Abstract
Many degenerative disease processes associated with aging result from enhanced extracellular matrix (ECM) breakdown. Concomitant with aberrant matrix destruction are alterations in levels of reactive oxygen species (ROS) generating and detoxification systems. ROS function as second messengers due to their ability to react with wide range of biomolecules resulting in modification of an array of signaling networks. ROS can activate upstream kinases (MKK) responsible for MAPK activation and restrict the activity of their inhibitory phosphatases. Here we focus on the redox-sensitive signaling components that control the expression of MMP-1, which is largely responsible for maintaining ECM homeostasis. Numerous disease processes are associated with shifts in steady-state ROS levels that influence overall ECM degradation. This review highlights the redox-sensitive regulatory signals that control the expression of the primary initiating protease MMP-1 and provides strong rational for the use of antioxidant based therapies for treatment of degenerative disorders associated with aberrant matrix destruction.
1. Introduction
1.1 Metalloproteinases
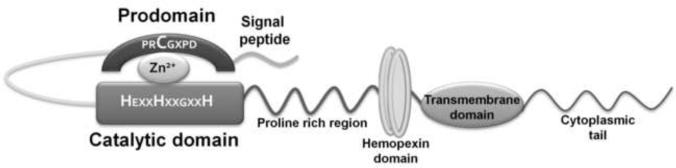
Metalloproteinases (MMPs) are Zn2+ and Ca2+ dependent endopeptidases involved in extracellular matrix (ECM) turnover. Of the 24 known human MMPs (hMMPs), many share a five domain architecture comprising a signal peptide, prodomain region, catalytic domain, hinge region and the hemopexin domain (Fig. 1). MMP activation can be achieved either by proteolytic cleavage of the prodomain or by disruption of thiol interactions. In vitro this can be accomplished by using organomercurials, detergent and free radicals (Springman et al. 1990). Both the hinge region and hemopexin domain are responsible for bringing substrate in close proximity to the catalytic domain for cleavage.
Fig. 1. Structure of Matrix Metalloproteinases.
At the N-terminal of all MMPs is a signal peptide that directs it to the extracellular space/membrane. Following this signal peptide is a prodomain region that caps the catalytic site as a result of “thiol interaction” between the cysteine in the prodomain with the Zn2+ in the catalytic domain, thereby keeping the enzyme inactive (residues highlighted in red). For activation of the enzyme, the prodomain can be cleaved by various proteases or the thiol interaction can be perturbed. The catalytic domain comprises an aspartic acid residue that binds Zn2+ and is essential for the enzyme's activity. The hemopexin domain and proline rich region aid in substrate binding and are responsible for determining substrate specificity. Some MMPs are membrane-bound with the help of a transmembrane domain and a cytoplasmic tail.
The MMPs are broadly classified into five groups – collagenases, gelatinases, stromelysins, matrilysins and membrane-type MMPS (MT-MMPs), based on their substrate specificity and structural differences. MMPs that differ from the general five domain structure are: gelatinases (MMP-2 and 9), which have a fibronectin domain present within the catalytic domain; matrilysins (MMP-7 and 26), which lack the hinge and hemopexin domain; and MT-MMPs that have a transmembrane domain and cytoplasmic tail. All MMPs are secreted as zymogens into the extracellular space with the exception of MT-MMPs, which have a furin recognition sequence at the end of prodomain region that allows for MT-MMPs intracellular activation (Lemaitre and D'Armiento, 2006).
MMP levels in tissues are extremely low and strictly regulated due to their ability to cleave a wide variety of substrates as they modulate many physiological processes including wound healing, cellular growth, embryogenesis, immune surveillance and bone remodeling (Pardo and Selman, 2005). This regulation occurs at the level of transcription, post-transcription and post-translation. Elevated MMP levels have been associated with a variety of pathological conditions such as rheumatoid and osteo-arthritis, lung emphysema, atherosclerosis, Alzheimer's, and cancer. Aging is also associated with an increase in many degenerative diseases involving ECM breakdown and aberrant MMP expression. Recent work by Fisher et al. has established a redox-component to age associated increases in MMP-1 (Fisher et al. 2009). The involvement of reactive oxygen species (ROS) in age-associated MMP expression provides a mechanistic link between the free radical theory of aging and many age-associated degenerative diseases. This paper reviews the molecular triggers that drive this relationship.
MMPs have been categorized into three groups based on promoter conformation(Yan and Boyd, 2007). MMPs in group 1 have promoters that contain a TATA box in the -30bp region with a proximal Activator Protein-1 (AP-1 site) at -70bp (MMP-1, 3, 7, 9, 10, 12, 13, 19 and 26). AP-1 was the first cis-promoter element identified in the MMP-1 promoter to be responsible for its gene expression (Angel et al. 1987). Since then many AP-1 binding sites have been identified within MMP promoters that are co-regulated by growth factors, cytokines, integrin binding and FAK activation. Members in group 1 are transcriptionally activated following IL-1, TNF-α and phorbol ester treatment (Clark et al. 2008). MMPs in group 2 have a TATA box without an AP-1 binding site (MMP-8, 11 and 21) whereas MMPs in group 3 lack all of the above elements (MMP-2, 14 and 28) (Yan and Boyd, 2007).
MMPs are also regulated at the post-transcriptional level by the presence of AU rich cis-elements in the 5'- and 3'- untranslated region (UTR) that dictate mRNA stability. The binding of trans-acting factors such as microRNA (miR) or RNA binding proteins to these cis-elements regulates mRNA stability (Garneau et al. 2007). The 3'-UTR of MMP-1 was reported to be involved in destabilization of transcript and following IL-1 treatment of rabbit synovial fibroblasts, MMP-1 transcripts were stabilized. Therefore, IL-1 is responsible for both transcriptional activation of MMP-1 and stabilization of its transcript thereby increasing MMP-1 expression (Vincenti et al. 1996). Additional studies also revealed p38 MAPK pathway to mediate the stability of MMP-1 transcript (Reunanen et al. 2002). Similarly cortisol was shown to stabilize steady state MMP-13 mRNA levels (Rydziel et al. 2004).
