Gaucher disease is caused by mutations in GBA1, the gene encoding for glucocerebrosidase (Gcase), an enzyme responsible for the final step of degradation of glycosphingolipids to ceramide and glucose.1 It is the most common lysosomal storage disease and manifests with a range of phenotypes, partially explained by the location of mutations in the protein. The N370S mutation is common in Ashkenazi Jewish patients. Many N370S homozygotes can be asymptomatic, whereas others carrying this mutation or different ones have phenotypes ranging from enlarged spleens and livers and low platelet counts, to bone infarcts, brain damage and the loss of skin barrier function.2 In recent years, GBA1 mutations were found to be a major risk factor for the development of Parkinson disease.3 Parkinson disease is a movement disorder caused by the disruption of the dopamine pathway in the brain, usually as a result of damage to the dopaminergic neurons in the substantia nigra.4 The hallmark brain pathology in Parkinson disease is the presence of Lewy bodies in the substantia nigra and other areas of the brain. Lewy bodies were also found in brains from patients with both Gaucher and Parkinson diseases, but not in patients with Gaucher disease without parkinsonism.5
Molecular link between Gaucher disease and Parkinson disease
Since 2002, when the likely connection between Gaucher and Parkinson disease was first noted, scientists and clinicians have been scrambling to find a molecular explanation for this phenomenon. Since there was no immediate connection between the classic clinical courses of the two diseases, it was clear that the parkinsonism could not be viewed as a natural consequence of Gaucher disease. Two competing theories evolved to explain this connection.
1. The misfolded protein theory
The mutant protein made in cells of patients with Gaucher disease is misfolded and missorted. As a result, it accumulates and burdens the proteasomal and lysosomal systems in charge of the disposal of misfolded proteins. This could cause the death of particularly sensitive cells, such as substantia nigra neurons, hence resulting in parkinsonism.
2. The offensive metabolite theory
The substrates of the enzyme, glucocerebrosides and glucosylsphingosines, accumulate in cells of patients with Gaucher disease, inhibiting lysosomal function in particularly sensitive cells. Alternatively, an increase in glucocerebrosides causes activation of the ryanodine receptor, leading to an increase in intracellular free calcium, followed by cell death and parkinsonism.6
Both of these theories invoke a second hit to explain why only a minority of patients with Gaucher disease would develop Parkinson disease in the course of their lifetime. The misfolded protein theory is better at this, suggesting that any other mutant protein going through the same cellular degradation pathway would add to the burden. The pathway suggested was the “unfolded protein response” and indeed it is activated in cells with certain GBA mutations.7
Weakness of proposed models
Both theories have weaknesses and can be refuted. The misfolded protein theory cannot explain why the risk for Parkinson disease is increased also in cases where mutations in GBA result in no protein product at all.8 Examples of this are a frameshift mutation in exon 2 called c.84dupG, which is relatively common, and several other mutations in which the GBA allele results from recombination with pGBA (the pseudogene located close to GBA on chromosome 1), resulting in multiple stop codons and no translation of a protein product.
The offensive metabolite theory is problematic because the carriers of Gaucher mutations do not accumulate the substrates, but still carry a 5 fold increased risk of developing Parkinson disease.
