Abstract
Objective
Loss of lean body mass with aging may contribute to falls and fractures. The objective of this analysis was to determine if taking postmenopausal hormone therapy (HT: estrogen plus progestogen therapy, EPT or estrogen therapy alone, ET) favorably affects age-related changes in lean body mass and if these changes partially account for decreased falls or fractures with HT.
Methods
Participants randomly assigned to either EPT (n=543) or control (n=471) and ET (n= 453) or control (n= 474) and receiving dual-energy X-ray absorptiometry (DXA) scans to estimate body composition during the Women’s Health Initiative (WHI) were evaluated. Falls and fracture occurrence were obtained by annual self-report. Fractures were confirmed by clinical chart review.
Results
At 6yrs post-randomization, lean body mass was not different between HT and control groups. Although lean body mass positively influenced BMD, independent of HT status, the preserved lean body mass observed in the HT arms in the first 3 years did not significantly contribute to models evaluating HT influence on falls and fractures between years 3 and 6. Women taking at least 80% of their medication in the HT arms demonstrated fewer falls compared to placebo; this difference was not attributable to change in lean body mass.
Conclusions
Despite early preservation of lean body mass with HT (3years), HT did not ameliorate long-term (6 years) loss in lean body mass with aging.
Keywords: estrogen therapy, estrogen plus progestogen therapy, menopause, muscle, falling, fractures
Women after menopause often experience a decrease in lean body mass,1 an increase in body fat mass and a shift to central or android fat distribution 2, which may increase their risk for sarcopenia, diabetes and cardiovascular diseases. These body composition changes are believed to be, at least in part, due to a sudden decline in endogenous estrogen production at the time of menopause. Therefore, it has been hypothesized that menopausal hormone therapy (HT) may help counter these changes in body composition among postmenopausal women.2–4 most of the previous investigations in this area have been limited to observational studies and clinical trials, with small sample sizes or short intervention times. Techniques for assessing body composition have varied widely across the studies and many findings have been based on anthropometric measurements, such as body mass index or hip and waist circumferences, as proxies for obesity and body fat distribution, but lacking assessments of lean body mass.
Recently, a subsample of the Women’s Health Initiative (WHI) Estrogen Plus Progestogen (EPT) Trial was used to investigate the affect of EPT on body composition.5 This subsample included women who had body composition measurements at baseline and year 3 from the three WHI bone mineral density (BMD) centers. The findings from the subsample indicated that a 3-year EPT intervention significantly helped to maintain lean body mass and prevented the shift toward android fat distribution in postmenopausal women. The effect size of EPT on lean body mass was relatively small (< 1 kg), however, we should expect that the beneficial treatment effect of E alone from the WHI ET trial on body composition to be at least as large as the findings from the WHI EPT trial, because previous studies have suggested that the magnitude of the impact of estrogen alone therapy on body composition might be larger than that of the EPT therapy6. It has been reported that both EPT and E alone interventions significantly reduced fracture risk among postmenopausal women.7,8 In order to understand the clinical implications of the beneficial effect of HT on lean body mass, we proposed to investigate whether the preserved lean body mass by HT contributed to the reduction of fracture risks among women assigned to hormone interventions in both the EPT and the ET trials. Most fractures occur because of a fall. Hence, we also tested whether the lean body mass effects of HT also influence fall risk.
We hypothesized that long-term use of postmenopausal HT (estrogen plus progestogen therapy, EPT; estrogen therapy alone, ET) would favorably affect age-related changes in lean body mass and that this favorable effect on lean body mass would contribute to a decrease in fractures.
METHODS
STUDY PARTICIPANTS AND PROCEDURES
Between 1993 and 1998 years a total of 10,739 postmenopausal women with prior hysterectomy were recruited and enrolled in the WHI ET trial, and 16,608 postmenopausal women with an intact uterus were recruited and enrolled into the WHI EPT trial, at 40 WHI clinical centers in the United States. Both trials were randomized, double-blind, placebo-controlled trials. The inclusion criteria were similar between the EPT and ET trials with the exception of hysterectomy among women in the ET trial; women were between 50–79 years and postmenopausal. The protocol and consent forms were approved by each institutional review board at each site and all participants provided written informed consent prior to participation. Details of the HT trials have been described previously.7,8
The participants were stratified by age and block randomized by clinical center to either hormone treatment or placebo group for both the ET and the EPT trials, where estrogen treatment in the ET trial was 0.625mg/d conjugated equine estrogen daily (Premarin, Wyeth, Philadelphia, PA) and estrogen plus progesterone treatment in the EPT trial was 0.625mg/d conjugated equine estrogen plus 2.5mg/d medroxyprogesterone acetate daily (Prempro, Wyeth, Philadelphia, PA). The washout period for prior hormone therapy use was 3 months prior to study initiation. Further design and participant details may be found in previously published articles. 9–11 The average length of participation in the ET trial was 7.7 ± 1.8 years and the EPT trial was 6.3 ± 1.5 years for those participating at the BMD clinical centers. Unblinding occurred in 2002 for the EPT trial and 2004 for the ET trial.
Body composition by dual energy X-ray absorptiometry (DXA) scans was assessed at 3 WHI BMD clinical centers (Pittsburgh, PA; Birmingham, AL; and Tucson-Phoenix, AZ) at baseline and every three years thereafter. Participants who completed the baseline, year 3 and year 6 DXA scans were included in this analysis. Diversity based on race/ethnicity was maximized in the WHI clinical centers where DXA measurements were conducted and therefore in this substudy, the distribution differed from the WHI cohort at large.
