INTRODUCTION
Ertapenem (MK-0826, Invanz®) is one of the newest carbapenem antibiotics to be approved for use in the United States and throughout the world. It differs structurally from imipenem by the presence of a β-methyl substitution on carbon 1 of the core β-lactam structure and from both imipenem and meropenem by the presence of a meta-substituted benzoic acid on the functional group at position two.1,2 With these variations, ertapenem retains excellent activity against the enterobacteriaceae, including extended-spectrum β-lactamase producers, and demonstrates comparable activity against Gram positive and anaerobic organisms.3–6 However, ertapenem possesses diminished activity against the non-fermentative Gram negative bacilli including Pseudomonas spp, Acinetobacter spp and Stenotrophomonas maltophilia.4 These structural modifications confer improved stability to cleavage by dehydropeptidase-I as compared with imipenem along with a markedly greater degree of protein binding and a longer half-life than either imipenem or meropenem which presents the opportunity for less frequent dosing.
With its broad spectrum of activity and favorable pharmacokinetic profile, once-daily administration of ertapenem is indicated for, and has been demonstrated effective in, the management of complicated intra-abdominal infections, acute pelvic infections, urinary tract infections, skin and skin structure infections, including diabetic foot infections without osteomyelitis, and community acquired pneumonia. Ertapenem is also indicated for the prophylaxis of surgical site infection following elective colorectal surgery. In controlled adult clinical trials, ertapenem demonstrates efficacy comparable to that of ceftriaxone, ceftriaxone + metronidazole and piperacillin/tazobactam.7–12 In pediatric patients, ertapenem appears to possess comparable efficacy to ceftriaxone in the treatment of urinary tact infections, skin and soft tissue infections and community acquired pneumonia.13,14 It also demonstrates efficacy comparable to that of ticarcillin/clavulanate in the treatment of pediatric intra-abdominal and pelvic infections.15,16 Notably, both pediatric investigations employed twice daily dosing regimens in the ertapenem arm for children 12 years and younger. This investigation was designed to evaluate the dose-exposure profile of ertapenem at varying doses in children from infancy through adolescence.
METHODS
Study Design
The study was conducted as an open label, multi-center, parallel-group, single dose evaluation of ertapenem pharmacokinetics in children 3 months to 17 years of age. Children were enrolled in 3 age cohorts (3 to 23 months, 2 to 12 yr, 13 to 17 yr) and received a single 15 mg/kg, 20 mg/kg (maximum 1 gram) or 40 mg/kg (maximum 2 grams) dose of ertapenem over one-half hour by intravenous infusion.
Subjects
Hospitalized children were eligible for enrollment if they were judged to be clinically stable, were currently requiring antibiotic therapy and were within the 3rd and 97th percentile for weight and height. Subjects were ineligible for enrollment if they met any of the following criteria: (1) a history of seizures or central nervous system infection, (2) a known or suspected CNS infection, (3) evidence of hemodynamic instability, (4) concurrent treatment with ceftriaxone therapy as a twice-daily regimen (due to potential for interference), (5) a history of significant drug or food allergies including intolerance or allergy to the β-lactam antibiotics, (6) a history of psychiatric disorders, (7) a history of cystic fibrosis, (8) clinically significant abnormalities on pre-study clinical examination or laboratory safety tests, (9) concomitant illness or medications which might potentially affect the pharmacokinetics of ertapenem (eg, highly protein bound drugs or drugs which compete for tubular secretion, including but not limited to, probenecid, cefoperazone, itraconazole, phenytoin, valproic acid, nafcillin) and (10) participation in the study of another investigational agent within 4 weeks of receiving ertapenem. Additionally, female subjects who had attained menarche were required to have a negative serum pregnancy test prior to administration of the study drug.
A medical history, physical examination, ECG and clinical laboratory tests (serum chemistry panel, complete blood count and urinalysis) were performed in each subject before enrollment and at completion of the study. The study protocol was approved by the Investigational Review Boards of each participating institution and all subjects were enrolled via informed parental permission and patient assent when appropriate (ie > 7 years of age).
