Abstract
Nonalcoholic fatty liver disease (NAFLD) is now recognized as both an important component of the metabolic syndrome and the most prevalent liver disease in the United States. Although the mechanisms for development of steatosis and chronic liver injury in NAFLD remain unclear, recent investigations have indicated that overactivation of c-Jun N-terminal kinase (JNK) is critical to this process. These findings, together with evidence for the involvement of JNK signaling in other manifestations of the metabolic syndrome such as obesity and insulin resistance, have suggested that JNK may be a novel therapeutic target in this disorder. This review details findings that JNK mediates lipid accumulation and cell injury in fatty liver disease and discusses the possible cellular mechanisms of JNK actions.
Nonalcoholic fatty liver disease is an important component of the metabolic syndrome
The metabolic syndrome has been defined classically by the clinical features of obesity, glucose intolerance, dyslipidemia and hypertension [1]. This disorder is highly prevalent, affecting 50 million people in the United States alone, and its incidence is increasing with the rising rates of obesity and diabetes. The metabolic syndrome is a significant cause of morbidity and an independent predictor of mortality in older adults [2]. Although interest has largely focused on cardiovascular complications resulting from accelerated atherosclerosis [3], the importance of increased lipid accumulation in other organs that characterizes the metabolic syndrome is now being recognized. One such manifestation is nonalcoholic fatty liver disease (NAFLD) [4]. NAFLD contributes to the morbidity and mortality of the metabolic syndrome, and one long-term study demonstrated a higher mortality rate in type 2 diabetics from liver disease than cardiovascular disease [5]. In addition to effects from the actual liver disease, fatty liver and the associated hepatic insulin resistance contribute to the metabolic syndrome because of the liver's central role in glucose and lipid homeostasis. The presence of liver injury predicts the development of cardiovascular disease [6,7], suggesting a central function for NAFLD in clinical manifestations of the metabolic syndrome.
The pathological spectrum of NAFLD ranges from simple hepatic steatosis to steatosis combined with varying degrees of necroinflammation, apoptosis and fibrosis termed nonalcoholic steatohepatitis (NASH). Although steatosis alone is a benign condition, NASH progresses to fibrosis or cirrhosis in 15–30% of patients [8]. The fact that a minority of individuals with steatosis progress to NASH has suggested that separate factors initiate the development of steatosis and the progression to liver injury and inflammation. Alternatively, simple steatosis and NASH may represent distinct entities such that steatosis is not a precursor to the more serious NASH which develops by its own separate mechanism(s). Obesity and insulin resistance are strong risk factors for NAFLD [8]; however, the mechanisms underlying lipid accumulation, insulin resistance, cellular injury and inflammation remain unclear. A recent advance in understanding the pathophysiology of this disease has been the demonstration of the central involvement for the c-Jun N-terminal kinase (JNK)/activator protein 1 (AP-1) signaling pathway in the pathogenesis of NAFLD.
JNK signaling pathway
JNK is a mitogen-activated protein kinase (MAPK) family member that mediates cellular responses to a variety of intra- and extracellular stresses. The JNK MAPKs are encoded by three genes, of which two, jnk1 and jnk2, are expressed in all cells including hepatocytes [9]. The genes are alternatively spliced to create multiple protein isoforms of 46 or 54 kDa size [10]. The functions of the multiple isoforms are unknown, but they may be required for interactions with different substrates.
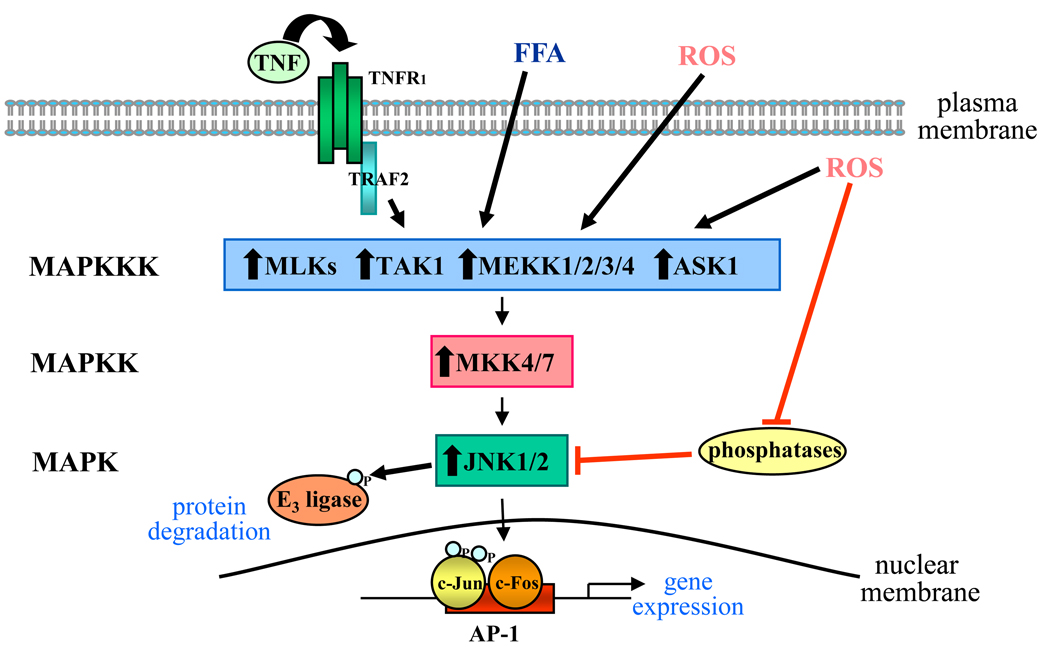
Activation of JNK involves a complex kinase cascade (Figure 1). The events initiating this kinase cascade remain unclear, but G-proteins such as Rac and cdc-42 and the TRAF group of adaptor proteins mediate activation from some stimuli. More than a dozen MAPK kinase kinases converge on the MAPK kinases MKK4 and MKK7, which preferentially phosphorylate JNK on tyrosine 185 and threonine 183, respectively [9]. Levels of phospho-JNK also depend on phosphatase activity [9], and the state of JNK activation represents an equilibrium between stimulation by upstream kinases and down-regulation by phosphatases.