BODY COMPOSITION ASSESSMENT
Anthropometric measures included height, weight, and waist circumference. Weight was measured without shoes on a balance-beam scale to the nearest 0.1 kg. Height was measured by wall-mounted stadiometer to the nearest 0.1 cm. BMI was calculated as weight (kg)/height (m)2. Waist circumference was measured by non-elastic tapes on all women at baseline and on a subset for years 3 (n=1622) and 6 (n=1429).
Body composition measurements were performed by DXA scans (QDR2000, 2000+, or 4500W; Hologic Inc, Bedford, MA). Whole body DXA scans were used to determine both regional and total body composition. Measurements included bone mineral density, lean body mass (lean soft tissue mass), fat mass, percentage of fat mass and lean body mass.
Standard WHI protocols were used for the positioning and analysis of DXA scans by radiology technicians, trained and certified by Hologic and the WHI Bone Density Coordinating Center at the University of California, San Francisco. Daily and weekly phantom scans were performed at each site for quality control. Additionally, Hologic spine, hip, and block calibration phantoms were circulated and scanned across all WHI BMD sites and instruments. The WHI quality assurance program also included machine and technician performance monitoring by reviewing phantom scans, random sampling of scans, and review of scans with specific problems. The replacement of an older dual-energy X-ray absorptiometer with a newer model (QDR2000 to QDR4500W) was accounted for by linear regression equations developed from an independent sample of 50 women scanned with both DXA machines on the same day; adjustments for the total and regional body-composition assessments were made accordingly, as previously published.5
FALLING AND FRACTURE ASSESSMENTS
Falls were assessed by self-report semi-annually. Fractures that occurred after study initiation were reported and confirmed for WHI BMD sites with clinical chart review by trained physicians in the WHI. Total fractures were defined as all reported clinical fractures except those of the ribs, chest/sternum, skull/face, fingers, toes, and cervical vertebrae. These fractures were confirmed by radiographic or operative reports. All hip fractures were confirmed by central adjudication and other fractures by local adjudication.
ASSESSMENT OF COVARIATES
Covariates of age, time since menopause, energy intake and other dietary variables, energy expenditure, ethnicity, smoking and alcohol habits, physical function, and prior HT use were assessed from baseline questionnaires. The last reported menstrual bleeding, time of bilateral oophorectomy, or initiation of menopausal HT was used to determine age at menopause and years of menopause. A validated food frequency questionnaire was used to assess energy intake, macronutrients, vitamins and minerals.12 To assess energy expenditure, the Compendium of Physical Activities by Ainsworth et al was used to assign metabolic equivalent values to reported weekly recreational activities (ratio of work metabolic rate to resting metabolic rate by activity type).13 Physical function was assessed by the Medical Outcomes Study Scale, where the higher the score, the higher physical function indicated.14
STATISTICAL ANALYSIS
Student’s independent t-tests for continuous variables and Chi-square tests for categorical variables were used to compare characteristics, falls, fractures and body composition between intervention groups for each trial at baseline, as well as for changes in body composition. Mixed models were used to test the average differences in lean mass and differences in the slope of change with time between the intervention and the placebo groups. Models further evaluating the contribution of lean body mass on falls and fractures were run with the intervention and control groups from each trial combined (i.e. ET plus EPT versus ET placebo group combined with EPT placebo group to form active HT versus placebo) based on the similarity of baseline characteristics between HT and control groups in each trial, as well as the lack of HT effect on body composition at the primary endpoint of 6 years in this substudy. This collapse of groups to form active HT versus placebo yielded greater power and was soundly based on the lack of between group differences at baseline and lack of difference between each HT group and its respective placebo for lean body mass at 6 years.
Cox proportional hazards survival models were used to investigate whether changes in lean body mass partially explained the treatment effect of HT on fracture risk by comparing the models with and without lean body mass. A similar logistic regression approach was used to examine whether HT was related to risk of falling and whether lean body mass is an explanatory factor in this relationship. In agreement with other large trials, a binary outcome variable of “faller” was created with fallers defined as those with ≥2 falls per year 15,16 because a history of ≥2 falls per year is a significant predictor of a recurrent faller 17 and a higher rate of falling is more highly associated with frailty related fractures.18 Based on prior evidence of a positive effect of HT on lean body mass at year 3 in the EPT trial,5 logistic regression and Cox proportional hazards models were created using change in lean body mass data from baseline to year 3 to predict falls and fractures between years 3 and 6. Potential confounders such as, baseline age, weight, ethnicity, geographic region of enrollment, number of years since menopause, HT use history, fracture history, and broken bone at or after age 55 years, fall history, energy expenditure on recreational activities, physical function score, change in fat and change in weight were evaluated by comparing unadjusted and adjusted associations of HT with outcomes; greater than 10% relative change between unadjusted and adjusted models was the a priori cut-point to identify a confounder. No potential confounders were identified, thus covariates were not included in analyses. Due to unblinding of some subjects prior to the year 6 DXA scan, analyses were run to compare those unblinded prior to year 6 DXA versus those that maintained blinded status at year 6; there was no significant effect of unblinding on fall or fracture analyses, therefore intent-to-treat analyses were used including all participants.
Both intent-to-treat and sensitivity analyses were performed in comparisons between active HT (E and EPT) and placebo groups to account for potential differences in the primary body composition outcomes based on medication compliance. Those that were ≥80% compliant were compared to the total study cohort. All analyses were conducted using STATA software, version 10 (STATA Corp. College Station, TX). Significance was set for 2-tailed tests at α = 0.05.