Synuclein and Parkinson disease
In order to generate a more convincing theory, the various mechanism proposed to explain Parkinson disease pathogenesis were explored, starting with the involvement of alpha-synuclein. Alpha-synuclein is a small lipophilic protein abundant in the brain and blood. It is thought to be involved in presynaptic neurotransmitter vesicles in the brain. Aggregates of alpha-synuclein are associated with brain disease with increasing age. Mutations in alpha-synuclein are a rare cause of Parkinson disease. Mice overexpressing human mutant alpha-synuclein develop neurological disease, and alpha-synuclein aggregates are a major component of Lewy bodies. These observations raise the likelihood that synuclein is the offending metabolite in Parkinson disease.9
Alpha-synuclein and protein misfolding
The alpha-synuclein pathogenesis hypothesis supports the misfolding protein theory. Mutant alpha-synuclein increases the sensitivity of cultured dopaminergic cells to proteasome inhibitors.10 Over-expressed mutant alpha-synuclein degrades slowly in cells. Mutant alpha-synuclein aggregates in cells.11 Mutations in other proteins cause alpha-synuclein aggregation. This would make it a candidate to interact with mutant glucocerebrosidase to facilitate the pathogenesis of Parkinson disease. Alpha-synuclein could aggregate together with other misfolded proteins, forming a complex containing both proteins. This requires co-localization of synuclein and the misfolded protein in the Lewy bodies. Indeed, immunoreactive Gcase was discovered in Lewy bodies of patients with both Gaucher and Parkinson disease, and aggregates containing mutant Gcase and alpha-synuclein were found in co-transfected cells. (Goker Alpan et al submitted).
The interaction of alpha synuclein with lipids
The interaction of alpha-synuclein with lipid may provide the basis for an alternate theory. Alpha-synuclein changes structure in a lipid environment, and tends to aggregate on the surface of lipid vesicles. Changes in alpha-synuclein structure depend on the types of lipids in the vesicle; both the hydrophobic chain length and the hydrophilic head-group are important.12 One could speculate that glucocerebroside vesicles are likely to be suitable for alpha-synuclein aggregation. Glucocerebroside vesicles may be abundant in brain cells of patients. Co-localization of abnormal lipid vesicles and synuclein provides the seed for the formation of synuclein aggregates.
The prion theory
Recently, Dr. Stanly Prusiner introduced the theory that alpha-synuclein is a prion.13 This theory is based on14 and15 which demonstrate that synuclein aggregation is transmitted between neurons in the brain. Prions are known to be small hydrophobic proteins that, under certain conditions, can change conformation and cause neurodegenration. They are the first known proteinous infectious agents; and can also cause brain disease when they are mutated in genetically inherited cases.16 Prusiner proposes that alpha-synuclein changes conformation to aggregate in certain cells, and then moves between nerve cells through the brain, infecting alpha-synuclein in more and more cells, eventually reaching the substantia nigra where alpha-synuclein is abundant, and rendering havoc in a manner similar to the known prion proteins. Lewy bodies can occur in other regions of the brain in non-affected individuals, indicating that the localization of the Lewy bodies in the substantia nigra is causative to parkinsonian symptoms.17 Some of the clinical features of prion diseases such as Creutzfeldt-Jakob disease include parkinsonism. Interestingly, patients with prion disease display myoclonic seizures, which can also be a feature of neuronopathic Gaucher disease.18 The importance of this new theory in explaining the relationship of Parkinson disease and Gaucher disease is surprisingly crucial.
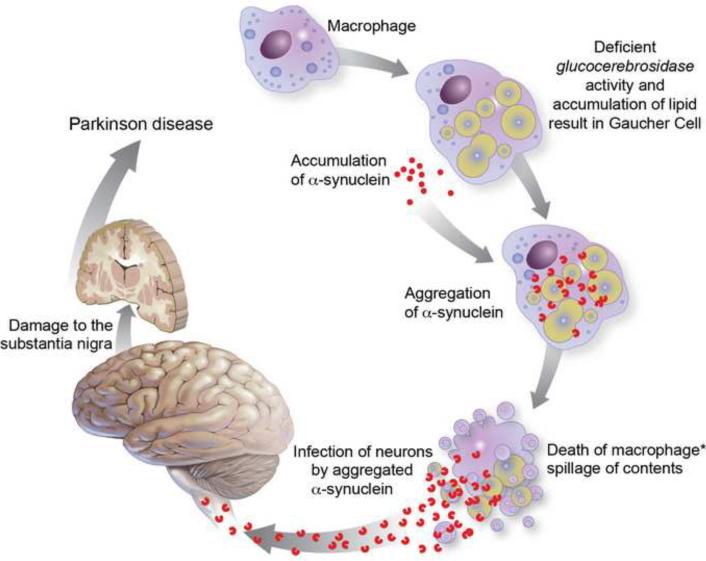
The prion hypothesis provides a mean to unlink the site of the damage from the initiation of Parkinson disease. This hypothesis works in favor of the offensive metabolite theory (Fig 1).
