Abstract
Purpose
The pathogenic mechanisms of fungal infection during human keratomycosis were investigated in an ex vivo corneal model that used strains of Fusarium oxysporum differing in the production of a fungal transcription factor.
Methods
A pacC- loss-of-function mutant and a pacCc dominant-activating mutant were constructed from a wild-type isolate of F. oxysporum, and the three strains were characterized by in vitro growth kinetics. Twenty-seven human donor corneas maintained in tissue culture were superficially scarified and topically inoculated with the wild-type, the pacC- loss-of-function mutant, or the pacCc dominant-activating strain. Relative hyphal invasion into the stroma was compared histopathologically in corneal sections.
Results
F. oxysporum strains demonstrated comparable exponential growth rates in vitro. Wild-type F. oxysporum invaded into corneal tissue within one day and penetrated through the anterior stroma during the next 4 days. The pacC- loss-of-function mutant invaded explanted corneas significantly less than the wild-type on day 1 (P<0.0001) and on day 3 (P=0.0003). The pacCc dominant-activating strain adhered and penetrated explanted corneas similar to the wild-type strain.
Conclusion
The PacC pathway regulating the transcription of fungal genes allows fungal adaptation to the ocular surface and enables invasion of the injured cornea by F. oxysporum.
Keywords: filamentous fungi, fungal keratitis, keratomycosis, ocular mycology
Fusarium species are widespread environmental moulds, important community-acquired pathogens, and prevalent causes of fungal keratitis.1-3 Trauma, contact lens wear, and immunosuppression can overcome the eye's natural defenses and predispose to Fusarium keratitis.4, 5 Infection ensues when Fusarium spp. produce filamentous forms that invade compromised tissues. The pathogenesis of keratomycosis involves a dynamic relationship between host susceptibility and fungal virulence factors.6
Hyphal invasion relies on fungal metabolic pathways that have an effect on pathogenicity.7 Several genetically directed processes influence fungal infection.8 One pathway involves a pH-responsive signaling cascade leading to activation of the PacC/Rim101p transcription factor.9 Orthologues of this regulatory intermediate have been found in several hyphomycetes including Acremonium chrysogenum,10 Aspergillus nidulans,11 Aspergillus niger,12 Colletotrichum acutatum,13 Fusarium oxysporum,14 Fusarium verticillioides,15 Penicillium chrysogenum,16 Sclerotinia sclerotiorum,17 and Trichoderma harzianum.18
Once activated, this transcription factor regulates genes involved in infection.14, 19 In studies on experimental Candida albicans keratitis, proteolytic activation of Rim101p, a homologue of the PacC transcription factor, promotes hyphal formation and fungal invasion.20,21 A comparable mechanism appears to be pivotal in the pathogenesis of infections caused by Fusarium and Aspergillus.22, 23
We hypothesized that PacC is important for filamentous fungal infection of the cornea. We used F. oxysporum genetic mutants in an ex vivo model of fungal adaptation and corneal infection to study whether pacC mutation would attenuate fungal invasion.24 We further determined the relative invasiveness of a strain with a dominant-activating pacC allele.
Materials and Methods
Fungal Strains
F. oxysporum f. sp. lycopersici strain 4287 (race 2) is a wild-type phytoisolate from the Instituto Nacional de Investigación y Tecnología Agraria y Almentaria, Madrid. The pacC gene is present as a single copy in the F. oxysporum genome and encodes a 610-amino acid protein (GenBank accession no. AY125958). A pacC- loss-of-function mutant (strain pacC+/-12) was developed from this wild-type strain by targeted gene replacement as reported previously, and complementation of the mutated strain with the wild-type pacC gene was able to restore pathogenicity.14 A pacCc dominant-activating merodiploid (strain pacCc9) was also constructed that carried both the wild-type pacC gene and an ectopic pacCc allele containing a mutation that removed the carboxy terminus of a truncated transcript.14
Fungal strains were stored as microconidial suspensions in glycerol at -80°C and then grown in potato-dextrose broth (PDB, Difco, Detroit, MI) at 27°C. Aliquots of each strain were harvested during exponential growth and suspended in sterile phosphate-buffered saline (PBS). Triplicate samples of 5,000 culturable units (CU) of each strain were inoculated into 25 mL M199 liquid medium (Invitrogen, Grand Island, NY) and buffered with Tris-HCl to pH 6.0, pH 7.3, and pH 8.0 to produce acidic, physiologic, and slightly alkaline conditions. A pH of 7.3 was chosen to approximate the pH of the normal human cornea; a pH of 8.0 was used to ensure adequate environmental activation of the PacC pathway;8 and an acidic pH was selected to determine whether this environment would result in altered fungal pathogenicity since the PacC pathway is typically selectively activated at normal-to-alkaline conditions.