RESULTS
DESCRIPTIVE CHARACTERISTICS OF PARTICIPANTS AT BASELINE
Baseline descriptive characteristics for participants in the WHI ET and EPT trials at the WHI BMD sites are presented in Table 1. Key demographic characteristics and habits were evaluated, in addition to physical function and fracture history. Within each trial, there were no differences between placebo and active HT intervention arms, with the exception of a trend toward differences in ethnic distribution between arms in the EPT trial, due to low sample sizes in some minority groups. Table 2 describes baseline body composition by anthropometry and DXA derived tissue specific measures, such as percent fat mass and lean body mass. All body composition measures were found to be similar between active HT and placebo groups for both the ET and EPT trials. Secondarily, although the mean age (63.3 ±7.4yrs) and energy intake (1699.0 ± 863.1 kcal) were similar across all treatment and placebo groups at baseline, energy expenditure was higher overall in the EPT trial versus the ET trial (11.67 ± 14.56 and 9.70 ± 12.14 METs per week respectively, p=0.005) and the time since menopause in the ET trial was greater than that in the EPT trial (22.21 ± 8.38 and 13.57 ± 8.52, respectively, p<0.0001). Similarly, physical functioning was not significantly different between intervention and control arms, but the percentage of women with physical function scores >90 was higher in the EPT trial (28% ET versus 38% EPT, p<0.001). BMI and waist circumference were also significantly lower in the EPT trial versus the ET trial (BMI: 29.93 ± 5.57 ET, 28.22 ± 5.45 EPT kg/m2; Waist: 90.01 ± 13.12ET, 85.85 ± 12.64 EPT cm, p<0.0001), while prior hormone use was higher in the ET trial than in the EPT trial (p<0.0001). In addition, baseline variables in completers versus dropouts were compared by t-tests and demonstrated statistically significant differences between age at screening, time since menopause, weekly energy expenditure, physical functioning scores > 90, smoking status, supplemental vitamin d, and whole body lean tissue mass (kg) (data not presented). However, these variables were not found to be confounders in further analyses.
Table 1.
Baseline characteristics by intervention group.
| Estrogen Trial (ET) |
Estrogen Plus Progestogen Trial (EPT) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active n=453 | Placebo n=474 | p1 | Active n=543 | Placebo n=471 | p2 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (y) | 63.3 | 7.6 | 63.4 | 7.6 | 0.78 | 63.2 | 7.2 | 63.4 | 7.1 | 0.76 |
| Time since menopause (y) | 21.9 | 8.1 | 22.5 | 8.7 | 0.43 | 13.3 | 8.9 | 13.8 | 8.1 | 0.44 |
| Dietary energy intake (kcal) | 1717.3 | 957.7 | 1660.9 | 865.5 | 0.35 | 1731.6 | 823.9 | 1682.3 | 808.5 | 0.34 |
| Dietary protein (g) | 70.4 | 37.0 | 67.9 | 36.6 | 0.31 | 71.7 | 34.4 | 69.9 | 35.6 | 0.40 |
| Dietary calcium (mg) | 791.4 | 481.8 | 767.7 | 466.5 | 0.45 | 839.0 | 466.0 | 819.5 | 505.4 | 0.53 |
| Dietary vitamin d (mcg) | 4.0 | 2.8 | 4.2 | 3.2 | 0.48 | 4.3 | 3.0 | 4.3 | 3.1 | 0.78 |
| Total weekly recreational energy expenditure (MET) | 10.1 | 12.8 | 9.3 | 11.4 | 0.43 | 11.4 | 14.6 | 11.9 | 14.6 | 0.65 |
| N | % | N | % | p1 | N | % | N | % | p2 | |
| Physical functioning score ≥ 90 | 0.31 | 0.89 | ||||||||
| no | 323 | 73.6 | 328 | 70.5 | 332 | 61.9 | 285 | 62.4 | ||
| yes | 116 | 26.4 | 137 | 29.5 | 204 | 38.1 | 172 | 37.6 | ||
| History of pre-study fracture | 0.15 | 0.89 | ||||||||
| no | 202 | 57.39% | 216 | 62.79% | 232 | 62.70% | 237 | 63.20% | ||
| yes | 150 | 42.61% | 128 | 37.21% | 138 | 37.30% | 138 | 36.80% | ||
| Ethnicity | 0.26 | 0.06 | ||||||||
| White | 338 | 74.8 | 337 | 71.1 | 447 | 82.5 | 385 | 81.7 | ||
| Black | 76 | 16.8 | 98 | 20.7 | 45 | 8.3 | 54 | 11.5 | ||
| Hispanic | 32 | 7.1 | 34 | 7.2 | 39 | 7.2 | 22 | 4.7 | ||
| American Indian/Alaskan | ||||||||||
| Native | 3 | 0.7 | 4 | 0.8 | 4 | 0.7 | 8 | 1.7 | ||
| Asian/Pacific Islander | 0 | 0.0 | 1 | 0.2 | 4 | 0.7 | 0 | 0.0 | ||
| Other | 3 | 0.7 | 0 | 0.0 | 3 | 0.6 | 2 | 0.4 | ||
| Hormone use | 0.11 | 0.83 | ||||||||
| Never | 268 | 59.2 | 295 | 62.2 | 429 | 79.2 | 369 | 78.3 | ||
| Past | 162 | 35.8 | 144 | 30.4 | 95 | 17.5 | 83 | 17.6 | ||
| Current3 | 23 | 5.1 | 35 | 7.4 | 18 | 3.3 | 19 | 4.0 | ||
| Smoking | 0.58 | 0.87 | ||||||||
| Never | 257 | 57.1 | 253 | 54.1 | 294 | 54.5 | 244 | 53.3 | ||
| Past | 145 | 32.2 | 157 | 33.5 | 189 | 35.1 | 168 | 36.7 | ||
| Current | 48 | 10.7 | 58 | 12.4 | 56 | 10.4 | 46 | 10.0 | ||
| Alcohol use | 0.57 | 0.56 | ||||||||
| Non-drinker | 199 | 44.4 | 223 | 47.5 | 210 | 39.0 | 176 | 37.8 | ||
| <7 drinks per week | 224 | 50.0 | 218 | 46.5 | 282 | 52.3 | 240 | 51.5 | ||
| >7 drinks per week | 25 | 5.6 | 28 | 6.0 | 47 | 8.7 | 50 | 10.7 | ||
comparison between active and placebo for ET trial
comparison between active and placebo for EPT trial
required 3 month washout period
MET, metabolic equivalent
Table 2.
