Abstract
Background
Factors driving the trend of earlier dialysis initiation for persons with end-stage renal disease are unknown. We wished to determine the association of the number and type of signs and symptoms with timing of initiation of dialysis among US nursing home residents.
Study design
Observational study
Setting and participants
We used data from the United States Renal Data System linked with the Minimum Data Set, a national registry of nursing home residents. The cohort consisted of 2402 nursing home residents who initiated dialysis between 1998 and 2000 and had at least two recorded clinical assessments in the year prior to dialysis initiation.
Predictors
We evaluated seven clinical signs and symptoms: dependence in activities of daily living, cognitive function, edema, dyspnea, nutritional problems, vomiting and body size.
Outcomes
Earlier dialysis initiation was defined as an estimated glomerular filtration rate ≥15 ml/min/1.73m2 at the start of dialysis.
Results
The median (25th percentile, 75th percentile) eGFR at the start of dialysis was 9.8 (7.4, 13.4) ml/min/1.73m2 [ND1]. After adjustment for age, sex, race and comorbid conditions, each additional sign or symptom was associated with a higher odds for earlier dialysis initiation (OR, 1.16 per symptom; 95% CI, 1.06-1.28), as was each adversely changing sign or symptom (OR, 1.26 per symptom; 95% CI, 1.16-1.38). The population attributable risk for earlier dialysis initiation associated with having one or more signs and symptoms of volume overload, cognitive decline, increasing activities of daily living dependence and weight loss was 31%; volume overload had the largest aggregate population attributable risk.
Limitations
We lacked information about metabolic indications for dialysis initiation.
Conclusions
Volume overload, cognitive decline, increasing activities of daily living dependence and weight loss were associated with earlier dialysis initiation; however, these factors explained less than one third of cases of earlier dialysis initiation in nursing home residents.
Keywords: dialysis initiation, end-stage renal disease, elderly
Over the last decade, persons with end-stage renal disease (ESRD) have started dialysis with progressively higher levels of estimated glomerular filtration rate (eGFR) 1, 2, generally assumed to represent an intervention earlier in the course of kidney disease. In general, patients who are older and patients with more comorbidity are more likely to start dialysis with a higher eGFR 3, 4. One proposed explanation for this observation is that these patients may develop symptoms earlier in the course of their kidney disease as compared to younger healthier patients. A related rationale is that creatinine-based GFR estimating equations are less accurate in elderly patients, leading to concerns they may underestimate the severity of kidney failure in this population. Non-uremic symptoms may also mimic symptoms of uremia in frail elderly patients. Alternatively, earlier initiation of dialysis may be driven by a preemptive management approach based predominantly on decline in eGFR below a specific threshold rather than on the development of uremic symptoms or other complications of ESRD.
In a recent clinical trial, planned initiation of dialysis when the Cockcroft Gault eGFR [ND2]fell between 10-14 ml/min did not prolong survival or improve other clinically meaningful outcomes compared to a strategy of dialysis initiation when symptoms develop or when eGFR falls below 7 ml/min 5. Preemptive earlier initiation of dialysis may impose a substantial burden on patients, caregivers and the health care system. Some have suggested that earlier initiation of dialysis has contributed to the recent dramatic rise in ESRD incidence among the elderly 2, 6, 7.
Studies of timing of dialysis initiation have largely utilized ESRD registries that fail to capture the events leading up to dialysis initiation 3, 8-12, thus the extent to which clinical signs or symptoms influence the timing of dialysis initiation is unknown. Further, it is also unclear which signs or symptoms are most strongly associated with timing of dialysis initiation in the elderly. The National Kidney Foundation’s Kidney Disease Quality Outcomes Initiative (KDOQI) clinical practice guidelines suggest that in addition to several metabolic complications, certain clinical signs and symptoms may justify initiation of dialysis when eGFR is greater than 15 ml/min/1.73m2, including neurologic dysfunction, refractory volume overload, weight loss, nausea and vomiting, or an otherwise unexplained decline in functioning 13.
We studied the association of seven commonly assessed signs and symptoms with timing of dialysis initiation: dependence in activities of daily living (ADL), cognitive function, edema, dyspnea, nutritional problems, vomiting, and body size. We hypothesized that the presence of these signs and symptoms and their trajectory would be independently associated with earlier initiation of dialysis, and that collectively these signs and symptoms would account for a large fraction of earlier initiation of dialysis among elderly nursing home residents.
Methods
Subjects
We used data from the United States Renal Data System (USRDS) that had been linked with data from the Minimum Data Set (MDS) using name, date of birth, social security number, health insurance claim number and beneficiary identity code. In this manner, we identified all persons who were residents of a nursing home at the start of dialysis and who started dialysis between June 1, 1998 and December 31, 2000, as previously described 14. The MDS is a national registry of residents of nursing homes in the US. We included all individuals who were residents of a nursing home on the first ESRD service date and with a length of stay of at least 90 consecutive days (not including departures of 15 days or less) or a length of stay of less than 90 consecutive days and culminating in death. Since nursing home residence can be transitional, we included persons who had resided in a nursing home for 90 consecutive days and were discharged no more than 15 days prior to the first ESRD service date and then returned to a nursing home within 90 days. For the primary analyses, we limited the cohort to individuals with at least two recorded clinical assessments within the year prior to dialysis initiation, resulting in 2509 individuals who were residents of a nursing home at the start of dialysis. Of these, we excluded 107 with missing values for eGFR at dialysis initiation, leaving 2402 individuals in the analytic cohort.
