Abstract
Actin-dependent finger-like protrusions such as filopodia and microvilli are widespread in eukaryotes, but their assembly mechanisms are poorly understood. Filopodia assembly requires at least three biochemical activities on actin: actin filament nucleation, prolonged actin filament elongation, and actin filament bundling. These activities are shared by several mammalian formin proteins, including mDia2, FRL1 (also called FMNL1), and FRL2 (FMNL3). In this paper, we compare the abilities of constructs from these three formins to induce filopodia. FH1-FH2 constructs of both FRL2 and mDia2 stimulate potent filopodia assembly in multiple cell types, and enrich strongly at filopodia tips. In contrast, FRL1 FH1-FH2 lacks this activity, despite possessing similar biochemical activities and being highly homologous to FRL2. Chimeric FH1-FH2 experiments between FRL1 and FRL2 show that, while both an FH1 and an FH2 are needed, either FH1 domain supports filopodia assembly but only FRL2’s FH2 domain allows this activity. A mutation that compromises FRL2’s barbed end binding ability abolishes filopodia assembly. FRL2’s ability to stimulate filopodia assembly is not altered by additional domains (GBD, DID, DAD), but is significantly reduced in the full-length construct, suggesting that FRL2 is subject to inhibitory regulation. The data suggest that the FH2 domain of FRL2 possesses properties not shared by FRL1 that allow it to generate filopodia.
Keywords: microvilli, FMNL3, mDia2, FH2 domain, bundling, FRL1
Introduction
Finger-like protrusions from the plasma membrane are common features of many eukaryotic cells, and have been most often described as “filopodia” and “microvilli”. While these terms have no formal definitions to our knowledge, filopodia most often describe finger-like protrusions that have at least occasional contact with a substratum, such as protrusions emanating from the leading edge of many motile cells. The term microvillus often refers to structures having no regular contact with a substratum, such as protrusions from epithelial brush border, hair cells, or circulating lymphocytes. These definitions for filopodia and microvilli may be imperfect, and certainly many gray areas exist given the diverse occurrence of finger-like protrusions (Chhabra and Higgs 2007; DeRosier and Tilney 2000; Faix et al. 2009; Gupton and Gertler 2007; Mellor 2010).
Despite these nomenclature issues, there are several commonalities between filopodia and microvilli from diverse sources. These finger-like structures (with diameters of 100-300 nm generally) contain parallel bundles of actin filaments, with the barbed ends of the filaments uniformly oriented to the distal tip. In almost all cases, the actin filaments appear to extend the length of the protrusion (DeRosier and Tilney 2000; Faix et al. 2009; Gupton and Gertler 2007; Mellor 2010). The actin filaments within both microvilli and filopodia grow by addition of actin monomers at the distal tips, and shrink by monomer loss at their base (Mallavarapu and Mitchison 1999). In some cases, such as brush border microvilli or stereocilia, monomer addition occurs continuously even though the length of the protrusion is relatively stable (Rzadzinska et al. 2004; Tyska and Mooseker 2002). Thus, length in these structures remains constant due to a remarkable balancing act between polymerization at the tip and depolymerization at the base, up to 100 microns away.
The molecular mechanisms controlling assembly and growth of filopodia and microvilli are controversial at present. One model for filopodia assembly favors initial nucleation of actin by Arp2/3 complex (Svitkina et al. 2003). Ordinarily, Arp2/3 complex-generated filaments are quickly capped at their barbed ends. In this model, however, filaments destined to generate filopodia are protected from capping by formin proteins and/or VASP, allowing them to extend (Svitkina et al. 2003; Yang et al. 2007). These elongating filaments are bundled by proteins such as fascin, allowing them to protrude into the finger-like structure (Vignjevic et al. 2006). This model was originally proposed for filopodia protruding from the leading edge lamellipodium, which is enriched in active Arp2/3 complex. Other data question this mechanism, and point instead to Arp2/3 complex-independent nucleation of actin filaments in filopodia (Steffen et al. 2006).
One potential nucleator is the formin protein, mDia2, whose expression in a constitutively active form or along with the active Rho GTPase, Rif, can lead to filopodia assembly (Block et al. 2008; Pellegrin and Mellor 2005; Yang et al. 2007). mDia2 can promote actin filament assembly and elongation, and bundle actin filaments (Harris et al. 2006). It is unclear which of these activities are required for mDia2’s ability to assemble filopodia.. mDia2 is highly enriched at filopodia tips (Block et al. 2008; Pellegrin and Mellor 2005; Yang et al. 2007), which does not exclude a role for nucleation or bundling, but suggests that a role in elongation is possible. It is unknown whether other formins, such as FRL1 and FRL2, which posses actin filament bundling activities similar to mDia2 (Harris et al. 2006; Vaillant et al. 2008), also promote filopodia formation.
Other actin-binding proteins are also enriched at filopodia tips and make up a “tip complex”, including VASP and myosin X (Berg et al. 2000; Svitkina et al. 2003). Myosin X expression can induce filopodia (Bohil et al. 2006), which is dependent on its rapid motility towards the tip (Kerber et al. 2009; Watanabe et al. 2010). The membrane-deforming I-Bar proteins, including IRSp53, are also capable of generating filopodia-like structures in an actin-independent manner, with actin filaments subsequently polymerizing in the protrusion and supporting its structure (Mattila et al. 2007; Millard et al. 2005; Yamagishi et al. 2004; Yang et al. 2009).
Thus, there may be several mechanisms mediating assembly of filopodia. In this article, we focus on filopodia assembly in mammalian cells by three formins: mDia2, FRL1 (also known as FMNL1), and FRL2 (also known as FMNL3). We focus on these formins because all three have been shown to bundle actin filaments in vitro (Harris et al. 2006; Vaillant et al. 2008), and filopodia contain bundled filaments of similar morphology. To minimize confusion, we will refer to the finger-like protrusions studied in this paper as “filopodia”, adopting a practice used by others (Bohil et al. 2006), and reserving the term “microvilli” for the more regular structures such as brush border microvilli.
We find that the FH1-FH2 construct of FRL2 induces potent filopodia assembly in multiple cell lines, similar to an FH1-FH2 construct of mDia2 (Yang et al. 2007). Remarkably, an FH1-FH2-containing construct of FRL1 does not cause filopodia assembly in any situation tested, despite being highly homologous to FRL2 and having similar biochemical bundling activity. Chimera experiments show that FRL2’s FH2 domain is required for filopodia assembly. A mutation in FRL2’s FH2 domain that renders it defective in barbed end binding also abolishes FRL2’s ability to stimulate filopodia assembly and localize to the plasma membrane. The full-length FRL2 construct has reduced ability to assemble filopodia, suggesting that it is subject to negative regulation. Our results suggest that the FH2 domain of FRL2 possesses special characteristics, not shared by FRL1, enabling it to assemble filopodia.
Materials and Methods
Plasmids
Constructs for FRL1, FRL2, mDia1, mDia2, and FHOD1 (sequences from mouse) were cloned into the eGFP-C1 vector (Clonetech) in which the A206K mutation in the GFP sequence was incorporated to reduce GFP dimerization (Zacharias et al. 2002). Constructs used were: FRL2 FH2 (amino acids 561-945), FRL2 FH1-FH2 (504-945), FRL2 FH1-FH2-C (504-1027), FRL2 45-1027, FRL1 FH2 (627-1020), FRL1 FH1-FH2-C (449-1094), mDia2 FH1-FH2-C (521-1171), mDia1 FH1-FH2-C (549-1255), and FHOD1 FH1-FH2-C (570-1042). Chimeric FRL1/FRL2 FH1-FH2 constructs were constructed by first producing constructs containing regions including the FH1 domains for each formin (FRL1 = amino acids 582-627 (which is a partial FH1 but made to match the number of profilin-binding sites in FRL2. FRL2 = 502-561) in the BamH1 site of eGFP-C1, so that the BamH1 site 5′ of the FH1 sequence was rendered resistant to further digestion. The DNA sequences corresponding to FH2 domains of each formin (domain boundaries above) were then produced with flanking BamH1 sites and ligated into the BamH1 site 3′ of the FH1 sequence. Thus, a BamH1 site between the FH1 and FH2 persisted. This site did not reduce the ability of FRL2 FH1-FH2 to produce filopodia (Figure 4 and Table 1), so was considered to not affect function. Full-length human FRL2 (1-1027) and deltaDAD FRL2 (1-987) were cloned into the pcDNA3.1+Zeo vector (Invitrogen) for expression in mammalian cells without GFP or other tag. The bacterial expression vector for FRL2 FH1-FH2 used the pGEX-KT ext plasmid (Guan and Dixon 1991). The I649A mutation was produced in FRL2 constucts using the QuickChange system (Stratagene). All constructs were verified by DNA sequencing. Mammalian expression constructs were also analyzed by Western blot after transfection into HeLa cells to determine whether bands of correct size were produced. Most constructs produced doublet bands by this analysis (Supplementary Figure 7), but these doublets corresponded to the expected masses. The mRFP-utrophin-CH construct, containing amino acids 1-261 of human utrophin, was a kind gift from Bill Bement (University of Wisconsin) and described in (Burkel et al. 2007). The full-length and deltaGBD (amino acids 258-1171) constructs of mouse mDia2, containing N-terminal GFP tags, were gifts from Tatyana Svitkina (University of Pennsylvania) and described in (Yang et al. 2007).