Baseline body composition measurements by intervention group and change in body composition from baseline to year 6 (B-Y6)
| Estrogen Trial (ET) |
Estrogen plus Progestogen Trial (EPT) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active (n=453) | Placebo (n=474) | p1 | Active (n=543) | Placebo (n=471) | p2 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Baseline Anthropometric measures | ||||||||||
| Weight (kg) | 77.71 | 15.61 | 78.01 | 15.63 | 0.77 | 73.36 | 14.73 | 73.94 | 15.24 | 0.54 |
| BMI (kg/m2) | 30.00 | 5.61 | 29.86 | 5.53 | 0.70 | 28.14 | 5.40 | 28.30 | 5.50 | 0.65 |
| Waist (cm) | 90.11 | 13.80 | 89.92 | 12.45 | 0.82 | 85.45 | 12.22 | 86.32 | 13.11 | 0.28 |
| Baseline Tissue specific measures | ||||||||||
| Fat Mass (kg) | 35.91 | 11.41 | 35.77 | 11.42 | 0.85 | 32.52 | 11.00 | 32.51 | 11.19 | 0.99 |
| Percent Fat Mass (%) | 46.02 | 6.76 | 45.67 | 6.69 | 0.43 | 43.96 | 7.37 | 43.62 | 7.30 | 0.46 |
| Lean Mass (kg) | 38.49 | 5.45 | 38.82 | 5.77 | 0.36 | 37.73 | 5.17 | 38.23 | 5.42 | 0.13 |
| Percent Lean Mass (%) | 51.26 | 6.52 | 51.54 | 6.31 | 0.50 | 53.23 | 7.06 | 53.58 | 7.05 | 0.43 |
| Change in Tissue specific measures from Baseline to Year 6 | ||||||||||
| (n=323) | (n=350) | (n=392) | (n=337) | |||||||
| Fat Mass (kg) | 0.05 | 5.68 | 0.12 | 5.50 | 0.87 | 0.64 | 5.43 | 0.43 | 5.33 | 0.59 |
| Percent Fat Mass (%) | 0.01 | 0.16 | 0.01 | 0.16 | 0.96 | 0.03 | 0.23 | 0.02 | 0.18 | 0.47 |
| Lean Mass (kg) | −0.44 | 2.28 | −0.50 | 2.45 | 0.72 | −0.29 | 1.99 | −0.40 | 2.15 | 0.46 |
| Percent Lean Mass (%) | −0.01 | 0.06 | −0.01 | 0.06 | 0.98 | −0.01 | 0.05 | −0.01 | 0.05 | 0.33 |
comparison between active and placebo for ET trial
comparison between active and placebo for EPT trial
BMI, Body Mass Index
CHANGES IN BODY COMPOSITION OVER 6 YEARS
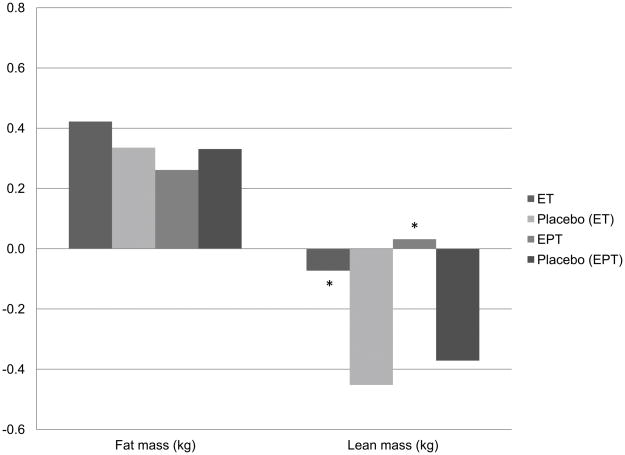
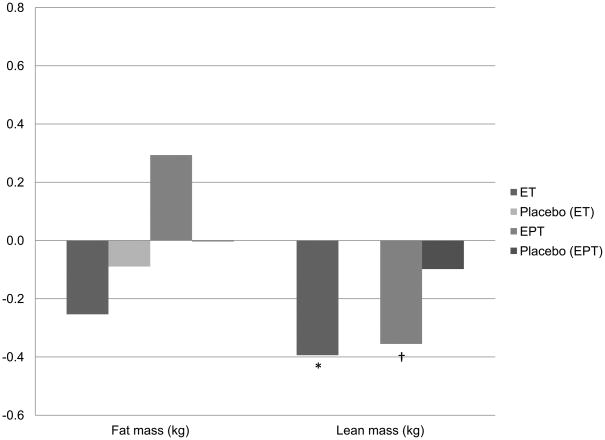
Figure 1 reflects the change in lean and fat mass (kg) over the first 3 years of each trial, with Figure 2 spanning years 3 to 6. Table 2 also demonstrates the overall change in lean and fat mass between intervention groups from baseline to 6 years. Fat mass change was not significantly different between intervention and control groups at any of the time points examined. Lean body mass loss was significantly less in each of the active HT intervention arms of the ET and EPT trials compared to their respective placebo groups at 3 years (Figure 1). Between years 3 and 6 the relationship was reversed; the active ET group lost significantly more lean body mass than the respective placebo group (p<0.05); there was a trend towards a greater loss of lean body mass among active EPT users versus the respective placebo group, as well (Figure 2, p=0.06). Thus, the effects over the full 6 years, presented in Table 2, appear to be null; no significant differences between active and placebo groups were detected in either trial for change in lean body mass (kg) over 6 years. Changes in appendicular lean body mass followed the same pattern at years 3 and 6 (data not presented). Overall, we saw a small decrease in lean body mass across all groups ranging from −0.29kg to −0.50kg over 6 years, with great variance; standard deviations ranged from 1.99kg to 2.45kg. The loss of lean body mass over 6 years on HT was less than lean body mass loss without HT, but the difference was not significant. When treatment arms were compared between trials, there was no significant difference between active ET and active EPT treatments for loss of lean body mass at 6 years. These findings were confirmed by mixed models (adjusted for age, weight, ethnicity, geographic region of enrollment, number of years since menopause, HT use prior to enrollment (ever), energy expenditure on recreational activities), where no significant effect of HT on lean body mass at 6 years was seen in either the ET or EPT trial (data not presented). These body composition findings for the overall group were in agreement with the findings in the subset of women taking at least 80% of their medication (data not presented); there were no treatment effects detected for either HT regimen on lean or fat mass compared to placebo over 6 years.