Exposure variables
MDS assessments are completed by nursing staff at admission and then every three months, as well as at the time of acute changes in status and readmission from hospital. From these assessments, we selected seven clinical signs and symptoms identified by practice guidelines as potential indications for earlier dialysis initiation: dependence in activities of daily living (ADL), cognitive function, edema, dyspnea, nutritional problems, vomiting, and underweight. Dependence in each of seven ADLs (eating, dressing, toileting, personal hygiene, locomotion, transferring and bed mobility) was rated 0-4 as follows: 0=independent, 1=needs supervision, 2=needs limited assistance, 3=needs extensive assistance, 4=dependent. The total MDS-ADL score ranges from 0-28, higher scores indicate more extensive functional impairment. An MDS-ADL score >20 can be interpreted as the need for extensive assistance in each of seven ADLs. Cognitive function was evaluated with the MDS Cognitive Performance Scale (CPS). The scale is derived from MDS items that assess consciousness, decision making skills, short term memory, ability to make oneself understood, and functional dependence in eating. MDS-CPS scores range from 0-6, higher scores indicate poorer cognitive function. An MDS-CPS score >1 is approximately equal to a Mini-Mental State Exam (MMSE) score <20, indicating moderate to severe cognitive impairment. The MDS-ADL and MDS-CPS scales have been previously validated 15-17. Edema, dyspnea, vomiting, and nutritional problems, defined as complaints about the taste of food or hunger or leaving >25% of meals uneaten, were evaluated as present or not present at each assessment. Body mass index at each assessment was categorized as ≤18.5 kg/m2, 18.5 to ≤25 kg/m2, 25 to ≤30 kg/m2, and >30 kg/m2.
We determined the change in each of these signs and symptoms using the first and last assessment conducted within one year prior to dialysis initiation. Increasing ADL dependence was defined as an increase in MDS-ADL score of 1 point or more. Cognitive decline was defined as an increase in the MDS-CPS of 1 point or more. The percentage change in body weight was categorized into quartiles (body weight decline >5.7%, decline ≤5.7%, increase ≤3.8%, increase >3.8%). Change in edema was categorized as no edema at baseline or follow-up, resolved edema at follow-up, new edema at follow-up and persistent edema at follow-up. Changes in symptoms of dyspnea, vomiting, and nutritional problems were categorized in a similar manner.
Outcome
Kidney function at the start of dialysis was obtained from the Centers for Medicare and Medicaid Services Medical Evidence form. Estimated GFR was calculated using the Modification of Diet in Renal Disease Study[ND3] equation, incorporating age, sex, race, and serum creatinine concentration 18. Earlier dialysis initiation was defined as an eGFR ≥15 ml/min/1.73m2 at the time of dialysis initiation.
Covariates
Residents were considered to have the following comorbid conditions if a “yes” was coded on either the USRDS Medical Evidence Report or on the MDS assessments prior to dialysis initiation: diabetes, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, cancer, and chronic obstructive pulmonary disease 19.
Statistical analysis
Resident characteristics at the start of dialysis were compared according to the number of symptoms prior to initiation using t-tests or chi-square tests, as appropriate. We used logistic regression to determine the association, expressed as an odds ratio (OR) and 95% confidence interval (95% CI), of signs and symptoms with earlier dialysis initiation. We first determined the association of the number of signs and symptoms present at the last assessment with earlier dialysis initiation. Adjusted models included age, sex, race, diabetes, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, cancer, and chronic obstructive pulmonary disease in addition to the number of signs and symptoms present. Next, we determined the association of individual signs or symptoms present at the last assessment with earlier dialysis initiation. Similar analyses were conducted to determine the association of changes in each sign and symptom during the year prior to dialysis with earlier dialysis initiation. For signs or symptoms measured on an ordinal scale, we then simultaneously adjusted for the follow-up severity of each sign in addition to its trajectory.
Because changes in body weight may be confounded by volume overload, we repeated these analyses after stratifying by the presence or absence of edema. We also stratified the analyses according to hospitalization status at the start of dialysis. We hypothesized that dialysis initiation would be more strongly associated with acute events rather than clinical signs and symptoms among inpatients.
We conducted several sensitivity analyses to assess the robustness of these results. First, we used several alternate definitions of earlier initiation: Cockcroft-Gault creatinine clearance ≥15 ml/min and CKD-EPI eGFR ≥15 ml/min/1.73m2. We also limited the analyses to residents whose last assessment was within 30 days before dialysis initiation (n=1099), and to those who were not hospitalized between baseline and follow-up assessments (n=2147). Finally, we expanded the cohort to include all individuals with at least one clinical assessment prior to dialysis initiation.
We calculated the population attributable risk percentage for selected signs and symptoms using the following equation: Pe × (RR-1)/RR, where Pe is the prevalence of each exposure, and RR is the multivariable adjusted relative risk (in this case odds ratio). This equation is felt to yield less bias when confounding may exist 20. Analyses were conducted with SAS v9.1 (www.sas.com).