Sample Collection
In children > 2 yr, venous blood samples (3.5 mL) for the determination of total and unbound ertapenem concentrations were collected from an indwelling venous catheter into sodium heparin containing tubes. Samples were collected immediately before the start of the infusion and at 0.5, 1, 2, 4, 8, 12 and 24 hours after the end of the infusion. An additional 6 hr sample was drawn in children receiving a 15 mg/kg dose. Due to blood volume restrictions, children < 2 yr were randomized to one of two sampling schemes. For the quantification of free and total ertapenem in children under 2 years of age, blood samples (3.5 mL) were collected at either 0.5 or 1 hr and at 12 hr after the infusion. In the remaining children, only total ertapenem concentrations were determined and blood samples (1.25 mL) were drawn at the post-dose intervals described above for the older children. Plasma was separated by centrifugation (2000 g × 10 minutes at 4°C) within 1 hour of collection and stored in polypropylene tubes at −70°C until analysis.
Analytic Procedures
For the determination of total ertapenem concentrations, plasma samples were thawed in a room temperature water bath and vortexed. A 100-μL aliquot of plasma was combined with an equal volume of 0.1 M 2-[N-Morpholino]ethanesulfonic acid (pH 3.5):ethylene glycol (1:1), vortexed and centrifuged under refrigeration (5°C) at 3000 rpm for 5 minutes. The supernatant was transferred into a glass autosampler vial, capped and placed in a temperature-controlled autosampler rack maintained at 5°C until injection onto a high-performance liquid chromatograph (HPLC) for analysis. Unbound ertapenem concentrations were determined according to a previously published method.17
Samples were analyzed by reverse-phase HPLC with column switching. Samples (50 μL) were injected onto a Maxil C18 extraction column (50 × 4.6mm, 10 μm, Phenomenex, Torrence, CA) using a 25 mM sodium phosphate buffer (pH 6.5) mobile phase pumped at 1.5 mL/min. Analytes were flushed off of the extraction column onto a Hypersil C18 analytical column (100 × 4.6 mm, 5 μm, Keystone Scientific, Bellefonte, PA) with 10.5% methanol in 25 mM sodium phosphate buffer (pH 6.5) delivered at a flow rate of 2.0 mL/min. The eluate was monitored with UV detection at 300 nm.
A standard curve using the peak area of active compound was prepared daily and used to calculate all plasma ertapenem concentrations. The analytic method demonstrated linearity (with 1/y weighting, r2>0.99) at ertapenem concentrations ranging from 0.125 to 50 μg/mL (total) and 0.25 to 100 μg/mL (unbound). Inter- and intra-day assay variability (CV) was less than 10% for all standards in the range including lower limit of quantification (LLOQ).
Pharmacokinetic and Statistical Analysis
Plasma ertapenem concentration versus time data were evaluated using a model-independent approach. The concentration at the end of the infusion (Ceoi) was obtained directly from the plasma concentration vs. time profile. The area under the plasma concentration versus time curve (AUC0-n) was determined using the mixed log-linear rule. Extrapolation of the AUC to infinity (AUC0-∞) was calculated by summation of AUC0-n + Cn/λz, where Cn represents the observed plasma concentration at the last quantifiable post-dose time point and λz is the apparent terminal elimination rate constant calculated from a curve fit of the apparent terminal elimination phase with 1/y weighting. Total body clearance (Cl) and steady-state distribution volume (Vss) were calculated from the AUC0-∞.
Ertapenem pharmacokinetic indices were examined using standard descriptive statistics. Univariate analysis of variance and nonlinear regression techniques were used to evaluate the relationship between demographic variables and pharmacokinetic parameter estimates. The significance limit for all statistical analyses was set at α=0.05. Pharmacokinetic and statistical analyses were conducted using WinNonlin Enterprise® Version 4.1 (Pharsight Corporation, Mountain View, CA) and SPSS version 11.5 (SPSS, Chicago, IL).