Figure 1.
Change in kilograms of total body fat and lean body mass from baseline to year 3.
ET Active n= 367, Placebo n=395; EPT Active n=438, Placebo n= 398.
*p<0.05 for comparisons between active ET or EPT and respective placebo.
Figure 2.
Change in kilograms of total body fat and lean body mass between years 3 and 6.
ET Active n= 306, Placebo n=330; EPT Active n=369, Placebo n= 322.
*p<0.05; †p=0.06 for comparisons between active ET or EPT and respective placebo.
THE RELATIONSHIP BETWEEN HORMONE THERAPY, CHANGE IN LEAN BODY MASS, AND FALLS OR FRACTURES
Table 3 presents odds ratios for falls occurring between years 3 and 6 for active hormone therapy versus placebo. There was a non-significant reduction in annual fall risk (≥2 falls per year) between years 3 and 6 with HT use. These logistic regression models in the total sample did not show an independent effect of the early lean body mass change, baseline to 3 years, on decreased risk of falling between year 3 and year 6. The relationship between HT and fall risk was not altered by change in lean body mass between baseline and year 3 or by baseline lean body mass. Upon exploration of HT trials separately, neither ET nor EPT alone significantly altered falls (≥2 falls per year) between years 3 and 6 compared to placebo in logistic regression models. The potential trend towards a reduction in falls for ET alone (ET OR 0.48, 95% CI: 0.22–1.04, p=0.06) was not explained by change in lean from baseline to year 3 or by baseline lean body mass. The relationship between EPT and falls was not significant (p=0.46, data not presented). The median number of falls per person between years 3 and 6 differed between treatment and control by Wilcoxon rank-sum test for ET alone and HT combined (p=0.005 and 0.03 respectively, supplemental table), however, the contribution of lean body mass to these differences could not be evaluated by this non-parametric test.
Table 3.
Odds ratios for falls occurring between 3 to 6 years for active hormone therapy versus placebo.
| Total Sample | OR | 95% CI | p | |
|---|---|---|---|---|
| Model 1 | ||||
| Hormone | 0.78 | 0.46 | 1.33 | 0.36 |
| Model 2 | ||||
| Hormone | 0.72 | 0.40 | 1.27 | 0.25 |
| Change in Lean Body Mass (B to Y3) | 1.08 | 0.97 | 1.21 | 0.16 |
| Model 3 | ||||
| Hormone | 0.71 | 0.40 | 1.27 | 0.25 |
| Change in Lean Body Mass (B to Y3) | 1.08 | 0.96 | 1.21 | 0.22 |
| Baseline Lean Body Mass | 0.99 | 0.94 | 1.04 | 0.71 |
CI, confidence interval, B to Y3, Baseline to Year 3
The odds ratio for Change in Lean Mass (B to Y3) corresponds to a 1-kg increase in lean mass.
Table 4 presents hazard ratios for total fractures occurring between years 3 and 6, comparing active HT versus placebo. In this sub-analysis of the WHI BMD sites, there was a non-significant reduction in fracture risk between years 3 and 6 with HT use. Change in lean body mass over the first 3 years of the trials was not independently associated with reduced risk of fractures between years 3 and 6, as evaluated by Cox proportional hazard models. The early change in lean body mass, baseline to year 3, did not alter the relationship between HT and fracture risk between years 3 and 6. Baseline lean mass did not alter the relationship between HT and fractures between years 3 and 6 either. When ET and EPT trials were explored separately, the results were not appreciably different from that of the HT combined analyses with respect to fractures.
