Abstract
In embryonic hearts explanted on collagen gels, epicardial cells delaminate and form vascular tubes, thus providing a model for coronary tubulogenesis. Using this model, we show that FGFs 1, 2, 4, 8, 9 and 18 contribute to tubulogenesis and that the availability of multiple FGFs provides the optimal tubulogenic response. Moreover, the FGF effects are VEGF dependent, while VEGF-induced tubulogenesis requires FGF signaling. The number of endothelial cells (ECs) is increased by all of the FGFs, while EC migration is significantly enhanced only by FGF-2 and FGF-18. Finally, addition of embryonic mesenchymal stem cells (EMSC) to the explants markedly enhances EC numbers and a 23-fold increase in SDF-1α, which is FGF dependent. Both explants and EMSCs produce SDF-1α. In conclusion, coronary tubulogenesis of embryonic epicardium: 1) is responsive to many FGF family members, 2) requires both FGF and VEGFA signaling, and 3) is responsive to EMSCs.
Keywords: embryonic mesenchymal stem cells, epithelial-mesenchymal transformation, epicardium, FGF, VEGF
Introduction
FGFs 1 and 2 have long been recognized as angiogenic and arteriogenic growth factors (reviewed in Eswarakumar et al., 2005; Presta et al., 2005; Murakami et al., 2008b). More recently, additional FGF family members have been implicated in vessel formation and growth. This is not surprising, since most FGF proteins activate FGFR-1, a receptor that is required for normal blood vessel development (Lee et al., 2000). For example, angiogenic activity has been ascribed to FGF-4 (Dell'Era et al., 2001), FGF8b (Mattila et al., 2001), FGF-9 (Lavine et al., 2006) and FGF-18 (Sonvilla et al., 2008). Our previous studies have documented the importance of FGF-2 in coronary vasculogenesis/angiogenesis and arteriogenesis during embryonic (Tomanek et al., 1998; Tomanek et al., 2001b; Tomanek et al., 2008) and postnatal (Tomanek et al., 2001a) development. Moreover, we noted that both FGF-2 and VEGF are required for vascular tube formation in the embryonic heart (Tomanek et al., 2001b). The elegant study of Lavine (2006) documented the requirement for FGF-9 in coronary vascular development and also showed that FGF-9 promotes HH signaling and that both promote VEGF ligand expression. These studies suggest that coronary vessel development may be regulated by a relatively broad spectrum FGF family members, which are linked to VEGF signaling.
Eighteen FGFs (FGFs 1-10 and 16-23) are known to activate FGF receptors (Beenken et al., 2009). These ligands activate 4 tyrosine kinase receptors (FGFR-1-FGFR-4) that have 3 extracellular Ig domains, i.e. D1-D3. Alternative splicing in the D3 domain of FGFR-1, -2 and -3 yields b and c isoforms (e.g. FGFR-1 IIIb and FGFR-1 IIIc) with specific binding characteristics (Johnson et al., 1991; Zhang et al., 2006). The b and c splice isoforms of FGFRs are usually specific for epithelial and mesenchymal cells, respectively. These findings, taken together, support the concept that multiple FGFs and receptor splice variants facilitate the formation of vascular tubes. Accordingly, the current study addressed three hypotheses. First, multiple FGF ligands are able to stimulate tubulogenesis from the epicardium of the embryonic heart. Second, 1) tubulogenesis requires both FGF and VEGF signaling and, 2) VEGF-induced epicardial-derived tubulogenesis requires FGF signaling. Third, EMSCs stimulate epicardial-derived tubulogenesis through a paracrine effect, probably mediated by SDF-1 and dependent on FGF signaling.
Results
Data are based on in vitro experiments that utilized the apical portions of embryonic quail and mouse hearts, explanted and cultured on collagen gels (Tomanek et al., 2001b; Tomanek et al., 2002). In this model, epicardial and subepicardial cells migrate into the gels and form tubes. Group means are based on 7-32 explants.
As documented earlier in quail explants by immunohistochemistry and electron microscopy (Yue et al., 2001), and in the current study, all cells that incorporate into vascular tubes in the explant gel are endothelial cells. In the first set of experiments, we explanted embryonic day 6 quail hearts to determine: 1) the efficacy of 6 FGF family members in stimulating tubulogenesis, and 2) whether tubulogenesis induced by the 6 FGFs requires VEGF signaling. The remaining experiments addressed key aspects of vascular formation, i.e. tube and cell densities and apoptosis embryonic day 14 mouse heart explants. In these experiments, we addressed: 1) the specific roles of the 6 FGF proteins, 2) the dependency of VEGF stimulation of tubulogenesis on FGFR signaling, and 3) the role of embryonic mesenchymal stem cells in coronary tubulogenesis. The optimal dose of each FGF in stimulating tubulogenesis was determined by dose-response curves. The utilization of 4 soluble adv FGF traps (FGFR1-IIIb, FGFR1-IIIc, FGFR3-IIIb and FGFR3-IIIc) and a dominant negative of all FGF signaling (FGF1-DN) provided some insight into the importance of FGF ligand overlap in this model of tubulogenesis. These soluble and dominant negative receptors, previously described (Ornitz et al., 1996; McDowell et al., 2006), were recently validated (Murakami et al., 2008a). The degree of receptor activation by the six FGF ligands used in our experiments is listed in the Table.
Table. Receptor Activation by FGF ligands*.
| Ligand | FGFR1-IIIb | FGFR1-IIIc | FGFR3-IIIb | FGFR3-IIIc |
|---|---|---|---|---|
| FGF-1 | +++ | +++ | +++ | +++ |
| FGF-2 | + | ++ | + | ++ |
| FGF-4 | + | ++ | - | + |
| FGF-8 | - | ++ | - | +++ |
| FGF-9 | - | + | +++ | +++ |
| FGF-18 | - | - | + | ++ |
Based on data from Zhang et al., 2006.