Flasks were incubated at 27°C with continuous shaking. Growth was estimated using optical density (OD) at a wavelength of 600 nm in an Ultraspec 2000 spectrophotometer (Pharmacia Biotech, Princeton, NJ), applying a conversion factor of one OD600 unit equivalent to 5.3 × 105 CU/mL that was determined from in vitro growth of Fusarium solani.4 These inocula based on OD values consisted of suspensions of hyphal spherules from log-phase growth in shaken cultures in PDB.
Ex Vivo Cornea Model
Human corneas were obtained from the Lions Eye Bank of Texas, Houston, after informed consent for research use was obtained from decedents' next-of-kin. Donor corneas were initially stored at 4°C in Optisol-GS (Bausch & Lomb, Irvine, CA) then transferred to modified supplemented hormonal epithelial medium (SHEM), consisting of equal volumes of Dulbecco's modified Eagle's medium and Ham's F12 medium supplemented with epidermal growth factor, insulin, transferrin, sodium selenite, hydrocortisone, cholera toxin A, dimethylsulfoxide, 50 μg/mL gentamicin, and 5% fetal bovine serum that was buffered with 2 M Tris-HCL to pH 7.3 or pH 8.0. An artificial anterior chamber (Refractive Technologies, Cleveland, OH) stabilized corneal buttons during superficial scarification performed by a 22-gauge needle, similar to a protocol previously described for an experimental fungal keratitis model.25 Ten μL of 1×105 CU F. oxysporum in PDB were topically applied to the corneal surface.4 Inoculated corneas were transferred into 6-well culture dishes (Corning, Corning, NY), immersing each corneoscleral rim in modified SHEM. Tissues were incubated at 34°C in 5% CO2 with 95% humidity, changing SHEM daily. After 24, 72, and 120 hours, corneas were removed, embedded in OCT compound (Sakura Finetec, Torrance, CA), placed in liquid nitrogen, and stored overnight in a -80°C freezer.
Histopathology
Ten μm-thick sections were stained with periodic acid-Schiff (PAS) reagent (Sigma-Aldrich, St. Louis, MO). Three sections were examined for each cornea, and images were captured at 10-μm intervals from the corneal mid-point with a DS-Fil digital camera (Nikon, Tokyo, Japan) attached to a Nikon Y-FL microscope. The depth of hyphal penetration of the corneal thickness was obtained at 5 equidistant points along the corneal length, using the NIS-Element 3.0 image analysis system (Nikon). The maximal percentage of hyphal penetration was estimated at regions demonstrating the greatest depth of corneal involvement, and the three largest hyphal-depth percentages were averaged from 5 measurements of each histological section. Three corneas were pooled to calculate the mean ± standard deviation, and collated results were compared by the Student t-test.
Results
In Vitro Comparison
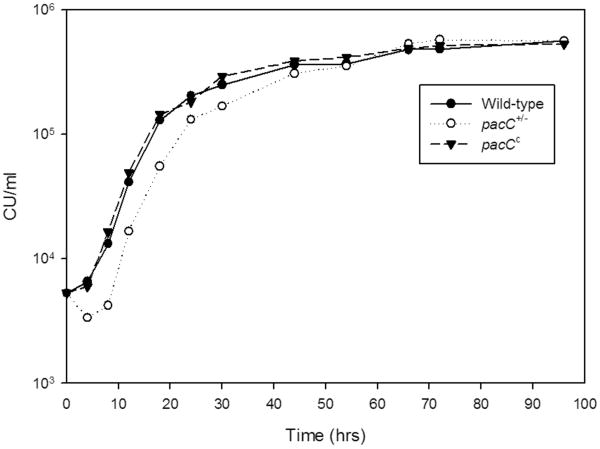
F. oxysporum strains demonstrated similar lag, log-growth, and plateau phases in vitro (Fig. 1). The doubling times among fungal strains were similar at pH 7.3 and at pH 8.0 (Table 1). At pH 6.0, the wild-type strain doubled at a mean of 4.4 ± 0.2 hrs, and generation times of the pacC- loss-of-function strain and of the dominant-activating strain pacCc9 averaged 3.3 ± 0.3 hrs and 4.6 ± 0.3 hrs, respectively.
