Abstract
Background
Improved kidney preservation methods are needed to reduce ischemia-reperfusion (IR) injury in kidney allografts. Lifor is an artificial preservation solution comprised of nutrients, growth factors, and a non-protein oxygen and nutrient carrier. The current study compared the effectiveness of Lifor to University of Wisconsin solution (UW) in protecting rat kidneys from warm IR and cold storage injury.
Materials and Methods
In a warm IR model, rat kidneys were perfused in situ with either saline, UW, or Lifor for 45 minutes. Renal function and histology were assessed 24 hours later. In a cold IR model, kidney slices were cold stored in saline, UW, or Lifor at 4°C. Kidney injury was assessed by the release of lactate dehydrogenase (LDH) and immunoblot analysis for cleaved caspase-3.
Results
Lifor perfusion significantly mitigated renal dysfunction and tubular injury at 24 hours compared to saline or UW. Lifor and UW prevented LDH release in hypoxic kidney slices in vitro, however activation of caspase-3 following hypoxia-reoxygenation was attenuated only with Lifor. Cold storage with Lifor or UW significantly decreased LDH release from kidney slices or normal rat kidney cells in comparison to storage in saline or culture media. After 24 hours of cold storage there was a significant decrease in cleaved caspase-3 in Lifor stored slices compared that seen following cold storage in saline or UW solution.
Conclusions
Lifor solution mitigates both warm and cold renal IR and appears to provide greater protection from apoptosis as compared to UW solution.
Keywords: renal ischemia-reperfusion, cold preservation, UW solution, Lifor, apoptosis
INTRODUCTION
Renal ischemia-reperfusion (IR) injury contributes to the development of delayed graft function (DGF) following kidney transplantation. Despite advances in cold storage and organ transport, DGF remains an important complication of kidney transplantation and is a major risk factor for acute rejection and long term kidney allograft dysfunction.[1, 2] Organ preservation solutions play an important role in the prevention of DGF, especially in organs exposed to prolonged cold ischemia or from expanded criteria donors.[3–5]
University of Wisconsin (UW) solution is the most commonly used cold storage solution for kidney transplantation but other solutions are also used.[6, 7] The effectiveness of these solutions is variable and DGF still occurs in a large proportion of kidney transplants.[8] The limited effectiveness of current preservation solutions may be due to inadequate protection from disparate pathologic mechanisms activated during periods of warm ischemia, cold storage, and rewarming.[9] For example, Salahudeen et. al. demonstrated that cold storage primarily induces necrosis of renal tubular epithelial cells whereas rewarming results in an increase in apoptotic cell death which could be mitigated by antioxidants.[10] This suggests that there is a need for preservation solutions that limits both warm IR injury as well as cold storage injury.
Lifor is a new organ preservation solution comprised of nutrients, growth factors, and a non-protein oxygen and nutrient carrier.[11, 12] Recently, Lifor was shown to preserve guinea pig hearts in a coronary recirculation system for up to 20 hours and to improve renal perfusion in a porcine model of room temperature perfusion.[12, 13] However, the effect of Lifor on renal function and renal tubular epithelial survival following either warm IR injury or cold storage is unknown. Therefore, the current study aimed to compare the effectiveness of Lifor to UW solution in protecting rat kidneys from warm IR and cold storage injury and to determine the effect of Lifor on renal tubular cell apoptosis.
MATERIALS AND METHODS
Warm renal ischemia-reperfusion model
This set of experiments was performed on 24 male Lewis rats weighing 220–240 grams that were purchased from Taconic Farms (Germantown, NY). The rats were housed in the Animal Care Facility at the Medical College of Wisconsin which is approved by the American Association for the Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin and carried out according to the 'Principles of Laboratory Animal Care' published by the US National Institutes of Health.
Four groups of rats (n=6/group) were used in this protocol. The renal failure control group underwent bilateral nephrectomy. The remaining groups underwent warm renal IR by in situ perfusion of the left kidney with either sterile 0.9% saline, UW (Duramed Pharmaceuticals, Inc, Cincinnati, OH), or Lifor (Lifeblood Medical, Inc, Adelphia, NJ). The composition of Lifor and UW solution have been previously described.[11]
Fasted male Lewis rats were anesthetized with isoflurane and transferred to a heated sterile surgical table to maintain body temperature at 37°C. A right nephrectomy was performed. Next, a 4-0 silk tie was placed to occlude the left renal artery and a micro-clamp was placed across the left renal vein. Then, a 27g needle was placed in the left renal artery and the kidney was then infused with sterile 0.9% saline at a flow rate of 1 ml/min. A 25g needle attached to an outflow catheter filled with heparinized saline (100 U/ml) was also inserted into the renal vein to allow drainage of the perfusion solution. These procedures were all performed in <1 minute. The infusion solution was switched to one of the test agents and the left kidney was then perfused in situ at 1 ml/min with either saline, UW, or Lifor for 45 minutes. At the end of the perfusion period the kidney was flushed with saline for 3 minutes. The needles in the artery and vein were removed and the holes sealed with VetBond adhesive and the micro-clamp and tie on the renal artery were removed. Reperfusion was confirmed by visual inspection. The abdominal incision was closed and the rats were allowed to recover for 24 hours. Rats were then euthanized with sodium pentobarbital (100 mg/kg, i.p.) and blood was collected from the aorta for measurement of plasma creatinine concentration using the Jaffe reaction (Ace Clinical Chemistry System, Alfa Wassermann, West Caldwell, NJ) and the left kidney was collected for histological analysis.