Levels of MMP protein and activity are also dependent upon the presence of both initiator proteases and tissue inhibitors of metalloproteinases (TIMPS). TIMPs are produced by cells that express MMPs and are transcriptionally regulated by growth factors and cytokines(Yan and Boyd, 2007). Presently, there are four different isoforms of TIMPs (TIMP 1–4) (Baker et al. 2002), which inhibit MMP activity through a non-covalent interaction with Zn2+ in the catalytic domain (Pardo and Selman, 2005;Baker et al. 2002). Mice deficient in TIMPs have shown to be at a higher risk of developing cardiovascular disorders such as atherosclerosis, aneurysms and dilated cardiomyopathies associated with impaired lung development (Lemaitre and D'Armiento, 2006).
1. 2. MMP-1
MMP-1 was the first human interstitial collagenase identified, and is responsible for tissue remodeling under physiological and pathological conditions. MMP-1 cleaves collagens type I, II and III with its highest affinity for type III (Lemaitre and D'Armiento, 2006) that is found primarily in cartilaginous and connective tissues of the body. Additionally, it can also degrade non-matrix constituents (aggrecan and perlecans) and activate cytokines such as IL-1β and TNF-α (Lemaitre and D'Armiento, 2006;Gearing et al. 1994;Schonbeck et al. 1998). Under normal physiological conditions MMP-1 levels are low or undetectable, however, in response to stress or a wide array pathophysiological processes MMP-1 levels are elevated dramatically.
MMP-1 specific transcriptional regulation is dependent on the degree of environmental stimuli responsible for inducing downstream signaling pathways along with the presence of variable MMP cis-promoter elements. Furthermore, various single nucleotide polymorphisms (SNPs) can also increase gene transcription. A single guanine insertion at position -1607 (1G/2G) creates an Ets-1 binding site that cooperates with AP-1 at -1602 to enhance MMP-1 transcription and is associated with a plethora of disease pathologies associated with aberrant matrix turnover (Rutter et al. 1998).
The primary role of MMP-1 is to cleave triple helical collagen and increase its susceptibility to degradation by other MMPs and proteases. While the absence of a complete homolog of human MMP-1 (hMMP-1) in murine models has made it difficult to evaluate its role in physiological and pathological processes it is clear that many age-associated degenerative disease processes such as emphysema, atherosclerosis, Alzheimer's, skin aging and rheumatoid/osteo-arthritis are associated with inceases in MMP-1.
Development of hMMP-1 transgenic mice helped shed light on the physiological importance of MMP-1 in disease pathologies. Transgenic expression of hMMP-1 in mouse lungs results in massive destruction of normal lung architecture due to cleavage of type III collagen in alveolar walls, resulting in emphysema (D'Armiento et al. 1992). Additional clinical studies further demonstrate high MMP-1 levels in type II pneumocytes of emphysematous patient whereas levels in healthy patients are non-detectable (Imai et al. 2001). In vitro studies also revealed that MMP-1 is induced by cigarette smoke which is a major risk factor for emphysema (Mercer et al. 2004).
Constitutive hMMP-1 expression in mouse heart leads to the development of dilated cardiomyopathy. Older mice demonstrate a significant decrease in diastolic and systolic functions and eventually succumb to cardiac failure (Kim et al. 2000) . Additionally, MMP-1 is also involved in the process of atherosclerosis. Excised diseased arteries reveal elevated levels of MMP-1 in smooth muscle cells and macrophages that underlie the fibrous cap (Nikkari et al. 1995). There is also a strong positive co-relation between the presence MMP-1 and increased plaque instability, strongly suggesting that MMP-1 might lead to plaque expansion, hemorrhage and eventual rupture (Nikkari et al. 1995;Libby, 2002).
Senescent dermal fibroblasts also display elevated levels of MMP-1, 2 and 3 that are closely associated with low levels of TIMP-1(Hornebeck, 2003). Expression of recombinant TIMP-1 was seen to increase the proliferative capacity of psoriatic and sclerotic dermal fibroblasts (Bertaux et al. 1991;Buisson-Legendre et al. 2000;Kikuchi et al. 1997). TIMP-1 mediated inhibition of MMPs and its growth promoting activity implicates TIMP-1 as an essential growth factor required to maintain skin homeostasis (Hornebeck, 2003). Low levels of TIMP-1 in senescent fibroblasts with enhanced MMP-1 activity may further clarify the underlying mechanism for skin aging and wrinkling. Burrage et al. also established that chondrocytes and synovial cells from diseased joints of patients suffering from rheumatoid and osteoarthritis show high level expression of MMP-1 and 13 (Burrage et al. 2006). This marked expression of MMP is responsible for generalized joint destruction, swelling and inflammation that are characteristic hallmarks of the disease. Additionally, 50% of cortical brain tissues obtained from deceased Alzheimer's patients were shown to display marked increases in MMP-1 (Leake et al. 2000). The blood brain barrier is comprised of endothelial cells in close communication with astrocytes and microglial cells. Elevated MMP-1 expression may disrupt blood brain barrier integrity and drive demyelination (multiple sclerosis) and degenerative dementia. Understanding the molecular mechanisms by which MMP-1 levels are increased may provide potential targets for the prevention of diseases and degenerative conditions associated with dysregulation of MMP-1.