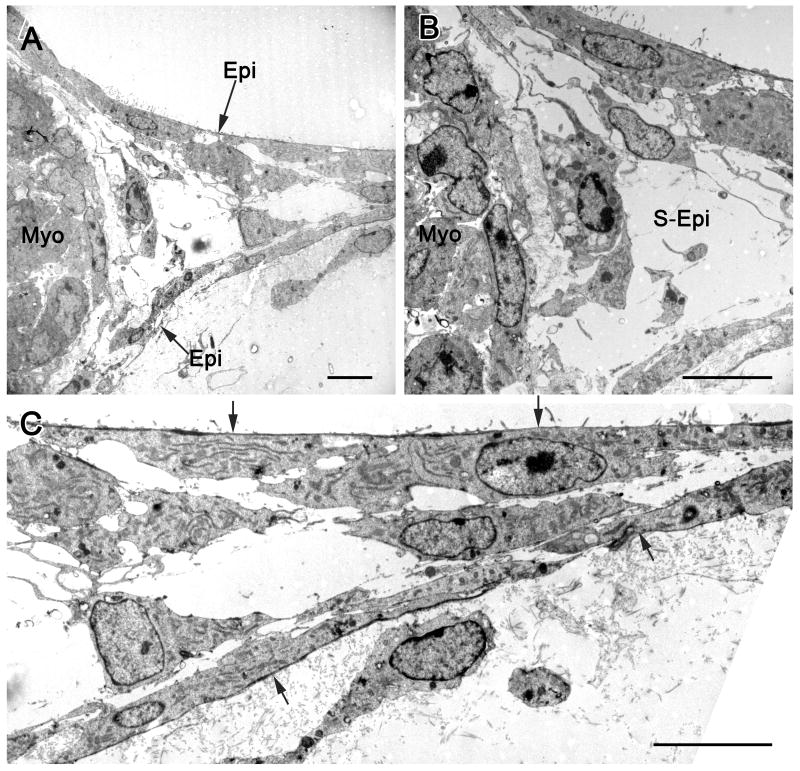
Figure 1, an electron microscopic image, provides documentation regarding tube formation originating from the epicardial surface. This image from a mouse heart explant is similar to those obtained from quail explants and reveals that cells forming the tubes are epicardial cells. Thus, the epicardial mesothelial cells delaminate to form the walls of the tubular network.
Figure 1.
Transmission electron micrographs illustrating epicardial cell formation of vascular tubes in a collagen matrix. In A, the edge of the embryonic mouse heart explant with myocardium (myo) is seen along with epicardial (epi) cells emerging from the explant. The region between the emerging epicardial cells is an extension of the subepicardium. A detail of this micrograph (B) shows subepicardial cells (S-Epi) in the lumen of the vascular tube. In C, the components of vascular tube distal to the explant are seen, with epicardial cells indicated by arrows. The lumen, containing subepicardial cells (S-Epi), is between the epicardial cells, which by immunohistochemistry are positive for the endothelial cell marker BS-I. These images are typical of tube formation in both mouse and quail explants, i.e., epicardial cells delaminate and form the walls of the tubes.
Multiple FGF Ligands Depend on VEGF (Quail Explants)
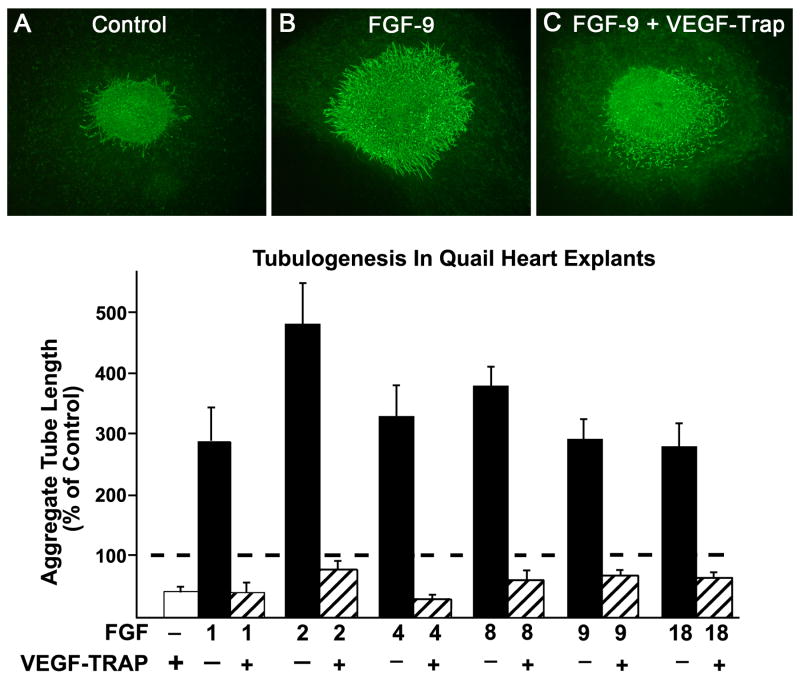
Using the quail endothelial cell specific antibody, QH1, we quantified aggregate tube length in heart explants treated with FGF proteins 1, 2, 4, 8, 9 and 18. In preliminary experiments, we determined the optimal doses (ng/ml) for each FGF ligand: 400 for FGF-1, 150 for FGF-2 and 900 for FGFs 4, 8, 9 and 18. As seen in Figure 2, aggregate tube length was 2.7-4.7 higher than controls when the FGFs were added to the explants. The greatest increase occurred with the addition of FGF-2. We then added VEGF-trap, (VEGF-intercept, Regeneron) a chimera of VEGFR-1 and VEGFR-2 in order to test the hypothesis that tubulogenesis induced by any of the FGFs required VEGF. In all cases, aggregate tube length was reduced below control (non-stimulated) values. As seen in Figure 2 (A, B, C), the main effect of VEGF-trap was inhibition of tube formation, and to a lesser degree on the number of ECs. Figure 2C illustrates an explant treated with FGF-9 and VEGF-trap. Although there are almost no tubes, numerous free ECs (those not incorporated into tubes) are seen. In contrast, the control explant (Figure 2A) has tubes, but few free ECs.
Figure 2.
Tubulogenesis in quail embryonic heart explants. Tube formation induced by each of 6 FGFs is 2.7-4.7 fold greater than in the controls and is prevented when VEGF is inhibited by exposure of the explants to VEGF-trap (a soluble VEGFR-1/VEGFR-2 chimera). Endothelial cells and tubes are identified by fluorescence immunoreactivity with quail QH-1 antibody, as shown in A, B, C. Although VEGF-trap clearly inhibits tubulogenesis, its effect on cell number is not as marked, as indicated by numerous free EC in the FGF-9 + VEGF-Trap samples (C). Data are means ± SEM and are expressed as a percent of control indicated by the broken line.
