Abstract
Toll-like receptors (TLRs) are typically expressed in immune cells to regulate innate immunity. Here we report that functional TLR7 is expressed in C-fiber primary sensory neurons and important for inducing itch (pruritis) but not necessary for eliciting mechanical, thermal, inflammatory and neuropathic pain in mice. Thus, we have uncovered TLR7 as a novel itch mediator and a potential therapeutic target for anti-itch treatment in skin disease conditions.
TLRs play a critical role in triggering innate immune responses to pathogen-associated molecular patterns (PAMPs) in mammals. Except for TLR3, TLRs engage downstream signaling cascades via MyD88 to produce cytokines and chemokines and fight against pathogenic infection1. Mammalian TLR family comprises at least 13 members (TLR1 to TLR13). TLR7 recognizes single-stranded RNAs from RNA viruses1. As innate immunity is strongly implicated in abnormal pain hypersensitivity2, we first examined whether thermal and mechanical pain sensitivity or pathological pain is altered in Tlr7 knockout (Tlr7−/−) mice.
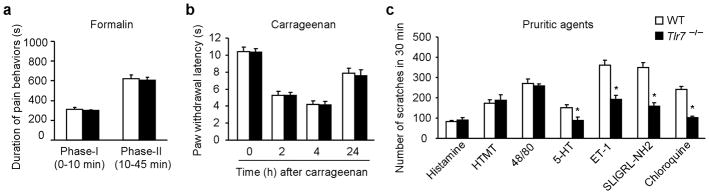
Compared to wild type (WT) mice, Tlr7−/− mice exhibited normal thermal pain sensitivity, assessed by Hargreave’s test and tail-flick test (Supplementary Fig. 1a, b), and normal mechanical pain sensitivity, assessed by graded von Frey filaments and Randall-Selitto test (Supplementary Fig. 1c, d). Acute inflammatory pain elicited by intraplantar injection of capsaicin, mustard oil (Supplementary Fig. 1e, f), or formalin in both the first and second phases (Fig. 1a), as well as carrageenan-induced persistent inflammatory pain (Fig. 1b) and spinal nerve ligation-induced neuropathic pain (Supplementary Fig. 1g) was unaltered in Tlr7−/− mice. Consistently, Tlr7−/− mice did not show any developmental defects in the dorsal root ganglia (DRG) and spinal cord and the expression of neurochemical markers such as TRPV1, CGRP, and IB4 was normal (data not shown).
Figure 1. Intact pain but impaired itch in Tlr7−/−mice.
(a, b) Acute and persistent inflammatory pain induced by intraplantar formalin (5%; n=5) and carrageenan (1%, n=5). P >0.05 compared with WT control. (c) Total number of scratches in 30 min following intradermal injection of 50 μl of pruritic agents including histamine (500 μg), HTMT (H1 agonist, 100 μg), compound 48/80 (48/80; 100 μg), serotonin (5-HT; 20 μg), endothelin-1 (ET-1, 25 ng), SLIGRL-NH2 (PAR2 agonist; 100 μg), and chloroquine (CQ; 200 μg) in Tlr7−/− and WT mice. *P<0.05, versus WT control, n = 5~8 mice. All data are means ± s.e.m.
Recent studies have revealed distinct molecular mechanisms underlying pain and itch 3–5. We next evaluated whether TLR7 plays a role in itch sensation. We counted the number of scratches (bouts) by a hindpaw of mouse following intradermal injection of pruritogenic agents in the nape of the neck. Notably, scratches induced by histamine-dependent pruritogens, such as histamine, HTMT (histamine H1 receptor agonist), and compound 48/80 which is known to release histamine from mast cells, were comparable in Tlr7−/− and WT mice (Fig. 1c and Supplementary Fig. 2a-c). Strikingly, Tlr7−/− mice showed a marked reduction in scratching behaviors in response to nonhistaminergic pruritogens, including chloroquine (CQ), an antimalaria drug5, and SLIGRL-NH2, an agonist of protease-activated receptor 2 (PAR2, Fig. 1c and Supplementary Fig. 2d, e). CQ-induced scratching in both sexes was reduced in Tlr7−/− mice (Supplementary Fig. 3a). Notably, CQ induced a bell-shaped dose response curve, but scratching elicited by the highest dose (600 μg) was TLR7-independent (Supplementary Fig. 3b). Further, scratches induced by serotonin (5-HT) and endothelin-1 (ET-1) were also impaired in Tlr7−/− mice (Fig. 1c and Supplementary Fig. 2f, g).