Table 4.
Total fracture hazard ratios for fractures occurring between 3 to 6 years comparing active HT versus control.
| HR | 95% CI | p | ||
|---|---|---|---|---|
| Model 1 | ||||
| Hormone | 0.79 | 0.50 | 1.24 | 0.30 |
| Model 2 | ||||
| Hormone | 0.76 | 0.48 | 1.22 | 0.26 |
| Change in Lean Body Mass (B to Y3) | 1.05 | 0.93 | 1.19 | 0.42 |
| Model 3 | ||||
| Hormone | 0.76 | 0.48 | 1.21 | 0.25 |
| Change in Lean Body Mass (B to Y3) | 1.05 | 0.92 | 1.19 | 0.46 |
| Baseline Lean Body Mass | 0.99 | 0.95 | 1.03 | 0.64 |
CI, confidence interval, B to Y3, Baseline to Year 3
The hazard ratio for Change in Lean Mass (B to Y3) corresponds to a 1-kg increase in lean mass.
DISCUSSION
The WHI ET and EPT trials are of the largest and longest randomized controlled trials to examine the affects of postmenopausal HT, providing an opportunity to evaluate the affects of HT on lean body mass and the lean body mass relationship to falls and fractures post-administration of HT. Based on a prior evaluation of the WHI EPT trial (year 3)5 the ongoing preservation of lean body mass via EPT was considered likely and the affect of E alone on lean body mass was hypothesized to match or exceed that of EPT over 6 years.6 We were able to confirm that lean body mass loss was less with either ET or EPT treatment at 3 years compared to placebo, however, despite plausible biological linkages and prior short-term evidence, the early preservation of lean body mass was lost by 6 years and there were no differences between placebo and HT treatment groups. Thus, there was a delay of lean body mass loss with HT, but the benefit did not persist long-term.
Others have also found short term enhancement of lean body mass with HT. In the Bone Estrogen and Strength Training trial, postmenopausal women using and not using HT who were randomly assigned to the no exercise group were followed during the intervention year for changes in body composition. Women in the no exercise, no HT group lost significant lean body mass, whereas women in the no exercise, HT group preserved lean body mass and demonstrated a trend towards gain in lean body mass (p=0.08) over one year. A similar study by Sipila et al,19 also found that lean body mass was enhanced by one year of HT compared to control without exercise. Sorensen et al conducted a short-term crossover study of HT among postmenopausal women which implicated a role for HT in increasing lean body mass.4 These results from smaller trials support the early preservation of lean body mass noted in the WHI report of EPT at year 35 and in ET at year 3 from this analysis.
In contrast, several other 2–3 year trials with various measures of lean body mass following hormone therapy in postmenopausal women have found no effect of HT. Sites et al found no effect of EPT on lean body mass compared to placebo over 2 years.20 Kenny et al found no change in lean body mass over 3 years with ultra-low dose ET.21 The Women’s Health, Osteoporosis, Progestin, Estrogen (HOPE) Trial, a randomized, double-blind, placebo-controlled trial of women (n=749) randomized to various HT regimens (4 doses of ET, 3 doses of EPT, plus 1 placebo group), found no differences between groups for DXA derived changes in lean body mass over 2 years.22 All groups experienced similar, small losses in lean body mass. Finally, Tanko et al found no change in appendicular lean body mass with hormone replacement therapy over 3 years and noted that the trend towards a decrease in appendicular lean body mass in HT groups compared to placebo between years 2 and 3 may suggest a catabolic rather than anabolic effect of HT over time.23 Although not a focus of this manuscript, upon evaluation of the WHI HT intervention cohorts, appendicular lean body mass followed the same pattern as overall lean body mass, i.e. preservation at year 3, but no differences between treatment and control arms at 6 years, and thus is not in agreement with the findings of Tanko et al and does not support a catabolic affect of HT on lean body mass. In agreement with our findings, a 5 year randomized controlled trial of HT, the Danish Osteoporosis Prevention Study (DOPS), found no significant difference between treatment and control for change in lean body mass over 5 years.24
Importantly, the rapid loss of lean body mass seen by others during early menopausal years1 was not evident in these cohorts, regardless of treatment arm. All groups within the WHI BMD sites, regardless of assignment to HT or placebo, lost ≤0.5kg over six years, as opposed to 2–3kg per year in the 3 year randomized placebo controlled trial by Aloia et al.1 Our 3 year results contrast with that of Aloia et al, in that we found that HT was associated with preservation of lean body mass, but by year 6 of our analysis there were no differences between the HT and placebo arms for change in lean body mass. Although the DOPS study in younger women (45–58 years) also demonstrated minimal lean body mass loss over 5 years,24 we may speculate that the relatively long time since menopause (13–22 years) among the women of the WHI HT trials included in the WHI BMD sites and the small degree of change in lean body mass overall during this window of time may have affected our ability to detect a long term effect of HT on lean body mass. Data from women participating in The Third National Health and Nutrition Examination Survey (NHANES III) evaluation of lean body mass by bioelectrical impedance supports a general decline in the pace of lean body mass loss as menopause progresses if we compare by age groups spanning typical menopausal years. The prevalence of normal lean body mass decreased by 20% when comparing age groups 40–49 years to 50–59 years; the prevalence of normal lean body mass declined by another 11% by ages 60–69 years and then remained level until 80+ years.25 The average age of our cohorts at baseline was 63 years. Thus, it is also possible that if investigated earlier in the menopausal years, the affect of HT on lean body mass over 6 years may be more in line with our hypothesis of longer term preservation with HT administration.