Results
Cohort characteristics
There were 431 individuals (18%) who had none of the seven signs or symptoms, 1495 individuals (62%) who had 1-2 signs or symptoms and 476 individuals (19%) with 2 or more signs and symptoms prior to dialysis initiation (Table 1). Those with more signs or symptoms prior to dialysis were older and more likely to be female. They also had a higher prevalence of most comorbid conditions, with the exception of diabetes and cancer.
Table 1.
Characteristics of nursing home residents at start of dialysis, stratified by number of signs and symptoms prior to the start of dialysis.
| Number of signs or symptoms | ||||
|---|---|---|---|---|
| Characteristic | None (n=431) | 1-2 (n=1495) | >2 (n=476) | P-value |
| Age (years) | 72.4 ± 12.0 | 73.7 ± 10.3 | 75.0 ± 10.1 | <0.001 |
| Body mass index (kg/m2) | 26.5 ± 6.5 | 26.2 ± 7.6 | 24.9 ± 7.9 | 0.001 |
| Albumin (g/dL) | 2.9 ± 0.6 | 2.9 ± 0.6 | 2.8 ± 0.6 | <0.001 |
| Blood urea nitrogen (mg/dL) | 91.5 ± 34.7 | 90.8 ± 35.2 | 94.3 ± 36.6 | 0.2 |
| Creatinine (mg/dL) | 6.0 ± 2.4 | 5.8 ± 2.4 | 5.6 ± 2.7 | 0.01 |
| Hemoglobin (g/dL) | 10.0 ± 1.8 | 9.9 ± 1.7 | 9.8 ± 1.7 | 0.2 |
| Female | 56.6 | 60.2 | 70.0 | <0.001 |
| Non-white race | 35.7 | 35.3 | 36.3 | 0.9 |
| Diabetes | 77.5 | 68.2 | 64.9 | <0.001 |
| Congestive heart failure | 60.6 | 71.0 | 72.9 | <0.001 |
| Coronary artery disease | 43.9 | 46.4 | 46.9 | 0.6 |
| Peripheral vascular disease | 38.8 | 38.3 | 39.1 | 0.9 |
| Cerebrovascular disease | 34.6 | 39.7 | 47.3 | <0.001 |
| Chronic obstructive pulmonary disease | 20.4 | 26.8 | 26.9 | 0.02 |
| Cancer | 10.2 | 13.4 | 11.8 | 0.2 |
| Hospitalized at start of dialysis | 67.1 | 71.3 | 74.2 | 0.06 |
N=2402. Values shown as Mean ± standard deviation or Frequency (%)
Note: Conversion factors for units: albumin in g/dL to g/L, x10, blood urea nitrogen in mg/dL to mmol/L, x0.357, creatinine in mg/dL to μmol/L, x88.4, hemoglobin in g/dL to g/L, x10.
Association of number of signs and symptoms with earlier dialysis initiation
There were 440 individuals (18%) who initiated dialysis earlier (with an eGFR ≥15 ml/min/1.73m2). After adjustment for age, sex, race and comorbid conditions, each additional sign or symptom present at the last assessment prior to dialysis initiation was associated with a 16% higher odds for earlier initiation of dialysis (OR, 1.16; 95% CI, 1.07-1.28). Similarly, each additional adversely changing sign or symptom was associated with a 24% higher odds for earlier initiation of dialysis (OR, 1.24; 95% CI, 1.14-1.34) after adjustment. Those with 2 or more adversely changing signs or symptoms had a significant, 1.5 to 3-fold higher odds of earlier dialysis initiation (Figure 1).
Figure 1.
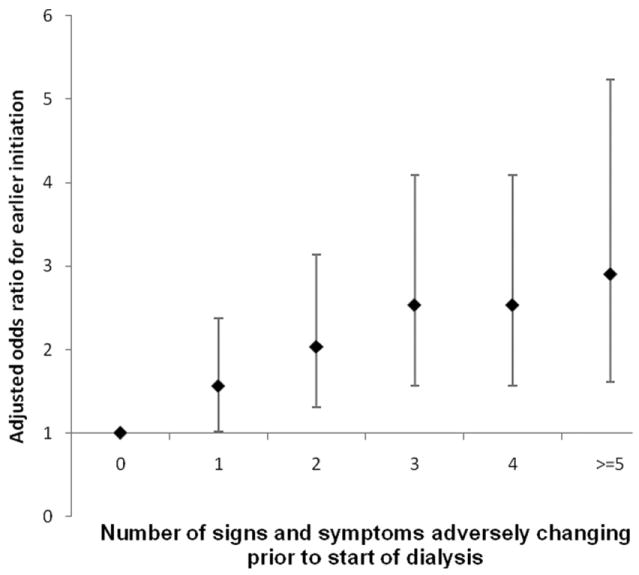
A[ND4]djusted association of number of adversely changing signs and symptoms with earlier dialysis initiation (estimated glomerular filtration rate ≥15 ml/min/1.73m2) among nursing home residents. Error bars indicate 95% confidence intervals. Note: Signs and symptoms included the following: increasing dependence in activities of daily living, cognitive decline, persistent or new edema, persistent or new dyspnea, persistent or new nutritional problem, persistent or new vomiting, weight loss >5.7% of body weight or weight gain >3.8% of body weight. Model adjusted for age, sex, race, diabetes, congestive heart failure, coronary artery disease, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, and cancer.
