repir Crit Care Med. 2004b;169:A400. [Google Scholar]
- 135.Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 137.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 138.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waypa GB, Schumacker PT. O2 sensing in hypoxic pulmonary vasoconstriction: the mitochondrial door re-opens. Respir Physiol Neurobiol. 2002;132:81–91. doi: 10.1016/s1569-9048(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 140.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol. 2005;98:404–414. doi: 10.1152/japplphysiol.00722.2004. [DOI] [PubMed] [Google Scholar]
- 141.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol. 2005;289:L5–L13. doi: 10.1152/ajplung.00044.2005. [DOI] [PubMed] [Google Scholar]
- 142.Weigand LA, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2006;290:L284–L290. doi: 10.1152/ajplung.00449.2004. [DOI] [PubMed] [Google Scholar]
- 143.Weissmann N, Ebert N, Ahrens M, Ghofrani HA, Schermuly RT, Hanze J, Fink L, Rose F, Conzen J, Seeger W, Grimminger F. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. Am J Respir Cell Mol Biol. 2003;29:721–732. doi: 10.1165/rcmb.2002-0217OC. [DOI] [PubMed] [Google Scholar]
- 144.Weissmann N, Zeller S, Schafer RU, Turowski C, Ay M, Quanz K, Ghofrani HA, Schermuly RT, Fink L, Seeger W, Grimminger F. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2006;34:505–513. doi: 10.1165/rcmb.2005-0337OC. [DOI] [PubMed] [Google Scholar]
- 145.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 146.Whorton AR, Willis CE, Kent RS, Young SL. The role of calcium in the regulation of prostacyclin synthesis by porcine aortic endothelial cells. Lipids. 1984;19:17–24. doi: 10.1007/BF02534603. [DOI] [PubMed] [Google Scholar]
- 147.Wu S, Haynes J, Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. Cav3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res. 2003;93:346–353. doi: 10.1161/01.RES.0000087148.75363.8F. [DOI] [PubMed] [Google Scholar]
- 148.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–L1276. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 149.Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L338–L348. doi: 10.1152/ajplung.00328.2004. [DOI] [PubMed] [Google Scholar]
- 150.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- 151.Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- 152.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol. 2003;285:L680–L690. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- 153.Zheng YM, Mei QB, Wang QS, Abdullaev I, Lai FA, Xin HB, Kotlikoff MI, Wang YX. Role of FKBP12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium. 2004;35:345–355. doi: 10.1016/j.ceca.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 154.Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]