RESULTS
Eighty-four patients (42 male, 42 female; 25 African-American, 36 Caucasian, 22 Hispanic, 1 multiracial) ranging in age from 3 months to 16 years and in weight from 6.1 to 82.6 kg (31.1 ± 22.7 kg) were enrolled in this multicenter trial. The study participants were receiving antibiotics for infections and/or conditions that included: lower respiratory tract infection (n=23), skin/skin structure infection (n=15), upper respiratory tract infection (n=14), pyelonephritis (n=8), appendicitis (n=4), central line infection (n=3), post-operative fever (n=3), septic arthritis (n=2), fever with neutropenia (n=2), urinary tract infection (n=2) and osteomyelitis, sepsis, gastrointestinal infection, endocarditis (n=1 each). The reason for antibiotic use was unspecified in an additional four children.
Overall, a single parenteral dose of ertapenem was well tolerated in the study participants. No child withdrew from the study as a consequence of adverse events and in only three children were the adverse events (nausea n=2, infiltration at the injection site n=1) considered to be related to study drug administration.
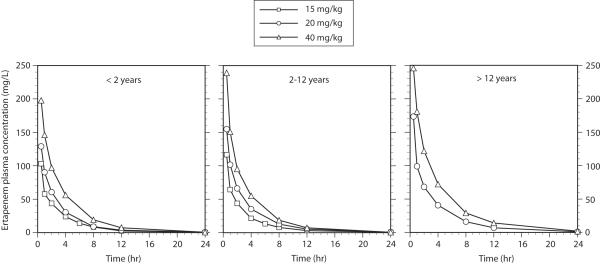
A complete pharmacokinetic profile was available in 70 study participants. In 13 children partially evaluable pharmacokinetic data were available owing to incomplete sample collection and/or problems that arose during sample processing. One additional child withdrew from the study prior to administration of their ertapenem dose. The mean ertapenem plasma concentration versus time profiles are illustrated in Figure 1 and the pharmacokinetic indices are summarized in the Table. For ease of comparison, the data have been segregated by the age cohorts determined at the time of enrollment.
FIGURE 1.
Ertapenem plasma concentration versus time data by age cohort. Dose levels are represented by (■) 15 mg/kg, (•) 20 mg/kg and (▲) 40 mg/kg.
In evaluating the relationship between ertapenem dosing arm (15 vs 20 vs 40 mg/kg) and exposure, a significant positive association was observed for both Ceoi (108.3 ± 47.0 vs 144.2 ± 37.7 vs 221.9 ± 48.9 mg/L, P<0.01) and AUC0-∞ (273.9 ± 114.3 vs 406.7 ± 133.4 vs 762.8 ± 339.5 mg*hr/L, P<0.01) in the study population (Figure 2). When evaluated along the continuum of actual administered dose (12.1–50.6 mg/kg), a significant linear relationship between weight-normalized dose and Ceoi can be described (r2=0.46, P<0.01). A weaker, albeit significant linear relationship was observed between weight-normalized dose and AUC0-∞; however, the relationship was best fit to a nonlinear function (r2=0.40, P<0.01) with AUC0-∞ disproportionately lower at higher doses. Introducing age into these regression models resulted in a modest improvement for both Ceoi (r2=0.61, P<0.01) and AUC0-∞ (r2=0.61, P<0.01) suggesting that the variability observed in the relationship between dose and exposure can be accounted for, in part, by changes in biodisposition that occur as a consequence of normal growth and development.
FIGURE 2.
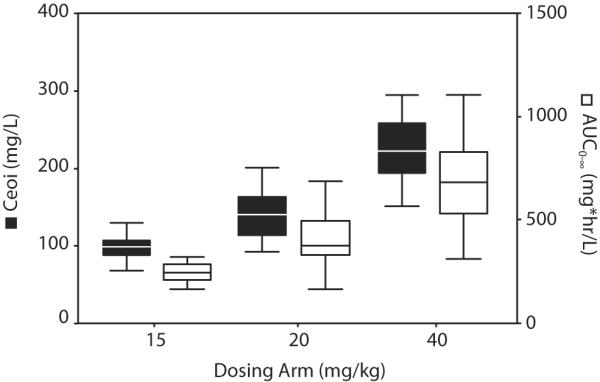
Box plots representing the median and quartiles for Ceoi and AUC0-∞ by dosing arm.