Figure 1.
JNK signaling pathway. A number of stimuli activate the JNK signaling cascade including TNF, free fatty acids (FFA) and extra- or intracellular reactive oxygen species (ROS). Upstream events are poorly defined but result in the activation of one or more MAPK kinase kinases (MAPKKK), several of which are indicated. MAPKKKs activate the JNK MAPK kinases (MAPKK), MKK4 and MKK7, which phosphorylate and activate JNK. The level or duration of JNK activation is negatively regulated by phosphatase action, and ROS may stimulate JNK activation through the inhibition of phosphatase activity. Activated JNK1 and JNK2 isoforms phosphorylate the AP-1 subunit c-Jun, increasing its transcriptional activity. As a heterodimer with another subunit such as c-Fos, c-Jun binds to AP-1 promoter regions, increasing gene expression. Alternatively, JNK may also alter protein levels through the phosphorylation and activation of ubiquitin E3 ligase that promotes protein degradation. Black lines indicate stimulatory pathways and red lines indicate inhibitory pathways. ASK1, apoptosis signal-stimulating kinase 1; MLK, mixed-lineage protein kinases; TAK-1, TGF-β-activated kinase 1, TNFR1, TNF receptor 1, TRAF2, TNF receptor associated protein 2.
The availability of jnk1 and jnk2 knockout mice has led to an examination of the separate physiological functions of these two kinases. Redundant function was suggested by the absence of a significant phenotype in the single knockouts and embryonic lethality in the jnk1/jnk2 double knockout secondary to severe dysregulation of brain apoptosis. The classical JNK effector is the AP-1 component c-Jun which is activated by JNK phosphorylation to regulate gene expression. Alternatively, JNK can alter protein degradation either indirectly through phosphorylation of E3 ligases or through direct target protein phosphorylation [11]. Distinct functions for JNK1 and JNK2 have now been described in vivo which include the ability of JNK1 to phosphorylate c-Jun; JNK2 lacks this function and may even block c-Jun phosphorylation [12]. However, subsequent in vitro studies employing a chemical genetic approach rather than gene ablation to obviate the upregulation of compensatory responses to JNK loss have suggested that the two isoforms may have the same effect on c-Jun [13]. As will be discussed, distinct functions of JNK1 and JNK2 have been clearly delineated in the pathogenesis of fatty liver disease.
JNK1 mediates the development of obesity and insulin resistance
The first indication that JNK signaling has a mechanistic role in NAFLD was the finding that JNK mediates two of the principal risk factors for NAFLD development – obesity and insulin resistance. These studies were prompted by the known relationship between insulin resistance and obesity and the ability of JNK overactivation to impair insulin signaling [14]. Hotamisligil and colleagues demonstrated that in diet-induced and genetic models of obesity, JNK activity was increased in adipose tissue, muscle and liver [15]. A functional role for JNK1 in obesity was shown by the finding that standard or high fat diet (HFD)-fed jnk1−/− but not jnk2−/− mice had decreased weight gain attributed to a reduction in adipose tissue mass. jnk1 null mice also had decreased serum glucose and insulin levels and increased hepatic insulin signaling, indicating improved insulin sensitivity. Effects on hepatic lipid accumulation were not examined. Although these studies suggested no JNK2 involvement in obesity and insulin resistance, the ability of JNK2 to oppose JNK1 phosphorylation of c-Jun suggested that the effect of a loss of JNK2 may be compensated for by increased JNK1 function. To address whether JNK2 contributed to this metabolic phenotype, jnk2 null mice lacking one jnk1 allele were examined. Mice lacking one jnk1 and both jnk2 alleles were protected from HFD-induced obesity but jnk1+/− mice were not, indicating that JNK2 contributed to obesity [16]. Although not quantitated, histological fat accumulation was noted to be decreased in mice lacking one or both jnk1 genes, suggesting for the first time that JNK1 regulates hepatic steatosis.