Association of individual signs and symptoms with earlier dialysis initiation
In unadjusted analyses, the presence of edema or dyspnea was associated with a significantly increased odds of earlier dialysis initiation, while the presence of moderate to severe cognitive impairment was associated with a lower odds of earlier dialysis initiation (Table 2). These associations were similar after adjustment for age, sex, race and comorbid conditions. When we expanded the cohort to include all individuals with at least one clinical assessment prior to dialysis initiation, ADL impairment, nutritional problems, and underweight were also significantly associated with earlier dialysis initiation (Tables S1-S3, available as online supplementary material).
Table 2.
Association of signs and symptoms prior to dialysis initiation with earlier initiation of dialysis among nursing home residents
| OR for earlier initiation (95% CI) | ||
|---|---|---|
| Sign or symptom | Unadjusted | Adjusted for resident characteristics* |
| Extensive assistance needed in all ADL | 1.12 (0.87-1.45) | 1.23 (0.95-1.61) |
| Moderate to severe cognitive impairment | 0.80 (0.65-0.99) | 0.84 (0.67-1.04) |
| Edema | 1.57 (1.28-1.94) | 1.54 (1.24-1.92) |
| Dyspnea | 2.07 (1.51-2.85) | 1.95 (1.40-2.73) |
| Nutritional problem | 1.05 (0.84-1.30) | 1.13 (0.91-1.42) |
| Vomiting | 0.71 (0.44-1.14) | 0.71 (0.43-1.15) |
| Body mass index (kg/m2) | ||
| ≤18.5 | 1.16 (0.76-1.77) | 1.34 (0.86-2.10) |
| >18.5 and ≤25 | 0.90 (0.70-1.16) | 0.93 (0.71-1.21) |
| >25 and ≤30 | 0.79 (0.60-1.05) | 0.81 (0.60-1.08) |
| >30 | 1.00 (Referent) | 1.00 (Referent) |
Earlier initiation of dialysis defined as estimated glomerular filtration rate >15 ml/min/1.73m2
Abbreviations: ADL (activities of daily living), OR, odds ratio; CI, confidence interval
Model adjusted for age, sex, race, diabetes, congestive heart failure, coronary artery disease, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, and cancer.
The associations differed somewhat when we evaluated the change in signs and symptoms over time. Cognitive decline, persistent or newly developed edema, persistent or resolved dyspnea, and weight gain ≥3.8% of body weight were associated with earlier dialysis initiation after adjustment for resident characteristics (Table 3). Increasing dependence in ADLs and weight loss >5.7% of body weight had borderline significant associations with earlier dialysis initiation. In stratified analyses, weight gain >3.8% of body weight (OR 1.72, 95% CI 1.15-2.57) remained associated with earlier initiation among those with edema, whereas there was not a statistically significant association of weight changes with earlier initiation among those without edema. In models adjusted for the severity of signs or symptoms in addition to their trajectory, weight gain and cognitive decline remained associated with a higher odds for earlier initiation (Tables S1-S3), whereas moderate to severe cognitive impairment at the last assessment prior to dialysis initiation was associated with a lower odds for earlier initiation.
Table 3.
Association of change in signs and symptoms prior to dialysis initiation with earlier initiation of dialysis among nursing home residents
| OR for earlier initiation (95% CI) | ||
|---|---|---|
| Sign or symptom | Unadjusted | Adjusted for resident characteristics* |
| Increasing dependence in ADL | 1.19 (0.97-1.47) | 1.22 (0.99-1.51) |
| ADL dependence maintained | 1.00 (Referent) | 1.00 (Referent) |
| Cognitive decline | 1.41 (1.12-1.78) | 1.48 (1.17-1.88) |
| Cognitive function maintained | 1.00 (Referent) | 1.00 (Referent) |
| Edema | ||
| Persistent | 1.75 (1.35-2.26) | 1.71 (1.31-2.24) |
| Newly developed | 1.52 (1.15-2.03) | 1.48 (1.11-1.99) |
| Resolved | 1.28 (0.90-1.82) | 1.23 (0.86-1.77) |
| No edema | 1.00 (Referent) | 1.00 (Referent) |
| Dyspnea | ||
| Persistent | 2.27 (1.38-3.74) | 2.05 (1.23-3.43) |
| Newly developed | 1.10 (0.62-1.94) | 0.94 (0.53-1.69) |
| Resolved | 1.97 (1.33-2.92) | 1.89 (1.26-2.85) |
| No dyspnea | 1.00 (Referent) | 1.00 (Referent) |
| Nutritional problem | ||
| Persistent | 1.08 (0.81-1.43) | 1.17 (0.87-1.56) |
| Newly developed | 1.29 (0.90-1.85) | 1.31 (0.90-1.89) |
| Resolved | 1.04 (0.76-1.42) | 1.11 (0.81-1.53) |
| No nutritional problem | 1.00 (Referent) | 1.00 (Referent) |
| Vomiting | ||
| Persistent | 0.44 (0.10-1.87) | 0.51 (0.12-2.21) |
| Newly developed | 0.89 (0.47-1.67) | 0.87 (0.46-1.64) |
| Resolved | 0.76 (0.45-1.26) | 0.74 (0.44-1.24) |
| No vomiting | 1.00 (Referent) | 1.00 (Referent) |
| Weight change (% of body weight) | ||
| loss >5.7% | 1.29 (0.96-1.74) | 1.31 (0.97-1.77) |
| loss 0-5.7% | 1.12 (0.82-1.52) | 1.16 (0.85-1.58) |
| gain 0-3.8% | 1.00 (Referent) | 1.00 (Referent) |
| gain >3.8% | 1.45 (1.09-1.95) | 1.43 (1.06-1.92) |
Earlier initiation of dialysis defined as estimated glomerular filtration rate >15 ml/min/1.73m2
Abbreviations: ADL (activities of daily living), OR, odds ratio; CI, confidence interval
Model adjusted for age, sex, race, diabetes, congestive heart failure, coronary artery disease, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, and cancer.