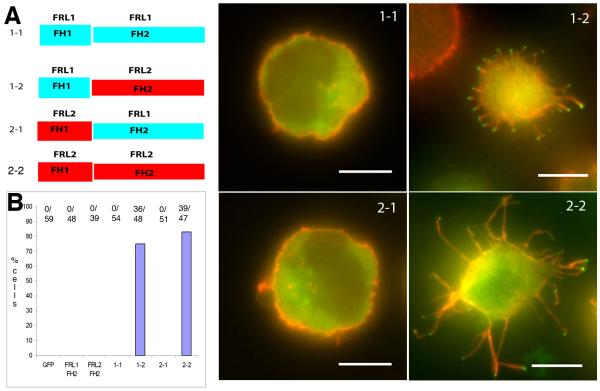
Figure 4. The FH2 domain of FRL2 is essential for filopodia assembly.
A) Chimeric constructs used in this experiment, expressed with an N-terminal GFP tag. B) Quantification of filopodia phenotype in Jurkat cells, compiled from three independent experiments. Numbers at the top of each column represent number of positive cells for microvilli/total number of cells counted. C-F) Examples of individual Jurkat cells for each of the chimeras. Scale bars, 5 μm.
Table 1.
Ability of transfected constructs to induce filopodia
| Cell Line | Formin | Construct | % Induction | N | Figure |
|---|---|---|---|---|---|
| GFP Fusion Consructs | |||||
| Jurkat | None | -- | 0 | 75 | S1 |
| Jurkat | FRL2 | FH1-FH2 | 84 | 67 | 1, 6, 7 |
| Jurkat | FRL1 | FH1-FH2-C | 0 | 46 | 1 |
| Jurkat | mDia2 | FH1-FH2-C | 86 | 22 | 1 |
| Jurkat | mDia1 | FH1-FH2-C | 0 | 27 | S1 |
| Jurkat | FHOD | FH1-FH2 | 0 | 23 | S1 |
| Jurkat | FRL2 | FH1-FH2 I649A |
0 | 33 | 7 |
| Jurkat | FRL2 | FH1-FH2-C | 80 | 20 | 7 |
| Jurkat | FRL2 | DeltaN45* | 73 | 15 | 7 |
| 300.19 | None | -- | 0 | 50 | S2 |
| 300.19 | FRL2 | FH1-FH2 | 97 | 34 | 2 |
| 300.19 | FRL1 | FH1-FH2-C | 0 | 34 | 2 |
| 300.19 | mDia2 | FH1-FH2-C | 63 | 35 | 2 |
| 300.19 | mDia1 | FH1-FH2-C | 0 | 41 | S2 |
| 300.19 | FHOD | FH1-FH2 | 0 | 49 | S2 |
| 3T3 | None | -- | 0 | 12 | S3 |
| 3T3 | FRL2 | FH1-FH2 | 93 | 14 | S3 |
| 3T3 | FRL1 | FH1-FH2-C | 0 | 16 | S3 |
| 3T3 | mDia2 | FH1-FH2-C | 89 | 9 | S3 |
| 3T3 | mDia1 | FH1-FH2-C | 0 | 16 | S3 |
| 3T3 | FHOD | FH1-FH2 | 0 | 17 | S3 |
| Chimeric GFP Fusion Constructs | |||||
| Jurkat | FRL1 | FH2 | 0 | 48 | --- |
| Jurkat | FRL2 | FH2 | 0 | 39 | --- |
| Jurkat | FRL1/FRL1 | FH1/FH2 | 0 | 54 | 4 |
| Jurkat | FRL1/FRL2 | FH1/FH2 | 75 | 48 | 4 |
| Jurkat | FRL2/FRL1 | FH1/FH2 | 0 | 51 | 4 |
| Jurkat | FRL2/FRL2 | FH1/FH2 | 83 | 47 | 4 |
| Untagged Constructs | |||||
| Jurkat | FRL2 | Full-length | 38 | 32 | 8 |
| Jurkat | FRL2 | Delta-DAD | 82 | 33 | 8 |
% induction refers to % of cells displaying at least five prominent filopodia, with GFP enrichment at tips. For 3T3 cells, % induction refers to % transfected cells showing enriched GFP staining at filopodial tips.
DeltaN45 contains the GBD/DID/dimerization domain/FH1/FH2/and C-terminus.
N refers to number of cells counted.
Results are representative of at least three experiments.
For Jurkats and 300.19 cells, cells were adhered to coverslips before fixation. However, where tested, similar results were obtained when cells were fixed prior to coverslip mounting.
Domain boundaries for FRL2
We designated domain boundaries based on the following criteria. The GBD, DID, and dimerization domains were defined based on the alignment given in (Otomo et al. 2005). The FH1 was defined as the proline-rich region N-terminal to the FH2, and its boundaries defined from the first proline in this region to the last proline in this region. The FH2 and DAD were defined by the alignments conducted in (Higgs and Peterson 2005). The FH1 definition is certainly open to interpretation, but we cannot at this time find a better definition. In most formins, including FRL1 and FRL2, intron/exon boundaries are not ideal to define the FH1, since there is often a splice site in the middle of the FH1, and often the first exon of the FH1 also includes extensive N-terminal sequence, including the dimerization domain. Our definitions of domain boundaries for mouse FRL2 (1027 amino acids) are: GBD = 45-236, DID = 79-395, DD = 398-452, FH1 = 505-548, FH2 = 561-945, DAD = 991-1000.
Mammalian cell culture and transfection
Jurkat T leukemic cells (human) were obtained from American Type Culture Collection and cultured in RPMI 1640 with L-glutamine, 5 % fetal bovine serum (Alanta Biologicals), 1 mM sodium pyruvate, 2.5 mg/mL glucose, and 0.05 mM 2-Mercaptoethanol at 37°C and 5% CO2. Cells were not allowed to grow more densely than 1×106 cells/mL and were split to 2×105 cells/mL. DNA constructs were introduced into cells by electroporation. Briefly, cells were washed twice in PBS and resuspended to 12.5 × 106 cells/mL in PBS. 400 μL of cells were mixed with 20 μg of plasmid DNA in a 4 mm gap electroporation cuvette (BTX Harvard Apparatus) and electroporated at 250 V, 975 μF capacitance in a Gene Pulser II (BioRad). Pre-warmed medium (1 mL) was added, cell debris was removed, and the remaining liquid transferred to 6 mL of pre-warmed medium. After 6 hrs expression, cells were centrifuged at 300xg for 5 min, and the cell pellet brought up in 1 mL pre-warmed medium. 0.5 mL of suspension was placed on a 12 mm round coverslip coated with 0.01% poly-L-lysine (>300,000 MW, Sigma) and incubated for 10 min prior to fixation. In cases in which cells were fixed in suspension, they were added to 9 volumes of fix solution for 1 hr at room temperature (described below), washed three times in 10 volumes of PBS, resuspended in ½ volume of 0.1x PBS, and spotted onto poly-L-lysine coated coverslips in a humidified chamber. After 30 min, one volume of fixative was added and the cells were fixed on the coverslips for 30 min, followed by staining as described below.
300.19 pre-B leukemic cells (mouse) were obtained from Dr. Jeffrey Kansas (Northwestern School of Medicine) and cultured in RPMI 1640 with L-glutamine, 5 % fetal bovine serum (Atlanta Biologicals), and 0.05 mM -Mercaptoethanol at 37°C and 5% CO2. Cells were electroporated in the same manner as Jurkats.
Swiss 3T3 cells (mouse) were a kind gift from Dr. David Cheresh (Fox Chase Cancer Center) and cultured in Dulbecco’s Modification of Eagle’s medium with 4.5 g/L glucose, L-glutamine, sodium pyruvate and 10 % calf serum (Atlanta Biologicals). Cells were transfected using Lipofectamine 2000 (Invitrogen).
HeLa cells were obtained from American Type Culture Collection and cultured in Dulbecco’s Modification of Eagle’s medium with 4.5 g/L glucose, L-glutamine, sodium pyruvate and 10 % calf serum (Atlanta Biologicals). Cells were transfected using Lipofectamine (Invitrogen).
Cell fixation and fluorescence microscopy
Cells were fixed on 12 mm round coverslips by removal of medium and addition of one volume fixative (PBS with 4% formaldehyde and 0.1% saponin) on ice for one hr. Cells were washed 3x with PBS, then blocked with PBS + 10% calf serum + 0.1% saponin for 30 min at 23°C. Removal of saponin from the solutions, and permeabilization using 0.25% Triton X-100 during blocking, had no noticeable effect on filopodia morphology. Cells were stained with 0.5 mM rhodamine-phalloidin (Sigma TRITC-phalloidin) and 0.4 ng/μL DAPI in 0.1X block solution diluted in PBS for 1 hr at 23°C. Cells were washed 3x with PBS, then mounted on slides in PVA/DABCO or Vectashield (Vector Laboratories). Immunofluorescence with anti-FRL2 was carried out by primary incubation with 0.5 ug/mL affinity-purified anti-FRL2 followed by 1/500 of Alexa 488-labeled goat anti-guinea pig IgG (Invitrogen).