JNK mediates lipid accumulation and liver injury in experimental NAFLD
JNK1 and not JNK2 functions in the development of steatosis and hepatitis
Subsequent studies examining JNK function in the hepatic manifestations of the metabolic syndrome revealed a critical mechanistic role for JNK in NAFLD development (Box 1). Investigations in methionine- and choline-deficient (MCD) diet-induced murine steatohepatitis demonstrated that increased hepatic JNK, c-Jun and AP-1 signaling occurred in parallel with the development of lipid over accumulation and hepatitis [17]. The MCD diet model of NASH is limited by the lack of the extrahepatic manifestations of the metabolic syndrome including obesity, dyslipidemia and peripheral insulin resistance [18,19]. However, the diet is frequently employed for studies of NASH because unlike other dietary models such as the HFD, significant liver injury and progression to fibrosis occurs with the MCD diet. MCD diet-fed jnk1 null mice had significantly reduced levels of steatosis, triglyceride accumulation, inflammation, lipid peroxidation, hepatocyte injury and apoptosis. Since MCD diet-induced NAFLD occurs in the setting of weight loss rather than weight gain, and in the absence of peripheral insulin resistance [20], the finding that jnk1−/− mice had decreased steatosis and hepatitis suggests that JNK activation in the liver, rather than or in addition to that in muscle or adipose tissue, regulates the development of fatty liver disease.
Box 1. Studies delineating the specific functions of the JNK isoforms in NAFLD
Steatosis
JNK1 mediates steatosis in a HFD-fed mouse [16]
JNK1 but not JNK2 mediates the development of hepatic steatosis in MCD diet-induced NASH [17]
JNK1 but not JNK2 mediates the development of HFD-induced hepatic steatosis [21]
JNK1 and not JNK2 knockdown reverses HFD-induced steatosis [21]
Hepatocyte loss of JNK1 does not alter steatosis in starved hepatocyte-specific knockout mice [57]
Liver injury
Fibrosis
Myeloid cell JNK1 mediates progression to fibrosis in bone marrow transplant chimeric mice [24]
This critical function of JNK in NAFLD development has been confirmed in HFD-induced murine NAFLD. Studies in JNK null mice demonstrated that jnk1 but not jnk2 promoted the development of HFD-induced NAFLD, since jnk1 null mice had significantly decreased steatosis and liver injury [21]. Consistent with prior studies [14], mice lacking JNK1 had reduced body weight and improved insulin sensitivity [21]. In contrast, jnk2 null mice had increased body weight, more severe insulin resistance and more advanced steatohepatitis. Findings in two dietary models of NAFLD therefore demonstrate a specific function for JNK1 and not JNK2 in the development of murine steatohepatitis.
JNK1 mediates continued lipid overaccumulation and hepatitis
In the MCD diet model of steatohepatitis, hepatic JNK/c-Jun activation occurred early after the start of diet feeding and was maintained throughout the course of the diet [17]. This suggested that JNK signaling may mediate not only initial NAFLD development but also continued lipid accumulation and liver injury. To examine the contribution of JNK to established disease, mice fed a HFD for 12 weeks were randomized to receive antisense oligonucleotides directed against JNK1 or JNK2. A strength of these studies is that an acute JNK knockdown presumably did not lead to confounding compensatory changes that may occur in JNK knockout mice. Acute knockdown of JNK1 or JNK2 markedly improved insulin sensitivity, but only the JNK1 knockdown displayed reduced steatosis or liver injury. On the other hand, the jnk2 knockdown mice had markedly increased liver injury despite improved insulin sensitivity and no change in steatosis. The increased injury was secondary to elevated protein levels of the pro-apoptotic Bcl-2 member Bim in jnk2 null mice that resulted from a lack of JNK phosphorylation of Bim necessary to trigger its proteasomal degradation. These studies demonstrated for the first time that an inhibition of JNK signaling can halt and reverse established steatohepatitis, further suggesting that JNK is a potential therapeutic target in human NAFLD. However, the findings raise a note of caution over the safety of JNK inhibition in the metabolic syndrome. By defining a novel hepatoprotective effect of JNK2, the studies indicate that a therapeutic approach of global JNK inhibition may be problematic because a block in JNK2 may exacerbate liver disease.