Ligands for FGFR1-IIIc Contribute to Coronary Tubulogenesis
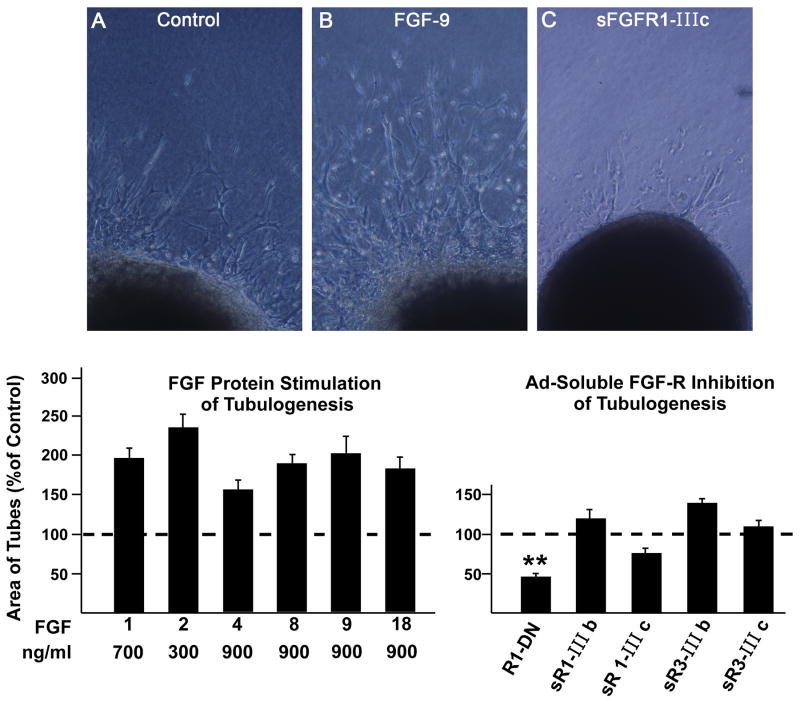
Based on these data from our quail explant model, we utilized embryonic mouse heart explants. All data in Figures 3-8 are based on mice. The following optimal doses of FGFs (ng/ml) in mouse explants were: FGF-1 700; FGF-2 300; FGFs 4, 8, 9 and 18 900. All FGFs significantly enhanced tubulogenesis (1.6-2.4-fold) as evidenced by tube density (Figure 3). In order to determine the role of FGF signaling in tubulogenesis, we added FGFR1-DN (an adenoviral construct encoding a cytoplasmic domain-deleted FGFR1 that inhibits signaling by all four FGF receptors) or soluble FGFR1-IIIb, FGFR1-IIIc, FGFR3-IIIb or FGFR3-IIIc. FGFR1-DN significantly attenuated vascular tube density by 54%. The only soluble splice variant that tended to inhibit tubulogenesis was sFGFR1-IIIc, which has the broadest ligand specificity as seen in Figure 3C.
Figure 3.
Each of the 6 FGF proteins used in this study stimulate tubulogenesis in mouse heart explants (all increases are statistically significant (ρ ≤ 0.01). Soluble FGFR1-DN (dominant negative) reduces tube formation to 40% of control (* *, ρ ≤ 0.01). Inverted microscope images (A, B, C) illustrate vascular tubes projecting from the surface of explants. Data are means ± SEM.
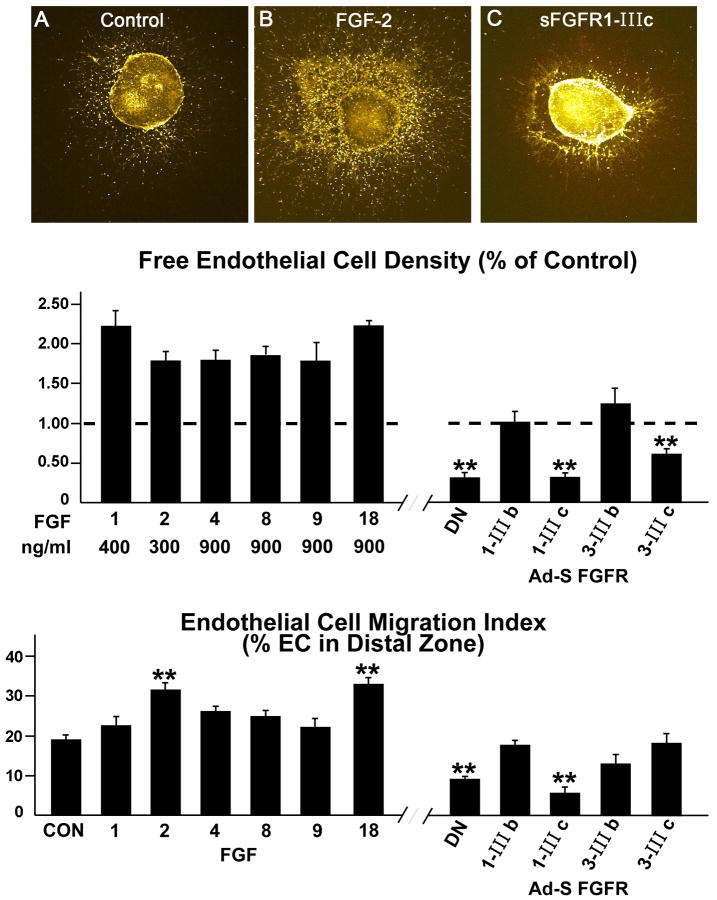
Next, we examined the roles of the 6 FGF ligands on EC density and migration in the collagen gels. The number of free live EC (those not incorporated into tubes) was enhanced 1.8-2.24-fold over control values by the FGF ligands (Figure 4). When exposed to FGFR1-DN or sFGFR1-IIIc, free cell numbers were reduced by 69%. Ad sFGFR3-IIIc also attenuated free cell numbers, but by only 39%, while the b isoforms of FGFR1 and FGFR3 had no effect. The effects of the IIIc isoforms, again, are consistent with the fact that they bind more FGFs than their IIIb counterparts. To evaluate migration effectiveness of the explant ECs, we counted the number of free cells in a zone distal to the explanted heart (Figure 4). The percentage of cells that migrated to this zone (% of total cells) was increased by the addition of FGFs, but only FGF-2 and FGF-18 affected a significant increase (>30%). Like free cell density, migration was significantly inhibited by sFGFR1-IIIc; as well as by adv FGF1-DN. These data document: 1) the impact of all 6 FGF ligands on EC number, and 2) the role of FGF signaling in EC migration. Moreover, although sFGFR3-IIIc attenuated EC density, it had no effect on EC migration. Thus, ligand combinations captured by soluble FGF3-IIIc play a role in EC numbers, but not migration. The fact that sFGF3-IIIc binds FGF-18 may be related to that ligand's ability to increase EC density.
Figure 4.
Free endothelial cell (EC) density and migration index in response to various FGFs and soluble receptor splice variants. EC free cells are those not incorporated into tubes. A, B and C are confocal images. As seen in B, free EC density (cells not incorporated into tubes) is enhanced by FGFs compared to controls (A). All 6 FGFs significantly increased cell density. EC density is significantly reduced by FGFR1-DN, sFGFR1-IIIc and sFGFR3-IIIc. **, ρ ≤ 0.01. As seen in the bottom panel, EC migration index tends to be increased when FGF ligands are added to the explants with FGF-2 and FGF-18 affecting significant increases. EC migration is reduced to values much lower than controls when sFGFR1-IIIc or FGFR1-DN are added to the explants. Data are means ± SEM. **, p ≤ 0.01.