Antibody Production
Anti-FRL2 was raised in guinea pig against a bacterially-expressed FH1-FH2-containing construct (amino acids 447-945 of the mouse protein) by Covance Inc. Anti-FRL2 was affinity-purified from serum over FRL2 FH1-FH2 covalently linked to Sulfolink resin (Pierce, Invitrogen).
Confocal Microscopy
For live cell movies, coverslips were mounted into Rose chambers, then onto a Wave FX spinning disc confocal microscope (Quorum Technologies, on a Nikon Eclipse microscope) with Bionomic Controller (20/20 Technology, Inc) temperature-controlled stage set to 37°C. After equilibrating to temperature for 10 min, cells were imaged through the 60x 1.4 NA Plan Apo objective (Nikon) using the 491 nm laser and 525/20 filter for GFP and the 561 nm laser and 593/40 filter for mRFP. Z-stacks of 0.4 μm were collected for each color at 10 sec intervals. Maximum intensity projections from five Z slices were assembled using Metamorph software. For 3D reconstructions of Z-stacks of fixed cells, coverslips were mounted using Vectashield, and Z-stacks of 100 nm steps were acquired through the 60x 1.4 NA objective. Stacks were assembled into 3D movies using Elements software (Nikon).
Quantification of phenotypes
Fixed cell coverslips were examined in a blinded manner for microvillar phenotype. For Jurkats, cells were scored as “positive” for filopodia phenotype if they displayed five or more filopodia with enriched staining for the formin (GFP or antibody) at the tips. For 300.19 cells, positive cells contained five or more abnormally long filopodia (against the background of a short microvillar surface which 300.19 cells normally possess) with enriched formin tip staining. For 3T3 cells, filopodia induction was difficult to quantify, due to their heterogeneous nature. Cells were scored positive if they displayed enriched GFP signal at the tips of filopodia. Filopodia lengths were measured using Elements software (Nikon), with at least 70 filopodia measured for each condition.
Biochemical assays on FRL2 FH1-FH2
Buffers
The following buffers are used frequently. G-buffer: 2 mM Tris pH 8, 0.5 mM DTT, 0.2 mM ATP, 0.1 mM CaCl2, and 0.01% NaN3. G-Mg buffer: same as G- buffer but with 0.1 mM MgCl2 instead of CaCl2. 10xKMEI: 500 mM KCl, 10 mM MgCl2, 10 mM EGTA, and 100 mM imidazole pH 7.0. 10xNaMEI: same as 10xKMEI but with 500 mM NaCl instead of KCl. Polymerization buffer: G-Mg buffer plus either 1xKMEI or 1xNaMEI.
Protein Purification
We expressed FRL2 FH1-FH2 (amino acids 447-945 of the mouse protein) and the I649A mutant as a glutathione S-transferase (GST) fusion protein in E. coli, as previously described (Harris et al. 2006). After cleavage from GST and subsequent purification, all proteins migrated as a single band on coomassie-stained SDS-PAGE, with no significant additional bands. Briefly, Rosetta 2, non-DE3 cells (Novagen 71402) containing expression construct were grown to OD600 of 1.0 in TB (12 g/liter Tryptone, 24 g/liter yeast extract, 4.5 mL/liter glycerol, 14 g/liter dibasic potassium phosphate, and 2.6 g/liter monobasic potassium phosphate) with 100 μg/mL ampicillin and 34 μg/mL chloramphenicol at 37°C. After reduction to 16°C, 0.5 mM ITPG was added and the cultures grown overnight. All subsequent purification steps were performed at 4°C or on ice. Bacteria were pelleted, resuspended in EB (50 mM Tris-HCl pH 8.0, 500 mM NaCl, 5 mM EDTA, 1 mM DTT and 1 pill/50 mL Complete protease inhibitors (Roche)), and extracted by sonication. After ultracentrifugation, supernatants were loaded onto glutathione-Sepharose 4B (Amersham), which was subsequently washed with WB (EB without protease inhibitors but with 0.05% thesit (Sigma P-9641)). Thrombin (Sigma T-4265) was added to a 50% slurry of beads to 10 U/mL, and the suspension mixed for 1 hour. Cleaved protein was washed from the column with WB, and thrombin was inactivated with 1 mM phenylmethylsulfonyl fluoride and 5 mM diisopropyl fluorophosphate for 15 minutes, after which DTT was added to 10 mM. FRL2 constructs were further enriched by step elution from SP Sepharose Fast Flow, and stored in 2 mM NaPO4 pH 7.0, 50 mM NaCl, 0.5 mM EGTA, 0.5 mM MgCl2, and 0.5 mM DTT. FRL2 protein was stored at 4°C. 6xHis-tagged FRL2 constructs and FRL2 point mutants were purified in the same manner. Rabbit skeletal muscle actin was purified from acetone powder (Spudich and Watt 1971), and labeled with pyrenyliodoacetamide (Cooper et al. 1983). Both unlabeled and labeled actin were gel filtered on S200 (MacLean-Fletcher and Pollard 1980) and stored in G-buffer at 4°C.
Analytical Ultracentrifugation
Analytical ultracentrifugation was conducted using a Beckman Proteomelab XL-A and an AN-60 rotor. For sedimentation velocity analytical ultracentrifugation of FRL2 403-910, 24 μM of FRL2 in 5 mM NaPO4 pH 7.0, 150 mM NaCl, 0.5 mM EGTA, 0.5 mM MgCl2, 1 mM DTT was centrifuged at 35,000 rpm at 20°C, and 280 nm absorbance monitored every minute by continuous scan at 0.003 cm steps. Protein partial specific volume, buffer density, and buffer viscosity were determined using Sednterp (program by David Hayes & Tom Laue). Scans 1-200 were analyzed using Sedfit87 (www.analyticalultracentrifugation.com).
Actin Polymerization by Fluorescence Spectroscopy
Unlabeled and pyrene labeled actin were mixed in G buffer to produce an actin stock of the desired pyrene-labeled actin percentage (5% unless otherwise stated). This stock was converted to Mg2+ salt by 2 min incubation at 23°C in 1 mM EGTA/0.1 mM MgCl2 immediately prior to polymerization. Polymerization was induced by addition of 10xKMEI to a concentration of 1x, with the remaining volume made up by G-Mg. Additional proteins were mixed together for 1 minute prior to their rapid addition to actin to start the assay. Pyrene fluorescence (excitation 365 nm, emission 407 nm) was monitored in a PC1 spectrofluorimeter (ISS, Champaign, IL). The time between mixing of final components and start of fluorimeter data collection was measured for each assay and ranged between 12 and 15 seconds.
Barbed End Elongation Assays
Unlabeled actin (10 μM) was polymerized 1 hr at 23°C, then diluted to 5 μM in the presence of 10 μM phalloidin and centrifuged at 100,000 rpm for 20 min in a TLA-120 rotor. The pellet was resuspended to 5 μM in 3X polymerization buffer (G-Mg with 3xKMEI), and then sheared by two passes through a 30G needle. 37.5 μl of this mixture was aliquoted into eppendorf tubes and allowed to re-anneal overnight at 23°C. 1X polymerization buffer (37.5 μl) containing formin protein was added to filaments, mixed by gentle flicking, and incubated at 23°C for 2 min. After 2 min at 23°C, 75 μl of 2 μM monomers (5% pyrene, Mg2+-converted) were added to the filaments with a cut p200 tip, mixed by pipetting up and down two times, and placed into the fluorimeter cuvette. Fluorescence (365/407 nm) was recorded for 180 sec. Elongation velocity was obtained by linear fitting the initial 100 sec of elongation. Final concentrations in the assay were 1.25 μM phalloidin-stabilized polymerized actin and 1 μM monomer.
Actin Filament Bundling Assays
Actin (5 μM) was polymerized for 2 hours at 23°C in polymerization buffer followed by addition of 5 μM phalloidin (Sigma P-2141). This actin stock was diluted to desired concentration in polymerization buffer, in the absence or presence of formin, in 1.5 mL eppendorf tubes to a final volume of 200 μl. After 5 min at 23°C samples were centrifuged in a microfuge at 16,000 xg for 5 minutes at 4°C. 160 μl of supernatant was removed, lyophilized, and resuspended in 16 μl SDS-PAGE sample buffer. After removal of the remaining supernatant, pellets were washed briefly with 200 μl polymerization buffer, then resuspended in 20 μl SDS-PAGE sample buffer. Supernatants and pellets were analyzed by Coomassie-stained SDS-PAGE.
Results
FH1-FH2 constructs of FRL2 and mDia2 promote filopodia
We tested the abilities of three bundling formins (FRL1, FRL2, and mDia2) as well as the non-bundling formin, mDia1, to assemble filopodia in three cell types possessing different surface protrusion characteristics. Jurkat cells grow in suspension and possess abundant surface ruffles in ~95% of the population (Nicholson-Dykstra and Higgs 2008). 300.19 cells grow in suspension and predominantly possess short (0.3-0.4 micron) filopodia (Majstoravich et al. 2004). Swiss 3T3 cells are adherent and possess both lamellipodial sheets and filopodia at their protrusive edges (Nicholson-Dykstra and Higgs 2008; Small and Celis 1978).