Histologic analysis of renal injury
Kidneys were fixed in 10% formalin and paraffin sections (3 μm) were prepared and stained with hematoxylin and eosin. The intensity of eosin autofluorescence is increased in necrotic tissue.[14] Therefore, eosin autofluorescence was used to assess the degree of renal tubular necrosis and cast formation as previously described by our group.[15, 16] The hematoxylin and eosin sections were examined using a Nikon 55i (Nikon Instruments Inc., Melville, NY) epifluorescent microscope equipped with a 540-nm excitation filter and a 590-nm emission filter. Five randomly chosen outer medullary fields were photographed for each specimen using a digital color camera (100× total magnification) and following background thresholding, the area containing autofluorescent cast material and necrotic tubular epithelium was quantified using Nis-Elements image analysis software (version 3.03, Nikon Instruments Inc., Melville, NY).
Apoptosis was detected by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay with ApopTag technology (Chemicon International) according to manufacturer's instructions. Apoptosis was quantified by counting positive cells per 40× field as previously described.[15] A minimum of twenty fields per slide and three slides per group were counted by this method.
Hypoxia-reoxygenation in tissue slices
This set of experiments was performed on 30 male Sprague-Dawley (SD) rats weighing 220–240 grams that were purchased from Taconic Farms (Germantown, NY). Rats were anesthetized with ketamine and sodium pentobarbital and a midline abdominal incision was made to expose the kidneys. The kidneys were then decapsulated, harvested, and thin 200 um kidney slices were prepared with a Stadie–Riggs microtome.[17] Kidney slices were rinsed in ice-cold 0.9% saline and then placed in individual wells and covered in 2 ml of either saline, UW, or Lifor. The tissue slices were then placed in a hypoxic incubator with 1% O2 and 5% CO2 at 37°C for 30 minutes, 1 hour, or 2 hours. An oxygen sensor was used to ensure that hypoxia was maintained throughout the duration of the experiment. Following the hypoxic interval, the tissue slices were returned to an incubator containing 95% air and 5% CO2 for 2 hours at 37°C. After this treatment the media and tissue were harvested for measurement of LDH levels.
Cold storage of tissue slices
Kidney slices were excised and rinsed in ice cold 0.9% saline immediately after cutting into 200 um sections. The slices were then placed in individual wells and covered in 2 ml of either saline, UW, or Lifor, and stored at 4°C for 2 hours, 4 hours, 8 hours or 24 hours. The media and tissue were harvested for analysis at the end of the various time points.
Cold storage of normal rat kidney (NRK) cells
Normal rat kidney-52E (NRK) cells were obtained from ATCC (Manassas, VA). NRK cells were grown in D-MEM containing 5% FBS, at 37°C in a 5% CO2, 95% air-humidified incubator. The experiments were carried out 24 hours post confluence. The cells were washed with dPBS and treated with 2 ml of serum free D-MEM, UW, or Lifor and stored at 4°C for 4, 8, 16, or 24 hours. The media was collected for measurement of lactate dehydrogenase (LDH) release. The cells were then harvested at various time points, lysed in RIPA lysis buffer with the addition of protease and phosphatase inhibitors and stored at −80°C prior to Western blot analysis for cleaved caspase-3.
Lactate Dehydrogenase Assay
LDH levels were measured as an indicator of cell toxicity in the tissue slice and cell culture experiments.[18, 19] LDH was detected using a commercially available kit (Diagnostic Chemicals, Oxford, CT). This assay is based on the conversion of L-lactate and NAD to pyruvate and NADH by the released LDH. The rate of increase in absorbance at 340 nm due to formation of NADH is directly proportional to the LDH levels. LDH levels were corrected to the total protein concentration of the tissue or cell lysate obtained from the same well.