JNK mediates the fibrotic response in NAFLD
JNK signaling may also promote the development of hepatic fibrosis in response to chronic injury through hepatic stellate cell activation [22]. In two non-steatotic models of fibrosis, bile duct ligation and carbon tetrachloride, JNK was activated in hepatic stellate cells, and fibrosis decreased in jnk1 null mice but increased or was unchanged in the absence of jnk2 [23]. In a steatotic fibrosis model induced by a choline-deficient L-amino acid-defined diet, fibrosis was also decreased in jnk1 but not jnk2 null mice [24]. Studies of chimeric mice lacking JNK1 specifically in the hematopoietic compartment that supplies the hepatic macrophages or Kupffer cells revealed that JNK1 expressed in hematopoietic-derived cells mediates fibrosis [24]. Thus, strong evidence concludes that macrophage JNK1 and not JNK2 mediates the fibrotic response to fatty liver disease.
Sources of JNK activation
Findings of increased JNK activity in the liver and other tissues in the setting of obesity, insulin resistance and fatty liver raise the question as to the mechanism of sustained JNK activation. As described, JNK activation results from initiation of a kinase cascade. How environmental stimuli trigger the activation of upstream kinases remains unclear; however, a number of hepatic JNK activators have been identified. Possible sources of JNK activation include lipids and sugars. Serum fatty acids are increased with insulin resistance, and saturated fatty acids induce JNK activation in cultured hepatocytes [25,26]. In contrast, hyperglycemia, another consequence of insulin resistance, failed to activate JNK [26], while other dietary sugars may upregulate JNK. Considerable interest has arisen recently over the contribution of high fructose-containing foods to NAFLD development [27]. The mechanism of the fructose effect is undetermined, but the ability of this sugar to activate JNK in hepatocytes is an interesting possibility [28].
A second potential activator of hepatic JNK is the inflammatory condition that underlies the metabolic syndrome. The metabolic syndrome is associated with chronic inflammation, particularly in adipose tissue, that leads to the production of cytokines such as TNF that may promote obesity and insulin resistance [29]. As NAFLD progresses from steatosis alone to hepatitis, hepatic inflammation is also present. TNF induces JNK, and TNF has been implicated as a mediator of NAFLD development [30]. Under certain physiological conditions, TNF induces sustained JNK activation in hepatocytes and mouse liver [31,32], and the continuous production of TNF by hepatic or adipose tissue inflammatory cells may induce JNK activation in fatty livers as well. Alternatively, JNK activation may result from the direct actions of gut-derived lipopolysaccharide (LPS) that drives inflammation in the metabolic syndrome [33]. LPS binds to toll-like receptor 4 (TLR4), triggering a signaling pathway that culminates in JNK activation [34]. Studies of TLR4-deficient mice have established that TLR4 mediates NAFLD development from MCD [35], high fat [36] and fructose-containing [37] diets. Further studies are needed in these models to determine whether TLR4 promotes disease through JNK activation.
Another frequent source of JNK activation is cellular oxidative stress. Human and rodent NAFLD are associated with oxidative stress [38,39], suggesting that oxidants may activate JNK in this disease. A potential mechanism of oxidative stress is the overexpression of the cytochrome P450 family member CYP2E1 which occurs in NAFLD [40]. In a cultured hepatocyte model of chronic oxidative stress from stable CYP2E1 overexpression, oxidants generated by this enzyme induced a sustained elevation in JNK activity [41]. The level of oxidative stress-induced JNK overactivation markedly impaired insulin signaling in these hepatocytes [42], suggesting that oxidant-dependent JNK activity mediates NAFLD development.
A central mechanism to unify these diverse JNK activators is through the induction of ER stress. The disruption of ER homeostasis and function termed the ER stress response has been implicated in the pathogenesis of NAFLD as well as in obesity and insulin resistance [43,44]. ER stress in these conditions can activate JNK through inositol-requiring kinase-1 (IRE1) [45]. Compatible with hepatic JNK activation resulting from metabolic syndrome-associated ER stress are findings that the prevention of ER stress by a pharmacological inhibitor or liver-specific deletion of protein-tyrosine phosphatase 1B blocked hepatic JNK overactivation in obese mice [46,47]. Fatty liver disease was also decreased by this approach [47], identifying the ER stress pathway as a possible convergence point for a variety of JNK activators in fatty liver disease and a potential therapeutic target.