2. Redox Signaling and MMP-1
MMP-1 is induced in response to growth factors, cigarette smoke and radiation, all of which are associated with increased ROS production. ROS can impact MMP-1 activity and expression indirectly through the modulation of signaling networks that contibute to its transcription or through direct oxidative activation of the enzyme.
Activation of Mitogen-Activated Protein Kinase (MAPK) signaling by ROS is achieved through multiple mechanisms. Glutathionylation of Cys118 in Ras inhibits its GTP/GDP exchange mechanism, keeping Ras in its active state, thus driving MAPK activation (Adachi et al. 2004). MAPK signaling is also prolonged by the restriction of activity of a number of inhibitory MAP Kinase Phosphatases (MKPs) by ROS.
Many phosphatases such as phosphatase and tensin homolog (PTEN) (Lee et al. 2002), protein tyrosine phosphatase 1B (PTB1B) (Lou et al. 2008), MAPK phosphatases (Seth and Rudolph, 2006) and low molecular weight (LMW) protein tyrosine phosphatases (Caselli et al. 1998) have been shown to be sensitive to oxidant inactivation at critical cysteine residues in their active site. These cysteines have a lower pKa due to the presence of positively charged amino acids surrounding them, resulting in their sensitivity to oxidative inactivation (Barford, 2004). Enhancement of signaling pathways by oxidants can therefore affect the recruitment of proteins at the promoter leading to increased transcription. The role of oxidants in enhancing MMP-1 transcription has been shown to be due to activation of ERK and JNK MAPK signaling. Treatment with either ERK or JNK inhibitors causes a decrease in MMP-1 promoter activity (Ranganathan et al. 2001).
Post-transcriptional activation of MMPs is also driven by ROS. Oxidative stress has been shown to modulate the thiol interaction between the prodomain and catalytic domain of MMP-7 leading to its activation. Work by Suschek and coworkers indicates that small molecular weight thiols including glutathione restrict MMP-1 activity and oxidation of the MMP-1 bound inhibitory thiol is required for its activation (Koch et al. 2009).
3. Redox-Signaling and Age-associated MMP-1Expression
Recent work has established that age-dependent increases in MMP-1 expression are also ROS-dependent (Dasgupta et al. 2010). Using a cellular aging model, the senescence associated increases in MMP-1 expression were shown to be redox-dependent. Age-dependent increases in oxidant production were linked to the augmented expression of MMP-1 that was sensitive to inhibition by the glutathione precursor, N-acetyl cysteine (NAC). Increases in both oxidant production and MMP-1 expression were also correlated with similar age-dependent increases in phospho Jun N-terminal Kinase (JNK) levels that were also sensitive to NAC treatment. The JNK-dependent transcription factor c-Jun is a key player in MMP-1 gene expression(Nelson et al. 2006;Auble et al. 1992). Increased binding of c-Jun to the MMP-1 promoter in senescent cells relative to young cells further implied that JNK contributes to age-dependent MMP-1 expression.
Serial activation of JNK is dependent upon upstream MAPK Kinases (MKKs) MKK-4 and MKK-7. Interestingly, MKK-4 levels are also shown to be regulated in an age and redox-dependent fashion. Negative regulators of JNK are MKP-1, 2, 5 and 7, of which MKP-1 is known to be induced in response to oxidative stress (Zhou et al. 2006).
Despite being induced by oxidants, phosphatases are highly susceptible to oxidative modification and form high molecular weight aggregates that are targeted for degradation (Kamata et al. 2005). This differential regulation by ROS can be dictated by the degree of activating stimulus i.e. low levels of ROS can impact gene expression of MKP-1 but excessively high levels are inhibitory in nature. Analysis of MKP-1 levels from aged cells revealed that it is targeted for ubiquitin dependent proteolysis that is prevented when cells are exposed to low oxygen. Moreover, both the age and redox-dependent expression of MMP-1 can be blocked by overexpression of a dominant negative JNK or MKP-1, both of which restrict JNK activation.
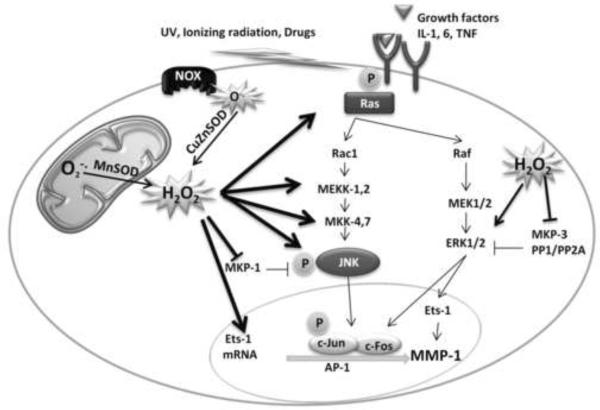
To date studies measuring general antioxidant and DNA repair capability in aging model in response to oxidative stress has revealed a gradual and consistent decline with age (Sohal et al. 1993;Carney et al. 1991;Barnett and King, 1995) suggesting that old cells are incapable of preventing oxidative damage of biomolecules. As summarized in Fig. 3, MMP-1 expression is increased with age and is regulated by shifts in intracellular steady-state H2O2 levels. In addition, redox-dependent JNK activation and simultaneous inhibition of MKP-1 points to ROS as the critical trigger in the process of age-dependent MMP-1 expression.
Fig. 3. Model of redox-dependent induction of senescence associated MMP-1.