Figure 1.
The prion theory model for the development of parkinson in patients with Gaucher disease.
The asterisk (*) denotes the spot likely to be the least probable step in the pathway, restricting the frequency of Parkinsonism among patients with Gaucher disease. Like in other prion disorders, it is not clear how the infectious agent enters the neuronal tissue in this particular case.
Synuclein prions formed in macrophages
Suppose that the damaged cell is a macrophage. The role of macrophages in prion disease is not clear. In some cases, they are believed to carry the prion protein to the nervous system, and in others, they may function to slow down the infection by destroying the prion protein.19 In any case, diseased macrophages are unpredictable. Macrophages are the most affected cells in adult patients with Gaucher disease. The Gaucher cell is a morphological definition for a macrophage type cell found in tissues of patients with Gaucher disease. Under light microscopy it looks foamy, and on EM it contains large lipid laden vesicles. It is formed when circulating macrophages eat dying RBC. They cannot digest the membrane glycolipids and accumulate intracellular membranes (Fig 1).20
There is also evidence that alpha-synuclein is a blood protein.21 One can speculate that if a macrophage ingests large quantities of alpha-synuclein found in the vicinity of lipid vesicles containing glucocerebrosides, it could acquire the prion form (Fig 1.a). Indeed, there is evidence that in the presence of lipids, alpha- synuclein tends to change conformation from an unordered form to an alpha-helix form that can aggregate.22 All these events would be highly probable in the lipid laden environment of the Gaucher cell. In a small number of patients, these cells will accidentally spill their toxic content in the vicinity of a neuron (Fig 1b) and start the vicious cycle that will culminate in Parkinson disease (Fig1c–g). The infection of neurons by macrophage alpha-synuclein aggregates would be the rate-limiting step for the development of parkinsonism in this model. It is possible that multiple traumatic events related to immune system activation,23 may contribute to this process and increase its likelihood with age.
How can the prevalence of Parkinson disease be explained in Gaucher carriers? In this case, the two hit theory can still apply. Autosomal dominant polycystic kidney disease (adPKD) can serve as an example here. In this disease, patients are born with one mutated allele of the gene (PKD1 or PKD2). At some point a spontaneous mutation occurs in the other allele in an epithelial cell. The doubly hit cell loses replication control and starts forming cysts in the kidney and other organs. The probability of a spontaneous somatic mutation correlates with the number of replications, and hence with age, explaining the increased prevalence of cysts and increased severity of the disease with age. In all, this is a very prevalent disease which occurs at a rate of close to 0.5% in the general population,24 indicating that a second mutation in somatic cells is not that rare. A similar explanation was proposed to explain the occurrence of sporadic prion disease.25
Somatic mutations and the double hit theory
In the case of Parkinson disease in Gaucher carriers, a double-hit would require a somatic second mutation in GBA1 in one macrophage precursor of a Gaucher mutation carrier. Notably, it could be any of the 300 and more mutations already reported to cause Gaucher disease. This cell and its small number of progeny would not be detectable, because most of the cells in the carrier are heterozygous for the original mutation. The alpha-synuclein would, however, be there, and the time bomb ticking. The argument that aggregated alpha-synuclein is sufficient to cause Parkinson disease is testable using the same experimental techniques used to demonstrate infection by the prion protein. The possibility of evaluating whether this cascade is more probable in Gaucher cells is also within reach. For example, a simple animal experiment could demonstrate whether the injection of aggregated alpha-synuclein can cause additional aggregation. Further experiments could determine whether the injection of Gaucher cells into animals can cause alpha-synuclein aggregation in the host cells. If the prion theory holds, it would make sense to prevent aggregation stimulating conditions in people at risk, in order to prevent the induction of Parkinson disease.