FIGURE 1.
Estimation of in vitro growth kinetics of F. oxysporum strains. Triplicate samples of each strain were inoculated into liquid M199 media at pH 7.3. Mean fungal concentrations (culturable units/ml) are plotted with standard deviations.
TABLE 1.
Estimation of Generation Time (hrs) of F. oxysporum Strains at Different pH Conditions
| Strain | Genotype | pH 7.3 | P | pH 8.0 | P |
|---|---|---|---|---|---|
| Wild-type | 4287 | 4.1 ± 0.2 | - | 4.7 ± 0.2 | - |
| pacC+/-12 | pacC- loss-of-function | 3.3 ± 0.5 | 0.09 | 5.0 ± 0.5 | 0.33 |
| pacCc9 | pacCc dominant-activating | 4.7 ± 0.4 | 0.11 | 5.0 ± 0.3 | 0.16 |
A fungal spherule suspension was used as the inoculum. Values expressed as mean ± standard deviation. P value compares growth of each mutant strain to wild-type.
Ex vivo Corneal Virulence
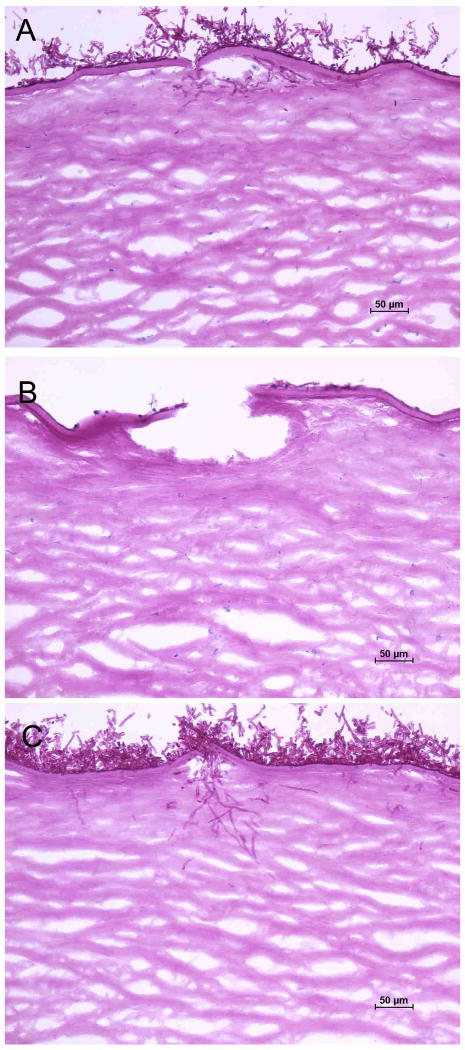
One day after inoculation of F. oxysporum onto human corneas incubated in SHEM buffered to pH 7.3, wild-type fungi adhered to the disrupted epithelial surface and invaded Bowman's layer into the anterior stroma through scarification marks, producing branched, septate hyphae that extended across and between stromal lamellae (Fig. 2). The pacC- loss-of-function mutant, on the other hand, formed a mycelial mat on the corneal surface but produced no fungal hyphae within corneal tissue by one day of incubation. The pacCc dominant-activating strain formed invasive hyphae similar to the wild-type (Table 2).
FIGURE 2.
F. oxysporum invasion into human corneas incubated at pH 7.3 (periodic acid-Schiff; original magnification, ×200). A, Wild-type fungi invaded the superficial corneal surface within one day. B, The wild-type strain progressed into the deeper corneal stroma by the third day following inoculation. C, The loss-of-function mutant pacC+/-12 produced no fungal hyphae within corneal tissue on day 1. D, On the third day after inoculation the F. oxysporum pacC+/-12 mutant produced hyphae that invaded into the anterior stroma. E, The dominant-activating strain pacCc9 formed hyphae in the superficial stroma on day 1. F, The pacCc9 strain proliferated on the corneal surface and continued to extend into the corneal stroma on day 3.