Our data suggests that age associated MMP-1 expression is redox-sensitive. The redox-dependence is initially due to the activation of c-Jun N-terminal kinase pathway. Previous results have also shown that the activation of JNK pathway involves activation of upstream MAPKK, MKK-4 and MKK-7. Our lab has shown that MKK-4 is similarly regulated in an age- and redox-dependent manner. Furthermore, with age there is decrease in total levels of MKP-1 which may implicate a process of “oxidative inactivation”. Use of kinase specific pharmacological and molecular inhibitor indicate that other MAP kinases such as Erk, p38 and PI-3-Kinase may also participate in oxidant-dependent regulation of MMP-1 transcription. To conclude, JNK is one of the key mediators of redox dependent MMP-1 induction and the senescence-dependent MMP-1 induction is a complex signaling process dependent on ROS regulating a number of distinct signaling networks that converge to drive MMP-1 expression. Dashed lines indicate sites of redox regulation.
4. Redox-dependent control of MMP-1 transcription
As described previously, the level of MMP-1 expression is influenced by a genetic variation in the MMP-1 promoter. An SNP located at −1607 bp, creates an Ets family transcription factor binding site of the sequence 5'-GGAT-3' by the insertion of a guanine base (G). Ets transcription factors normally do not bind DNA alone, but preferentially form coactivator complexes with transcription factors, such as AP-1. The Ets and AP-1 coactivating complex may also play a critical role in regulating the expression of various MMP family members, particulary that of MMP-1 (Westermarck et al. 1997). The protooncoproteins Fos and Jun, which comprise the AP-1 complex, can homo or heterodimerize and bind its cognate consensus sequence (TGACTCA) in the regulatory domains of many genes including various MMP family members (Whitmarsh and Davis, 1996). Numerous reports indicate that transcription factors important for MMP-1 expression are sensitive to redox-activation that occurs, in part, through activation of the MAPK family members ERK1/2 and JNK (Frost et al. 1994;Clerk et al. 1998;Westermarck and Kahari, 1999). JNK phosphorylates the AP-1 family members including c-Jun and JunB (Reddy and Mossman, 2002), while c-Jun, c-Fos, FosB, Fra-1 are sensitive to ERK activation. We have established that JNK confers redox-sensitivity to the MMP-1 promoter while both ERK and/or JNK are required for maximal basal promoter activity and the expression of AP-1 and Ets-1 (Nelson et al. 2006). In addition, both c-Jun and Ets-1 are recruited to the region of the MMP-1 SNP in response to alterations in the steady state production of H2O2.
Jun also forms complexes with other proteins to bind to distinct elements on the MMP-1 promoter (Parra et al. 1998). For example, c-Jun can complex with Fra-1 and bind NFκB elements. This mode of regulation needs to be further explored due to redox-sensitivity of NFκB binding and the location of a binding site close to the TATA box on the MMP-1 promoter.
Several lines of evidence suggest that H2O2-generated by the mitochondrial superoxide dismutase (Sod2) is important to enhanced expression of MMP-1. Scharffetter-Kochanek and coworkers demonstrated that overexpression of Sod2 in dermal fibroblasts caused a profound increase in the expression of MMP-1 when superoxide was provided to the cells (Wenk et al. 1999). The investigators established that the induction of MMP-1 is due to the increase in the production of H2O2 as a result of the dismuting function of Sod2. This is further supported by work from our group that indicates binding of Ets-1 to the MMP-1 promoter and sustained JNK signaling requires the enzyme activity of Sod2. Furthermore, Sod2 is sufficient and required for the induction of MMP-1 and potentially other MMP family members via the H2O2-dependent activation of MAPK signaling (Nelson et al. 2003). Thus, Sod2-dependent alterations in steady state H2O2 levels play an important role in regulating the upstream signaling that leads to optimal MMP-1 transcriptional activity and likely parallels the signiling scheme outlined in Fig 3.
5. Regulation of Chromatin Remodeling by ROS and RNS
Histone deacetylases (HDACs) are important histone modifying proteins that are generally involved in gene repression due to their deacetylating activity (Kuo and Allis, 1998). Removal of acetyl groups from histones by HDACs restores the positive charge of the histones and allows for their tighter interaction with DNA, possibly preventing access of transcriptional factors to promoter binding sites. HDAC2 has been previously shown to be involved in MMP-9 inhibition by metastasis associated gene 1 (MTA-1) and HDAC1 and 3 and to play a role in suppression of MMP-9 in HeLa cells (Yan et al. 2003).
Modification of HDACs by ROS and/or RNS results in reduced HDAC protein and activity levels, thus altering their function in transcriptional repression. This reduction is caused by nitrotyrosine modifications and aldehyde-adduct formation in HDACs in response to ROS or RNS. Exposure of the human macrophage cell line MonoMac6 to cigarette smoke, which itself is a potent generator of ROS and RNS, stimulates an imflammatory response resulting from a decline in HDAC1, HDAC2 and HDAC3 activity and protein, and a concomitant increase in the expression and release of proinflammatory cytokines (Yang et al. 2006). In human alveolar cells A549, both H2O2 and cigarette smoke result in HDAC2 nitration and a decrease in protein levels and activity accompanied by increase in histone H4 acetylation (Moodie et al. 2004). Ito et al estabished that H2O2 significantly enhances cytokine production in BEAS-2B cells as a result of increased tyrosine nitration and decreased activity of HDAC2 (Ito et al. 2004).
HDACs 1 and 2 have been shown to play an important role in cell proliferation as well as mediating repression of pro-inflammatory cytokine expression along with corticosteroids. In COPD patients, the oxidative stress caused by cigarette smoke modifies HDAC2 and renders corticosteroid treatments ineffective in treating inflammation (Moodie et al. 2004). Moodie et al have demonstrated that increasing concentrations of cigarette smoke can reduce HDAC2 levels as well as activity leading to increased histone H4 acetylation. Moreover H2O2 as well as TNF-α treatment also decreased HDAC2 levels and activity (Moodie et al. 2004).