The pattern of signs and symptoms associated with earlier dialysis initiation differed among those starting dialysis as an inpatient versus an outpatient. For example, more extensive ADL dependence at follow-up and increasing ADL dependence were associated with outpatient but not inpatient earlier dialysis initiation (P-value for interaction = 0.03 and 0.04, respectively, Table 4). Nutritional problems were more strongly associated with earlier initiation of dialysis among outpatients, whereas edema was less strongly associated with earlier dialysis initiation in these patients. However, these differences did not reach statistical significance (P-values for interaction 0.3 and 0.06, respectively).
Table 4.
Adjusted association of severity at last assessment and change in signs and symptoms with earlier dialysis initiation, stratified by hospitalization status at the start of dialysis.
| Sign or symptom at last assessment prior to dialysis initiation | dialysis initiation |
P* | Change in sign or symptom | dialysis initiation |
P* | ||
|---|---|---|---|---|---|---|---|
| Inpatient (n=1708) | Outpatient (n=694) | Inpatient (n=1708) | Outpatient (n=694) | ||||
| Extensive assistance needed in all ADL | 1.04 (0.76-1.43) | 1.90 (1.14-3.16) | 0.03 | Increasing dependence in ADL | 1.06 (0.82-1.36) | 1.68 (1.13-2.50) | 0.04 |
| ADL dependence unchanged | 1.00 (Referent) | 1.00 (Referent) | |||||
| Moderate to severe cognitive impairment | 0.73 (0.57-0.95) | 1.12 (0.75-1.68) | 0.06 | Cognitive decline | 1.35 (1.02-1.79) | 1.95 (1.25-3.05) | 0.2 |
| Cognitive function maintained | 1.00 (Referent) | 1.00 (Referent) | |||||
| Edema | 1.77 (1.37-2.30) | 1.11 (0.74-1.66) | 0.06 | Edema | 0.09 | ||
| Persistent | 1.92 (1.40-2.65) | 1.28 (0.77-2.14) | |||||
| Newly developed | 1.84 (1.29-2.61) | 0.93 (0.54-1.60) | |||||
| Resolved | 1.37 (0.89-2.11) | 0.98 (0.51-1.88) | |||||
| No edema | 1.00 (Referent) | 1.00 (Referent) | |||||
| Dyspnea | 1.99 (1.34-2.96) | 1.94 (1.04-3.59) | 0.9 | Dyspnea | 0.7 | ||
| Persistent | 1.98 (1.05-3.74) | 2.20 (0.90-5.39) | |||||
| Newly developed | 0.89 (0.46-1.72) | 1.14 (0.31-4.18) | |||||
| Resolved | 1.97 (1.21-3.19) | 1.78 (0.81-3.91) | |||||
| No dyspnea | 1.00 (Referent) | 1.00 (Referent) | |||||
| Nutritional problem | 1.05 (0.81-1.38) | 1.33 (0.87-2.03) | 0.4 | Nutritional problem | 0.7 | ||
| Persistent | 1.05 (0.71-1.59) | 1.77 (1.01-3.10) | |||||
| Newly developed | 1.24 (0.77-2.02) | 1.58 (0.78-3.22) | |||||
| Resolved | 1.19 (0.79-1.78) | 1.46 (0.77-2.76) | |||||
| No nutritional problem | 1.00 (Referent) | 1.00 (Referent) | |||||
| Vomiting | 0.70 (0.39-1.26) | 0.73 (0.30-1.79) | 0.9 | Vomiting | 0.8 | ||
| Persistent | 0.73 (0.16-3.30) | ** | |||||
| Newly developed | 0.89 (0.41-1.97) | 0.83 (0.28-2.47) | |||||
| Resolved | 0.69 (0.37-1.30) | 0.85 (0.34-2.09) | |||||
| No vomiting | 1.00 (Referent) | 1.00 (Referent) | |||||
| BMI (kg/m2) | 0.009 | Weight change (% of body weight) | 0.6 | ||||
| ≤18.5 | 1.09 (0.63-1.88) | 2.29 (1.02-5.12) | loss >5.7% | 1.22 (0.85-1.76) | 1.53 (0.90-2.63) | ||
| >18.5 and ≤25 | 0.76 (0.56-1.04) | 1.52 (0.90-2.56) | loss 0-5.7% | 1.37 (0.95-1.98) | 0.76 (0.42-1.38) | ||
| >25 and ≤30 | 0.74 (0.53-1.05) | 1.07 (0.61-1.90) | gain 0-3.8% | 1.00 (Referent) | 1.00 (Referent) | ||
| >30 | 1.00 (Referent) | 1.00 (Referent) | gain >3.8% | 1.47 (1.03-2.09) | 1.41 (0.81-2.45) | ||
Values shown are adjusted odds ratios (95% confidence intervals). Earlier initiation of dialysis defined as estimated glomerular filtration rate >15 ml/min/1.73m2. Model adjusted for age, sex, race, diabetes, congestive heart failure, coronary artery disease, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, and cancer.