TLR7 was originally identified to recognize imidazoquinoline derivatives such as imiquimod and resiquimod (R848) and guanine analogues such as loxoribine, and all have anti-viral and anti-tumor properties1, 6, 7. Intradermal injection of imiquimod, R848, and loxoribine induced inverted-U-shaped dose response curve of scratching in mice (Fig. 2a and Supplementary Fig. 4a, b). As expected, scratches induced by imiquimod, R848, and loxoribine were reduced in Tlr7−/− mice (Fig. 2b and Supplementary Fig. 2h–j, 4c, d).
Figure 2. Scratches induced by imiquimod.
(a) Dose-dependent scratches after intradermal imiquimod (n=5~8). Inset, structure of imiquimod. (b) Imiquimod-induced scratches in WT and Tlr7−/− mice (n=5). (c) Imiquimod-induced scratches after RTX and vehicle treatment, in Trpv1−/− and their WT control mice, as well as in mast cell-deficient SASH mice and their WT control mice. *P<0.05, versus saline (a), #P<0.05. N.S., not significant. n=5. All data are means ± s.e.m.
To further investigate a unique role of TLR7 in itch versus pain, we used a recently developed “cheek model” of itch8. The TLR7 ligands imiquimod, R848, and loxoribine all elicited itch-like scratching but not pain-indicative wiping behavior (Supplementary Fig. 5), supporting a specific role of TLR7 in mediating itch rather than pain.
C-fibers, express transient receptor potential subtype V1 (TRPV1) and are indispensable for itch sensation by various pruritogens9. Pretreatment with resiniferatoxin (RTX), an ultra potent TRPV1 agonist, resulted in a loss of heat sensitivity (Supplementary Fig. 6a) and almost abolished imiquimod-induced scratches (Fig. 2c). Despite a marked reduction of histamine-induced scratching in Trpv1−/− mice10, imiquimod-induced scratching remained unaltered in Trpv1−/− mice (Fig. 2c). Thus, TRPV1-expressing C-fibers but not TRPV1 per se are required for imiquimod-elicited itch. We also tested itch responses in mast cell-deficient SASH mice5 and only found a moderate reduction (28%) in imiquimod-induced scratching (Fig. 2c).
It is virtually unknown whether primary sensory neurons express functional TLR7. Immunohistochemistry revealed that TLR7 was mainly expressed in small-size dorsal DRG neurons (Fig. 3a and Supplementary Fig. 7a). TLR7 is highly co-localized with TRPV1 and gastrin-releasing peptide (GRP), a neuropeptide that is known to elicit itch via GRP receptor expressed by spinal cord superficial dorsal horn neurons3 (Supplementary Fig. 7b, c). Absence of TLR7 staining in DRG sections of Tlr7−/− mice confirmed the specificity of TLR7 antibody (Supplementary Fig. 7d). In situ hybridization also showed TLR7 mRNA expression in DRG neurons (Supplementary Fig. 7, e). Single cell RT-PCR analysis, conducted selectively in small-size DRG neurons, indicated that TLR7+ population is within GRP+ population which is within TRPV1+ population (Fig. 3b and Supplementary Fig. 8a, d). Interestingly, the G protein-coupled receptor (GPCR) MrgprA3, which is known to mediate CQ-induced itch5, was co-localized with TLR7 (Fig. 3c). In contrast, TLR8, a family member that is phylogenetically most similar to TLR7, was not expressed in adult mouse DRG (Fig. 3c and Supplementary Fig. 8b, c).
Figure 3. Expression of functional TLR7 in DRG neurons.