Western blot analysis
Anti-cleaved caspase 3 antibody (Cat #9661) was obtained from Cell Signaling Technology (Danvers, MA). Anti-β-actin antibody (Cat. #A5441) was obtained from Sigma-Aldrich (St. Louis, MO). Kidney tissue was homogenized in 1× RIPA buffer (Millipore, Billerica, MA) with 2% Halt™ Protease Inhibitors and 1% Halt™ Phosphatase Inhibitors (Pierce Biotechnology). The lysates were spun down at 11,000g for 15 minutes and the protein concentrations of the supernatant were measured using the Bradford Method (Bio-Rad, Hercules, CA). 80 μg of protein was size-separated on a 4–12% NuPAGE® Bis-Tris Gradient Gel (Invitrogen), and transferred to a supported nitrocellulose membrane (Bio-Rad, Hercules, CA). The membranes were blocked in Odyssey Blocking Buffer (Licor, Lincoln, NE) and blotted with the primary antibodies (1:500 for cleaved caspase 3; 1:5000 for β-actin) overnight at 4° C. After subsequent washing steps with TBS-0.1% Tween-20, the membranes were incubated with HRP coupled secondary anti-rabbit (1:2000) (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-mouse (1:5000) (Bio-Rad, Hercules, CA) antibodies at room temperature for 1 hour. The bands were detected on film following incubation in Amersham ECL Plus solution (GE Healthcare, Piscataway, NJ). Band densities were determined using the NIH ImageJ software and normalized to corresponding β-actin signal.
Statistical analysis
Mean values ± SE are presented. The significance of differences in mean values between groups was analyzed using a one-way or repeated measures ANOVA followed by a Dunn's post-hoc test. A P value < 0.05 was considered to be statistically significant.
RESULTS
Effect of Lifor on severity of warm renal IR injury
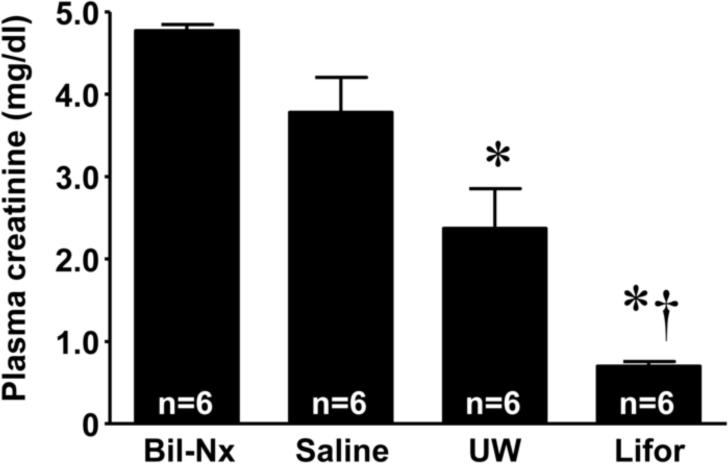
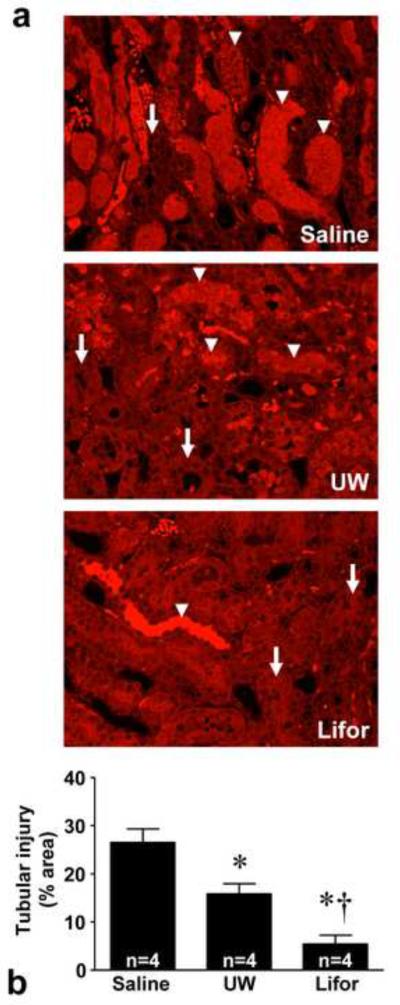
Following 45 minutes of warm in situ perfusion with saline, mean plasma creatinine values were 3.8 ± 0.4 mg/dl (Figure 1) and did not significantly differ from the value seen in the bilateral nephrectomy group (plasma creatinine 4.8 ± 0.1 mg/dl). Warm in situ perfusion with either Lifor or UW solution significantly mitigated renal dysfunction at 24 hours compared to saline (plasma creatinine 0.7 ± 0.1 and 2.4 ± 0.5, respectively), with Lifor being significantly more protective than UW (P <0.05). Histopathological evaluation (Figure 2) confirmed that the degree of tubular necrosis and cast formation was significantly (P < 0.05) reduced in the kidneys perfused with Lifor as compared to kidneys perfused with either UW solution or saline.
Figure 1. Effect of Lifor solution on renal dysfunction 24 hours after warm renal IR injury.
Male Lewis rats underwent right nephrectomy followed by in situ perfusion of the left kidney with saline, UW, or Lifor at 1 ml/min for 45 minutes. Bilateral nephrectomy (Bil-Nx) served as an absolute renal failure control group. Administration of Lifor significantly mitigated renal dysfunction compared to perfusion with saline or UW solution. Mean values ± SE are presented. * P < 0.05 vs. saline group. † P < 0.05 vs. UW group.