We initially tested FH2-only GFP-fusion constructs for the ability to assemble filopodia. None of the FH2-only constructs promote filopodia assembly, and all except mDia1, which localizes mainly to the nucleus, are diffuse and cytoplasmic (Table I for FRL1 and FRL2, data not shown for other constructs). None of these FH2-only constructs cause any significant increase in actin filament levels, as judged by TRITC-phalloidin staining, in any of the three cell types tested (not shown). This result is consistent with previous work showing that the FH2 domain of mDia1 is ineffective at causing cellular actin polymerization (Copeland and Treisman 2002; Watanabe et al. 1999).
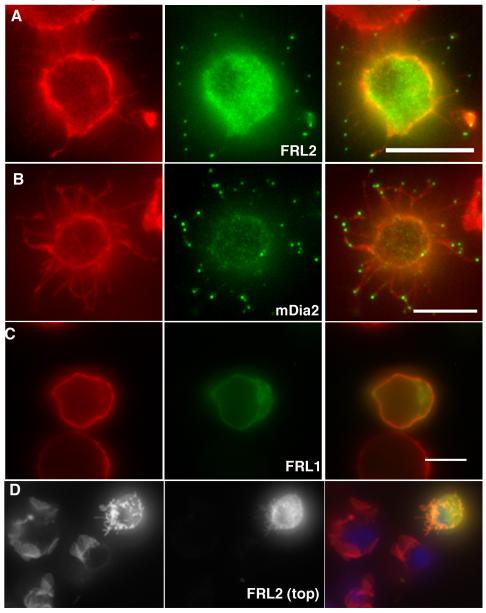
Since the FH1 domain causes rapid actin-based motility of FH2 domain-containing structures in cells (Higashida et al. 2004), and is required for profilin-mediated elongation acceleration biochemically (Kovar et al. 2006; Romero et al. 2004), we tested constructs containing both the FH1 and FH2 domain for filopodia assembly. In Jurkat cells, which normally do not posses finger-like protrusions, both FRL2 and mDia2 FH1-FH2-containing constructs cause potent filopodia assembly (Figure 1A and 1B), with the GFP enriching at filopodia tips. This effect is most easily quantified by allowing the cells to adhere to poly-lysine-coated coverslips briefly before fixation, in which case the filopodia adhere to the coverslip. Movie 1 shows a deconvolved 3-D reconstruction of a cell transfected with FRL2 FH1-FH2 and adhered prior to fixation. Using this technique, over 85% of cells possess the filopodia phenotype (Table I). However, filopodia assembly is not dependent on surface adhesion, since FRL2-transfected cells that are fixed in suspension display robust filopodia surfaces, and ablation of ruffles (Figure 1D). In this image, one can also observe that FRL2-FH1-FH2 causes the complete transformation of the Jurkat surface from ruffles to filopodia. Interestingly, the GFP-FRL2 enrichment at filopodia tips is not as pronounced when cells are fixed in suspension rather than after surface adhesion.
Figure 1. FRL2 and mDia2 FH1-FH2 domain-containing constructs induce filopodia in Jurkat cells.
GFP-fusion constructs containing the FH1-FH2 domains of FRL2 (A, FH1-FH2 construct), mDia2 (B, FH1-FH2-C construct) or FRL1 (C, FH1-FH2-C construct) were expressed in Jurkat cells for six hours, followed by formaldehyde fixation and staining with rhodamine-phalloidin and DAPI. Panels A-C represent cells that were allowed to adhere to poly-L-lysine coated coverslips prior to fixation. Panel D represents cells that were transfected with FRL2 FH1-FH2-C construct, then fixed in suspension prior to adhesion to poly-L-lysine coated coverslips. Scale bar represents 5 μm.
Surprisingly, the FRL1 FH1-FH2-containing construct, which bundles robustly in vitro (Harris et al. 2006), does not have this effect, showing no filopodia production in Jurkat cells (Table I, Figure 1C). The FRL1 construct localizes diffusely in the cytoplasm, with occasional accumulation in regions of cytoplasm, but no apparent enrichment at the plasma membrane (Figure 1C, Movie 2). It is worth noting here that addition of the C-terminal region, including the DAD, to FH1-FH2 constructs does not change the abilities of FRL1 or FRL2 to assemble filopodia, as will be shown subsequently. Neither mDia1 nor FHOD1 FH1-FH2 domains display filopodia phenotypes or plasma membrane enrichment (Supplementary Figure 1), with mDia1 again enriching in the nucleus.
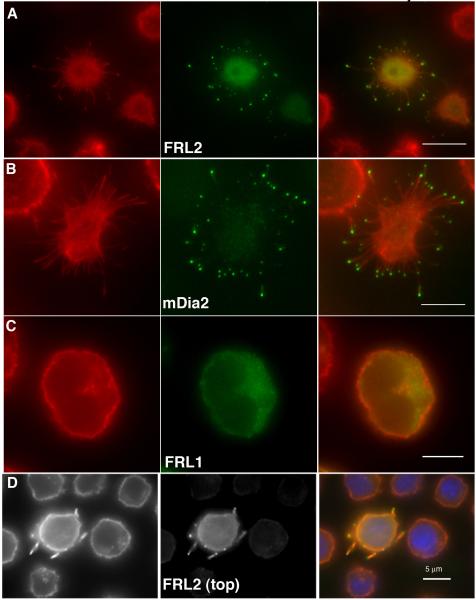
We tested the FH1-FH2 domain-containing constructs in 300.19 cells, which possess a uniform surface of filopodia that are too short to be resolved by conventional light microscopy ((Majstoravich et al. 2004), see untransfected cells in Figure 2D). Both FRL2 and mDia2 FH1-FH2-containing constructs cause assembly of abnormally long filopodia, with intense enrichment of GFP at the tips, in 97% and 63% of the cells for FRL2 and mDia2, respectively (Figure 2A, 2B and Table I). As with Jurkat cells, the FRL1 construct causes neither of these effects (Figure 2C). The filopodia phenotype is not due to surface adhesion, since cells fixed in suspension present the same characteristics (Figure 2D). In contrast to Jurkat cells fixed in suspension, 300.19 cells fixed in suspension still display strong GFP-FRL2 enrichment at filopodia tips. Neither the mDia1 nor FHOD1 constructs display the filopodia phenotype, with mDia1 again enriching in the nucleus (Supplementary Figure 2 and Table I).
Figure 2. FRL2 and mDia2 FH1-FH2 domain-containing constructs induce filopodia in 300.19 cells.
GFP-fusion constructs containing the FH1-FH2 domains of FRL2 (A, FH1-FH2), mDia2 (B, FH1-FH2-C) or FRL1 (C, FH1-FH2-C) were expressed in 300.19 cells for six hours, followed by formaldehyde fixation and staining with rhodamine-phalloidin and DAPI. Panels A-C represent cells that were allowed to adhere to poly-L-lysine coated coverslips prior to fixation. Panel D represents cells that were transfected with FRL2 FH1-FH2-C then fixed in suspension prior to adhesion to poly-L-lysine coated coverslips. Scale bars represent 5 μm.
To test whether a similar effect occurs in adherent cells, we transfected the GFP-FH1-FH2-containing constructs into Swiss 3T3 cells. Both FRL2 and mDia2 enrich at the plasma membrane in protrusive regions of the cell, with particular enrichment at the tips of filopodia protrusions (Supplementary Figure 3). Due to the heterogeneous nature of 3T3 morphology, as compared to Jurkats or 300.19 cells, it was difficult to quantify the abilities of the constructs to cause increased filopodia assembly. Our general impression, however, is that both FRL2 and mDia2 stimulate filopodia assembly in these cells. We quantify filopodia tip localization of the constructs in Table 1. The effect of the mDia2 construct is similar to that previously observed for mDia2 FH1-FH2 in melanoma cells (Yang et al. 2007), though not creating the bulbous filopodia seen for longer constructs of mDia2 (Block et al. 2008; Yang et al. 2007).
The FRL1 construct, despite showing some enrichment in protrusive plasma membrane regions, does not display the intense enrichment at filopodia tips (Supplementary Figure 3), nor does it seem to cause an increase in these structures. As with the other cell types, neither mDia1 nor FHOD1 enrich at plasma membrane, with mDia1 being primarily nuclear (Supplementary Figure 3).
One possibility is that these filopodia are actually “retraction fibers” that remain attached to the surface after partial cellular retraction (Mitchison 1992). To ensure that the structures we observed were not retraction fibers, we performed live-cell microscopy on HeLa cells co-transfected with GFP-FRL2 FH1-FH2 and mRFP-utrophin-CH domain to label actin filaments. FRL2 clearly generates filopodia that protrude from the plasma membrane, at a rate of 0.9 μm/min (Figure 3, Movie 3). We rarely observe shortening of the filopodia, and the most common tendency is that the filopodia elongate then maintain a stable length or detach from the coverslip. The tips of these filopodia remain enriched with GFP label throughout the course of the movie.
Figure 3. Dynamics of FRL2 FH1-FH2 induced filopodial protrusion in HeLa cells.