Treatment of A549 cells with the histone deacetylase inhibitor Trichostatin-A (TSA) increased expression of IL-8, the pro-inflammatory cytokine repressed by HDAC2. In addition, TSA treatment also decreased HDAC2 levels suggesting that inactivation of HDAC2 targets it for degradation. In addition to HDAC2, HDAC1 and 3 were also shown to be oxidatively modified by tyrosine nitration, which results in an increase in IL-8 expression in an NFκB dependent manner in MonoMac-6 cells (Yang et al. 2006). Concomitantly, upon TSA treatment, NFκB was activated to a greater extent which again suggests that HDAC inactivation decreases its protein levels.
Tyrosine nitration, in addition to inactivating an enzyme and targeting it for degradation may also prevent other tyrosine modifications such as phosphorylation that are required for optimal function (Turko and Murad,2002). Exposure of endothelial cells to peroxynitrite was shown to decrease the amount of tyrosine phosphorylated proteins and increase nitrotyrosine-containing proteins and target those proteins for degradation (Gow et al. 1996). However peroxynitrite mediated nitration affects signaling pathways in a biphasic manner; low levels of peroxynitrite ranging from 2 μM to 500 μM (depending on the cell type) deplete intracellular glutathione and other reducing agents leading to a more pro-oxidant environment that can inactivate protein phosphatases leading to enhanced signaling. At higher peroxynitrite concentrations tyrosine nitration predominates leading to complete inhibition of tyrosine phosphorylation (Monteiro et al. 2008). The presence of a mitochondrial nitric oxide synthase, which could potentially produce NO (Parihar et al. 2008;Ghafourifar and Richter,1997;Tatoyan and Giulivi,1998;Elfering et al. 2002) might mediate nitration as observed for mitochondrial proteins under ischemia/reoxygenation (Koeck et al. 2004). The amount of peroxynitrite produced in any cellular system and, therefore, the amount of tyrosine nitration depends on the flux between the two substrates, NO and superoxide, which form peroxynitrite at near diffusion rates. Increases or decreases in either of the two affect the rate of the reaction. Therefore, activities of NOS, SOD and NADPH oxidases as well as other superoxide producing processes need to be considered in a cell as a source of peroxynitrite generation.
Tyrosine nitration to date is still considered to be a dead end reaction resulting in the protein being targeted for degradation. However, there is evidence pointing towards a possible mechanism for reversing this reaction. Work done by Murad and co-workers has shown that homogenates from rat liver and spleen could modify nitrotyrosine-containing BSA (Kamisaki et al. 1998). Incubation with the homogenates resulted in a loss of the nitro-tyrosine epitope that is recognized by a specific monoclonal antibody. This activity of the homogenates was heat labile and sensitive to proteolytic degradation and was identified as a `denitrase'. More recently an in vivo substrate for the `denitrase' of histone H1.2 was identified in RAW 64.7 cells (Irie et al. 2003). In addition, various heme containing proteins in the presence of cellular thiols can non-enzymatically convert nitro tyrosines into amino tyrosine (Balabanli et al. 1999). The amino tyrosine modification can then be potentially removed by the action of nitro reductases in the cell. However, the role of nitro reductases in this process has not been clearly established. Whether either or both mechanisms exist to reverse tyrosine modifications still needs to be extensively characterized.
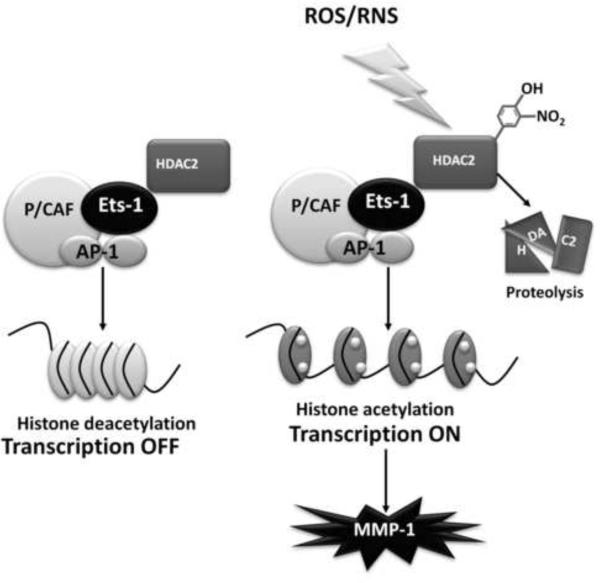
Overall nitration of HDAC's may result in their ubiquitination and degradation by the proteosome. Fig. 4 depicts a proposed model for how oxidants might impact the MMP-1 chromatin remodeling complex. Oxidants promote an increase in the recruitment of specific transcription factors (Ets-1 and AP-1) and histone modifying proteins (P/CAF) to the MMP-1 promoter. Nitration and subsequent degradation of HDAC2 allows for a more accessible chromatin. In addition, oxidants promote increases in JNK and ERK signaling which upregulate Ets-1, c-Jun and c-Fos expression in and play a critical role in driving high level MMP-1 transcription.
Fig. 4.
Schematic representation of the role of oxidants in modulating MMP-1 transcription by redox-sensitive chromatin remodeling proteins. Redox-shifts potentially lead to increases in HDAC2 nitration leading to its degradation. Loss of deacetylase activity would further propagate MMP-1 transcription by minimizing any suppressive activity of HDAC2.
5. MMP-1 Promoter Specific Redox responsive Chromatin Remodeling Factors
Zantema et al have defined the various chromatin remodeling factors and histone modifications that play a role in MMP-1 transcription in response to addition of serum (Martens et al. 2003). These results show a role for p300 (a histone acetyl transferase or HAT), Set9 (histone methyl transferase), Rsk2 (kinase), Brg1 (component of the SWI/SNF chromatin remodeling complex) as well as c-Jun and c-Fos (AP-1 transcription factor) in this process.