Mechanisms of JNK regulation of steatosis and hepatitis
Effects on insulin resistance and lipid metabolism
A possible mechanism by which JNK1 inhibition protects against NAFLD development and reverses established disease may be through increased insulin sensitivity. Insulin resistance is critical for the tissue-specific manifestations of the metabolic syndrome. Peripheral insulin resistance increases adipocyte lipolysis and release into the serum of free fatty acids that are taken up by the liver and stored as triglycerides. Adipose tissue JNK activation may promote steatosis through this mechanism, as most of the lipid that accumulates in NAFLD is derived from serum fatty acids [48]. Hepatic insulin resistance also promotes increased hepatic lipid storage. JNK mediates cellular insulin resistance through inhibitory serine phosphorylation of the insulin signaling molecule IRS-1 [49]. Inhibition of JNK signaling increases insulin sensitivity in diabetic mice, decreasing levels of gluconeogenic enzymes and hepatic glucose production [49]. As previously discussed, NAFLD-associated chronic oxidative stress induces JNK-dependent inhibition of both IRS-1 and IRS-2 signaling and hepatocyte insulin resistance [42]. Thus, inhibitory effects of JNK1 on the insulin signaling pathway leading to peripheral and hepatic insulin resistance may underlie the ability of JNK1 to promote hepatic steatosis.
An alternative mechanism by which JNK could regulate hepatic lipid accumulation is through a direct effect on pathways of lipid metabolism such as de novo fatty acid synthesis or fatty acid β-oxidation. JNK1 knockdown in cultured hepatocytes decreased fatty acid synthesis and increased β-oxidation [50]. An adenovirus-mediated knockdown of JNK1 in mouse liver not only improved insulin sensitivity but also upregulated genes involved in glycolysis, triglyceride secretion and β-oxidation [51]. Recent studies have also demonstrated that blocking JNK function increases fatty acid oxidation in muscle [52], so the possibility that JNK inhibition has a beneficial effect on steatosis by increasing fatty acid utilization in the liver or other organs needs further investigation.
Interestingly in vivo inhibition of JNK2 function had no effect on hepatic steatosis despite an even greater improvement in systemic insulin resistance than that achieved by JNK1 knockdown [21]. This finding suggests the possibility that promotion of steatosis by JNK1 is mediated through an effect other than that on insulin resistance, as loss of JNK2 failed to affect lipid accumulation despite significantly improving insulin sensitivity. The absence of an effect on hepatic lipid accumulation in vivo is in contrast to findings in adipocytes and muscle cells in which JNK2 inhibition increased β-oxidation and lipolysis, respectively [52,53]. This discrepancy may reflect an artifact of the in vitro studies in the non-hepatic cells or the tissue-specific nature of JNK regulation of lipid metabolism. The later possibility has important implications for the potential clinical use of JNK inhibitors.
Modulation of inflammation
Chronic inflammation, particularly in adipose tissue, leads to increased systemic cytokine production, thought to promote obesity and insulin resistance [29]. JNK promotes the production of proinflammatory cytokines such as TNF and IL-6 and therefore has the potential to up regulate adipocyte or macrophage generation of these proteins in inflamed adipose or liver tissue. JNK inhibition may reduce this proinflammatory state. Indeed, a selective knockout of JNK1 in adipose tissue but not myeloid cells resulted in increased hepatic insulin sensitivity [54]. In the absence of JNK1, increased adipose tissue production of IL-6 induced by HFD feeding was blocked. Hepatic steatosis was inhibited in the knockout mice, as evaluated by histology, but this effect was not quantified. Proinflammatory JNK signaling in adipose tissue may therefore promote NAFLD development by generating systemic mediators of insulin resistance.
Promotion of hepatocyte injury
In addition to promoting the initial stage of lipid accumulation, hepatocyte JNK activation may also mediate cellular injury through several mechanisms. The first is by triggering hepatocellular TNF toxicity. A discussed previously, TNF induces JNK activation [31,32], and this cytokine has been implicated as an injurious factor in NAFLD [30]. Sustained JNK activation in response to TNF mediates necrotic as well as apoptotic hepatocyte death [55]. Multiple JNK activators may act in concert in NAFLD to induce prolonged JNK activation that sensitizes hepatocytes to TNF cytotoxicity. Oxidative stress from CYP2E1 overexpression sensitized rat hepatocytes to necrotic death from TNF [41]. CYP2E1 overexpression by itself increased basal JNK activity slightly, but together with TNF, induced sustained JNK activation that led to cell death. Reactive oxygen species generated by CYP2E1 or other sources may therefore promote JNK-dependent TNF toxicity in NAFLD. CYP2E1-induced oxidative stress also causes a JNK-dependent inhibition of insulin signaling [42] that is an important survival pathway in hepatocytes. Alternatively, JNK may promote cell injury through the process of fatty acid toxicity or lipotoxicity. Saturated but not monounsaturated fatty acids increase hepatocyte JNK activity and induce apoptotic cell death that is JNK dependent [25]. Death occurs via the mitochondrial death pathway secondary to JNK1-mediated upregulation of the pro-apoptotic factor PUMA [56]. Findings of increased PUMA levels in the livers of patients with NAFLD [56] indicate that this JNK1-dependent mechanism of hepatocyte death may be relevant to human NAFLD. Animal studies examining the effects of chronic hepatocyte exposure to physiologically relevant levels of fatty acids are needed to further define the importance of lipotoxicity and its JNK dependence in vivo.