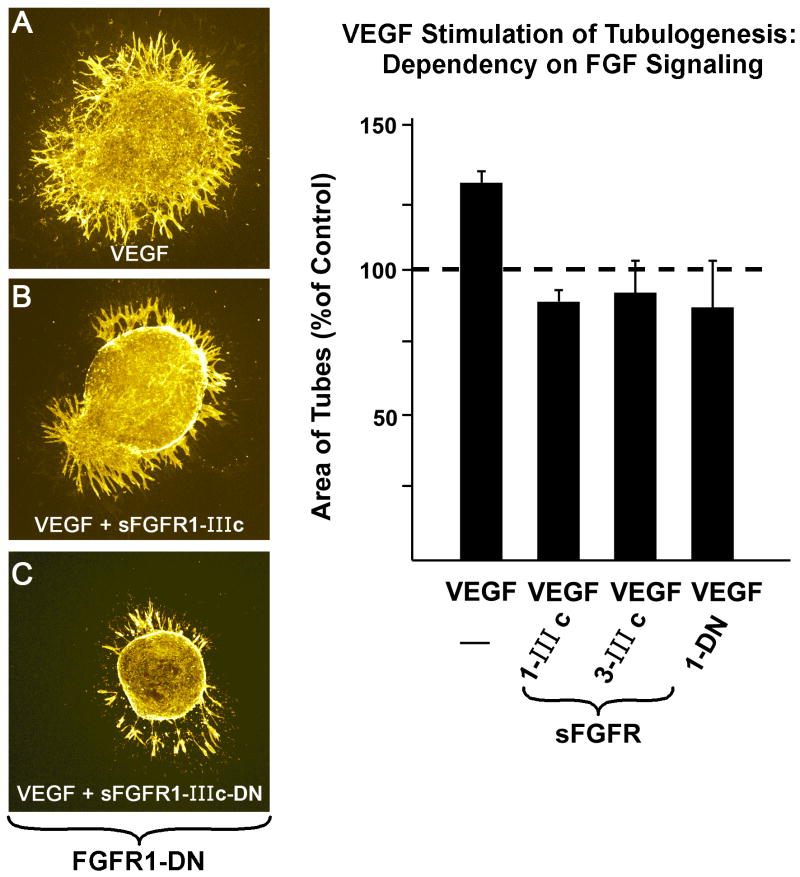
VEGF Stimulation of Tubulogenesis Requires FGF Signaling (Figure 5)
Figure 5.
FGF signaling is required for VEGF stimulation of tubulogenesis. sFGFR1-IIIc and sFGFR3-IIIc, as well as FGFR1-DN negate VEGF induction of tube formation. Micrographs (A-C) are confocal images displaying the relatively wide tubes typical of VEGF stimulation. Data are means ± SEM.
Having shown that all 6 FGFs studied in our quail experiments require VEGF signaling (Figure 2), we tested the hypothesis that VEGF-stimulated tubulogenesis (in mouse embryonic hearts) depends on FGFR signaling. VEGF affected a 30% increase in the density of tubes. However, as seen in Figure 5, the addition of FGFR1-DN, sFGFR1-IIIc or sFGFR3-IIIc completely prevented the increase, as evidenced by values that were similar to those of control (non-treated explants). Figure 5A, B and C illustrate that the effect of VEGF is primarily on tube formation rather than on free cell density and that FGF signaling is required for this function.
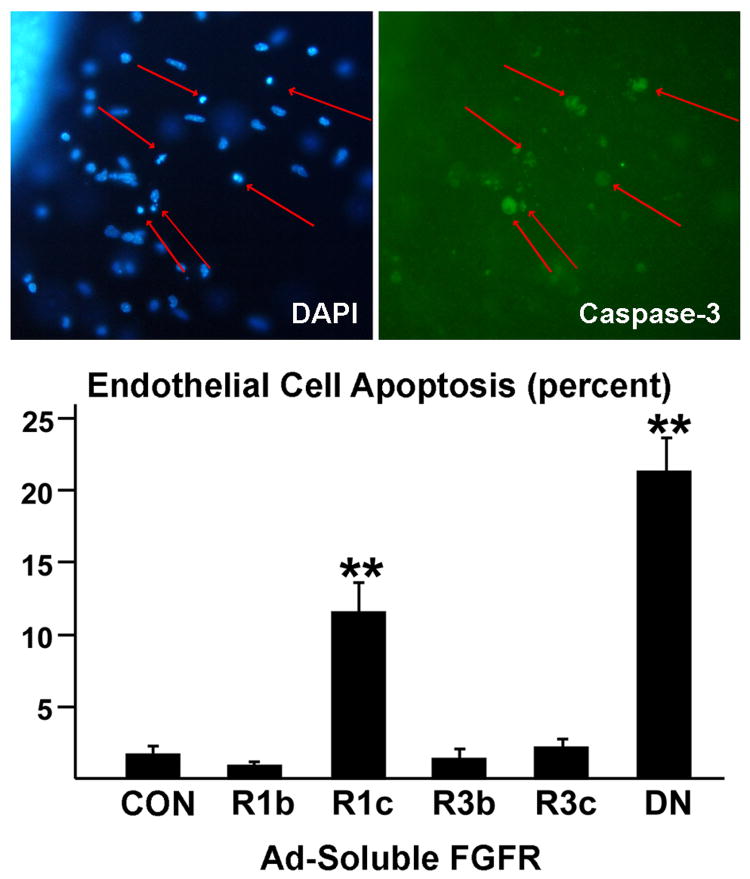
EC Apoptosis
Since inhibition of FGF reduces EC density (Figure 4), we evaluated apoptosis of EC using an immunohistochemical method for caspase-3 activation (Figure 6). The data revealed that FGFR-1DN and sFGFR-IIIc enhanced the number of apoptotic ECs, but that sFGFR1-IIIb and sFGF3-IIIb had no effect. These experiments reveal that FGF signaling is anti-apoptotic for coronary ECs.
Figure 6.
Apoptosis of endothelial cells using caspase-3 activation and secondary Cy2 immunostaining. Nuclei were visualized with DAPI. The data demonstrate that FGF signaling required to minimize apoptosis and this inhibition is primarily due to inhibition of the FGFs that bind to s FGFR1-IIIc. The arrows indicate apoptotic cells. **, ρ ≤ 0.01 Data are means ± SEM.
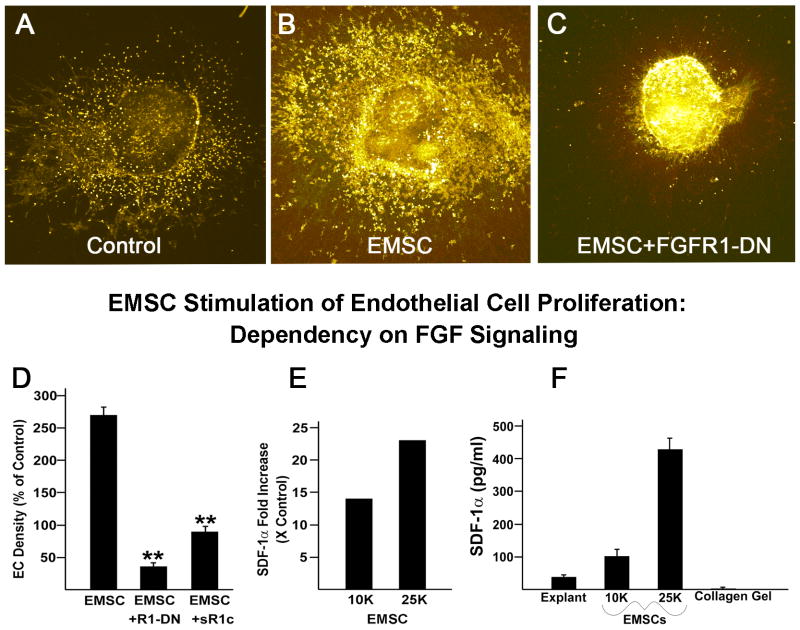
Embryonic Mesenchymal Stem Cell (EMSC) Enhanced EC Density Requires FGF Signaling
Our preliminary data revealed that murine embryonic mesenchymal stem cells (EMSC) had a significant effect on the number of free EC in the gels containing embryonic heart explants. The C3H/10T1/2 cells (ATCC, Manassas, VA) can differentiate into several cell types, including smooth muscle, endothelial, adipocytes, and osteocytes (Wang et al., 2005; Joo et al., 2010). In our model, EMSCs form a monolayer in the gel. Thus, these cells appear to exert a trophic/paracrine function in influencing ECs on the heart explants.