Various chromatin remodeling factors participate in the redox responsive transcriptional initiation from the MMP-1 promoter. Results from chromatin immunoprecipitation experiments, which examined the recruitment of various factors to the MMP-1 promoter in redox engineered cell lines suggest that c-Jun, c-Fos, Ets-1 as well as P/CAF recruitment to the redox sensitive regions of the MMP-1 promoter are likely to be key triggers in enhancing MMP-1 transcription (Nelson et al. 2003).
The recruitment of various chromatin remodeling proteins occurs at specific regions of the MMP-1 promoter. Ets-1, c-Jun and c-Fos recruitment is highly enhanced in the region near the 2GSNP. Brinckerhoff et al as well as Nelson et al have shown that this region plays an important role in augmenting MMP-1 expression and requires ERK1/2 signaling (Nelson et al. 2003;Tower et al. 2002). Indirectly it has been demonstrated that oxidants from cigarette smoke enhances expression from the −1607 2G polymorphism contained within the MMP-1 promoter as compared to the 1G sequence (Mercer et al. 2006). Importantly, ERK1/2 MAPK is required for this promoter activity in human small airway epithelial cells (Mercer et al. 2006). It was suggested that individuals with the 2G insertion polymorphism who smoke may be predisposed to enhanced MMP-1 expression in the lung, which can contribute to the development of chronic obstructive pulmonary disease.
A candidate histone modifiying factor that participates in the redox-dependent regulation of MMP-1 is the histone deacetylase HDAC2. HDAC2 has been shown to play a role in MTA-1 mediated inhibition of MMP-9 in HT1080 cells at a distal region of the promoter (Yan et al. 2003). HDAC2 was also shown to bind to the proximal promoter region even in the absence of MTA-1, which would suggest that HDAC2 represses MMP-9 independently of MTA-1. However, in HeLa cells HDAC1 and HDAC3 are involved in suppression of MMP-9 at proximal promoter regions which is relieved on PMA treatment (Ma et al. 2004). Therefore, recruitment of different chromatin modifying proteins depending on the cell type and signal used to activate transcription can impact MMP-1 expression. In summary, the distal promoter region of MMP-1 that contains the SNP is shown to play an important role in the oxidant dependent induction of MMP-1. Particularly, the recruitment of primary transcription factors Ets-1, c-Jun and c-Fos is increased at this site. Chromatin modifying proteins P/CAF and HDAC2 may be differentially recruited to the distal promoter element in an oxidant dependent manner. These concerted events are mediated by ROS and bring about a more transcriptionally active MMP-1 promoter complex.
6. Antioxidants and MMPs
The role of oxidants in enhancing MMP expression has been widely documented in the literature. H2O2 generation enhances the expression of MMP-1 in a variety of systems. Compounds that detoxify H2O2 or reduce its levels abrogate MMP-1 expression. Concomitantly MMP-1 expression is enhanced by compounds such as buthionine sulfoxime (a glutathione synthesis inhibitor) or aminotriazole (catalase inhibitor) that interfere with the ability to remove H2O2 (Wenk et al. 1999). In UVA irradiated fibroblasts, treatment with antioxidant constituents from Melothria heterophylla scavenged reactive oxygen species and reduced MMP-1 expression (Cho et al. 2006). Dermal fibroblasts exposed to ROS generated by the hypoxanthine-xanthine oxidase system and treated with catalase show decreased expression of MMP-1 (Zaw et al. 2006). Epigallocatechin-3-gallate, a polyphenol component of green tea that scavenges oxidants can suppress MMP-1 expression in hepatic stellate cells and reduce hepatic fibrosis (Nakamuta et al. 2005). Antioxidants, Vitamin A and E were used to reduce MMP-1 levels in a porcine model of atherosclerosis (Orbe et al. 2003). All trans retinoic acid treatment of skin prevents UV mediated MMP-1 expression by preventing accumulation of c-Jun which is necessary for MMP-1 transcription as a part of the AP-1 complex (Fisher et al. 1999;Fisher and Voorhees,1998). MMP-1 production is inhibited by reduced glutathione in transformed human heart fibroblast cell lines by reducing oxidant levels (Tyagi et al. 1996). Resveratrol, a polyphenolic compound found in grape skin can inhibit TNF-α induced MMP-9 expression in human vascular smooth muscle cells (Lee and Moon,2005). N-acetyl cysteine (Hozumi et al. 2001), a ROS scavenger, as well as catalase (Warner et al. 2000) can also block MMP-9 expression by TNF-α. It is clear that various ROS, in particular H2O2, play a major role in modulating the expression of MMP-1 in a variety of cell lines and tissues. ROS levels are increased in many different cancers that express MMP-1.
Conclusion
Taken together, it is clear that the redox-responsiveness of MMP-1 occurs at multiple levels. The redox-dependent shifts in MAPK signaling promote recruitment of transcription factors to the MMP-1 promoter. The specificity and sensitivity of the MMP-1 promoter to oxidative stress is very likely dependent on redox-dependent modifications of chromatin remodeling factors that are engaged in driving MMP-1 expression. Understanding the many potential redox modifications that control aberrant MMP-1 transcription will lead to the development of targeted antioxidant based therapies for treatment of the many pathologies associated with excessive matrix turnover.