Cell type specific JNK functions
Critical to understanding which of the biological effects of JNK promote NAFLD development and progression is a determination of the cell types in which JNK activation mediates these effects. Studies of systemic JNK inhibitors or global JNK knockout mice fail to distinguish between the effects of JNK inhibition on hepatic versus nonhepatic cell types. Direct effects of JNK inhibition on hepatocyte insulin sensitivity or lipid accumulation have been reported [50],suggesting that JNK-mediated effects in NAFLD occur principally in the hepatocyte. Other studies have suggested that it is adipocyte JNK activity that mediates NAFLD [54]. However, the finding that JNK inhibition was effective in the MCD diet model in which NAFLD develops in the absence of weight gain and systemic insulin resistance [17] strongly suggests important liver-specific effects of JNK in this disease (Figure 2). In contrast to previous studies, a recent investigation using hepatocyte-specific JNK1 knockout mice has suggested that loss of JNK1 had no effect on insulin sensitivity and in fact increased steatosis [57]. However, liver lipid accumulation in this study was measured only in regular diet-fed mice that were starved for a prolonged period in order to induce a massive, acute hepatic lipid accumulation. The physiological relevance of this model to the slow, chronic development of steatosis that occurs in NAFLD is uncertain. In addition, starvation induces macroautophagy, a critical regulator of hepatocyte lipid stores [58], and JNK can alter autophagic function [59]. Thus, the implications of these findings to NAFLD are uncertain until the studies are repeated in a diet-induced model of NAFLD.
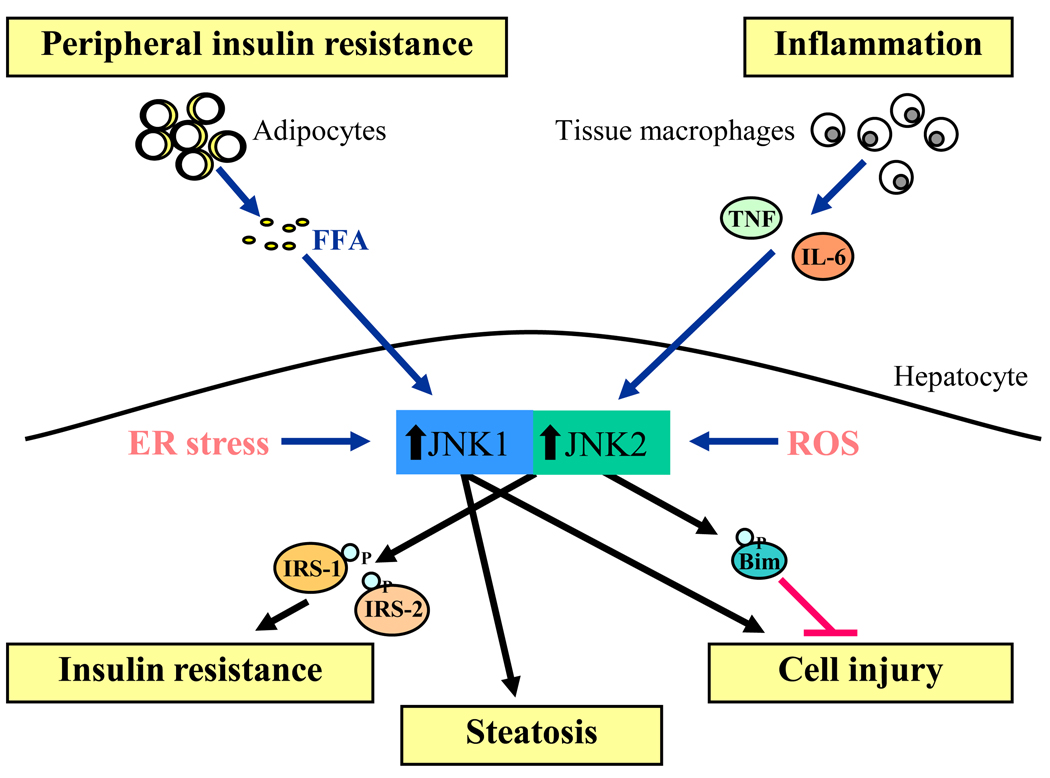
Figure 2.
Activators and biological effects of hepatocyte JNK in NAFLD. Characteristic of the metabolic syndrome are peripheral insulin resistance that leads to increased serum levels of free fatty acids (FFA) and adipose tissue inflammation that produces proinflammatory cytokines such as tumor necrosis factor (TNF) and interleukin 6 (IL-6). These factors together with intracellular ER stress and reactive oxygen species (ROS) combine to cause JNK overactivation in hepatocytes (blue arrows). Increased JNK1 and JNK2 contribute to hepatic insulin resistance in part through inhibitory serine phosphorylation of the insulin signaling molecules IRS1 and IRS2. JNK1 overactivation also leads to steatosis and hepatocyte injury. In contrast, JNK2 activation has no effect on hepatic lipid accumulation and inhibits hepatocellular injury through the phosphorylation and degradation of Bim.