Other possible diseases linked to Parkinsonism
The crucial weakness of the prion theory is that it introduces many different possible explanations for the pathogenesis of Parkinson disease, many of them equally probable. For example, does this situation apply to other lysosomal diseases? It is possible that aggregation of alpha-synuclein can be promoted in macrophages of patients with Niemann-Pick Type C (NPC) disease? In these patients, macrophages engorged with a variety of lipids accumulate in the body of the patients as a result of a defective transport mechanism. Patients who live long enough demonstrate pathological signs of both Parkinson disease and Alzheimer disease at autopsy. Whether there is a propensity to develop Parkinson disease in carriers of NPC is currently under investigation.26,27
More prions?
Are there other candidate prions in the lysosomal disease field? Both PrP and alpha-synuclein are small hydrophobic proteins capable of changing their conformation and aggregating under changes in their environment. The same is now explored in studies on the pathogenesis of Alzheimer disease. Researchers speculate that aggregated Tau protein “infects” more and more neurons causing the formation of neurofibrillary tangles and Alzheimer symptoms.28,29 Tau has also been shown to change conformation and aggregate in the presence of lipids.30 Another candidate that comes to mind is the subunit C of mitochondrial ATP synthase that accumulates in the cells of patients with Neuronal Ceroid Lipofuscinosis (NCL) as a result of lysosomal dysfunction.31 Patients with NCL suffer neurodegeneration and die at a young age, but it would be interesting to test the possibility of an association with a brain disease similar to Parkinson disease in older carriers of this disease. Other candidates could be the saposin subunits. Saposins are detergent-like, small, natural proteolytic products of prosaposin. They are cofactors in the catabolic process of sphingolipids in the lysosomes and are also involved in the immune system in antigen presentation. Saposin C, which is the cofactor for glucocerebrosidase, accumulates in tissue from patients with Gaucher disease. Other saposins accumulate in several lysosomal diseases.32
Other possible explanations for the link between Gaucher disease and Parkinson disease
It is possible that even a slight reduction in glucocerebrosidase activity can result in some damage over time in patients with another genetic susceptibility. Two examples include the involvement of a second human beta glucosidase GBA2, and Saposin C. GBA2 may contribute by metabolizing excess glucosyl ceramides in the brain33, increasing the substrate load. Saposin C, increased in patients with Gaucher disease, results in a change in the lipid environment in cells in the brain, and might provide the right conditions for synuclein aggregation. Moreover, glucocerebrosidase may be marginally involved in removal of environmental lipophilic toxins, which may increase the incidence of Parkinson disease when its activity is reduced.34
Conclusions
Both the protein misfolding and the offensive metabolite theories are currently insufficient to explain the genetic link between Gaucher disease and Parkinson disease. Additional stipulations such as the prion theory and the second hit hypotheses may be essential in developing a valid model for the link.