In this analysis we also evaluated the contribution of the early preservation of lean body mass with HT (baseline to year 3) to the risk of annual falls and the incidence of fracture in later years. We hypothesized that the reduction in fractures known to accompany HT 10,11 may be partially explained by the preservation of lean body mass and that lean body mass preservation may also contribute to reduced falls. These hypotheses were based on earlier work by Baumgarter et al and Melton et al demonstrating associations between critically low lean body mass, termed sarcopenia, and falls and fractures.26,27 In the presence of HT, change in lean body mass has also been a predictor of BMD, which is related to fracture risk.24 These hypotheses were primarily rejected by results from this analysis. We did not find a relationship between the preservation of lean body mass up to year 3 and subsequent annual falls and fractures between years 3 and 6.
In agreement with the findings of a large observation study, The Study of Osteoporotic Fractures, we found that postmenopausal HT use did not significantly reduce the risk of falling.28 HT was associated with a non-significant risk reduction in both falls and fractures between years 3 and 6. However, there was a suggestion of decreased risk of total falls over 6 years with HT which may occur by an unknown mechanism and is worthy of further investigation.
Although not significant in this sub-sample, the fracture risk reduction was within range of the main WHI EPT 10 and ET trials.11 However, these findings were not explained by early preservation of lean body mass via hormone therapy for falls or for fractures. The WHI Observational Study points towards other soft tissue changes in risk of falling and fractures; they recently reported that the more obese may be at greater risk of falling and site specific fractures,29 which was not evaluated in this substudy of the hormone therapy intervention arms of WHI. In this analysis we found a non-significant 8% increase in risk of falling and 5% increase in total fractures with increased lean body mass, which may be an indication of increased overall body size and would support the recent WHI Observational Study results. When we evaluated change in weight and change in body fat in the respective statistical models, there was no independent effect of weight or body fat on falls or total fractures and the relationships between HT, lean body mass, and falls and fractures were not changed, however site specific fractures were not evaluated.
The present analysis was focused on change in lean body mass which was not predictive of falling and fractures and other long-term trials relating hormone therapy induced changes in lean body mass to risk of falling and fracture could not be located for comparison. However, there is evidence that exercise training can both improve muscle function and reduce risk of falling.30 A recent meta-analysis suggested that hormone therapy may also improve strength,31 which may lead to reduced risk of falling and subsequent fractures. Further research is needed to evaluate strength enhancement by HT and fall and fracture patterns over several years.
The randomized, placebo controlled trial design, relatively large sample size, and lengthy duration (6 years) of the analysis are major strengths of this substudy of the WHI HT trials. This substudy is an important addition to the previously published 3 year analysis of EPT affects on body composition5 which included baseline and year 3 measurements only. Herein we were able to demonstrate that ET alone conferred similar lean body mass benefits at 3 years. This 6 year analysis also allowed us to examine trends in HT affects on lean body mass, and although it may be informative to examine even longer periods of HT treatment, the analysis was limited to the time period in which we had the greatest number of blinded subjects in both the ET and EPT trials.
Importantly, the mean BMI and mean age in the subsample were representative of the entire WHI ET and EPT cohorts by intervention assignments at baseline.10,11 Although other baseline characteristics, such as ethnicity, varied slightly between the WHI cohorts at large and the WHI BMD sites, block randomization occurred by clinical center, such that we would expect the similar results if these analyses were repeated in the entire WHI cohort. Additionally, this study was limited by the small number of fractures for specific sites so the affects of lean body mass on fracture risk at hip, lumbar spine and forearms could not be assessed separately. Our relatively small number of events (falls and fractures) was a limitation of our study and future studies with larger sample sizes and longer follow-up may provide more insight into the relationship between lean mass, falls, and fractures because a greater number of events will be more likely. The use of DXA-derived lean body mass to proxy skeletal muscle mass is also a limitation of this study. Although a good relationship between DXA-derived lean body mass and skeletal muscle mass measured on MRI has already been reported,32 whether DXA is sensitive enough to detect changes in lean body mass is unknown.
CONCLUSIONS
In conclusion, hormone therapy conferred an early benefit of preserved lean body mass in postmenopausal women, but longer term preservation of lean body mass did not endure. Additionally, the early preservation of lean body mass in these cohorts did not predict risk of falling or fracture. In addition, there is some indication that HT may reduce the risk of falls, but further study will be needed to evaluate the threshold of post-menopausal lean body mass improvement, including strength changes, required to decrease risk or falls and fractures, with and without HT.
SHORT LIST OF WHI INVESTIGATORS
Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center
(Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers
(Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence S. Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon.
Women’s Health Initiative Memory Study
(Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Supplementary Material
Acknowledgments
Funded by: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. Dr. Bea was supported by an R25T Cancer Prevention and Control Fellowship (NIH/NCI CA-78447) during the writing of this manuscript.
We are thankful for the contribution of the WHI Investigators and staff at the clinical centers, clinical coordinating center, and project office. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. A short list of WHI Investigators is included below. Detailed information about the WHI investigators can be found at http://www.whi.org/about.
Footnotes
Disclaimers: The authors have no commercial, proprietary, or financial interest in the products or instruments described in this article.1
Reprint Requests: Reprints will not be made available.