The exposure estimates observed in this investigation, whether single point determinations of concentration or estimates of total body exposure, were lower in our pediatric population as compared to adults for every milligram dose administered per kilogram body weight (Table). This is addressed by exploring the influence of age on ertapenem biodisposition. A weak, albeit significant, association between age and weight-corrected apparent volume of distribution was evident (r2=0.10, P<0.01) with distribution volume decreasing with increasing age. A corresponding association between age and dose-normalized Ceoi (r2=0.16, P<0.01) was evident with a larger attainable Ceoi (per mg/kg dose administered) noted with increasing age.
TABLE.
Ertapenem pharmacokinetic parameters following a single intravenous dose.
| Parameter | <2 years (n=41) | 2 to 12 years (n=28) | >12 years (n=11) | Adulta (n=67) |
|---|---|---|---|---|
| Age (yr) | 1.0 ± 0.6 | 6.7 ± 3.4 | 14.3 ± 0.9 | NR |
| Dose (mg) | 241 ± 132 | 663 ± 442 | 1331 ± 519 | 1000 |
| Dose (mg/kg) | 25.56 ± 11.49 | 26.12 ± 10.98 | 24.58 ± 8.34 | 14.3 |
| Ceoi (mg/L) | 135.2 ± 51.8b | 173.1 ± 68.3c | 212.0 ± 57.4d | 161.5 (145.6–175.3) |
| Ceoi (mg/L per mg/kg dose) | 6.1 ± 2.2b | 6.9 ± 2.4c | 8.7 ± 1.9d | 11.3 (10.2–12.3) |
| AUC0-∞ (mg*hr/L) | 385.8 ± 175.2e | 469.8 ± 246.8c | 861.3 ± 478.2d | 600.6 (572.1–627.3) |
| AUC0-∞ (mg*hr/L per mg/kg dose) | 17.0 ± 5.4e | 18.4 ± 8.0c | 34.7 ± 14.7d | 42.0 (40.0–43.9) |
| C6 (mg/L) | 13.8 ± 4.0f | 11.7 ± 10.0g | NP | NR |
| C6 (mg/L per mg/kg dose) | 0.9 ± 0.3f | 0.8 ± 0.6g | NP | NR |
| C12 (mg/L) | 3.8 ± 3.0h | 5.5 ± 4.2c | 10.6 ± 8.2 | 10.2 (9.3–11.3) |
| C12 (mg/L per mg/kg dose) | 0.2 ± 0.1h | 0.2 ± 0.2c | 0.4 ± 0.2 | 0.7 (0.7–0.8) |
| CL (mL/min/kg) | 1.02 ± 0.27e | 1.02 ± 0.35c | 0.64 ± 0.16d | 0.41 (0.38–0.42) |
| Vss (L/kg) | 0.21 ± 0.05e | 0.21 ± 0.06c | 0.17 ± 0.02d | 0.14 (0.13–0.15) |
| t½ (hr) | 2.9 ± 0.7j | 3.0 ± 0.9 | 4.0 ± 0.8 | 4.0 (3.8–4.4) |
| λz (1/hr) | 0.262 ± 0.094j | 0.254 ± 0.092 | 0.182 ± 0.037 | 0.176 (0.158–0.182) |
Data are represented as the weighted pooled average (range) for the individual studies reported by Majumdar et al.[17] Weight corrected estimates assume an average adult weight of 70 kg
n=40
n=27
n=10
n=33
n=11
n=8
n=39
n=34
NR- not reported
NP- not performed
A modest relationship was observed for weight-corrected estimates of total body clearance (r2=0.21, P<0.01) and half-life (r2=0.20, P<0.01) the former decreasing and the latter increasing with increasing age. Consequently, a stronger association between age and dose-normalized AUC0-∞ (r2=0.34, P<0.01) was observed as compared to Ceoi, with larger estimates of AUC0-∞ achieved (per mg/kg dose administered) in older children.