TABLE 2.
Maximal Penetration of F. oxysporum Strains into Explanted Human Corneas under Physiological Conditions
| Day | Wild-Type | pacC+/-12 | pacCc9 | |||||
|---|---|---|---|---|---|---|---|---|
| n | Penetration (%) | n | Penetration (%) | P | n | Penetration (%) | P | |
| 1 | 3 | 9.1 ± 0.9 | 3 | 0 | <0.0001 | 3 | 8.9 ± 1.9 | 0.87 |
| 3 | 3 | 18.3 ± 1.0 | 3 | 7.5 ± 3.8 | 0.0003 | 3 | 27.8 ± 6.2 | 0.10 |
| 5 | 3 | 65.0 ± 9.1 | 3 | 65.0 ± 8.9 | 1.00 | 3 | 74.9 ± 2.5 | 0.14 |
Values expressed as mean ± standard deviation for pools of 3 corneas per group. P value compares corneal invasion of each mutant strain to wild-type.
By the third day following inoculation, wild-type F. oxysporum continued to penetrate into the corneal stroma, to an average maximal depth twice that at day 1 (Table 2). However, the pacC- loss-of-function mutant produced scanty hyphae, few of which invaded into the anterior stroma. In contrast, the dominant-activating pacCc strain showed progressive invasion which exceeded that of the wild-type isolate (Fig. 2). While no significant differences in hyphal penetration were found on the fifth day following fungal inoculation, interpretation was limited by mycelial overgrowth of some sections by the dominant-activating strain.
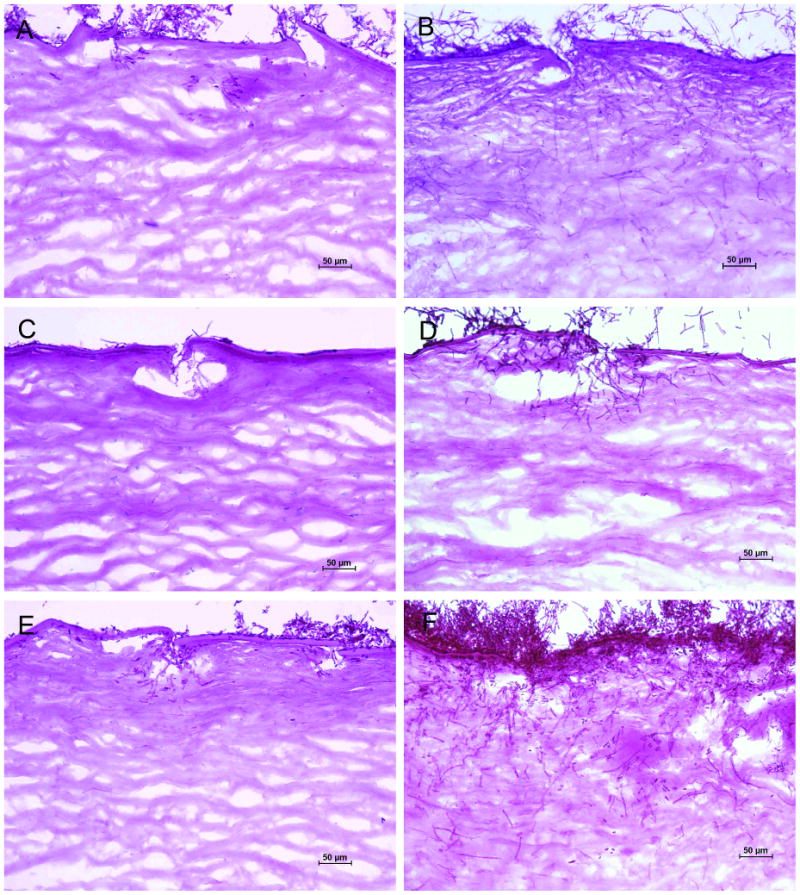
In SHEM buffered to pH 8.0, wild-type and dominant-activating strains showed similar initial invasiveness, averaging maximal penetration of 4.4% ± 1.5% and 6.7% ± 1.5%, respectively, after one day postinoculation. At day 1 postinoculation maximal penetration percentages of wild-type and dominant-activating strains (Fig. 3) were significantly less at pH 8.0 compared to those at pH 7.3 (P=0.005 and P=0.005). The loss-of-function mutant pacC+/-12 did not produce fungal hyphae in corneal tissue at day 1. In vitro fungal kinetics are not necessarily associated with tissue pathogenicity during infection, and the ex vivo data did not correlate with projections from the in vitro growth curves.