Figure 2. Renal histology 24 hours after warm renal IR injury.
(a) Fluorescence microscopy of representative H&E stained cross-sections of the renal outer medulla are presented. Necrotic tubules (arrowheads) exhibited marked autofluorescence compared to intact tubules (arrows). Original magnification, × 100. (b) Tubular injury scores 24 hours after warm renal IR injury. Administration of Lifor resulted in less severe renal injury compared to perfusion with saline or UW solution. Mean values ± SE are presented. * P < 0.05 vs. saline treated group. † P < 0.05 vs. UW.
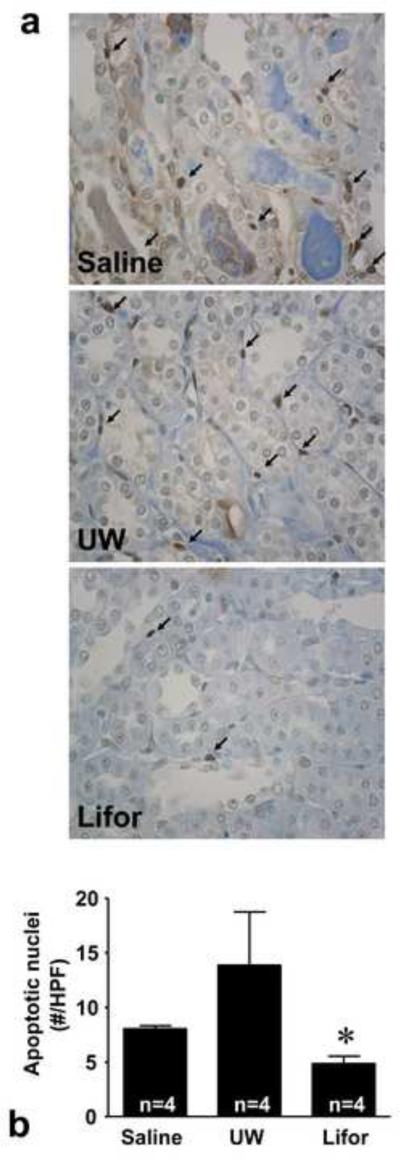
Apoptosis was measured by TUNEL staining in histological sections from saline, UW, and Lifor perfused rats. A large number of apoptotic nuclei in renal tubular epithelial cells and peri-tubular interstitial cells were detected in saline and UW perfused kidneys (Figure 3). Compared to UW, significantly fewer apoptotic nuclei were detected in rat kidneys perfused with Lifor (14.7 ± 4.5 and 4.7 ± 0.6 nuclei/HPF, respectively, P < 0.05).
Figure 3. Effect of Lifor on apoptosis 24 hours after warm renal IR injury.
Apoptosis was assessed by TUNEL staining. (a) Representative photomicrographs of TUNEL staining depicting apoptotic nuclei (arrows) in saline, UW, and Lifor perfused kidneys. Apoptotic nuclei were detected in renal tubular epithelial cells and peri-tubular interstitial cells. (b) The number of apoptotic nuclei was significantly less in rat kidneys perfused with Lifor compared to saline or UW solution. N=4 animals/group. At least 10 fields per section and 2 sections per animal were counted. Mean values ± SE are presented. * P < 0.05 vs. saline group.
Effect of Lifor on severity of ex vivo hypoxia-reoxygenation injury
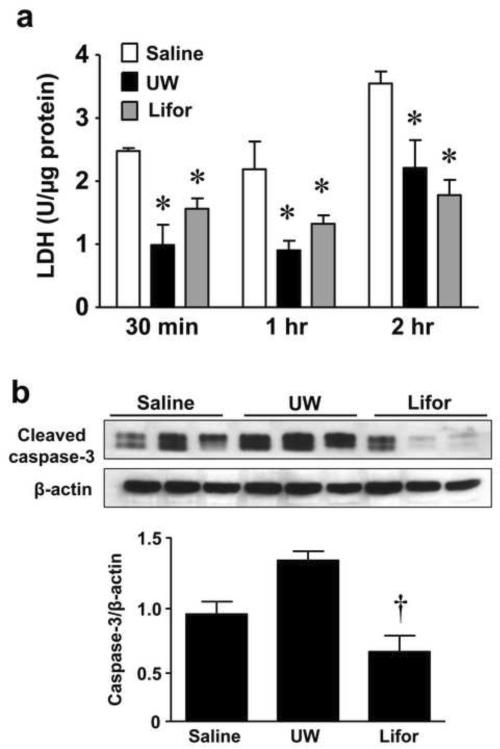
UW solution has been associated with microcirculatory abnormalities in models of organ transplantation.[20, 21] Therefore, to exclude the possibility that the effectiveness of Lifor was solely dependent on improved microcirculatory function, we explored the protective effect of Lifor solution in an ex vivo hypoxia-reoxygenation model. Rat kidney slices were incubated with either saline, UW, or Lifor in a hypoxic incubator with 1% O2, 5% CO2 at 37°C for 30 minutes, 1 hour, or 2 hours. The tissue slices were then reoxygenated in a 37°C, 5% CO2 incubator for 2 hours. Compared with kidney slices incubated in saline, incubation with either UW or Lifor significantly (P < 0.05) mitigated injury to the renal tissue in vitro as detected by LDH release at all time points (Figure 4a).