HeLa cells were transfected with GFP-FRL2 FH1-FH2 (green) and mRFP-utropin-CH (red, a gift from Bill Bement) to label actin filaments. Micrographs were acquired at 10 sec intervals as Z-series and maximum intensity projections generated. The montage shows selected frames from Movie 3. Two filopodia actively protrude in this sequence. The white arrows mark the initial positions of the GFP-enriched filopodial tips, and remain in the same positions in subsequent frames to show progressive elongation. Times are given in sec.
Filopodia assembly depends on the FH2 domain of FRL2
We were curious as to why the FH1-FH2 domain construct of FRL2 could promote filopodia assembly, whereas the corresponding FRL1 construct could not. These proteins are highly similar in their FH2 domains (59% identity over 392 amino acids, with one gap of one residue), and display very similar actin polymerization and bundling properties ((Harris et al. 2006) for FRL1, Figure 5 for FRL2). As with most formins, the FH1 domains diverge significantly between FRL1 and FRL2, to the point where sequence alignments become uninformative. The FH1 of FRL1 (amino acids 532-612 of mouse protein) contains 81 residues, of which 56% are proline, and has the potential to bind 4-5 profilins (based on the number of stretches of five or more consecutive prolines). The FH1 of FRL2 (amino acids 505-548 of mouse protein) contains 44 residues, of which 52% are prolines, and has the potential to bind 2-3 profilins.
Figure 5. I649A mutation disrupts barbed end binding but not bundling by FRL2.
A) Sedimentation velocity analytical ultracentrifugation of 24 μM wild-type (black) or I649A mutant (red) FRL2 FH1-FH2. One major species sediments at 4.25 S.
B) Polymerization of 2 μM actin monomers (10% pyrene-labeled) in the presence of the indicated concentrations of mDia1 or FRL2 FH1-FH2.
C) Elongation rates of 1 μM actin monomers (10% pyrene-labeled) from phalloidin-stabilized actin filaments (1.5 μM) in the presence of the indicated concentrationsof wild-type or I649A FRL2 FH1-FH2. Inset shows raw elongation curves in the presence of 30 nM of the indicated construct. D) Low-speed pelleting assays of actin filament bundling by FRL2 FH1-FH2. Pre-polymerized actin filaments (2 μM) were mixed with the indicated nM concentrations of FRL2 FH1-FH2 construct, then centrifuged at 16,000xg for 10 min. Pellet fractions (representing bundled filaments with bound FRL2) are shown.
To determine the crucial domain for filopodia assembly by FRL2 (the FH1 or FH2), we made chimeric FH1-FH2 constructs of FRL1 and FRL2 (Figure 4A) and transfected them into Jurkat cells. The FH2-only constructs of FRL1 and FRL2 have no effect on cell morphology (Figure 4B). The FRL2 FH2 domain, when coupled with the FH1 domain of either FRL1 or FRL2, causes robust filopodia assembly, whereas the FH2 domain of FRL1 has no effect with either FH1 domain (Table 1, Figure 4B-F). These results show that, while the FH1 domain is required for the filopodia effect, the specificity of the effect resides in the FH2 domain of FRL2. However, the lengths of the filopodia generated by the FRL1-FRL2 chimera (2.38 +/− 1.61 μm, n = 91) are noticeably shorter than those of the FRL2 construct (3.88 +/− 1.96 μm, n = 71). The nature of the FH1-FH2 construct used might, thus, have an effect on filopodia length. In these chimeras, we used a partial FH1 domain of FRL1, in order to balance the number of profilin binding sites (see Methods).
Barbed end binding is required for filopodia assembly by FRL2
To determine whether the ability of the FH2 domain to bind the barbed end was required for FRL2’s ability to induce filopodia we used a mutagenesis approach. For the budding yeast formin, Bni1p, mutation of I1431 to alanine strongly reduces its ability to accelerate actin polymerization (Xu et al. 2004), and mutation of the analogous residue has similar effects on mDia1, mDia2, and FRL1 (Harris et al. 2006). We made the corresponding mutation (I649A) in the FRL2 FH1-FH2 construct and tested its properties biochemically and in cells. Both wild-type and I649A FH1-FH2 constructs express well in bacteria, and sediment as single peaks of 4.25 S by velocity analytical ultracentrfugation (Figure 5A). The FH1-FH2 construct of FRL2 is extremely poor at accelerating polymerization of pyrene-actin (Figure 5B), similar to the FRL2 FH2-C construct tested previously (Vaillant et al. 2008). To test the ability of FRL2 FH1-FH2 to bind filament barbed ends, we performed filament elongation assays. Wild-type FH1-FH2 inhibits actin elongation rate by 70%, with an IC50 of 2 nM, similar to other FH2 domains, suggesting that FRL2 FH2 effectively binds barbed ends. In contrast, the I649A mutant does not affect filament elongation, at concentrations up to 100 nM (Figure 5C). Both wild-type and I649A mutant bundle filaments with similar potency in low-speed pelleting assays (Figure 5D). Therefore, the I649A mutant strongly reduces barbed end binding but does not affect bundling.
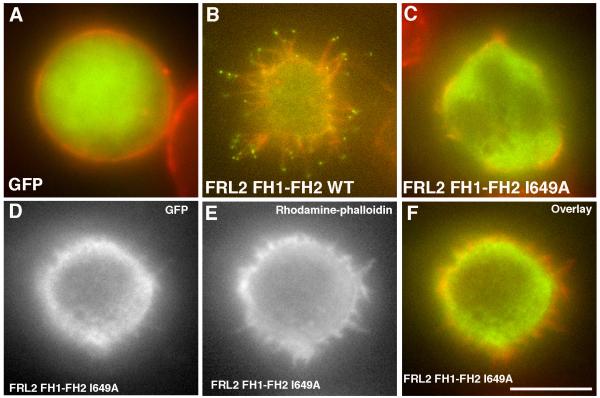
In Jurkat cells, the I649A mutation in the GFP-FRL2 FH1-FH2 construct eliminates filopodia assembly (Figure 6C and Table 1). A minority of cells transfected with the I649A construct (<20%) display a variable number of short, stubby protrusions. However, in no case do these cells display GFP staining at the tips of these structures (Figure 6D-E). Our interpretation is that these structures may be due to mutant’s bundling ability, but the lack of barbed end binding by the mutant causes them to get capped. The I649A construct shows no ability to generate long filopodia in 300.19 cells, nor does it enrich in filopodia in 3T3 cells (Supplementary Figure 4). Our conclusion is that barbed end binding ability is necessary for FRL2-induced filopodia assembly.
Figure 6. The FRL2 barbed end binding mutant is defective in filopodia assembly.
Jurkat cells were transfected withGFP (A), GFP-FRL2 FH1-FH2 (B), or GFP-FRL2 FH1-FH2 I649A (C-F). After 6 hrs, the cells were plated onto poly-lysine-coated coverslips for 10 min, fixed, and stained with rhodamine-phalloidin. GFP, green. Rhodamine-phalloidin, red. Panels D, E and F show an I649A-transfected cell that displays short actin-rich protrusions. Panel D (GFP) shows that there is no enrichment of I649A to the tips of these structures, In contrast to the intense enrichment for wild-type (panel B). The cells displaying the phenotype shown in D-F make up less than 20% of the population. Scale bar, 5 μm.
Effects of additional domains on filopodia assembly by FRL2
FRL2 belongs to the group of “diaphanous” formins, in that it contains clear sequences for DID and DAD, as well as a GBD. The DID and DAD interact tightly in several proteins (Li and Higgs 2003; Li and Higgs 2005; Wallar et al. 2006), and this interaction strongly inhibits the FH2’s ability to nucleate actin in mDia1 (Li and Higgs 2003). For mDia2, the full-length protein lacks the ability to assemble filopodia in the absence of other stimuli (Block et al. 2008; Pellegrin and Mellor 2005; Yang et al. 2007), suggesting that DID/DAD autoinhibition also regulates its activity. Previous studies using transfected constructs containing N-terminal Myc-tags have shown that full-length human FRL2’s effects on overall cellular actin polymerization in NIH 3T3 cells are similar to those of shorter constructs, suggesting that FRL2 is not regulated (Vaillant et al. 2008). Biochemical assays show that the DID-containing FRL2 N-terminus can bind to the DAD-containing FH2-C construct, but does not inhibit actin bundling or the weak actin polymerization induced by FH2-C (Vaillant et al. 2008).
To test the ability of other domains to influence the ability of FRL2 to induce finger-like protrusions, we made a series of FRL2 constructs containing N-terminal GFP tags (Figure 7A), including the DeltaN45 construct (containing GBD/DID/FH1/FH2/DAD), and the FH1-FH2-C construct (containing the FH1, FH2, DAD and entire C-terminus), and tested them for microvillar assembly in Jurkat cells. Both the DeltaN45 and FH1-FH2-C constructs cause filopodia assembly to a degree indistinguishable from that of the FH1-FH2 construct (Figure 7 and Table I). We did not detect any clear differences in filopodia length or morphology between the three FRL2 constructs tested (FH1-FH2, FH1-FH2-C, and DeltaN45). Filopodia lengths fall in a broad distribution from <0.5 μm to about 10 μm for all constructs, with mean lengths between 2 and 3 μm (Figure 7E). Thus, inclusion of the GBD/DID region, which would be predicted to cause auto-inhibition of FRL2, does not result in a decrease in ability to assemble filopodia, in support of previously published results suggesting that FRL2 is not auto-inhibited (Vaillant et al. 2008).