FIGURE 3.
F. oxysporum infection at pH 8.0 on first day following inoculation (periodic acid-Schiff; original magnification, ×200). A, The wild-type strain invaded into the anterior cornea. B, The oss-of-function mutant pacC+/-12 did not produce invasive fungal hyphae. C, The dominant-activating strain pacCc9 invaded the superficial cornea similar to the wild-type.
Discussion
Fusarium is a leading cause of fungal keratitis that can complicate corneal injury and contact lens wear.3, 5, 26 Fusarium solani, F. oxysporum, F. verticillioides, and related species are phytopathogens dispersed throughout the environment.27, 28 These filamentous fungi have several cellular and molecular attributes that allow colonization and infection of plants and animals.29 This investigation examined a fusarial virulence pathway associated with fungal attachment and penetration into host tissues.
We modified an ex vivo system to study the pathogenic mechanisms of hyphal invasion into human corneas.24 Explanted donor corneas, lacking ocular surface defenses and systemic immunity, were used to analyze the relative pathogenicity of fungi for the corneal stroma. Similar to other alternative models,30 tissue culture of eye-bank eyes provides a method to selectively examine the initial events during microbial adherence and invasion of ocular tissue.
We used F. oxysporum, a plant pathogen and occasional human corneal isolate that is moderately less virulent than F. solani.31-33 Selected genetic mutants of pathogenic fungi aid in identifying specific virulence factors required for mycotic infection.34, 35 Therefore, applying molecular disruption techniques, a single gene of F. oxysporum was altered that encodes a sequence-specific protein potentially involved in disease pathogenicity.36
We found that deletion of the pacC gene retarded invasiveness of F. oxysporum, indicating that the transcription factor PacC may be involved in filamentous fungal survival and growth at the ocular surface. Slightly more fungal penetration of the cornea was observed with a dominant-activating PacC strain, but this was not statistically significant. Our findings are consistent with studies demonstrating that PacC is needed to establish invasive fusariosis or aspergillosis.22, 23 PacC-regulated genes appear to be involved in fungal adaptation to the corneal microenvironment and in filamentous growth into stromal tissue.
PacC is part of an intracellular signaling system in several filamentous fungi that responds to ambient pH.37 Synthesized as an inactive polypeptide, PacC is activated at neutral to alkaline pH through enzymatic proteolysis.38 Zinc-finger DNA-binding domains then regulate the expression of several fungal genes, including phosphatases and proteases.9 The PacC-regulated phenotype could thereby affect adaptive filamentous growth at the ocular surface and facilitate opportunistic fungal invasion into the traumatized cornea.
The cornea is susceptible to diverse fungi, and experimental models provide ways to study the virulence of yeasts and filamentous moulds. The emergence of fungal genomics offers additional opportunities for examining the multifactorial processes of fungal growth and pathogenicity in oculomycoses.39 Molecular mechanisms of tissue invasion by Fusarium spp., such as the PacC pathway, present potential targets for improved control of fungal eye disease.
Acknowledgments
Supported by grants from the National Eye Institute (EY02520), the Research to Prevention Blindness, and the Sid W. Richardson Foundation. The funding organizations had no role in the design or conduct of this research.
Footnotes
The authors have no proprietary or commercial interest in any of the material discussed in this article.