Figure 4. Effect of Lifor on ex vivo warm renal IR injury.
Kidney slices (n=3 at each timepoint) from Sprague-Dawley rats were incubated with either saline, UW, or Lifor and placed in a hypoxic incubator with 1% O2, 5% CO2 at 37°C for 30 minutes, 1 hour, or 2 hours. The tissue slices were then reoxygenated in a 37°C, 5% CO2 incubator for 2 hours. (a) Incubation of kidney slices with either UW or Lifor significantly mitigated LDH release compared to incubation with saline. (b) Incubation of kidney slices in Lifor significantly attenuated activation of caspase-3 compared to UW following 2 hours hypoxia and 2 hours reoxygenation. Mean values ± SE are presented. * P < 0.05 vs. saline group. † P < 0.05 vs. UW group.
In order to determine whether Lifor acted by reducing apoptosis following hypoxia-reoxygenation, we compared the activation of caspase-3 in tissue slices incubated in the presence of saline, UW and Lifor kidney slices. Western blot analysis revealed a strong 17 kDa cleaved caspase-3 band in both saline and UW treated slices after 2 hours hypoxia/2 hours reoxygenation. Incubation of kidney slices in Lifor significantly (P < 0.05) attenuated activation of caspase-3 compared to UW solution (Figure 4b).
Effect of Lifor on severity of ex vivo cold preservation injury
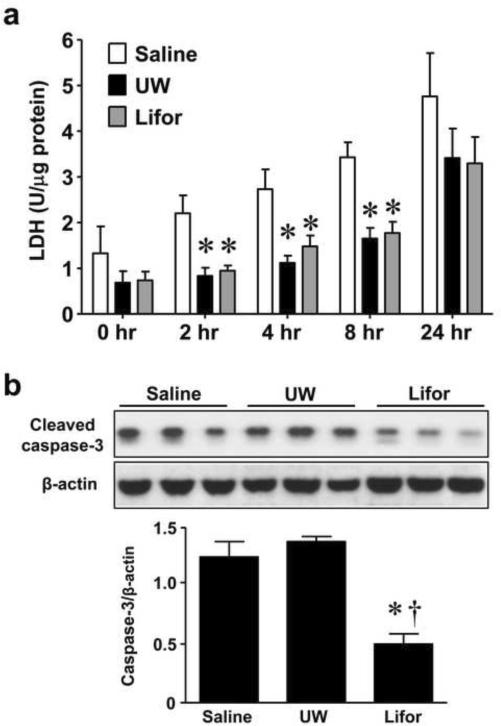
Kidney slices from SD rats were stored for 2, 4, 8, 16 or 24 hours in saline, UW or Lifor solutions at 4° C. Compared to saline, both UW and Lifor significantly (P < 0.05) mitigated kidney tissue injury as measured by LDH release following storage of kidney slices at 4°C for 2 hours, 4 hours, and 8 hours (Figure 5a).
Figure 5. Effect of Lifor on cold storage injury in kidney slices.
Kidney slices (n=3 at each timepoint) from Sprague-Dawley rats were cold stored at 4°C for 2 hours, 4 hours, 8 hours or 24 hours. Tissue injury was measured by LDH release. (a) Storage of kidney slices in either UW or Lifor significantly mitigated LDH release compared to media. Activation of caspase-3 was detected by western blot analysis of cleaved caspase-3. (b) Lifor significantly attenuated activation of caspase-3 in kidney slices after 24 hours of cold storage compared to UW. Mean values ± SE are presented. * P < 0.05 vs. saline group. † P < 0.05 vs. UW group.
Western blot analysis for cleaved caspase-3 was performed to examine the effect of Lifor on apoptosis in cold stored tissue slices. Western blot analysis for cleaved caspase-3 at earlier time points revealed that caspase-3 was activated as early as 2 hours in UW solution but not in Lifor stored slices (data not shown). Cold storage of kidney slices in Lifor significantly (P < 0.05) attenuated activation of caspase-3 compared to both saline or UW after 24 hours (Figure 5b).