Acknowledgments
Thanks to Dr. Ellen Sidransky, Dr. Andrew Singleton, Dr. Bryan Traynor and Dr. Raphael Schiffmann for critically reading the manuscript. This work is supported by the Intramural Research Program of the National Human Genome Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beutler E, Grabowski GA. Gaucher disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th ed. McGraw-Hill; New York: 2001. pp. 3635–3668. [Google Scholar]
- 2.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29:567–83. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 3.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–61. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 5.Wong K, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Pelled D, Trajkovic-Bodennec S, Lloyd-Evans E, et al. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol Dis. 2005;18:83–8. doi: 10.1016/j.nbd.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz M, Alfalah M, Aers JM, et al. Impaired trafficking of mutants of lysosomal Glucocerebrosidase in Gaucher's disease. Int J Biochem Cell Biol. 2005;37:2310–20. doi: 10.1016/j.biocel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 9.Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:4–9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrucelli L, O'Farrell C, Lockhart PJ, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–19. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 11.Cuervo AM, Stefanis L, Fredenburg R, et al. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 12.Uversky VN, Eliezer D. Biophysics of Parkinson's disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–99. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olanow CW, Prusiner SB. Is Parkinson's disease a prion disorder? Proc Natl Acad Sci U S A. 2009;106:12571–2. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 16.Belay ED. Transmissible spongiform encephalopathies in humans. Annu Rev Microbiol. 1999;53:283–314. doi: 10.1146/annurev.micro.53.1.283. [DOI] [PubMed] [Google Scholar]
- 17.Tong J, Wong H, Guttman M, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–88. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- 18.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Lwin S, Inoshima Y, Atoji Y, et al. Immune cell types involved in early uptake and transport of recombinant mouse prion protein in Peyer's patches of calves. Cell Tissue Res. 2009;338:343–54. doi: 10.1007/s00441-009-0879-6. [DOI] [PubMed] [Google Scholar]
- 20.Schueler UH, Kolter T, Kaneski CR, et al. Correlation between enzyme activity and substrate storage in a cell culture model system for Gaucher disease. J Inherit Metab Dis. 2004;27:649–58. doi: 10.1023/b:boli.0000042959.44318.7c. [DOI] [PubMed] [Google Scholar]
- 21.Barbour R, Kling K, Anderson JP, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5:55–9. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 22.Kjaer L, Giehm L, Heimburg T, Otzen D. The influence of vesicle size and composition on alpha-synuclein structure and stability. Biophys J. 2009;96:2857–70. doi: 10.1016/j.bpj.2008.12.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, O'Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol. 2008;167:90–5. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 24.Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med. 2001;7:151–6. doi: 10.1016/s1471-4914(01)01953-0. [DOI] [PubMed] [Google Scholar]
- 25.Prusiner SB. Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle. Dev Biol Stand. 1991;75:55–74. [PubMed] [Google Scholar]
- 26.NP-C Guidelines Working Group. Wraith JE, Baumgartner MR, Bembi B, et al. Recommendations on the diagnosis and management of Niemann-Pick disease type C. Mol Genet Metab. 2009;98:152–65. doi: 10.1016/j.ymgme.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Ludolph AC, Kassubek J, Landwehrmeyer BG, et al. Reisensburg Working Group for Tauopathies With Parkinsonism Tauopathies with parkinsonism: clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur J Neurol. 2009;16:297–309. doi: 10.1111/j.1468-1331.2008.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J Cell Sci. 2010;123:1191–201. doi: 10.1242/jcs.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010 May 20; doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Elbaum-Garfinkle S, Ramlall T, Rhoades E. The role of the lipid bilayer in tau aggregation. Biophys J. 2010 Jun 2;98(11):2722–30. doi: 10.1016/j.bpj.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer DN, Oswald MJ, Westlake VJ, Kay GW. The origin of fluorescence in the neuronal ceroid lipofuscinoses (Batten disease) and neuron cultures from affected sheep for studies of neurodegeneration. Arch Gerontol Geriatr. 2002;34:343–57. doi: 10.1016/s0167-4943(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 32.Chang MH, Bindloss CA, Grabowski GA, et al. Saposins A, B, C, and D in plasma of patients with lysosomal storage disorders. Clin Chem. 2000;46:167–74. [PubMed] [Google Scholar]
- 33.Walden CM, Sandhoff R, Chuang CC, et al. Accumulation of glucosylceramide in murine testis, caused by inhibition of beta-glucosidase 2: implications for spermatogenesis. J Biol Chem. 2007;282(45):32655–64. doi: 10.1074/jbc.M702387200. [DOI] [PubMed] [Google Scholar]
- 34.Caldwell KA, Tucci ML, Armagost J, et al. Investigating bacterial sources of toxicity as an environmental contributor to dopaminergic neurodegeneration. PLoS One. 2009;4:e7227. doi: 10.1371/journal.pone.0007227. [DOI] [PMC free article] [PubMed] [Google Scholar]