References
- 1.Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dignani MC, Anaissie E. Human fusariosis. Clin Microbiol Infect. 2004;10(Suppl 1):67–75. doi: 10.1111/j.1470-9465.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 3.Dóczi I, Gyetvai T, Kredics L, et al. Involvement of Fusarium spp. in fungal keratitis. Clin Microbiol Infect. 2004;10:773–776. doi: 10.1111/j.1469-0691.2004.00909.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu TG, Keasler VV, Mitchell BM, et al. Immunosuppression affects the severity of experimental Fusarium solani keratitis. J Infect Dis. 2004;190:192–198. doi: 10.1086/421300. [DOI] [PubMed] [Google Scholar]
- 5.Ahearn DG, Zhang S, Stulting RD, et al. Fusarium keratitis and contact lens wear: facts and speculations. Med Mycol. 2008;46:397–410. doi: 10.1080/13693780801961352. [DOI] [PubMed] [Google Scholar]
- 6.van Burik JA, Magee PT. Aspects of fungal pathogenesis in humans. Annu Rev Microbiol. 2001;55:743–772. doi: 10.1146/annurev.micro.55.1.743. [DOI] [PubMed] [Google Scholar]
- 7.Lengeler KB, Davidson RC, D'Souza C, et al. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 2000;64:746–785. doi: 10.1128/mmbr.64.4.746-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson BE, Wilhelmus KR, Mitchell BM. Genetically regulated filamentation contributes to Candida albicans virulence during corneal infection. Microb Pathog. 2007;42:88–93. doi: 10.1016/j.micpath.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peñalva MA, Tilburn J, Bignell E, et al. Ambient pH gene regulation in fungi: making connections. Trends Microbiol. 2008;16:291–300. doi: 10.1016/j.tim.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt EK, Kempken R, Kück U. Functional analysis of promoter sequences of cephalosporin C biosynthesis genes from Acremonium chrysogenum: specific DNA-protein interactions and characterization of the transcription factor PACC. Mol Genet Genomics. 2001;265:508–518. doi: 10.1007/s004380000439. [DOI] [PubMed] [Google Scholar]
- 11.Tilburn J, Sarkar S, Widdick DA, et al. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 1995;14:779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacCabe AP, Van den Hombergh JP, Tilburn J, et al. Identification, cloning and analysis of the Aspergillus niger gene pacC, a wide domain regulatory gene responsive to ambient pH. Mol Gen Genet. 1996;250:367–374. doi: 10.1007/BF02174395. [DOI] [PubMed] [Google Scholar]
- 13.You BJ, Choquer M, Chung KR. The Colletotrichum acutatum gene encoding a putative pH-responsive transcription regulator is a key virulence determinant during fungal pathogenesis on citrus. Mol Plant Microbe Interact. 2007;20:1149–1160. doi: 10.1094/MPMI-20-9-1149. [DOI] [PubMed] [Google Scholar]
- 14.Caracuel Z, Roncero MIG, Espeso EA, et al. The pH signalling transcription factor PacC controls virulence in the plant pathogen Fusarium oxysporum. Mol Microbiol. 2003;48:765–779. doi: 10.1046/j.1365-2958.2003.03465.x. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty JE, Pirttilä AM, Bluhm BH, et al. PAC1, a pH-regulatory gene from Fusarium verticillioides. Appl Environ Microbiol. 2003;69:5222–5227. doi: 10.1128/AEM.69.9.5222-5227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suárez T, Peñalva MA. Characterization of a Penicillium chrysogenum gene encoding a PacC transcription factor and its binding sites in the divergent pcbAB-pcbC promoter of the penicillin biosynthetic cluster. Mol Microbiol. 1996;20:529–540. doi: 10.1046/j.1365-2958.1996.5421065.x. [DOI] [PubMed] [Google Scholar]
- 17.Rollins JA. The Sclerotinia sclerotiorum pac1 gene is required for sclerotial development and virulence. Mol Plant Microbe Interact. 2003;16:785–795. doi: 10.1094/MPMI.2003.16.9.785. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Mateos MA, Delgado-Jarana J, Codón AC, et al. pH and Pac1 control development and antifungal activity in Trichoderma harzianum. Fungal Genet Biol. 2007;44:1355–1367. doi: 10.1016/j.fgb.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol. 2009;12:1–6. doi: 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Martin SJ, Bruno VM, et al. Candida albicans Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot Cell. 2004;3:741–751. doi: 10.1128/EC.3.3.741-751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell BM, Wu TG, Jackson BE, et al. Candida albicans strain-dependent virulence and Rim13p-mediated filamentation in experimental keratomycosis. Invest Ophthalmol Vis Sci. 2007;48:774–780. doi: 10.1167/iovs.06-0793. [DOI] [PubMed] [Google Scholar]