Effect of Lifor on in vitro cold storage injury
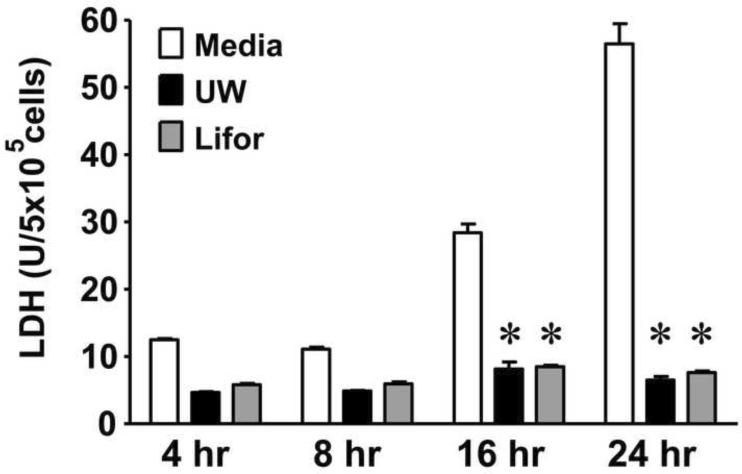
We next examined the effects of Lifor on cold stored renal epithelial cells. The immortalized NRK-52E rat epithelial cell line was chosen since our in vivo and ex vivo studies were performed in the rat. NRK cells were stored at 4°C for 4, 8, 16, or 24 hours in serum free media, UW, or Lifor. LDH release in the media was then examined as an indicator of cytotoxicity. Beginning at 16 hours, there was increase in cytotoxicity as measured by LDH release in media stored cells (Figure 6). UW and Lifor significantly (P < 0.05) mitigated cell injury as measured by LDH release compared to storage in media alone for both 16 and 24 hours (Figure 6).
Figure 6. Effect of Lifor on cold storage injury in NRK cells.
NRK cells were cold stored at 4°C for 4, 8, 16, or 24 hours in serum free D-MEM, UW, or Lifor. N=3 (4, 8 and 16 hours) and N=6 (24 hours). Cell injury was measured by LDH release. Storage of NRK cells in either UW or Lifor significantly mitigated LDH release compared to media. * P < 0.05 vs. media group.
DISCUSSION
The present study evaluated the effectiveness of Lifor, a novel organ preservation solution, in protecting rat kidneys from warm IR injury and cold storage injury. The results indicate that Lifor provided superior protection from warm in vivo renal IR injury compared to UW following 45 minutes of warm ischemia. Although the mechanism by which Lifor provides protection from IR injury is unclear, the results of the ex vivo hypoxia-reoxygenation and cold storage experiments suggest that Lifor may limit activation of apoptotic pathways. Taken together, these findings provide a rationale for further study of Lifor as an alternative preservation solution for storage of kidneys prior to transplantation.
The efficacy of Lifor as an organ preservation solution was assessed in two previous studies in isolated perfused hearts.[11, 12] Stowe et al first evaluated Lifor in a model of low-flow perfused guinea pig hearts. Under ambient air and temperature conditions, perfusion with Lifor for 10 hours provided superior protection against cardiac injury compared to UW perfusion. Whereas UW perfused hearts were non-functional upon reperfusion, the hearts preserved with Lifor exhibited near complete recovery of cardiac function.[11] In a follow up study, Stowe et al perfused isolated guinea pig hearts for 20 hours with Lifor or cardioplegia solution. Hearts perfused with cardioplegia solution were nonfunctional upon reperfusion. Lifor perfusion provided marked protection from cardiac injury as indicated by near complete return of cardiac contractility.[12] Recently, Gage et al evaluated Lifor in a porcine model of room temperature renal perfusion. In comparison to hypothermic or room temperature machine perfusion with UW solution, kidneys perfused with Lifor at room temperature demonstrated significant improvements in output flow and intrarenal resistance.[22] However, the effect of Lifor on glomerular filtration rate and tubular injury was not explored. Notably, in these studies Lifor provided protection from cardiac injury and renal hemodynamic alterations when used at room temperature. In the present study, we provide further evidence of the efficacy of Lifor in warm ischemia and provide new evidence supporting the efficacy of Lifor under cold storage conditions.
Although both Lifor and UW prevented renal injury following hypoxia-reoxygenation of kidney slices, Lifor provided superior protection from renal dysfunction and renal tubular injury in the warm in vivo IR model. This discrepancy could be due to differences in the composition of Lifor and UW solutions that impact the post-ischemic microcirculation in the in vivo model. Lifor has been reported to contain cellular nutrients, buffers, salts, and lipid nanoparticles, but the proprietary nature of Lifor solution precludes a detailed comparison to UW.[11, 12] However, there are several reported differences between UW and Lifor that may affect post-ischemic microvascular function. The K+ content of UW (94 ± 2 mM) is substantially higher than that of Lifor (15.8 ± 0.4mM).[11] High K+ solutions are used to maintain intracellular ion balance during organ preservation but the high K+ concentrations can subsequently impair post-ischemic perfusion by promoting vasoconstriction.[3] Additionally, UW solution is highly viscous in comparison to Lifor and has been associated with red blood cell aggregation and microcirculatory abnormalities in models of organ transplantation.[20, 21] Taken together, these differences between Lifor and UW may explain the superior protection of Lifor in the in vivo model.