The contribution of macrophage JNK activation to NAFLD has been addressed in studies of chimeric bone marrow transplanted mice. These investigations have shown no effect of JNK activity in hematopoietically derived cells on body weight or fat mass but variable effects on insulin sensitivity, adipose tissue macrophage infiltration and the development of steatosis [60,61]. Discrepancies between these studies likely reflect differences in transplantation methods and dietary treatments. Bone marrow transplantation experiments are also complicated by the profound effects of radiation administered as part of the bone marrow transplantation on weight gain [61,62]. Studies to better delineate the mechanisms by which JNK promotes NAFLD development and progression are needed to further define the hepatic and peripheral cell types responsible for JNK's effects (Box 2).
Box 2. Summary of findings of cell type specific JNK involvement in NAFLD
Hepatocytes
JNK1 mediates hepatic steatosis in a non-obese, insulin sensitive mouse (MCD dietinduced NAFLD model) [17]
JNK1 decreases hepatocyte fatty acid synthesis and increases β-oxidation (gene knockdown in cultured hepatocytes) [50]
Hepatocyte loss of JNK1 had no effect on hepatic insulin resistance and steatosis (hepatocyte-specific knockout mouse) [57]
Adipocytes
JNK1-dependent adipose tissue IL-6 production induces hepatic insulin resistance (adipocyte-specific JNK1 knockout mouse) [54]
Myeloid cells (macrophages)
JNK1 in myeloid cells increases insulin resistance and adipose tissue macrophage infiltration but not hepatic steatosis (bone marrow transplant chimeric mice) [60]
JNK1 in non-myeloid cells regulates insulin sensitivity and adipose tissue macrophage infiltration but myeloid JNK1 increases steatosis (bone marrow transplant chimeric mice) [61]
JNK1 in myeloid cells does not affect insulin sensitivity (myeloid cell specific knockout mouse) [54]
Myeloid cell JNK1 mediates progression to fibrosis (bone marrow transplant chimeric mice) [24]
Conclusions and future directions
Following the demonstration of JNK involvement in the pathogenesis of obesity and insulin resistance, considerable interest has been focused on the function of JNK in the fatty liver disease that is a component of the metabolic syndrome. Studies have now demonstrated that JNK1 is a critical mediator of both lipid accumulation and hepatocellular injury in experimental NAFLD. The mechanism of this JNK1 effect remains unclear as does the pathogenesis of NAFLD. JNK1 has many functions relevant to NAFLD including effects on lipid metabolism, insulin sensitivity and inflammation. JNK1 may exert physiologically relevant effects in multiple cell types in NAFLD but this issue remains unresolved. Isoform-specific functions of JNK2 still remain unclear. JNK2 has also been implicated in promoting insulin resistance, but it may exert beneficial effects by blocking hepatocyte cell death pathways. Inhibition of JNK2 function may therefore improve peripheral insulin resistance but worsen liver disease. Nonetheless, novel therapeutic approaches to fatty liver disease and the clinical manifestations of the metabolic syndrome in other organs have been suggested by these studies. These agents target upstream events that stimulate JNK activation [47], modulators of JNK activity such as phosphatases [63], or JNK itself [21,64]. Other general therapeutic measures directed at the metabolic syndrome need to be examined for their effects on JNK. Fructose consumption may promote NAFLD in part through JNK activation. Exercise in obese rats decreased hepatic JNK activity, increasing insulin sensitivity in concert with a reduction in hepatic and systemic inflammation [65]. The effects of basic disease modifiers such as diet and exercise may therefore be mediated in part through JNK, qualifying JNK as a promising new therapeutic target in NAFLD, a disease for which there is currently no proven therapy.