Figure 7. Effects of additional domains on FRL2-induced filopodia.
Jurkat cells were electroporated with GFP-fusion constructs of varying lengths of FRL2, then fixed and stained with rhodamine-phalloidin. A) Bar diagram of FRL2, showing the lengths of each construct. The FH1-FH2 construct is 504-945, FH1-FH2-C is 504-1027, DeltaN45 is 46-1027, Full is 1-1027, and ΔDAD.is 1-987. Domain boundaries are: GBD - 45-236; DID - 79-395; DD (dimerization domain) 398-452; FH1 - 505-548; FH2 - 561-945; DAD - 991-1000. The basis for the boundary predictions is given in Materials and Methods. B) - D) are micrographs of individual cells transfected with each construct. See Table 1 for quantification of phenotype. E) Filopodial lengths for the three constructs tested. Values for minimum and maximumlengths (in μm) and number of filopodia measured are as follows. FH1-FH2: 0.3 μm, 7.5 μm, and n= 109. FH1-FH2-C: 0.6, 9.4, and 70. DeltaN45: 0.6, 11.6, and 115. Scale bars represent 5 μm.
FRL2 is predicted to be post-translationally modified with a myristoyl group at its N-terminus, based on its N-terminal sequence and the fact that FRL1 has been shown to be myristoylated (Han et al. 2009). We therefore wondered whether inclusion of a free N-terminus would be important for FRL2 auto-inhibition, since both our DeltaN45 construct and the constructs used in the previous study (Vaillant et al. 2008) contain N-terminal tags that would prevent myristoylation or might have other effects. To test this possibility, we made un-tagged constructs of full-length FRL2 and of “delta-DAD” FRL2, which is missing the C-terminal 40 amino acids. The delta-DAD construct is predicted to be constitutively active, since it no longer is capable of DID/DAD interaction. We transfected these constructs into Jurkat cells, then stained cells with anti-FRL2 to determine which cells were transfected. Western blots characterizing the antibody are in Supplementary Figure 5, and immunofluorescence staining of transfected cells is in Supplementary Figure 6. Despite recognizing additional bands on western, the antibody clearly distinguishes transfected from non-transfected cells, thus is suitable for the experiment.
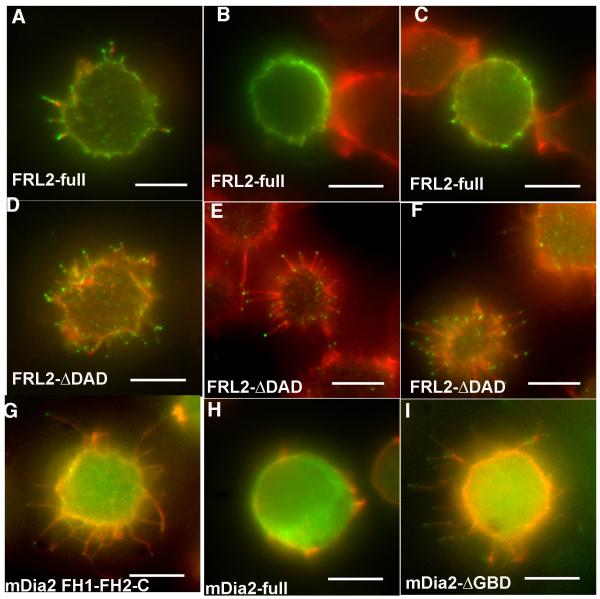
The delta-DAD FRL2 construct is much more potent at filopodia assembly than is the full-length construct (Figure 8, Table 1). Over 80% of the delta-DAD-transfected cells display abundant filopodia, similar to the GFP-fusion constructs of FRL2 (Figure 8D-F). In contrast, less than 40% of the full-length FRL2-transfected cells display filopodia (Figure 8A-C). In addition, many of the cells scoring “positive” for filopodia with the full-length construct had much fewer filopodia than did positive delta-DAD cells (for an example, see Figure 8A). The difference in filopodia generation is not due to differential expression levels, since quantification of fluorescence staining for individual cells shows that there is no correlation between expression level of the FRL2 construct and filopodia assembly (Supplementary Figure 6). This result suggests that, in constructs without an N-terminal tag, FRL2 is subject to some form of negative regulation in cells, although it maintains some activity. In comparison, we find that a full-length GFP fusion construct of mDia2 lacks filopodia assembly activity whereas the delta-GBD construct causes robust filopodia assembly (Figure 8G-I), similar to previous results (Block et al. 2008; Pellegrin and Mellor 2005; Yang et al. 2007). This finding suggests that mDia2 is effectively regulated even when an N-terminal GFP tag is present.
Figure 8. Full-length FRL2 has reduced ability to assemble filopodia.
A-F) Jurkat cells were transfected with un-tagged constructs containing either full-length FRL2 (A-C), or FRL2 DDAD (D-F). After 6 hrs, cells were plated onto poly-lysine for 10 min, fixed, and stained with rhodamine-phalloidin (red) and anti-FRL2 followed by fluorescently labeled secondary antibody (green). Three examples of transfected cells are shown for each FRL2 construct. A) shows an example of a full-length FRL2 cell scored “positive” for filopodia, whereas B) and C) are “negative”. D)-F) are all examples of “positive” cells transfected with FRL2 ΔDAD. G-H) Jurkat cells transfected with GFP-fusions of mDia2 constructs. G) is mDia2 FH1-FH2-C, H) is mDia2 full-length, I) is mDia2-ΔGBD. Scale bars represent 5 μm.
Discussion
In this paper, we show that a minimal construct containing the FH1-FH2 domains from FRL2 is capable of assembling filopodia, similar to mDia2 (Block et al. 2008; Pellegrin and Mellor 2005; Yang et al. 2007). Surprisingly, a similar construct of the highly related protein, FRL1, does not have this capability. These results lead us to discuss the biochemical activities necessary for filopodia assembly in general, and what activities FRL2 FH1-FH2 might be providing for this process. While we feel that cells are likely to have several different mechanisms for generating filopodia physiologically, we wish to use this admittedly non-physiological over-expression system to ask mechanistic questions about filopodia assembly. We also point out that the FH1-FH2 domain constructs used here do not necessarily provide accurate insight into cellular function, since full-length constructs of FRL1 localize to plasma membrane and play important roles in phagocytosis and adhesion (Seth et al. 2006) (Mersich et al.).
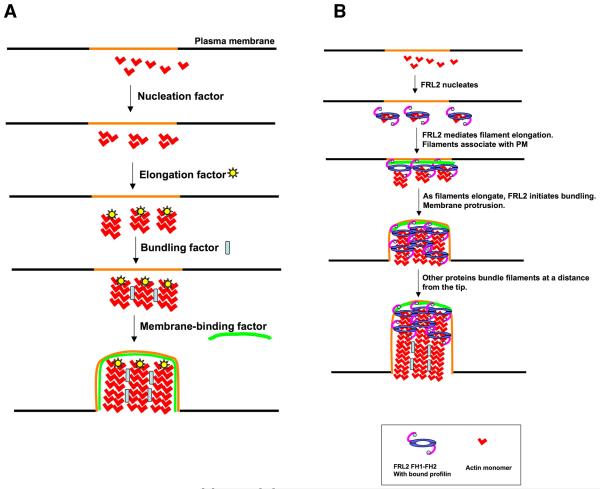
Theoretically, assembly of the filopodia observed in this paper requires several activities ((Faix et al. 2009), Figure 9A). First, something must nucleate filaments. Second, something must allow these filaments to elongate even in the presence of barbed end capping proteins. The abundance of capping proteins and their high affinity/low off-rate for barbed ends means that, in the absence of other factors, filaments would be capped long before reaching lengths observed here (>2 microns) (Pollard et al. 2000; Wear and Cooper 2004). Third, something must bundle the filaments, or else they would splay due to the pressure exerted by the membrane. Forth, something must maintain the filament bundle at or near the plasma membrane. Membrane association could occur at any stage of the process, and could be effected by the same protein conducting one of the other activities. In the absence of a membrane-binding factor, we would expect the bundles to be less productive in producing protrusions. FRL2 has, in principle, the capacity to fulfill three of these activities (nucleation, elongation, bundling), and we discuss these activities here.
Figure 9. Filopodia assembly models.
A) Schematic representations of four biochemical activities needed for filopodia assembly from a designated region of the plasma membrane (orange). Theoretically, membrane binding could occur at any step, though we include it as the last step. Also, membrane binding could occur at the tips and/or the sides of filopodia.
B) Working model for FRL2 FH1-FH2-mediated filopodia assembly, taking into account its known biochemical activities of nucleation acceleration, elongation regulation, and bundling. FRL2 FH1-FH2 nucleates filaments. Barbed end-bound FRL2 FH1-FH2 facilitates filament elongation through profilin. Additional FRL2 molecules mediate bundling near the tip. As the filaments elongate, other proteins bundle the filaments further from the tip. An unknown factor mediates membrane association. We depict membrane association at the elongation step, but it could occur during another step.