We performed additional experiments to determine if Lifor has a direct effect on survival of renal tissue and tubular epithelial cells following hypoxic injury in vitro. The results of these experiments indicate that Lifor opposes activation of apoptotic pathways independent of an effect on the microcirculation. Renal tubular epithelial cell apoptosis contributes to renal dysfunction following kidney transplantation.[23, 24] In a mouse model of cold storage injury, Jani et al demonstrated that renal tubular apoptosis, rather than necrosis, was the dominant form of tubular pathology. Following 48 hours of cold storage in UW solution, caspase-3 activity and tubular apoptosis was markedly increased. However, addition of a pan-caspase inhibitor to the storage solution markedly reduced tubular injury.[25] Several studies suggest that the activation of apoptosis in transplanted kidneys is dependent on storage conditions and temperature. Burns et al found that post-reperfusion kidneys from deceased donors had significantly more apoptosis compared to living donor transplants. However, kidneys from living donors had significantly more apoptosis than kidneys from deceased donors in the pre-perfusion period indicating that longer warm ischemia times might enhance tubular apoptosis.[26] Salahudeen et. al. showed that cold storage primarily induces necrotic cell death but following rewarming there is an increase in apoptosis.[10] In the current study, both Lifor and UW mitigated cytotoxicity, as measured by a reduction in LDH release, in cold stored kidney tissue and NRK cells. However, Lifor provided superior protection from apoptosis as measured by caspase-3 activation under both warm ischemic and cold storage conditions. Further studies are required to determine whether inhibition of caspase-3 activation and apoptosis by Lifor improves renal function following cold storage.
In summary, Lifor solution mitigates both warm renal IR and cold storage injury and may provide superior protection from apoptosis compared to UW. This study provides further evidence of the efficacy of Lifor under warm ischemic conditions and is the first study to demonstrate a potential benefit of Lifor under cold storage conditions. This study provides additional rationale for further exploration of Lifor as a preservation solution in kidney and solid organ transplantation.
ACKNOWLEDGEMENTS
This research was funded in part by grants HL 036279 and HL 029587 from the NIH (RJR), a grant from the American Heart Association (VN), a grant from the Clinical Translational Science Institute at the Medical College of Wisconsin (KRR), and a contract with Lifeblood Medical, Inc (Adelphia, NJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boom H, Mallat MJK, De Fijter JW, Zwinderman AH, Paul LC. Delayed graft function influences renal function, but not survival. Kidney Int. 2000;58:859–66. doi: 10.1046/j.1523-1755.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 2.Gerd Rüdiger H, Barbara K, Matthias B, et al. Risk factors for delayed graft function after renal transplantation and their significance for long-term clinical outcome. Transplant International. 2002;15:10–6. doi: 10.1007/s00147-001-0368-7. [DOI] [PubMed] [Google Scholar]
- 3.Hauet T, Eugene M. A new approach in organ preservation: potential role of new polymers. Kidney Int. 2008;74:998–1003. doi: 10.1038/ki.2008.336. [DOI] [PubMed] [Google Scholar]
- 4.Stevens RB, Skorupa JY, Rigley TH, et al. Increased Primary Non-Function in Transplanted Deceased-Donor Kidneys Flushed with Histidine-Tryptophan-Ketoglutarate Solution. American Journal of Transplantation. 2009;9:1055–62. doi: 10.1111/j.1600-6143.2009.02624.x. [DOI] [PubMed] [Google Scholar]
- 5.Fieux F, Losser M-R, Bourgeois E, et al. Kidney retrieval after sudden out of hospital refractory cardiac arrest: a cohort of uncontrolled non heart beating donors. Critical Care. 2009;13:R141. doi: 10.1186/cc8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess. 2009;13:1–180. doi: 10.3310/hta13380. [DOI] [PubMed] [Google Scholar]
- 7.Stewart ZA, Lonze BE, Warren DS, et al. Histidine-Tryptophan-Ketoglutarate (HTK) Is Associated with Reduced Graft Survival of Deceased Donor Kidney Transplants. American Journal of Transplantation. 2009;9:1048–54. doi: 10.1111/j.1600-6143.2008.02545.x. [DOI] [PubMed] [Google Scholar]