A total of 154 paired observations for free and total ertapenem determined over the course of the dosing interval were available from 37 children. There was a direct relationship between plasma ertapenem concentration and the degree of protein binding observed in this study with the percent of unbound drug in the circulation increasing with increasing total plasma ertapenem concentration (r2=0.53, P<0.01). The addition of age into the regression model as a covariate did not alter the relationship suggesting that normal growth and development, after the age of 3 months, does not appear to influence the degree of protein binding observed with ertapenem. Rather, the findings are likely explained by concentration dependent protein binding. In fact, a strong nonlinear relationship between total and free ertapenem concentrations was evident over the spectrum of ertapenem concentrations observed in this study (P<0.01, Supplemental Digital Content 1, http://links.lww.com/INF/A523).
Considering the relevant pharmacodynamic profile for the β-lactams, the probability that total ertapenem concentration observed 12 hours after the end of a single infusion exceeded a range of minimum inhibitory concentration (MIC) was projected for each age group (Supplemental Digital Content 2, http://links.lww.com/INF/A524). As depicted in the figure, both dose and age influenced the likelihood that concentrations at the end of a 12-hour dosing interval exceeded the susceptibility breakpoint (≤ 2 μg/mL).18 Total ertapenem concentrations remained well in excess of the susceptibility breakpoint in adolescents receiving ≥20 mg/kg. Similarly, more than three-fourths of children less than the age of 2 years exceeded the susceptibility breakpoint, irrespective of dose. When treating pathogens with MIC values approaching 2 μg/mL, children aged 2–12 years may require a minimum of 20 mg/kg to achieve adequate ertapenem concentrations over the entire dosing interval; however, doses of 15 mg/kg appear suitable for susceptible organisms within one dilution of the established breakpoint.
DISCUSSION
In contrast to the existing carbapenems which require multiple daily doses, ertapenem has been demonstrated in adults to be effective with once-daily administration. The recent use of twice daily dosing (15 mg/kg twice daily) in pediatric clinical trials for children less than 12 years of age was based on preliminary results from this investigation which was designed to better understand the impact of ontogeny on ertapenem biodisposition.14,16 This study was designed to assess the impact of development on the pharmacokinetics of ertapenem and to define the dose-exposure profile of ertapenem in children at escalating doses.
The disposition of ertapenem following a 1 gram (~14 mg/kg) intravenous dose has been relatively well characterized in adults.19 As with most β-lactams, ertapenem distribution volume approximates extracellular fluid stores. Given the recognized developmental differences in body water spaces,20 it is not unexpected to find that the volume of distribution estimates observed in these children were larger than those reported in adults. As a result, the maximum attainable concentrations at the end of the infusion were lower for every mg/kg dose administered to children as compared with adults.
Similarly, with total plasma clearance of ertapenem principally restricted to glomerular filtration and active tubular secretion, we would predict more rapid elimination in younger children as was observed in this investigation. Consequently, clearance is more rapid, estimates of total body exposure (per mg/kg dose administered) are lower and the concentrations observed at the end of a given dosing interval are lower in children as compared with adults.
In murine thigh infection models, optimal ertapenem activity is observed against S. pneumoniae and the enterobacteriaceae when free drug concentrations exceed the MIC for approximately 30–40% of the dosing interval.21,22 Exploratory pharmacodynamic models for evaluating predicted free drug concentrations in our population (data not shown) suggests that children younger than 12 years require more frequent dosing to achieve optimal efficacy when treating organisms with an MIC near the susceptibility breakpoint. In contrast, the putative pharmacodynamic targets are readily achieved in children older than 12 years administered doses of 20 mg/kg or greater.