In order to determine if FGF signaling is required for the EMSC influence on ECs, we quantified EC density in the presence of EMSCs. As illustrated in Figure 7, EC density was enhanced 2.7-fold in the presence of embryonic EMSCs, but was less than non-treated controls when FGFR-1DN or sFGFR1-IIIc were added along with EMSCs. We then tested the hypothesis that the presence of EMSCs might activate chemokines which would serve to stimulate tubulogenesis. ELISA analysis of conditioned media revealed that the explants that contained 10K or 25K EMSCs had stromal derived factor-1α (SDF-1α) levels 14- and 23-fold higher, respectively, than controls (Figure 7E). These experiments further document the importance of FGF signaling in the induction of tubulogenesis from the embryonic epicardium. Finally, we addressed the issue of the source of the SDF-1α by culturing EMSCs on collagen in the absence of the heart explants (Figure 7F). The data indicate that 10 K and 25 K EMSCs cultured on collagen in the absence of the heart explant secrete 2.7 and 12 times as much SDF-1α, respectively, compared to the explant. A comparison of these data with those derived from experiments using both heart explants and EMSCs (Figure 7E) shows that the interaction of the two produces the greatest increase in SDF-1α.
Figure 7.
Coronary embryonic free EC density is enhanced in the presence of EMSCs and is dependent on FGF signaling (A, B, C). EC density is markedly reduced when FGFR1-DN or soluble FGFR1-IIIc are added to the explants containing EMSCs; **, ρ<0.001 (D). The conditioned media from wells containing both heart explants and EMSCs have much higher levels of SDF-1α (E). The SDF-1α level is dependent on the number of EMSCs added. When heart explants are cultured in the absence of EMSCs, the cultured medium contains about 35 ρg/ml of SDF-1α (F). If 10k and 25K EMSCs are cultured alone (no heart explant), the SDF-1α in the conditioned media is nearly 2.7 and 12 times higher, respectively, than that observed in heart explants.
Discussion
The embryonic epicardium is well established as the source of coronary vascular cells (reviewed in Majesky, 2004; Olivey et al., 2004; Tomanek, 2005). Our previous studies utilized embryonic heart explants to document roles for VEGF, FGF-2 and angiopoietins in the formation of a network of vascular tubes from the epicardium (Tomanek et al., 1998; Yue et al., 1999; Tomanek et al., 2001a; Tomanek et al., 2001b; Yue et al., 2001; Tomanek et al., 2002). More recently, Lavine and colleagues (2006) showed that FGF-9 plays a key role in hedgehog activation essential for coronary vessel development. Accordingly, we hypothesized that multiple FGFs can induce vasculogenic/angiogenic responses of the embryonic epicardium. Our explant model enables us to study coronary tubulogenesis from the epicardium/subepicardium since endothelial cells are derived directly from this portion of the ventricle. As noted in Figure 1, epicardial cells delaminate and form tubes in the collagen gel. Thus, our model: 1) has direct relevance to epicardial-mesenchymal transformation, and 2) allows the quantification of endothelial tubes and endothelial cells not incorporated into tubes in the collagen gel.
Our data support several novel and important conclusions. First, in addition to roles for FGFs 2 and 9, previously reported (Tomanek et al., 1998; Tomanek et al., 2001b; Lavine et al., 2006) in coronary tubulogenesis, we document the effectiveness of 4 additional FGFs, i.e. 1, 4, 8 and 18. At the same time, we show, via soluble FGF receptor splice variants, that the maximal effects of FGF signaling occur when the greatest number of FGF family member ligands available for binding to receptors, especially with respect to EC cell density and migration. Second, the data provide evidence that each of the 6 FGF ligands depends on VEGF signaling and that, in turn, VEGF induction of coronary tubulogenesis necessitates FGFR signaling. Third, we document a role for EMSC interaction with the embryonic heart that enhances EC proliferation. This enhancement is associated with large increases in SDF-1α derived from both the explant and the EMSCs.
FGFR Signaling is Required for Epicardial Cell Transformation
Recently, Pennisi and Mikawa (2009) documented novel roles for FGF-1 signaling in epicardial cell transformation and differentiation of coronary lineage cells. They showed that FGFR-1 is upregulated after epithelial-mesenchymal transformation (EMT) and that its overexpression affects an increase in EMT and enhances delamination of epicardial cells. Moreover, they provided evidence that knockdown of FGFR-1 limited the invasiveness of the epicardial-derived cells. Their data support our findings regarding the necessity of FGF signaling for all aspects of coronary tubulogenesis, i.e. delamination of the epicardial cells, their proliferation, migration and assembly into tubes. As illustrated in Figure 1, tube formation from the ventricular explants occurs by outward delamination of epicardial mesothelial cells into the collagen gel. These cells are positive for endothelial markers and form the walls of the vascular tubes or occur as free cells. Inhibition of FGFR signaling virtually negates both tube formation and cell proliferation. Accordingly, the failure to form tubes when FGFR1-DN is added to the explants is a consequence of limited or arrested mesothelial delamination and proliferation. The occurrence of a few tubes or cells in the FGFR1-DN treated explants is due to tubulogenesis during the first explant day, prior to treatment with inhibitors of FGF signaling.
The ability of exogenous FGFs to stimulate EC proliferation and tubulogenesis is likely mediated by an FGF-induced upregulation of FGFR1. Upregulation of FGFR1 mRNA and protein in response to increased FGF-2 has been previously documented (Estival et al., 1996). Moreover, FGF-1 or FGF-4 retroviral infection of the ventricular wall of embryonic quail caused an upregulation of FGFR1 and VEGFR-2 in epicardial and subepicardial cells adjacent to the FGF overexpressing sites (Pennisi et al., 2005).