Abbreviations: ADL (activities of daily living); BMI, body mass index
for interaction
odds ratio could not be estimated due to small number of subjects
In sensitivity analyses utilizing different definitions for earlier initiation of dialysis, the results were similar, except for the association of nutritional signs and symptoms with estimated creatinine clearance ≥15 ml/min (Tables S1-S3). When the analyses were limited to residents who were not hospitalized between assessments, or those whose last assessment was within 30 days of dialysis initiation, the results were also similar. For example, among those with a recent clinical assessment, cognitive decline (OR 1.63, 95% CI 1.16-2.28), persistent edema (OR 2.20, 95% CI 1.48-3.26), newly developed edema (OR 1.80, 95% CI 1.18-2.76) and persistent dyspnea (OR 2.06, 95% CI 1.03-4.12) all had significant associations with earlier dialysis initiation.
Population attributable risk
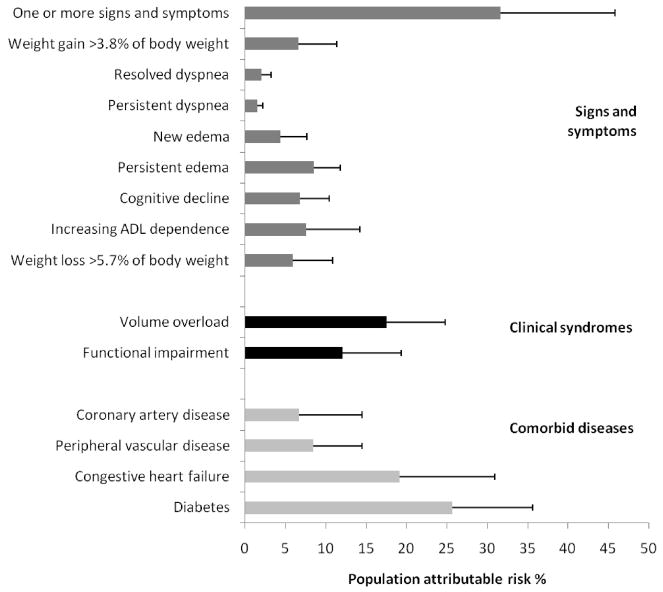
The population attributable risk was 25% for having one or more of the following signs and symptoms: cognitive decline, persistent or new edema, persistent or resolved dyspnea, or weight gain >3.8% of body weight. Of these signs and symptoms, persistent edema had the largest individual population attributable risk (9%), and the syndrome of volume overload (edema, dyspnea, or weight gain) had the largest aggregate poulation attributable risk (18%) (Figure 2). If increasing ADL dependence and weight loss were also included, the population attributable risk increased to 31%. Results were similar when population attributable risk was calculated using the Cockcroft Gault formula to define earlier initiation (35%). The population attributable risk for one or more of these signs and symptoms was higher among outpatients (42%) versus inpatients (26%), and was similar by age group, sex and race.
Figure 2.
Population attributable risk percent of selected signs and symptoms, clinical syndromes and comorbid conditions for earlier dialysis initiation (estimated glomerular filtration rate ≥15 ml/min/1.73m2). Volume overload defined as weight gain >3.8% of body weight, persistent or resolved dyspnea, or persistent or new edema. Functional impairment defined as increasing ADL dependence or cognitive decline. Bars indicate 95% confidence interval.
Discussion
The frequency of dialysis initiation with an eGFR ≥15 ml/min/1.73m2 has increased dramatically over the past decade in the absence of strong evidence in favor or against, and has very important policy and resource implications for the US ESRD program. In the IDEAL (Initiating Dialysis Early and Late) study, subjects in the planned early-start group initiated dialysis a mean of 6 months earlier but did not have prolonged survival or better quality of life compared to subjects in the late-start group who initiated dialysis based on the development of symptoms or when eGFR declined below 7 ml/min 5. These findings suggest that it may be possible to safely delay dialysis initiation in some patients with advanced CKD provided that symptoms of uremia or other ESRD-associated complications are not present 21. To understand whether these results might be applied to an older and sicker population, a better understanding of the drivers of earlier dialysis initiation is critical.