Nucleation Activity
Formins are often called “nucleation factors”, due to the ability of some formins to accelerate filament assembly from monomers. Most active in this respect are mDia1, mDia2, and Bnr1p, which are capable of assembling filaments stoichiometric to their own concentrations extremely rapidly (Harris et al. 2006; Li and Higgs 2003; Li and Higgs 2005; Moseley and Goode 2005). However, not all formins are potent nucleators (Goode and Eck 2007). Others have shown that FH2 domain of DAAM1 has low nucleation activity in vitro (Lu et al. 2007). We have shown the FH1-FH2-C construct of FRL1 is at least 80-fold weaker at nucleation than equivalent constructs of mDia1 and mDia2 (Harris et al. 2006).
Here we show that the FH1-FH2 domain of FRL2 is an extremely weak nucleator. It is striking that two formins with such widely differing nucleation activities, mDia2 and FRL2, can induce filopodia. Interestingly, the intensity of actin filament staining in the filopodia generated by mDia2 consistently appears stronger than for FRL2 (see Figures 1 and 2), although we have not examined this quantitatively. We wonder whether FRL2 is capable of nucleating the filaments in the filopodia it assembles. One possibility is that the FH1-FH2 domain has much higher nucleation activity in cells than the bacterially-expressed FH1-FH2 domain in vitro, perhaps by associating with another factor. Alternately, FRL2 FH1-FH2 could utilize filaments nucleated by other proteins, such as Arp2/3 complex or another formin. It is interesting in this context that FRL2 FH1-FH2 assembles filopodia in three very different cell types: adherent cells (3T3 and HeLa); non-adherent cells with large membrane ruffles (Jurkat); and non-adherent cells with short (300-400 nm) microvilli (300.19 cells). Arp2/3 complex is present in all of these cell types (Nicholson-Dykstra and Higgs 2008), and thus could be the nucleator. Studies aimed at inhibiting this highly abundant protein complex (>1 μM in these cell types) must be carried out to test this possibility.
Elongation and Anti-Capping Activity
Almost all formin FH2 domains tested bind filament barbed ends and move processively with the barbed end as monomers add. This processive barbed end binding effectively blocks capping protein (Harris et al. 2004; Moseley et al. 2004; Zigmond et al. 2003). In all cases studied, profilin accelerates elongation from formin-bound barbed ends at least 4-fold, as long as the formin FH1 domain is present in cis with the FH2 domain (Kovar et al. 2006; Romero et al. 2004). The basis for this profilin-mediated acceleration is that profilin binds the FH1 domain, positioning profilin-bound actin to add more efficiently to the FH2-bound barbed end (Paul and Pollard 2009).
We find that the presence of an FH1 domain is critical for FRL2-mediated filopodia assembly. We hypothesize the FH1 domain is necessary to allow rapid elongation of filament barbed ends. However, the exact sequence of the FH1 domain does not appear to be critical, since the FH1 domain of FRL1 or FRL2 is sufficient to allow filopodia assembly when in cis with FRL2’s FH2 domain. Therefore, we hypothesize that the major function of the FH1 domain in FRL2 FH1-FH2 domain-mediated filopodia assembly is to bind profilin and accelerate elongation. The fact that the barbed end binding mutant, I649A, completely eliminates the ability of FRL2 FH1-FH2 to cause filopodia assembly is strong evidence that this formin’s elongation/anti-capping activities are important for this function.
We do note, however, that we obtain differences in filopodia length in the FRL1-FRL2 chimera experiments, depending on whether the FRL1 partial FH1 or the FRL2 FH1 is used. This difference raises the possibility that differences in the ways in which FH1 and FH2 work together could influence filopodia length by changing elongation rates. Previous work by others suggests that profilin isoforms are highly specialized to interact with certain formins, and that this specificity is due to features of both the FH1 and FH2 domain (Neidt et al. 2009), so the geometry of the FH1-FH2 linkage could also play a role. However, the ability of FRL2’s FH2 domain to use its own FH1 in its normal linkage or with two extra amino acids (the inserted BamH1 site in the FRL2-FRL2 pseudo-chimera, adding Gly-Ser between FH1 and FH2) to assemble filopodia suggests that there is some leeway in the length of the linkage.
Filament Bundling
Both FRL2 and mDia2 FH2 domains bundle actin filaments (this paper, (Harris et al. 2006)), and both cause filopodia assembly. It is tempting to speculate that their bundling activities are necessary for filopodia assembly. Our future goal is to produce bundling-deficient mutants as a test of this hypothesis. If bundling is a necessary activity for FRL2-mediated filopodia assembly, it does not appear to be necessary throughout the filopodium, but more toward the tip, as we observe the most intense FRL2 localization at the tip and generally much less at regions distal to the tip. It is possible that FRL2’s bundling function is only required at the extreme distal tip.
One possibility is that FRL2-mediated bundling is required only for initiation of the filopodia bundle, and that a more potent bundler takes over subsequently. A similar scenario of sequential bundling has been proposed for several types of filopodia/microvilli, particularly in Drosophila bristles due to the sequential action of Forked and fascin (DeRosier and Tilney 2000; Tilney et al. 1998). Fascin is clearly involved in filopodia assembly in other systems (Vignjevic et al. 2006), however, fascin enriches at the tips of mDia2-generated filopodia (Yang et al. 2007), suggesting it may not be the bundler responsible for displacing or replacing formins along the filopodia shaft.
Based on the above discussion, we suggest the scheme outlined in Figure 9B as a possible working model. FRL2 FH1-FH2 nucleates new filaments, perhaps requiring a cellular activation step (eg. binding to other proteins) to do so. FRL2 remains at the filament barbed ends, allowing rapid elongation of these filaments. Additional FRL2 molecules initiate the bundling process near the tip. Other bundlers mediate bundling further away from the tip. While speculative, we propose this model as a starting point for future testing of individual steps. In particular, we wonder at the difference in ability of FRL1, FRL2, and mDia2 FH1-FH2 constructs to assemble filopodia, even though they possess similar biochemical bundling activities. Possibilities include the abilities of these proteins to bind other factors necessary for filopodia assembly. For instance, the mechanism for membrane association of these filopodia is not clear. Could FRL2 FH1-FH2 bind to a factor on the membrane, either protein or lipid, to enable filopodia assembly? There is evidence for mDia1 binding anionic phospholipids through multiple sites, including possibly a binding site in the FH2 (Ramalingam et al.).
We note that the full-length FRL2 protein is more complicated than the FH1-FH2 construct featured here, and a number of components of FRL2’s N-terminus could mediate membrane interaction, including its putative N-terminal myristoyl group, and its GBD. The hydrophobic myristoyl group could provide some membrane binding affinity, as it is proposed to do for FRL1 (Han et al. 2009). We note that currently there is no direct proof that FRL2 is myristoylated. The GBD could also mediate membrane binding through its interaction with a Rho GTPase. At present, the Rho GTPase that binds FRL2 is unknown, but its GBD is highly similar to those of other formins known to bind RhoA, Rac, Cdc42, or Rif (see alignment in (Otomo et al. 2005)). Since Rho family GTPases are membrane-bound, the combination of the myristoyl group and GTPase binding might enable stable membrane interaction. Interactions with other membrane components have also been identified for other formins. An N-terminal construct of FRL1 with a C-terminal GFP tag effectively interacts with cellular membranes (Seth et al. 2006), possibly through a combination of interactions. The N-terminus of mDia1 interacts with polyphosphoinositides in vitro through a basic region (Ramalingam et al.), and with IQGAP1 in an interaction important for mDia1 cellular localization (Brandt et al. 2007).
One difference between our results for FRL2 and mDia2 and previous results for constitutively active mDia2 constructs (Block et al. 2008; Yang et al. 2007) is that we do not observe a high proportion of bulbous, club-shaped filopodia with increased actin filament accumulation at the tips, although these do occur rarely. One reason could be our use of a minimal FH1-FH2 construct in most cases. However, we do not observe these structures in the majority of cells transfected with longer FRL2 constructs either, or with the delta-GBD mDia2 construct used in a previous study (Yang et al. 2007). It is possible that morphology of the filopodia generated may reflect differences in cell types used between these studies.
We show that full-length un-tagged FRL2 has less ability to assemble filopodia in Jurkat cells than does the delta-DAD construct. These data suggest that full-length FRL2 is subject to inhibitory interactions. Previous work showed that, while FRL2’s DID and DAD could interact in vitro and in cells, this interaction did not lead to inhibition of activities mediated by FRL2’s FH2 domain either in vitro or in cells (Vaillant et al. 2008). In these experiments, the FRL2 N-terminus was modified by an epitope tag. Thus, it is possible that a free N-terminus is important in the regulation of FRL2. One possibility is that FRL2 is myristoylated, as has been suggested for FRL1 (Han et al. 2009), and that myrystoylation plays a role in FRL2 regulation. Further biochemical work will be needed to test this possibility.