FGF Signaling Partners with other Coronary Signaling Events
A key study (Lavine et al., 2006) documenting hedgehog (HH) signaling as necessary for coronary growth found that FGF triggers HH activation essential for VEGF and angiopoietin-2 expression. Moreover, they found that FGF-9 is required for coronary development as evidenced by FGF-9 -/- mouse hearts that had a reduced vascular plexus. Consistent with these data is the evidence that FGF-9, which is expressed in the epicardium and endocardium (Lavine et al., 2005), and sonic hedgehog, regulate VEGF-A expression and capillary angiogenesis in the lung (White et al., 2007). Moreover, inhibition of VEGF in hexamethylene-bis-acetamide-inducible protein mutant mice resulting in decreased myocardial vascularization was associated with decreased expression of FGF-9 (Montano et al., 2008). Using embryonic quail heart explants, we previously found that tubulogenesis was inhibited by anti FGF-2 or VEGF-A antibodies or soluble Tie-2, and that the effects were additive (Tomanek et al., 2001b). Furthermore, tubulogenesis induced by FGF-2 or VEGF-A was interdependent and that induced by either growth factor was dependent on Tie-2 signaling. Thus, that study documented the importance of 3 signaling pathways, i.e., FGF, VEGF and angiopoietin/Tie-2 in coronary tubulogenesis. Subsequently, Lavine et al. (2006) found support for these findings by documenting an FGF-hedgehog-VEGF/angiopoietin signaling cascade that controls both epicardial and intramyocardial blood vessel growth. The well-documented expression of these molecules in the embryonic heart during coronary vascularization (Tomanek et al., 2002; Ward et al., 2002; Detillieux et al., 2003; Lavine et al., 2005) is consistent with the proposed signaling cascade. Tubulogenesis in the embryonic heart has also been shown to be dependent on other VEGFs (VEGF-B, VEGF-C) and on VEGFR-1 (Tomanek et al., 2002). In that study, a soluble VEGFR-1 receptor diminished coronary tubulogenesis by more than 80% and inhibition of its VEGF-B ligand attenuated tubulogenesis by 65%. Moreover, VEGF-C, as well as VEGF-A, have been shown to synergize with FGF-2 to induce angiogenesis in vitro (Pepper et al., 1998). These findings, taken together, indicate that coronary tubulogenesis in the embryonic heart requires multiple growth factors and signaling events.
Multiple FGFs Facilitate Embryonic Heart Tubulogenesis
We selected FGFs 4, 8 and 18 for our experiments because of evidence that they appear to be angiogenic in some cell lines or organs. FGF-4 protein, present by stage 15 in avians, has been found to regulate cardiogenesis and to promote differentiation of smooth muscle cells (Zhu et al., 1996; Kruithof et al., 2006). In adults, this FGF protein has been shown to induce angiogenesis in the ischemic heart (Fukuyama et al., 2007) and hindlimb (Rissanen et al., 2003). Most importantly, FGF-4 initiates the differentiation of embryonic stem cells (Kosaka et al., 2009). Evidence that FGF-8 is angiogenic came from a study that documented vessel growth in the chorion allantoic membrane (CAM) exposed to FGF-8b (Mattila et al., 2001). FGF-8 has been shown to be present in the secondary heart field/outflow myocardium (Waldo et al., 2001). FGF-18 has been documented in several embryonic tissues, including the myocardium (Cormier et al., 2005) and has been shown to be strongly chemotactic in human umbilical cord endothelial cells (Antoine et al., 2006).
The current study supports the conclusion that the 6 FGF proteins tested stimulate tubulogenesis in embryonic hearts of both quail and mouse, when administered at optimal doses. However, the finding that FGF-2 provided the strongest response at only 1/3 of the dose required by FGFs 4, 8, 9 and 18 supports previous data that document FGF-2 as a key angiogenic factor (Tomanek et al., 1998; Tomanek et al., 2001b; Tomanek et al., 2008).
The increased cell density in response to FGF-18 (Figure 4) suggests a possible role for FGF3-IIIc signaling, since FGF-18 binds preferentially to FGFR3-IIIc (Antoine et al., 2005; Davidson et al., 2005). This effect may be limited to embryonic hearts, since HUVEC proliferation was not enhanced by FGF-18 (Antoine et al., 2006). In contrast to the effectiveness of all 6 FGFs in tubulogenesis and EC density, only FGF-2 and FGF-18 significantly effected EC migration (Figure 4). This role for FGF-18 has previously been documented in HUVECs (Antoine et al., 2006). Another important function of FGFR signaling is the regulation of vascular integrity as noted in adults (Murakami et al., 2008a). That study noted increased vascular permeability due to a loss of EC to cell contact induced primarily by sFGFR1-IIIc and to a lesser extent, by sFGF3-IIIc. That sFGFR1-IIIc and sFGFR3-IIIc have some effect on these processes is likely due to the fact that these receptors are more responsive to activation by FGF-2 and that they bind more FGF ligands than the IIIb isoforms (Table).
EMSC Increase the Number of ECs
Endothelial cell progenitor cell numbers have been shown to increase in response to SDF-1 (De Falco et al., 2004). Such a role for SDF-1 is implied by our data indicating that the more than 2.5-fold increase in EC density was coupled with a more than 20-fold increase in SDF-1α when EMSCs were added to the cultures. Moreover, our data document that the heart explant and EMSCs both produce SDF-1α and that their interaction enhances SDF-1α production beyond the additive effect of that secreted by each. This finding indicates that the presence of EMSCs in the embryonic heart provides a vasculogenic stimulus. That SDF-1α and FGF pathways interact during morphogenesis is supported by the finding that FGF signaling modulates the expression of SDF-1α and its receptors during zebra fish fin regeneration (Bouzaffour et al., 2009). SDF-1 has been found to induce revascularization in ischemic hindlimbs of mice via its CXCR4+ receptor on hemangiocytes (Jin et al., 2006) and those data are consistent with data that document CDXCR4+ progenitor cell recruitment to regenerating tissues by hypoxia gradients via HIF-1 induced SDF-1 expression (Ceradini et al., 2004).
Summary and Conclusions
Our study provides direct evidence that tubulogenesis from the embryonic epicardium, i.e. formation of a primary capillary plexus, requires FGF signaling and that at least 6 FGF family members are effective in this role. We show that multiple FGFs increase EC density, migration and tube formation, and that these events depend on FGFR signaling. Some specific roles for different FGFs are suggested by the finding that sFGFR1-IIIc, but not sFGFR3-IIIc inhibits EC migration, while both traps inhibit EC numbers. Our major findings are that: 1) tubulogenesis, but not EC density, induced by any of the 6 FGFs is dependent on the presence of VEGF, and 2) VEGF-stimulated tubulogenesis requires FGF signaling. Finally, the presence of EMSCs enhances EC density and is associated with marked elevation of SDF-1α, which is derived from both heart explants and EMSCs.