In an international survey of more than 4000 nephrologists, uremic signs and symptoms were rated as the most important factor guiding the timing of dialysis initiation 22. This finding was confirmed by the IDEAL study, which reported that uremia was the primary reason for dialysis initiation with eGFR >7 ml/min in the late-start group 5. Yet few studies have prospectively evaluated in detail the clinical factors that influence the timing of dialysis initiation. In a study of 238 patients starting dialysis, Curtis et al. evaluated symptoms associated with uremia and timing of dialysis initiation 23. Similar to our findings, patients with a larger number of symptoms were more likely to start dialysis earlier. However, the association of individual symptoms and their trajectories with timing of dialysis initiation was not evaluated.
Our study evaluated many of the signs and symptoms suggested by clinical practice guidelines as potentially appropriate indications for earlier dialysis initiation 13. We considered not only the number of signs and symptoms present, but also their trajectory and severity at follow-up. Our results suggest that both the trajectory and the severity of signs and symptoms are being used as indications for earlier dialysis initiation, depending on the individual sign or symptom and the clinical setting.
Dyspnea, edema and weight gain were strongly and independently associated with earlier initiation of dialysis, suggesting that failing medical management or the relative ease of dialytic versus medical management for volume overload are to some extent driving the trend towards earlier dialysis initiation in the elderly. Serum creatinine concentration is dependent on the volume of distribution of creatinine (roughly equal to total body water), and hence, may not accurately reflect the severity of kidney failure when volume overload is present 24. We could not determine the prescribed medical and dietary therapy from the current data and thus we could not infer whether “maximal” medical therapy was prescribed prior to dialysis initiation. Others have speculated that concerns about worsening kidney function, medication side effects, and the need for more intensive monitoring are among the reasons why cardiovascular medications might be utilized less frequently in patients with advanced CKD 25, 26.
Our study also provides evidence that malnutrition and functional impairments may influence decision making. These findings highlight some of the difficulty in determining the appropriate indications for and timing of dialysis initiation in a frail elderly population, and the need for evidence-based clinical practice guidelines to aid decision making 27. For example, non-uremic symptoms such as delirium related to medication side-effects may be mistaken for uremia, especially when coupled with uncertainty surrounding the accuracy of GFR estimates, and lead to earlier dialysis initiation. Dialysis initiation in these situations may not be beneficial and may in fact, lead to its own complications and additional symptoms 14.
The population attributable risk of 31% can be interpreted as the fraction of cases of earlier dialysis initiation that would be prevented if signs and symptoms of volume overload, cognitive decline, increasing ADL dependence and weight loss were eliminated from this population of nursing home residents approaching ESRD. This figure is lower than what might be expected from self-reported practice patterns 22, highlighting the large fraction of earlier initiation that remains unexplained in this population. Clinical factors other than those evaluated in the current study may have influenced the timing of dialysis initiation in this cohort. For example, uremic symptoms such as pruritus or sleep disturbance, metabolic abnormalities, lack of psychosocial support or acute illness may play a role in determining the timing of dialysis initiation. In support of the latter hypothesis, we found that the population attributable risk of these signs and symptoms was lower among inpatients, suggesting that contextual factors associated with hospitalization may play an important role in determining the timing of dialysis initiation 28. Regional and possibly economic factors unrelated to patient characteristics appear to influence a range of dialysis and pre-dialysis practice patterns 29, 30, and may also influence timing of dialysis.
Several limitations of this study should be noted. First, some have proposed that dialysis initiation with a high eGFR reflects dialysis initiation in individuals with lower muscle mass rather than dialysis initiation earlier in the course of kidney disease. Our study was not designed to assess the relation of signs and symptoms with “true” GFR, but with the practice of dialysis initiation with a high estimated GFR. Second, we did not have information regarding other potential clinical indicators for dialysis initiation, such as hyperkalemia, acidosis, or refractory hypertension, although data from the IDEAL trial suggest these are infrequent causes for earlier dialysis initiation in a younger population 5. Third, although we utilized comorbidity data from both the MDS and USRDS registries, there may be residual confounding from unmeasured comorbid conditions. Finally, we did not have serial measures of eGFR. This information may have provided insight into whether these clinical syndromes are associated with slowly progressive declines in kidney function culminating in ESRD or with episodes of acute kidney injury leading to ESRD 31, 32.
In summary, signs and symptoms associated with uremia were independently associated with earlier dialysis initiation, but the clinical factors studied herein accounted for less than one third of all cases of earlier initiation in elderly nursing home residents and less than half of cases among outpatients, a group in whom dialysis initiation was less likely to be prompted by acute illness. Future research into these syndromes, especially volume overload and functional decline, and the impact of dialytic versus medical management on these conditions may yield insight into their appropriateness as indicators for earlier dialysis initiation and provide opportunities to improve clinical care.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Shu-Cheng Chen and Cheryl Arko at USRDS for their assistance merging the datasets. The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government or the Department of Veterans Affairs.
Support: This work was supported by a Paul B. Beeson Career Development Award in Aging (K23AG028952 to Dr Kurella Tamura and K23AG28980 to Dr O’Hare) from the National Institute of Aging, N01DK70005 from the National Institute of Diabetes and Digestive and Kidney Diseases, KL2RR024130 from the National Center for Research Resources, and a grant from the Centers for Disease Control and Prevention (Dr O’Hare).