Conclusions
A single intravenous dose of ertapenem appears to be well tolerated in children 3 months to 17 years of age. However, it does not appear that weight-based dosing of ertapenem can be administered to pediatric patients without regard for age. While doses of 20 to 40 mg/kg administered once daily appear to be suitable for children older than 12 years, children 12 years and younger would appear to benefit from a more frequent dosing interval (eg 15 mg/kg administered every 12 hours).
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contributions made by the following clinical collaborators who contributed patients to this clinical trial: Peter Adamson, M.D., Children's Hospital of Philadelphia, Philadelphia, PA, Joseph Bocchini, M.D., Louisiana State University Medical School, Shreveport, LA, John Bradley, M.D., Infectious Diseases, Children's Hospital & Health Center, San Diego, CA, Walter Ledermann, M.D., Hospital Luis Calvo Mackenna, Santiago, Chile, Lisa Bomgaars, M.D., Baylor College of Medicine, Texas Children's Cancer Center, Houston, TX. The authors also acknowledge Liwen Xi of Merck who provided statistical assistance.
This work was supported in part by a grant from Merck & Co., Inc. and grant # 5U10HD031323-13 (Network of Pediatric Pharmacology Research Units) from the National Institute of Child Health and Human Development, Bethesda, MD.
Footnotes
Presented, in part, at 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., December 2005.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hammond ML. Ertapenem: a Group 1 carbapenem with distinct antibacterial and pharmacological properties. J Antimicrob Chemother. 2004;53(suppl 2):7–9. doi: 10.1093/jac/dkh203. [DOI] [PubMed] [Google Scholar]
- 2.Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother. 2003;52:331–344. doi: 10.1093/jac/dkg375. [DOI] [PubMed] [Google Scholar]
- 3.Jacoby G, Han P, Tran J. Comparative in vitro activities of carbapenem L-749,345 and other antimicrobials against multiresistant gram-negative clinical pathogens. Antimicrob Agents Chemother. 1997;41:1830–1831. doi: 10.1128/aac.41.8.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs PC, Barry AL, Brown SD. In-vitro antimicrobial activity of a carbapenem, MK-0826 (L-749,345) and provisional interpretive criteria for disc tests. J Antimicrob Chemother. 1999;43:703–706. doi: 10.1093/jac/43.5.703. [DOI] [PubMed] [Google Scholar]
- 5.Livermore DM, Oakton KJ, Carter MW, Warner M. Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent beta-lactamases. Antimicrob Agents Chemother. 2001;45:2831–2837. doi: 10.1128/AAC.45.10.2831-2837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odenholt I, Löwdin E, Cars O. In vitro pharmacodynamic studies of L-749,345 in comparison with imipenem and ceftriaxone against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 1998;42:2365–2370. doi: 10.1128/aac.42.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham DR, Lucasti C, Malafaia O, et al. Ertapenem once daily versus piperacillintazobactam 4 times per day for treatment of complicated skin and skin-structure infections in adults: results of a prospective, randomized, double-blind multicenter study. Clin Infect Dis. 2002;34:1460–1468. doi: 10.1086/340348. [DOI] [PubMed] [Google Scholar]
- 8.Yellin AE, Hassett JM, Fernandez A, et al. Ertapenem monotherapy versus combination therapy with ceftriaxone plus metronidazole for treatment of complicated intra-abdominal infections in adults. Int J Antimicrob Agents. 2002;20:165–173. doi: 10.1016/s0924-8579(02)00160-7. [DOI] [PubMed] [Google Scholar]
- 9.Solomkin JS, Yellin AE, Rotstein OD, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg. 2003;237:235–245. doi: 10.1097/01.SLA.0000048551.32606.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells WG, Woods GL, Jiang Q, Gesser RM. Treatment of complicated urinary tract infection in adults: combined analysis of two randomized, double-blind, multicentre trials comparing ertapenem and ceftriaxone followed by appropriate oral therapy. J Antimicrob Chemother. 2004;53(suppl 2):67–74. doi: 10.1093/jac/dkh208. [DOI] [PubMed] [Google Scholar]