Experimental Procedures
Embryonic Heart Explants
Hearts from fertilized quail eggs (Coturnix Japonica) were removed following 6 days of incubation and the apical ventricular quarter placed on collagen gels in culture plates, as previously detailed (Yue et al., 1999; Tomanek et al., 2002). Following a 2 day incubation in M199 plus 10% fetal bovine serum, the explants were exposed to low (0.5%) serum for 1 day. Then the explants were exposed for 48 hours to various FGF proteins with or without the addition of VEGF-trap (VEGF-Intercep). To visualize vascular tubes and free ECs, the explants were immunostained with QH1 antibody (Hybridoma Bank, University of Iowa). A digital cameral was used to capture explant images by using fluorescein isothiocyanate (FITC)-labeled goat, anti-mouse secondary antibody. Tubulogenesis was quantified by measuring the aggregate length of vascular tubes and expressing this value per perimeter of the explant.
Embryonic mouse heart explants were placed on collagen gels, as described above for quail explants. They were obtained from FVB (Harlan) pregnant mice at 14 days gestation after anesthetizing the mother with a lethal dose of Ketamine HCl. On the third day of incubation in 10% bovine serum, FGF proteins, soluble receptors, VEGF protein or mouse EMSCs were added. Then, 3 days later (6 days after explantation), explants were viewed under an inverted microscope and images captured with a digital camera. Following fixation in 4% paraformaldehyde and immunoreaction with Bandeiraea Simplicifolia lectin (BS-I) conjugated with Alexa Fluor 594, images were captured with a confocal microscope. Endothelial cells and tubes were visualized by the BS-I staining. Some explants were fixed in a solution containing 1.5% glutaraldehyde, 0.2% paraformaldehyde and 0.03 M CaCl2 and 0.1 M cacodylate, and post-fixed in OsO4 (Tomanek et al., 1991). The dehydrated specimens were embedded in Spurr's plastic and ultra-thin sections cut with an ultramicrotome. Digital images were captured with a JOEL JEM-1230 transmission microscope.
Apoptosis in mice explants was evaluated 6 days after explantation. The method is that modified by Dr. Robert Thompson, Medical University of South Carolina. Caspase-3 activation was used to assess the degree of apoptosis. Explants fixed with 4% paraformaldehyde were exposed, overnight, to anti-activated caspase antibody, rinsed in Sanger buffer and then incubated with the secondary antibody, Cy2. DAPI immunostaining provided a marker for nuclei.
Reagents
Growth factor proteins
We utilized human recombinant FGF and VEGF proteins. FGFs 1 and 4 and VEGF165 were purchased from R&D Systems (Minneapolis); sources for the other proteins were: FGF-2 (BD Biosciences, San Jose, CA), FGFs 8, 9 and 18 (Invitrogen, Carlsbad, CA).
Soluble FGFR construction and adenovirus production
To generate sFGFR constructs as previously described (Murakami et al., 2008b), the extracellular domain of mouse FGFR-1 IIIc (gift from A. Mansukhani, New York University), mouse FGFR-3 IIIb and mouse FGFR-3 IIIc (gift from D. Ornitz, Washington University) were amplified by PCR with 5′ HindIII and 3′ BamHI sites, respectively. After digestion with these restriction enzymes, PCR products were fused in frame at the 5′ side of the hinge-CH2-CH3 region of human IgG1 (CDM7B-/CD5-Ig vector). For adenoviral production, sFGFR cDNAs were subcloned into an adenovirus shuttle vector, and nonreplicative, recombinant adenovirus vectors were generated and propagated to high titer using 293A cells. The expression and inhibitory effects of the sFGFR adenoviruses have been extensively tested both in vitro and in vivo.
VEGF-trap (aflibercept)
This VEGF R1, R2 chimera was provided by Regeneron Pharmaceuticals, Inc. (Tarrytown, NY) and used to bind ligands for VEGFR-1 and VEGFR-2 as previously utilized in our laboratory (Tomanek et al., 2002).
SDF-1α protein
We used the commercially available anti-mouse ELISA kit (Ray Biotech, Inc., Norcross, GA) according to the manufacturer's guidelines. Absorbance values plotted against standard curves yielded a correlation coefficient > 0.99.
Embryonic Mesenchymal Stem Cells (EMSC)
Mouse EMSCs (C3H/10T 1/2, Clone 8) were purchased from ATCC (Manassas, VA). The cells were subcultured in Eagle's Basal Medium with 2mM L-glutamine and modified Carle's BSS. We added either 10 or 25 K cells per tissue culture.
Quantitative Analysis and Statistics
Using a Pro-Image Program, we quantified tubulogenesis in 2 ways. For quail hearts, aggregate (total) tube length/perimeter of explant was used. For mouse explants, we scanned 4 fields obtained from 4 regions at 12, 3, 6 and 9 o'clock and noted the area occupied by tubes. Free EC density was quantified by counting the cells in the 16 fields comprising the 4 regions, while the migration index was based on the number of ECs in the distal zone of each of the 4 fields. All comparisons were between controls and each treatment group. Statistically significant differences (p ≤ 0.05) were determined by Dunnett's Method for multiple comparisons. In most cases, we adjusted the mean of the control group to 1 and expressed the values of the treatment groups as a ratio of the control.
Acknowledgments
This study was supported by funds from the National Institutes for Health grant R01HL075446. The authors gratefully acknowledge Dr. Robert Thompson, Medical University of South Carolina, for his advice and protocols regarding our experiments on apoptosis.