- 8.Bronzatto EJM, da Silva Quadros KR, Santos RLS, Alves-Filho G, Mazzali M. Delayed Graft Function in Renal Transplant Recipients: Risk Factors and Impact on 1-Year Graft Function: A Single Center Analysis. Transplantation Proceedings. 2009;41:849–51. doi: 10.1016/j.transproceed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Kosieradzki M, Rowinski W. Ischemia/Reperfusion Injury in Kidney Transplantation: Mechanisms and Prevention. Transplantation Proceedings. 2008;40:3279–88. doi: 10.1016/j.transproceed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Salahudeen AK, Joshi M, Jenkins JK. Apoptosis versus necrosis during cold storage and rewarming of human renal proximal tubular cells. Transplantation. 2001;72:798–804. doi: 10.1097/00007890-200109150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Stowe DF, Camara AKS, Heisner JS, Aldakkak M, Harder DR. Ten-hour preservation of guinea pig isolated hearts perfused at low flow with air-saturated Lifor solution at 26{degrees}C: comparison to ViaSpan solution. Am J Physiol Heart Circ Physiol. 2007;293:H895–901. doi: 10.1152/ajpheart.00149.2007. [DOI] [PubMed] [Google Scholar]
- 12.Stowe DF, Camara AKS, Heisner JS, Aldakkak M, Harder DR. Low-flow Perfusion of Guinea Pig Isolated Hearts With 26°C Air-saturated Lifor Solution for 20 Hours Preserves Function and Metabolism. The Journal of Heart and Lung Transplantation. 2008;27:1008–15. doi: 10.1016/j.healun.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gage F, Leeser DB, Porterfield NK, et al. Room temperature pulsatile perfusion of renal allografts with Lifor compared with hypothermic machine pump solution. Transplant Proc. 2009;41:3571–4. doi: 10.1016/j.transproceed.2009.06.228. [DOI] [PubMed] [Google Scholar]
- 14.von Overbeck J, Saraga P, Gardiol D. An autofluorescence method for the diagnosis of early ischaemic myocardial lesions. A systematic study on 732 autopsies, including 182 cases of sudden death. Virchows Arch A Pathol Anat Histopathol. 1986;409:535–42. doi: 10.1007/BF00705423. [DOI] [PubMed] [Google Scholar]
- 15.Liang HL, Hilton G, Mortensen J, Regner K, Johnson CP, Nilakantan V. MnTMPyP, a cell-permeant SOD mimetic, reduces oxidative stress and apoptosis following renal ischemiareperfusion. Am J Physiol Renal Physiol. 2009;296:F266–76. doi: 10.1152/ajprenal.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regner KR, Zuk A, Van Why SK, et al. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 2009;75:511–7. doi: 10.1038/ki.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadie WC, Riggs BC. Microtome for the preparation of tissue slices for metabolic studies of surviving tissues in vitro. The Journal of Biological Chemistry. 1944;154:687–90. [Google Scholar]
- 18.Chen G, Bridenbaugh EA, Akintola AD, et al. Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Am J Physiol Renal Physiol. 2007;293:F1272–81. doi: 10.1152/ajprenal.00138.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilakantan V, Maenpaa C, Jia G, Roman RJ, Park F. 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol Renal Physiol. 2008;294:F562–70. doi: 10.1152/ajprenal.00387.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreinemachers M-CJM, Doorschodt BM, Florquin S, et al. Improved preservation and microcirculation with POLYSOL after transplantation in a porcine kidney autotransplantation model. Nephrol Dial Transplant. 2009;24:816–24. doi: 10.1093/ndt/gfn559. [DOI] [PubMed] [Google Scholar]
- 21.van der Plaats A, t Hart NA, Morariu AM, et al. Effect of University of Wisconsin organ-preservation solution on haemorheology. Transpl Int. 2004;17:227–33. doi: 10.1007/s00147-004-0705-8. [DOI] [PubMed] [Google Scholar]
- 22.Gage F, Leeser DB, Porterfield NK, et al. Room Temperature Pulsatile Perfusion of Renal Allografts With Lifor Compared With Hypothermic Machine Pump Solution. Transplantation Proceedings. 2009;41:3571–4. doi: 10.1016/j.transproceed.2009.06.228. [DOI] [PubMed] [Google Scholar]
- 23.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, et al. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation. 2003;76:50–4. doi: 10.1097/01.TP.0000069835.95442.9F. [DOI] [PubMed] [Google Scholar]
- 24.Oberbauer R, Rohrmoser M, Regele H, Muhlbacher F, Mayer G. Apoptosis of Tubular Epithelial Cells in Donor Kidney Biopsies Predicts Early Renal Allograft Function. J Am Soc Nephrol. 1999;10:2006–13. doi: 10.1681/ASN.V1092006. [DOI] [PubMed] [Google Scholar]
- 25.Jani A, Ljubanovic D, Faubel S, Kim J, Mischak R, Edelstein CL. Caspase Inhibition Prevents the Increase in Caspase-3, -2, -8 and -9 Activity and Apoptosis in the Cold Ischemic Mouse Kidney. American Journal of Transplantation. 2004;4:1246–54. doi: 10.1111/j.1600-6143.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 26.Burns AT, Davies DR, McLaren AJ, Cerundolo L, Morris PJ, Fuggle SV. Apoptosis in ischemia/reperfusion injury of human renal allografts. Transplantation. 1998;66:872–6. doi: 10.1097/00007890-199810150-00010. [DOI] [PubMed] [Google Scholar]