We do, however, find evidence that FRL2’s regulation is not as absolute as it appears to be for mDia2. Similar to past results (Block et al. 2008; Pellegrin and Mellor 2005; Yang et al. 2007), we find that the full-length mDia2 construct shows no ability to induce filopodia, while full-length FRL2 still maintains partial activity. One possibility is that optimal regulation of FRL2 requires other cellular factors, and these factors are overwhelmed by over-expression of the full-length protein.
Supplementary Material
Acknowledgements
We are very grateful to John Copeland for sending us the full-length human FRL2 cDNA clone, Bill Bement for the mRFP-utrophin ABD construct, Tatyana Svitkina for the full-length and deltaGBD mDia2 constructs, Odio Pilaf for extending his wisdom to us, and to Angela Krackhardt for useful reagents and discussion. This work was funded by R01 GM69818 to HNH, an American Heart Association Pre-doctoral Fellowship to ESH, and a Ruth Kirschtein Pre-doctoral National Research Service Award (F31-GM089149) to EGH.
References
- Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113(Pt 19):3439–3451. doi: 10.1242/jcs.113.19.3439. [DOI] [PubMed] [Google Scholar]
- Block J, Stradal TE, Hanisch J, Geffers R, Kostler SA, Urban E, Small JV, Rottner K, Faix J. Filopodia formation induced by active mDia2/Drf3. J Microsc. 2008;231(3):506–17. doi: 10.1111/j.1365-2818.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U S A. 2006;103(33):12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178(2):193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64(11):822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9(10):1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Walker SB, Pollard TD. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983;4(2):253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Copeland JW, Treisman R. The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol Biol Cell. 2002;13(11):4088–4099. doi: 10.1091/mbc.02-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosier DJ, Tilney LG. F-actin bundles are derivatives of microvilli: What does this tell us about how bundles might form? J Cell Biol. 2000;148(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Faix J, Breitsprecher D, Stradal TE, Rottner K. Filopodia: Complex models for simple rods. Int J Biochem Cell Biol. 2009;41(8-9):1656–1664. doi: 10.1016/j.biocel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and Function of Formins in Control of Actin Assembly. Annu Rev Biochem. 2007 doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE 2007. 2007;(400):re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Han Y, Eppinger E, Schuster IG, Weigand LU, Liang X, Kremmer E, Peschel C, Krackhardt AM. Formin-like 1 (FMNL1) is regulated by N-terminal myristoylation and induces polarized membrane blebbing. J Biol Chem. 2009;284(48):33409–33417. doi: 10.1074/jbc.M109.060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ES, Li F, Higgs HN. The mouse formin, FRLa, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J. Biol. Chem. 2004;279(19):20076–20087. doi: 10.1074/jbc.M312718200. [DOI] [PubMed] [Google Scholar]
- Harris ES, Rouiller I, Hanein D, Higgs HN. Mechanistic differences in actin bundling activity of two mammalian formins, FRL1 and mDia2. J. Biol. Chem. 2006;281(20):14383–14392. doi: 10.1074/jbc.M510923200. [DOI] [PubMed] [Google Scholar]
- Higashida C, Miyoshi T, Fujita A, Oceguera-Yanez F, Monypenny J, Andou Y, Narumiya S, Watanabe N. Actin polymerization-driven molecular movement of mDia1 in living cells. Science. 2004;303:2007–2010. doi: 10.1126/science.1093923. [DOI] [PubMed] [Google Scholar]
- Higgs HN, Peterson KJ. Phylogenetic analysis of the Formin Homology 2 (FH2) domain. Mol Biol Cell. 2005;16(1):1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber ML, Jacobs DT, Campagnola L, Dunn BD, Yin T, Sousa AD, Quintero OA, Cheney RE. A novel form of motility in filopodia revealed by imaging myosin-X at the single-molecule level. Curr Biol. 2009;19(11):967–973. doi: 10.1016/j.cub.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124(2):423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Li F, Higgs HN. The mouse formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13(15):1335–1340. doi: 10.1016/s0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- Li F, Higgs HN. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J. Biol. Chem. 2005;280(8):6986–6992. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- Lu J, Meng W, Poy F, Maiti S, Goode BL, Eck MJ. Structure of the FH2 domain of Daam1: implications for formin regulation of actin assembly. J Mol Biol. 2007;369(5):1258–1269. doi: 10.1016/j.jmb.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S, Pollard TD. Mechanisms of action of cytochalasin B on actin. Cell. 1980;20:329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Majstoravich S, Zhang J, Nicholson-Dykstra S, Linder S, Friedrich W, Siminovitch KA, Higgs HN. Lymphocyte microvilli are dynamic, actin-dependent structures that do not require Wiskott-Aldrich syndrome protein (WASp) for their morphology. Blood. 2004;104(5):1396–1403. doi: 10.1182/blood-2004-02-0437. [DOI] [PubMed] [Google Scholar]
- Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol. 1999;146(5):1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol. 2007;176(7):953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H. The role of formins in filopodia formation. Biochim Biophys Acta. 2010;1803(2):191–200. doi: 10.1016/j.bbamcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Mersich AT, Miller MR, Chkourko H, Blystone SD. The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton (Hoboken) 67(9):573–585. doi: 10.1002/cm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard TH, Bompard G, Heung MY, Dafforn TR, Scott DJ, Machesky LM, Futterer K. Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. Embo J. 2005;24(2):240–250. doi: 10.1038/sj.emboj.7600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ. Actin based motility on retraction fibers in mitotic PtK2 cells. Cell Motil Cytoskeleton. 1992;22(2):135–151. doi: 10.1002/cm.970220207. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Goode BL. Differential activities and regulation of Saccharomyces cerevisiae formin proteins Bni1 and Bnr1 by Bud6. J Biol Chem. 2005;280(30):28023–33. doi: 10.1074/jbc.M503094200. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. A conserved mechanism for Bni1- and mDia1- induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell. 2004;116(5):711–723. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidt EM, Scott BJ, Kovar DR. Formin differentially utilizes profilin isoforms to rapidly assemble actin filaments. J Biol Chem. 2009;284(1):673–684. doi: 10.1074/jbc.M804201200. [DOI] [PubMed] [Google Scholar]
- Nicholson-Dykstra SM, Higgs HN. Arp2 depletion inhibits sheet-like protrusions but not linear protrusions of fibroblasts and lymphocytes. Cell Motil Cytoskeleton. 2008;65(11):904–922. doi: 10.1002/cm.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18(3):273–281. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Paul AS, Pollard TD. Review of the mechanism of processive actin filament elongation by formins. Cell Motil Cytoskeleton. 2009;66(8):606–617. doi: 10.1002/cm.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15(2):129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- Ramalingam N, Zhao H, Breitsprecher D, Lappalainen P, Faix J, Schleicher M. Phospholipids regulate localization and activity of mDia1 formin. Eur J Cell Biol. 89(10):723–732. doi: 10.1016/j.ejcb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119(3):419–429. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164(6):887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol. 2006;174(5):701–713. doi: 10.1083/jcb.200605006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Celis JE. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 1978;16(2):308–325. [PubMed] [Google Scholar]
- Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- Steffen A, Faix J, Resch GP, Linkner J, Wehland J, Small JV, Rottner K, Stradal TE. Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol Biol Cell. 2006;17(6):2581–2591. doi: 10.1091/mbc.E05-11-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160(3):409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM. Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J Cell Biol. 1998;143(1):121–133. doi: 10.1083/jcb.143.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82(4):1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant DC, Copeland SJ, Davis C, Thurston SF, Abdennur N, Copeland JW. Interaction of the N- and C-terminal auto-regulatory domains of FRL2 does not inhibit FRL2 activity. J Biol Chem. 2008 doi: 10.1074/jbc.M803156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174(6):863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallar BJ, Stropich BN, Schoenherr JA, Holman HA, Kitchen SM, Alberts AS. The basic region of the diaphanous-autoregulatory domain (DAD) is required for autoregulatory interactions with the diaphanous-related formin inhibitory domain. J. Biol. Chem. 2006;281(7):4300–4307. doi: 10.1074/jbc.M510277200. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1(3):136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Watanabe TM, Tokuo H, Gonda K, Higuchi H, Ikebe M. Myosin-X induces filopodia by multiple elongation mechanism. J Biol Chem. 2010 doi: 10.1074/jbc.M109.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear MA, Cooper JA. Capping protein: new insights into mechanism and regulation. Trends Biochem Sci. 2004;29(8):418–428. doi: 10.1016/j.tibs.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Xu Y, Moseley J, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal Structures of a formin homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–723. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- Yamagishi A, Masuda M, Ohki T, Onishi H, Mochizuki N. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J Biol Chem. 2004;279(15):14929–14936. doi: 10.1074/jbc.M309408200. [DOI] [PubMed] [Google Scholar]
- Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5(11):2624–2645. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hoelzle M, Disanza A, Scita G, Svitkina T. Coordination of membrane and actin cytoskeleton dynamics during filopodia protrusion. PLoS One. 2009;4(5):e5678. doi: 10.1371/journal.pone.0005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296(5569):913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- Zigmond SH, Evangelista M, Boone C, Yang C, Dar AC, Sicheri F, Forkey J, Pring M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr Biol. 2003;13(20):1820–3. doi: 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.