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Supplementary Material Note: The supplementary material accompanying this article (doi:–––––––) is available at www.ajkd.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins AJ, Foley RN, Herzog C, et al. United States Renal Data System 2008 Annual Data Report Abstract. Am J Kidney Dis. 2009 Jan;53(1 Suppl):vi–vii. S8–374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2.McDonald S, McCredie M, Williams S, Stewart J. Factors influencing reported rates of treated end-stage renal disease. Adv Chronic Kidney Dis. 2005 Jan;12(1):32–38. doi: 10.1053/j.ackd.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003 Sep;14(9):2305–2312. doi: 10.1097/01.asn.0000080184.67406.11. [DOI] [PubMed] [Google Scholar]
- 4.Kausz AT, Obrador GT, Arora P, Ruthazer R, Levey AS, Pereira BJ. Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol. 2000 Dec;11(12):2351–2357. doi: 10.1681/ASN.V11122351. [DOI] [PubMed] [Google Scholar]
- 5.Cooper BA, Branley P, Bulfone L, et al. A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis. N Engl J Med. 2010 Jun 27; doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 6.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007 Feb 6;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 7.Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009 May 20; doi: 10.1038/ki.2009.161. [DOI] [PubMed] [Google Scholar]
- 8.Bonomini V, Albertazzi A, Vangelista A, Bortolotti GC, Stefoni S, Scolari MP. Residual renal function and effective rehabilitation in chronic dialysis. Nephron. 1976;16(2):89–102. doi: 10.1159/000180589. [DOI] [PubMed] [Google Scholar]
- 9.Churchill DN. An evidence-based approach to earlier initiation of dialysis. Am J Kidney Dis. 1997 Dec;30(6):899–906. doi: 10.1016/s0272-6386(97)90102-5. [DOI] [PubMed] [Google Scholar]
- 10.Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005 Nov;46(5):887–896. doi: 10.1053/j.ajkd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Korevaar JC, Jansen MA, Dekker FW, et al. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet. 2001 Sep 29;358(9287):1046–1050. doi: 10.1016/S0140-6736(01)06180-3. [DOI] [PubMed] [Google Scholar]
- 12.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002 Aug;13(8):2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Am J Kidney Dis. 2006;(48):S1–S322. [Google Scholar]
- 14.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009 Oct 15;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawes C, Morris JN, Phillips CD, Mor V, Fries BE, Nonemaker S. Reliability estimates for the Minimum Data Set for nursing home resident assessment and care screening (MDS) Gerontologist. 1995 Apr;35(2):172–178. doi: 10.1093/geront/35.2.172. [DOI] [PubMed] [Google Scholar]
- 16.Snowden M, McCormick W, Russo J, et al. Validity and responsiveness of the Minimum Data Set. J Am Geriatr Soc. 1999 Aug;47(8):1000–1004. doi: 10.1111/j.1532-5415.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 17.Lawton MP, Casten R, Parmelee PA, Van Haitsma K, Corn J, Kleban MH. Psychometric characteristics of the minimum data set II: validity. J Am Geriatr Soc. 1998 Jun;46(6):736–744. doi: 10.1111/j.1532-5415.1998.tb03809.x. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the endstage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000 Mar;11(3):520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 20.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998 Jan;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunori G, Viola BF, Parrinello G, et al. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: a prospective randomized multicenter controlled study. Am J Kidney Dis. 2007 May;49(5):569–580. doi: 10.1053/j.ajkd.2007.02.278. [DOI] [PubMed] [Google Scholar]
- 22.Ledebo I, Kessler M, van Biesen W, et al. Initiation of dialysis-opinions from an international survey: Report on the Dialysis Opinion Symposium at the ERA-EDTA Congress, 18 September 2000, Nice. Nephrol Dial Transplant. 2001 Jun;16(6):1132–1138. doi: 10.1093/ndt/16.6.1132. [DOI] [PubMed] [Google Scholar]
- 23.Curtis BM, Barret BJ, Jindal K, et al. Canadian survey of clinical status at dialysis initiation 1998-1999: a multicenter prospective survey. Clin Nephrol. 2002 Oct;58(4):282–288. [PubMed] [Google Scholar]
- 24.Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. May 6;14(3):R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008 Nov 4;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Berger AK, Duval S, Manske C, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease. Am Heart J. 2007 Jun;153(6):1064–1073. doi: 10.1016/j.ahj.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Moss AH. Shared decision-making in dialysis: the new RPA/ASN guideline on appropriate initiation and withdrawal of treatment. Am J Kidney Dis. 2001 May;37(5):1081–1091. doi: 10.1016/s0272-6386(05)80027-7. [DOI] [PubMed] [Google Scholar]
- 28.Crews DC, Jaar BG, Plantinga LC, Kassem HS, Fink NE, Powe NR. Inpatient Hemodialysis Initiation: Reasons, Risk Factors and Outcomes. Nephron Clin Pract. 2009 Oct 9;114(1):c19–c28. doi: 10.1159/000245066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV. Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol. 2009 May;20(5):1078–1085. doi: 10.1681/ASN.2008060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’hare A, Rodriguez R, Hailpern S, Larson E, Kurella Tamura M. Regional healthcare intensity and treatment practices for end-stage renal disease in older adults. Jama. doi: 10.1001/jama.2010.924. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama. 2009 Sep 16;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 32.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009 Jan;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.