- 11.Vetter N, Cambronero-Hernandez E, Rohlf J, et al. A prospective, randomized, double-blind multicenter comparison of parenteral ertapenem and ceftriaxone for the treatment of hospitalized adults with community-acquired pneumonia. Clin Ther. 2002;24:1770–1785. doi: 10.1016/s0149-2918(02)80078-9. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz-Ruiz G, Caballero-Lopez J, Friedland IR, et al. A study evaluating the efficacy, safety, and tolerability of ertapenem versus ceftriaxone for the treatment of community-acquired pneumonia in adults. Clin Infect Dis. 2002;34:1076–1083. doi: 10.1086/339543. [DOI] [PubMed] [Google Scholar]
- 13.Arguedas A, Wang J, Snyder T, Wimmer W, Gesser R. Safety & Efficacy in a Double-Blind Study of Ertapenem (ETP) vs Ceftriaxone (CRO) in Pediatric Patients (pts) with Complicated Urinary Tract Infections (CUTI), Community Acquired Pneumonia (CAP), or Skin & Soft Tissue Infections (SSTI). Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 45th Annual Meeting; Washington, DC. December 2005; Washington, DC: American Society for Microbiology; 2005. Abstract G-1357. [Google Scholar]
- 14.Arguedas A, Cespedes J, Asensi Botet F, Blumer J, Yogev R, Gesser R, Wang J, West J, Snyder T, Wimmer W, for the Protocol 036 Study Group Safety and Tolerability of Ertapenem versus Ceftriaxone in a Double-Blind Study Performed in Children with Complicated Urinary Tract Infections, Community Acquired Pneumonia, or Skin and Soft Tissue Infections. Int J Antimicrob Agents. 2009;33:163–167. doi: 10.1016/j.ijantimicag.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J, Yellin A, Higareda I, Fernsler D, Gesser R. Safety & Efficacy in an Open-Label Study of Ertapenem (ETP) vs Ticarcillin/Clavulanate (T/C) in Pediatric Patients with Complicated Intra-Abdominal Infections (IAI) or Acute Pelvic Infections (API). Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 45th Annual Meeting; Washington, DC. 2005; Washington, DC: American Society for Microbiology; 2005. Abstract G-932. [Google Scholar]
- 16.Yellin AE, Johnson J, Higareda I, et al. Ertapenem or Ticarcillin/Clavulanate for Treatment of Intra-abdominal Infections or Acute Pelvic Infections in Pediatric Patients. Am J Surg. 2007;194:367–374. doi: 10.1016/j.amjsurg.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Musson DG, Birk KL, Kitchen CJ, et al. Assay methodology for the quantitation of unbound ertapenem, a new carbapenem antibiotic, in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783:1–9. doi: 10.1016/s1570-0232(02)00240-4. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute . Sixteenth Informational Supplement (S16) Clinical and Laboratory Standards Institute; Wayne, PA: Jan, 2006. Performance Standards for Antimicrobial Susceptibility Testing; p. M100. [Google Scholar]
- 19.Majumdar AK, Musson DG, Birk KL, et al. Pharmacokinetics of Ertapenem in Healthy Young Volunteers. Antimicrob Agents Chemother. 2002;46:3506–3511. doi: 10.1128/AAC.46.11.3506-3511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearns GL, Abdel-Rahman SM, Alander S, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology: the impact of ontogeny on drug disposition and action. New Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 21.Xuan D, Banevicius M, Capitano B, Kim MK, Nightingale C, Nicolau D. Pharmacodynamic assessment of ertapenem (MK-0826) against Streptococcus pneumoniae in a murine neutropenic thigh infection model. Antimicrob Agents Chemother. 2002;46:2990–2995. doi: 10.1128/AAC.46.9.2990-2995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maglio D, Banevicius MA, Sutherland C, Babalola C, Nightingale CH, Nicolau DP. Pharmacodynamic profile of ertapenem against Klebsiella pneumoniae and Escherichia coli in a murine thigh model. Antimicrob Agents Chemother. 2005;49:276–280. doi: 10.1128/AAC.49.1.276-280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]