Dr. LaCroix serves on the Scientific Advisory Board for the PEARL Trial, a multi-center international trial of lasofoxifene sponsored by Pfizer, Inc. Dr. LaCroix also serves as a consultant and site Principal Investigator to the University of Massachusetts on an unrestricted educational grant funded by the Alliance for Better Bone Health (Proctor and Gamble and Sanofi-Aventis) to design and implement the Global Longitudinal study of Osteoporosis in Women (GLOW).
References
- 1.Aloia JF, Vaswani A, Russo L, Sheehan M, Flaster E. The influence of menopause and hormonal replacement therapy on body cell mass and body fat mass. Am J Obstet Gynecol. 1995;172(3):896–900. doi: 10.1016/0002-9378(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 2.Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, Genazzani AR. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas. 2001;39(2):125–32. doi: 10.1016/s0378-5122(01)00194-3. [DOI] [PubMed] [Google Scholar]
- 3.Kristensen K, Pedersen SB, Vestergaard P, Mosekilde L, Richelsen B. Hormone replacement therapy affects body composition and leptin differently in obese and non-obese postmenopausal women. J Endocrinol. 1999;163(1):55–62. doi: 10.1677/joe.0.1630055. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9(10):622–6. doi: 10.1038/oby.2001.81. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition--a substudy of the estrogen plus progestin trial of the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):651–6. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 6.Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Campbell LV. Moderate alcohol consumption, estrogen replacement therapy, and physical activity are associated with increased insulin sensitivity: is abdominal adiposity the mediator? Diabetes Care. 2003;26(10):2734–40. doi: 10.2337/diacare.26.10.2734. [DOI] [PubMed] [Google Scholar]
- 7.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. Jama. 2003;290(13):1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RD, Wactawski-Wende J, LaCroix AZ, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the women’s health initiative randomized trial. J Bone Miner Res. 2006;21(6):817–28. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 9.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 12.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 15.Faulkner KA, Cauley JA, Roth SM, et al. Familial Resemblance and Shared Latent Familial Variance in Recurrent Fall-Risk in Older Women. J Appl Physiol. 2009:00128. doi: 10.1152/japplphysiol.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone KL, Ewing SK, Lui LY, et al. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc. 2006;54(8):1177–83. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 17.Stalenhoef PA, Diederiks JP, Knottnerus JA, Kester AD, Crebolder HF. A risk model for the prediction of recurrent falls in community-dwelling elderly: a prospective cohort study. J Clin Epidemiol. 2002;55(11):1088–94. doi: 10.1016/s0895-4356(02)00502-4. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AV, Nevitt MC, Brown BW, Jr, Kelsey JL. Increased falling as a risk factor for fracture among older women: the study of osteoporotic fractures. Am J Epidemiol. 2005;161(2):180–5. doi: 10.1093/aje/kwi023. [DOI] [PubMed] [Google Scholar]
- 19.Sipila S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001;101(2):147–57. [PubMed] [Google Scholar]
- 20.Sites CK, L’Hommedieu GD, Toth MJ, Brochu M, Cooper BC, Fairhurst PA. The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(5):2701–7. doi: 10.1210/jc.2004-1479. [DOI] [PubMed] [Google Scholar]
- 21.Kenny AM, Kleppinger A, Wang Y, Prestwood KM. Effects of ultra-low-dose estrogen therapy on muscle and physical function in older women. J Am Geriatr Soc. 2005;53(11):1973–7. doi: 10.1111/j.1532-5415.2005.53567.x. [DOI] [PubMed] [Google Scholar]
- 22.Thorneycroft IH, Lindsay R, Pickar JH. Body composition during treatment with conjugated estrogens with and without medroxyprogesterone acetate: analysis of the women’s Health, Osteoporosis, Progestin, Estrogen (HOPE) trial. Am J Obstet Gynecol. 2007;197(2):137, e1–7. doi: 10.1016/j.ajog.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 23.Tanko LB, Movsesyan L, Svendsen OL, Christiansen C. The effect of hormone replacement therapy on appendicular lean tissue mass in early postmenopausal women. Menopause. 2002;9(2):117–21. doi: 10.1097/00042192-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Jensen LB, Vestergaard P, Hermann AP, et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2003;18(2):333–42. doi: 10.1359/jbmr.2003.18.2.333. [DOI] [PubMed] [Google Scholar]
- 25.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 27.Melton LJ, 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–30. [PubMed] [Google Scholar]
- 28.Seeley DG, Cauley JA, Grady D, Browner WS, Nevitt MC, Cummings SR. Is postmenopausal estrogen therapy associated with neuromuscular function or falling in elderly women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1995;155(3):293–9. [PubMed] [Google Scholar]
- 29.Beck TJ, Petit MA, Wu G, Leboff MS, Cauley JA, Chen Z. Does Obesity Really Make the Femur Stronger? Bone Mineral Density, Geometry and Fracture Incidence in the Women’s Health Initiative-Observational Study. J Bone Miner Res. 2009 doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaStayo PC, Ewy GA, Pierotti DD, Johns RK, Lindstedt S. The positive effects of negative work: increased muscle strength and decreased fall risk in a frail elderly population. J Gerontol A Biol Sci Med Sci. 2003;58(5):M419–24. doi: 10.1093/gerona/58.5.m419. [DOI] [PubMed] [Google Scholar]
- 31.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64(10):1071–81. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Wang Z, Lohman T, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007;137(12):2775–80. doi: 10.1093/jn/137.12.2775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.