- 22.Ortoneda M, Guarro J, Madrid MP, et al. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect Immun. 2004;72:1760–1766. doi: 10.1128/IAI.72.3.1760-1766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bignell E, Negrete-Urtasun S, Calcagno AM, et al. The Aspergillus pH-responsive transcription factor PacC regulates virulence. Mol Microbiol. 2005;55:1072–1084. doi: 10.1111/j.1365-2958.2004.04472.x. [DOI] [PubMed] [Google Scholar]
- 24.Vermeltfoort PBJ, van Kooten TG, Bruinsma GM, et al. Bacterial transmission from contact lenses to porcine corneas: an ex vivo study. Invest Ophthalmol Vis Sci. 2005;46:2042–2046. doi: 10.1167/iovs.04-1401. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X, Mitchell BM, Wilhelmus KR. Expression of matrix metalloproteinases during experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2009;50:737–742. doi: 10.1167/iovs.08-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zapater RC, Arrechea A. Mycotic keratitis by Fusarium: a review and report of two cases. Ophthalmologica. 1975;170:1–12. doi: 10.1159/000307154. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, O'Donnell K, Sutton DA, et al. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehl HL, Epstein L. Fusarium solani species complex isolates conspecific with Fusarium solani f. sp. cucurbitae race 2 from naturally infected human and plant tissue and environmental sources are equally virulent on plants, grow at 37°C and are interfertile. Environ Microbiol. 2007;9:2189–2199. doi: 10.1111/j.1462-2920.2007.01333.x. [DOI] [PubMed] [Google Scholar]
- 29.Roilides E, Dotis J, Katragkou A. Fusarium and Scedosporium: emerging fungal pathogens. In: Kavanagh K, editor. New Insights in Medical Mycology. Dordrecht: Springer; 2007. pp. 267–286. [Google Scholar]
- 30.Gow NAR, Knox Y, Munro CA, et al. Infection of chick chorioallantoic membrane (CAM) as a model for invasive hyphal growth and pathogenesis of Candida albicans. Med Mycol. 2003;41:331–338. doi: 10.1080/13693780310001600859. [DOI] [PubMed] [Google Scholar]
- 31.Michielse CB, Rep M. Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol. 2009;10:311–324. doi: 10.1111/j.1364-3703.2009.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oechsler RA, Feilmeier MR, Ledee DR, et al. Utility of molecular sequence analysis of the ITS rRNA region for identification of Fusarium spp. from ocular sources. Invest Ophthalmol Vis Sci. 2009;50:2230–2236. doi: 10.1167/iovs.08-2757. [DOI] [PubMed] [Google Scholar]
- 33.Mayayo E, Pujol I, Guarro J. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J Med Microbiol. 1999;48:363–366. doi: 10.1099/00222615-48-4-363. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz MC. Genomic approaches to fungal pathogenicity. Curr Opin Microbiol. 2002;5:372–378. doi: 10.1016/s1369-5274(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 35.Alonso-Monge R, Navarro-García F, Roman E, et al. Strategies for the identification of virulence determinants in human pathogenic fungi. Curr Genet. 2003;42:301–312. doi: 10.1007/s00294-002-0364-1. [DOI] [PubMed] [Google Scholar]
- 36.Prados-Rosales RC, Serena C, Delgado-Jarana J, et al. Distinct signalling pathways coordinately contribute to virulence of Fusarium oxysporum on mammalian hosts. Microbes Infect. 2006;8:2825–2831. doi: 10.1016/j.micinf.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Peñalva MA, Arst HN., Jr Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu Rev Microbiol. 2004;58:425–451. doi: 10.1146/annurev.micro.58.030603.123715. [DOI] [PubMed] [Google Scholar]
- 38.Kurzai O, El Barkani A, Mühlschlegel FA. Adaptation of fungi to alterations in ambient pH. In: Calderone RA, Cihlar RL, editors. Fungal Pathogenesis: Principles and Clinical Applications. New York: Marcel Dekker; 2002. pp. 139–160. [Google Scholar]
- 39.Xu JR, Peng YL, Dickman MB, et al. The dawn of fungal pathogen genomics. Annu Rev Phytopathol. 2006;44:337–366. doi: 10.1146/annurev.phyto.44.070505.143412. [DOI] [PubMed] [Google Scholar]