Acknowledgements
This work was supported by National Institutes of Health Grant DK61498.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Eckel RH, et al. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Blazer DG, et al. Metabolic syndrome predicts mobility decline in a community-based sample of older adults. J. Am. Geriatr. Soc. 2006;54:502–506. doi: 10.1111/j.1532-5415.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 3.Hassinen M, et al. Metabolic syndrome and the progression of carotid intima-media thickness in elderly women. Arch. Intern. Med. 2006;166:444–449. doi: 10.1001/archinte.166.4.444. [DOI] [PubMed] [Google Scholar]
- 4.Powell EE, et al. Dangerous liaisons: the metabolic syndrome and nonalcoholic fatty liver disease. Ann. Intern. Med. 2005;143:753–754. doi: 10.7326/0003-4819-143-10-200511150-00015. [DOI] [PubMed] [Google Scholar]
- 5.de Marco R, et al. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22:756–761. doi: 10.2337/diacare.22.5.756. [DOI] [PubMed] [Google Scholar]
- 6.Gastaldelli A, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537–1544. doi: 10.1002/hep.22845. [DOI] [PubMed] [Google Scholar]
- 7.Soderberg C, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann. Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 10.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 11.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabapathy K, et al. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell. 2004;15:713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke A, et al. JNK2 is a positive regulator of the cJun transcription factor. Mol. Cell. 2006;23:899–911. doi: 10.1016/j.molcel.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre V, et al. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 15.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 16.Tuncman G, et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schattenberg JM, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 18.Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J. Gastroenterol. Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- 19.Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig. Dis. 2010;28:247–254. doi: 10.1159/000282097. [DOI] [PubMed] [Google Scholar]
- 20.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Singh R, et al. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2008;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J. Gastroenterol. Hepatol. 2007;22:S73–S78. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 23.Kluwe J, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodama Y, et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhi H, et al. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 26.Solinas G, et al. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim JS, et al. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y, et al. Fructose selectively modulates c-jun N-terminal kinase activity and insulin signaling in rat primary hepatocytes. J. Nutr. 2005;135:1642–1646. doi: 10.1093/jn/135.7.1642. [DOI] [PubMed] [Google Scholar]
- 29.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, et al. NF-κB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J. Biol. Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 34.Mencin A, et al. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera CA, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saberi M, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spruss A, et al. Toll-like receptor 4 is involved in the development of fructoseinduced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 38.Leclercq IA, et al. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanyal AJ, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 40.Lieber CS. CYP2E1: from ASH to NASH. Hepatol.Res. 2004;28:1–11. doi: 10.1016/j.hepres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, et al. Increased cytochrome P-450 2E1 expression sensitizes hepatocytes to c-Jun-mediated cell death from TNF-α. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G257–G266. doi: 10.1152/ajpgi.00304.2001. [DOI] [PubMed] [Google Scholar]
- 42.Schattenberg JM, et al. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J. Biol. Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- 43.Kapoor A, Sanyal AJ. Endoplasmic reticulum stress and the unfolded protein response. Clin. Liver Dis. 2009;13:581–590. doi: 10.1016/j.cld.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 45.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 46.Delibegovic M, et al. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakatani Y, et al. Modulation of the JNK pathway in liver affects insulin resistance status. J. Biol. Chem. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 50.Yu XX, et al. Reduction of JNK1 expression with antisense oligonucleotide improves adiposity in obese mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E436–E445. doi: 10.1152/ajpendo.00629.2007. [DOI] [PubMed] [Google Scholar]
- 51.Yang R, et al. Liver-specific knockdown of JNK1 up-regulates proliferators-activated receptor γ coactivator 1β and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice. J. Biol. Chem. 2007;282:22765–22774. doi: 10.1074/jbc.M700790200. [DOI] [PubMed] [Google Scholar]
- 52.Vijayvargia R, et al. JNK deficiency enhances fatty acid utilization and diverts glucose from oxidation to glycogen storage in cultured myotubes. Obesity. (Silver Spring) 2010 doi: 10.1038/oby.2009.501. EPub. [DOI] [PubMed] [Google Scholar]
- 53.Rozo AV, et al. Silencing Jnk1 and Jnk2 accelerates basal lipolysis and promotes fatty acid re-esterification in mouse adipocytes. Diabetologia. 2008;51:1493–1504. doi: 10.1007/s00125-008-1036-6. [DOI] [PubMed] [Google Scholar]
- 54.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schattenberg JM, Czaja MJ. TNF and TNF receptors. In: Dufour JF, Clavien P-A, editors. Signaling Pathways in Liver Diseases. 2nd edn. Springer-Verlag; 2010. pp. 161–179. [Google Scholar]
- 56.Cazanave SC, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J. Biol. Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabio G. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh R, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Y, et al. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Vallerie SN, et al. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ablamunits V, et al. Reduced adiposity in ob/ob mice following total body irradiation and bone marrow transplantation. Obesity. (Silver Spring) 2007;15:1419–1429. doi: 10.1038/oby.2007.170. [DOI] [PubMed] [Google Scholar]
- 63.Emanuelli B, et al. Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3545–3550. doi: 10.1073/pnas.0712275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaneto H, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat. Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 65.Kiraly MA, et al. Exercise maintains euglycemia in association with decreased activation of c-Jun NH2-terminal kinase and serine phosphorylation of IRS-1 in the liver of ZDF rats. Am. J. Physiol. Endocrinol. Metab. 2010;298:E671–E682. doi: 10.1152/ajpendo.90575.2008. [DOI] [PubMed] [Google Scholar]