References
- Antoine M, Wirz W, Tag CG, Gressner AM, Wycislo M, Muller R, Kiefer P. Fibroblast growth factor 16 and 18 are expressed in human cardiovascular tissues and induce on endothelial cells migration but not proliferation. Biochem Biophys Res Commun. 2006;346:224–233. doi: 10.1016/j.bbrc.2006.05.105. [DOI] [PubMed] [Google Scholar]
- Antoine M, Wirz W, Tag CG, Mavituna M, Emans N, Korff T, Stoldt V, Gressner AM, Kiefer P. Expression pattern of fibroblast growth factors (FGFs), their receptors and antagonists in primary endothelial cells and vascular smooth muscle cells. Growth Factors. 2005;23:87–95. doi: 10.1080/08977190500096004. [DOI] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzaffour M, Dufourcq P, Lecaudey V, Haas P, Vriz S. Fgf and Sdf-1 pathways interact during zebrafish fin regeneration. PLoS One. 2009;4:e5824. doi: 10.1371/journal.pone.0005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Cormier S, Leroy C, Delezoide AL, Silve C. Expression of fibroblast growth factors 18 and 23 during human embryonic and fetal development. Gene Expr Patterns. 2005;5:569–573. doi: 10.1016/j.modgep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, Buschmann MD, Henderson JE. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280:20509–20515. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- Dell'Era P, Belleri M, Stabile H, Massardi ML, Ribatti D, Presta M. Paracrine and autocrine effects of fibroblast growth factor-4 in endothelial cells. Oncogene. 2001;20:2655–2663. doi: 10.1038/sj.onc.1204368. [DOI] [PubMed] [Google Scholar]
- Detillieux KA, Sheikh F, Kardami E, Cattini PA. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57:8–19. doi: 10.1016/s0008-6363(02)00708-3. [DOI] [PubMed] [Google Scholar]
- Estival A, Monzat V, Miquel K, Gaubert F, Hollande E, Korc M, Vaysse N, Clemente F. Differential regulation of fibroblast growth factor (FGF) receptor-1 mRNA and protein by two molecular forms of basic FGF. Modulation of FGFR-1 mRNA stability. J Biol Chem. 1996;271:5663–5670. doi: 10.1074/jbc.271.10.5663. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fukuyama N, Tanaka E, Tabata Y, Fujikura H, Hagihara M, Sakamoto H, Ando K, Nakazawa H, Mori H. Intravenous injection of phagocytes transfected ex vivo with FGF4 DNA/biodegradable gelatin complex promotes angiogenesis in a rat myocardial ischemia/reperfusion injury model. Basic Res Cardiol. 2007;102:209–216. doi: 10.1007/s00395-006-0629-9. [DOI] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Lu J, Chen H, Werner S, Williams LT. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol Cell Biol. 1991;11:4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SY, Cho KA, Jung YJ, Kim HS, Park SY, Choi YB, Hong KM, Woo SY, Seoh JY, Cho SJ, Ryu KH. Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy. 2010;12:361–370. doi: 10.3109/14653240903502712. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Sakamoto H, Terada M, Ochiya T. Pleiotropic function of FGF-4: its role in development and stem cells. Dev Dyn. 2009;238:265–276. doi: 10.1002/dvdy.21699. [DOI] [PubMed] [Google Scholar]
- Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev Biol. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lee SH, Schloss DJ, Swain JL. Maintenance of vascular integrity in the embryo requires signaling through the fibroblast growth factor receptor. J Biol Chem. 2000;275:33679–33687. doi: 10.1074/jbc.M004994200. [DOI] [PubMed] [Google Scholar]
- Majesky MW. Development of coronary vessels. Curr Top Dev Biol. 2004;62:225–259. doi: 10.1016/S0070-2153(04)62008-4. [DOI] [PubMed] [Google Scholar]
- Mattila MM, Ruohola JK, Valve EM, Tasanen MJ, Seppanen JA, Harkonen PL. FGF-8b increases angiogenic capacity and tumor growth of androgen-regulated S115 breast cancer cells. Oncogene. 2001;20:2791–2804. doi: 10.1038/sj.onc.1204430. [DOI] [PubMed] [Google Scholar]
- McDowell LM, Frazier BA, Studelska DR, Giljum K, Chen J, Liu J, Yu K, Ornitz DM, Zhang L. Inhibition or activation of Apert syndrome FGFR2 (S252W) signaling by specific glycosaminoglycans. J Biol Chem. 2006;281:6924–6930. doi: 10.1074/jbc.M512932200. [DOI] [PubMed] [Google Scholar]
- Montano MM, Doughman YQ, Deng H, Chaplin L, Yang J, Wang N, Zhou Q, Ward NL, Watanabe M. Mutation of the HEXIM1 gene results in defects during heart and vascular development partly through downregulation of vascular endothelial growth factor. Circ Res. 2008;102:415–422. doi: 10.1161/CIRCRESAHA.107.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008a;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008b;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivey HE, Compton LA, Barnett JV. Coronary vessel development: the epicardium delivers. Trends Cardiovasc Med. 2004;14:247–251. doi: 10.1016/j.tcm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Pennisi DJ, Mikawa T. Normal patterning of the coronary capillary plexus is dependent on the correct transmural gradient of FGF expression in the myocardium. Dev Biol. 2005;279:378–390. doi: 10.1016/j.ydbio.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Pennisi DJ, Mikawa T. FGFR-1 is required by epicardium-derived cells for myocardial invasion and correct coronary vascular lineage differentiation. Dev Biol. 2009;328:148–159. doi: 10.1016/j.ydbio.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Mandriota S, Jeltsch M, Kumar V, Alitalo K. Vascular Endothelial Growth Factor (VEGF)-C Synergizes with Basic Fibroblast Growth Factor and VEGF in the Induction of Angiogenesis In Vitro and Alters Endothelial Cell Extracellular Proteolytic Activity. Journal of Cellular Physiology. 1998;177:439–452. doi: 10.1002/(SICI)1097-4652(199812)177:3<439::AID-JCP7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, Jauhiainen S, Cashion L, Gruchala M, Narvanen O, Taipale P, Kauppinen RA, Rubanyi GM, Yla-Herttuala S. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. Faseb J. 2003;17:100–102. doi: 10.1096/fj.02-0377fje. [DOI] [PubMed] [Google Scholar]
- Sonvilla G, Allerstorfer S, Stattner S, Karner J, Klimpfinger M, Fischer H, Grasl-Kraupp B, Holzmann K, Berger W, Wrba F, Marian B, Grusch M. FGF18 in colorectal tumour cells: autocrine and paracrine effects. Carcinogenesis. 2008;29:15–24. doi: 10.1093/carcin/bgm202. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis. 2005;8:273–284. doi: 10.1007/s10456-005-9014-9. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Aydelotte MR, Torry RJ. Remodeling of coronary vessels during aging in purebred beagles. Circ Res. 1991;69:1068–1074. doi: 10.1161/01.res.69.4.1068. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Hansen HK, Christensen LP. Temporally expressed PDGF and FGF-2 regulate embryonic coronary artery formation and growth. Arterioscler Thromb Vasc Biol. 2008;28:1237–1243. doi: 10.1161/ATVBAHA.108.166454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn. 2002;225:233–240. doi: 10.1002/dvdy.10158. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Lotun K, Clark EB, Suvarna PR, Hu N. VEGF and bFGF stimulate myocardial vascularization in embryonic chick. Am J Physiol. 1998;274:H1620–1626. doi: 10.1152/ajpheart.1998.274.5.H1620. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res. 2001a;88:1135–1141. doi: 10.1161/hh1101.091191. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Zheng W, Peters KG, Lin P, Holifield JS, Suvarna PR. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn. 2001b;221:265–273. doi: 10.1002/dvdy.1137. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin Cell Dev Biol. 2002;13:19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development. 2007;134:3743–3752. doi: 10.1242/dev.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Tomanek RJ. Stimulation of coronary vasculogenesis/angiogenesis by hypoxia in cultured embryonic hearts. Dev Dyn. 1999;216:28–36. doi: 10.1002/(SICI)1097-0177(199909)216:1<28::AID-DVDY5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Yue X, Tomanek RJ. Effects of VEGF(165) and VEGF(121) on vasculogenesis and angiogenesis in cultured embryonic quail hearts. Am J Physiol Heart Circ Physiol. 2001;280:H2240–2247. doi: 10.1152/ajpheart.2001.280.5.H2240. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Sasse J, McAllister D, Lough J. Evidence that fibroblast growth factors 1 and 4 participate in regulation of cardiogenesis. Dev Dyn. 1996;207:429–438. doi: 10.1002/(SICI)1097-0177(199612)207:4<429::AID-AJA7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]