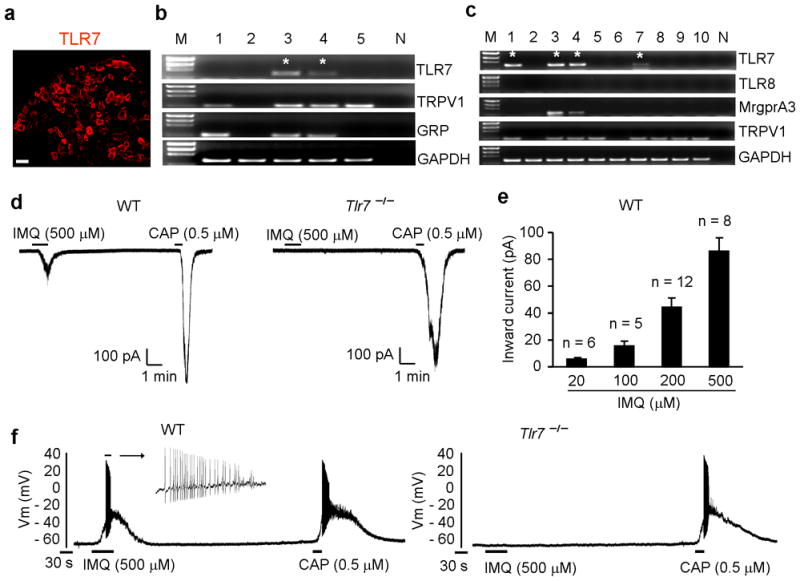
(a) Immunohistochemistry showing TLR7 expression in DRG neurons. Scale, 50 μm. (b, c) Single cell RT-PCR showing co-localization of TLR7 with TPRV1, GRP, and MrgprA3. M, molecular weights; N, negative controls from pipettes that did not harvest any cell contents but were submerged in the bath solution. * indicates TLR7+ neurons. (d) Inward currents evoked by imiquimod (500 μM) and capsaicin (0.5μM) in small DRG neurons from WT mice. Eight out of 17 neurons from WT mice respond to imiquimod. All 10 neurons from Tlr7−/− mice fail to respond to imiquimod. (e) Amplitude of inward currents evoked by imiquimod (20–00 μM). Numbers over bracket indicate the number of responsive neurons. (f) Action potentials evoked by imiquimod (500 μM) and capsaicin (0.5 μM) in small DRG neurons from WT mice. Note that imiquimod-induced action potentials are lost in Tlr7−/− mice (n=8 neurons).
Patch clamp recording showed that imiquimod (20–500 μM) induced dose-dependent inward currents in capsaicin-responsive DRG neurons of WT but not Tlr7−/− mice (Fig. 3d, e). Imiquimod also induced action potentials in DRG neurons of WT but not Tlr7−/− mice (Fig. 3f). Thus, imiquimod could directly excite DRG neurons in a TLR7-dependent manner. R848, a dual ligand for TLR7 and TLR8, was also able to induce TLR7-dependent inward currents (Supplementary Fig. 9).
Immunohistochemistry revealed TLR7 expression in nerve branches in the dermis and nerve terminals in the epidermis of skin tissues (Supplementary Fig. 10a-c). RTX treatment ablated TLR7+ fibers in the skin (Supplementary Fig. 6c). We also found TLR7 immunoreactivity in spinal cord axonal terminals (Supplementary Fig. 10d). Thus, it is likely that TLR7 is transported from DRG cell bodies to skin nerve terminals to mediate pruritus.
In summary, we identified TLR7 as a novel mediator for itch sensation, which is quite different from previously identified GPCR receptors 3, 5, 11. We found functional TLR7 receptors in small-size DRG neurons that co-express TRPV1, GRP, and MrgprA3. In particular, TLR7 is important for pruritus elicited primarily by nonhistaminergic pruritogens, although we do not exclude a partial role of TLR7 in histamine-dependent itch. Because TLR7 activation by imiquimod and R848 not only induced marked scratching but also generated inward currents and actions potentials in DRG neurons, TLR7 ligands are likely to elicit itch via a direct action on sensory neurons. However, we should not rule out a role of non-neuronal cells in the skin such as keratinocytes and mast cells in TLR7-mediated pruritus. Notably, topical application of imiquimod, a clinically used anti-viral and anti-tumor drug, frequently elicits pruritus in human12 and also induces psoriasis-like skin inflammation in mice13, indicating a conservative role of TLR7 in pruritus across different species. Chronic itch is frequently associated with skin diseases and is often resistant to antihistamine treatment 14, 15. Given a crucial role of TLR7 in pruritus, targeting TLR7 may be promising for anti-itch treatment under skin disease conditions.
Supplementary Material
Acknowledgments
The work was supported by US National Institutes of Health grants R01-DE17794, R01-NS54362 and R01-NS67686 to R.-R.J.
Footnotes
AUTHOR CONTRIBUTIONS
T.L. conducted behavioral tests for itch and acute pain and participated in experimental design and manuscript preparation. Z.-Z.X. performed immunohistochemistry and behavioral tests of pain; C.-K.P. conducted single cell PCR and electrophysiology, T.B. performed in situ hybridization; R.-R.J. supervised the project, designed experiments, and wrote the manuscript.
COMPTTING FINANTIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Takeuchi O, Akira S. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Marchand F, Perretti M, McMahon SB. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 3.Sun YG, Chen ZF. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 4.Sun YG, et al. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q, et al. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmi H, et al. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, et al. Proc Natl Acad Sci U S A. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada SG, LaMotte RH. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imamachi N, et al. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shim WS, et al. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim WS, Oh U. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan V, Lear JT, Szeimies RM. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 13.van der Fits L, et al. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 14.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 15.Paus R, Schmelz M, Biro T, Steinhoff M. J Clin Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.