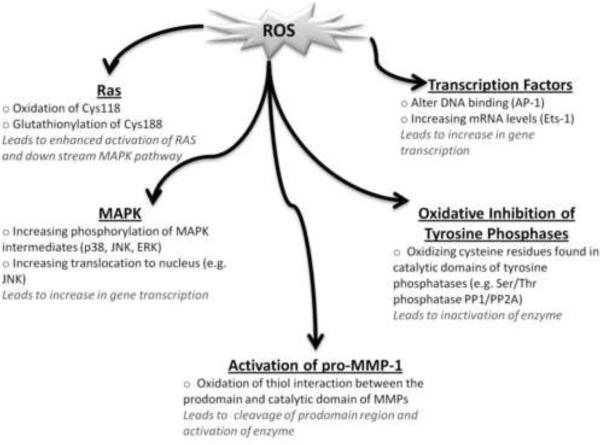
Fig. 2. Redox-regulation of MMP-1.
Matrix metalloproteinases are primarily regulated at the level of gene transcription by Ras and MAP kinases. Many mechanisms have been documented with evidence pertaining to redox-regulation of other members of matrix metalloproteinase family. Some of these mechanisms may play a role in redox regulation of MMP-1.
Acknowledgements
This article was supported by NIA (AG031067 + AG031067-02S1) (J.A.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004 doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- Angel P, Baumann I, Stein B, Delius H, Rahmsdorf HJ, Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987;7:2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auble DT, Sirum-Connolly KL, Brinckerhoff CE. Transcriptional regulation of matrix metalloproteinase genes: role of AP-1 sequences. Matrix Suppl. 1992;1:200. [PubMed] [Google Scholar]
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Balabanli B, Kamisaki Y, Martin E, Murad F. Requirements for heme and thiols for the nonenzymatic modification of nitrotyrosine. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13136–13141. doi: 10.1073/pnas.96.23.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 2004;14:679–686. doi: 10.1016/j.sbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Barnett YA, King CM. An investigation of antioxidant status, DNA repair capacity and mutation as a function of age in humans. Mutat. Res. 1995;338:115–128. doi: 10.1016/0921-8734(95)00017-z. [DOI] [PubMed] [Google Scholar]
- Bertaux B, Hornebeck W, Eisen AZ, Dubertret L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol. 1991;97:679–685. doi: 10.1111/1523-1747.ep12483956. [DOI] [PubMed] [Google Scholar]
- Buisson-Legendre N, Emonard H, Bernard P, Hornebeck W. Relationship between cell-associated matrix metalloproteinase 9 and psoriatic keratinocyte growth. J Invest Dermatol. 2000;115:213–218. doi: 10.1046/j.1523-1747.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Carney JM, Starke Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N- tert-butyl-alpha-phenylnitrone. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J. Biol. Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- Cho YH, Kim JH, Sim GS, Lee BC, Pyo HB, Park HD. Inhibitory effects of antioxidant constituents from Melothria heterophylla on matrix metalloproteinase-1 expression in UVA-irradiated human dermal fibroblasts. J. Cosmet. Sci. 2006;57:279–289. [PubMed] [Google Scholar]
- Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Clerk A, Michael A, Sugden PH. Stimulation of multiple mitogen-activated protein kinase sub-families by oxidative stress and phosphorylation of the small heat shock protein, HSP25/27, in neonatal ventricular myocytes. Biochem. J. 1998;333(Pt 3):581–589. doi: 10.1042/bj3330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell. 1992;71:955–961. doi: 10.1016/0092-8674(92)90391-o. [DOI] [PubMed] [Google Scholar]
- Dasgupta J, Kar S, Liu R, Joseph J, Kalyanaraman B, Remington SJ, Chen C, Melendez JA. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. Online ed. 2010 doi: 10.1002/jcp.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfering SL, Sarkela TM, Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J. Biol. Chem. 2002;277:38079–38086. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Talwar HS, Lin J, Voorhees JJ. Molecular mechanisms of photoaging in human skin in vivo and their prevention by all-trans retinoic acid. Photochem. Photobiol. 1999;69:154–157. doi: 10.1562/0031-8655(1999)069<0154:mmopih>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J. Investig. Dermatol. Symp. Proc. 1998;3:61–68. [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. Collagen Fragmentation Promotes Oxidative Stress and Elevates Matrix Metalloproteinase-1 in Fibroblasts in Aged Human Skin. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JA, Geppert TD, Cobb MH, Feramisco JR. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc. Natl. Acad. Sci. U. S. A. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–66. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- Hornebeck W. Down-regulation of tissue inhibitor of matrix metalloprotease-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol (Paris) 2003;51:569–573. doi: 10.1016/j.patbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hozumi A, Nishimura Y, Nishiuma T, Kotani Y, Yokoyama M. Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am. J. Physiol Lung Cell Mol. Physiol. 2001;281:L1444–L1452. doi: 10.1152/ajplung.2001.281.6.L1444. [DOI] [PubMed] [Google Scholar]
- Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D'Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir. Crit Care Med. 2001;163:786–791. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5634–5639. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem. Biophys. Res. Commun. 2004;315:240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S.i., Maeda S, Chang L, Hirata H, Karin M. Reactive Oxygen Species Promote TNF[alpha]-Induced Death and Sustained JNK Activation by Inhibiting MAP Kinase Phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Wada K, Bian K, Balabanli B, Davis K, Martin E, Behbod F, Lee YC, Murad F. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11584–11589. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Kadono T, Furue M, Tamaki K. Tissue inhibitor of metalloproteinase 1 (TIMP-1) may be an autocrine growth factor in scleroderma fibroblasts. J Invest Dermatol. 1997;108:281–284. doi: 10.1111/1523-1747.ep12286457. [DOI] [PubMed] [Google Scholar]
- Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D'Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest. 2000;106:857–866. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Volkmar CM, Kolb-Bachofen V, Korth HG, Kirsch M, Horn AH, Sticht H, Pallua N, Suschek CV. A new redox-dependent mechanism of MMP-1 activity control comprising reduced low-molecular-weight thiols and oxidizing radicals. J Mol Med. 2009;87:261–272. doi: 10.1007/s00109-008-0420-5. [DOI] [PubMed] [Google Scholar]
- Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J. Biol. Chem. 2004;279:27257–27262. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Leake A, Morris CM, Whateley J. Brain matrix metalloproteinase 1 levels are elevated in Alzheimer's disease. Neurosci. Lett. 2000;291:201–203. doi: 10.1016/s0304-3940(00)01418-x. [DOI] [PubMed] [Google Scholar]
- Lee B, Moon SK. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J. Nutr. 2005;135:2767–2773. doi: 10.1093/jn/135.12.2767. [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Lemaitre V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo. Today. 2006;78:1–10. doi: 10.1002/bdrc.20065. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, Tonks NK, Meng TC. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- Ma Z, Shah RC, Chang MJ, Benveniste EN. Coordination of cell signaling, chromatin remodeling, histone modifications, and regulator recruitment in human matrix metalloproteinase 9 gene transcription. Mol. Cell Biol. 2004;24:5496–5509. doi: 10.1128/MCB.24.12.5496-5509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, Verlaan M, Kalkhoven E, Zantema A. Cascade of distinct histone modifications during collagenase gene activation. Mol. Cell Biol. 2003;23:1808–1816. doi: 10.1128/MCB.23.5.1808-1816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer B, Brinckerhoff C, D'Armiento J. Activation of the MMP-1 promoter by cigarette smoke in human small airway epithelial cells requires ERK MAP kinase signaling: differential response of the 1G and 2G promoter sequences. Proc. Am Thorac. Soc. 2006;3:477. doi: 10.1513/pats.200603-041MS. [DOI] [PubMed] [Google Scholar]
- Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem. 2004;279:17690–17696. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- Monteiro HP, Arai RJ, Travassos LR. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid. Redox. Signal. 2008;10:843–890. doi: 10.1089/ars.2007.1853. [DOI] [PubMed] [Google Scholar]
- Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- Nakamuta M, Higashi N, Kohjima M, Fukushima M, Ohta S, Kotoh K, Kobayashi N, Enjoji M. Epigallocatechin-3-gallate, a polyphenol component of green tea, suppresses both collagen production and collagenase activity in hepatic stellate cells. Int. J. Mol. Med. 2005;16:677–681. [PubMed] [Google Scholar]
- Nelson KK, Ranganathan AC, Mansouri J, Rodriguez AM, Providence KM, Rutter JL, Pumiglia K, Bennett JA, Melendez JA. Elevated sod2 activity augments matrix metalloproteinase expression: evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin. Cancer Res. 2003;9:424–432. [PubMed] [Google Scholar]
- Nelson KK, Subbaram S, Connor KM, Dasgupta J, Ha XF, Meng TC, Tonks NK, Melendez JA. Redox-dependent matrix metalloproteinase-1 expression is regulated by JNK through Ets and AP-1 promoter motifs. J Biol Chem. 2006;281:14100–14110. doi: 10.1074/jbc.M601820200. [DOI] [PubMed] [Google Scholar]
- Nikkari ST, O'Brien KD, Ferguson M, Hatsukami T, Welgus HG, Alpers CE, Clowes AW. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995;92:1393–1398. doi: 10.1161/01.cir.92.6.1393. [DOI] [PubMed] [Google Scholar]
- Orbe J, Rodriguez JA, Arias R, Belzunce M, Nespereira B, Perez-Ilzarbe M, Roncal C, Paramo JA. Antioxidant vitamins increase the collagen content and reduce MMP-1 in a porcine model of atherosclerosis: implications for plaque stabilization. Atherosclerosis. 2003;167:45–53. doi: 10.1016/s0021-9150(02)00392-1. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37:283–288. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Nazarewicz RR, Kincaid E, Bringold U, Ghafourifar P. Association of mitochondrial nitric oxide synthase activity with respiratory chain complex I. Biochem. Biophys. Res. Commun. 2008;366:23–28. doi: 10.1016/j.bbrc.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra E, McGuire K, Hedlund G, Dohlsten M. Overexpression of p65 and c-Jun substitutes for B7-1 costimulation by targeting the CD28RE within the IL-2 promoter. J. Immunol. 1998;160:5374–5381. [PubMed] [Google Scholar]
- Ranganathan AC, Nelson KK, Rodriguez AM, Kim KH, Tower GB, Rutter JL, Brinckerhoff CE, Epstein CJ, Huang TT, Jeffrey JJ, Melendez JA. Manganese superoxide dismutase signals matrix metalloproteinase expression Via H2O2-dependent ERK1,2 activation. J. Biol. Chem. 2001;276:14264–14270. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- Reddy SP, Mossman BT. Role and regulation of activator protein-1 in toxicant-induced responses of the lung 1. Am. J. Physiol Lung Cell Mol. Physiol. 2002;283:L1161–L1178. doi: 10.1152/ajplung.00140.2002. [DOI] [PubMed] [Google Scholar]
- Reunanen N, Li SP, Ahonen M, Foschi M, Han J, Kahari VM. Activation of p38 alpha MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J Biol Chem. 2002;277:32360–32368. doi: 10.1074/jbc.M204296200. [DOI] [PubMed] [Google Scholar]
- Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, Brinckerhoff CE. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58:5321–5325. [PubMed] [Google Scholar]
- Rydziel S, Delany AM, Canalis E. AU-rich elements in the collagenase 3 mRNA mediate stabilization of the transcript by cortisol in osteoblasts. J Biol Chem. 2004;279:5397–5404. doi: 10.1074/jbc.M311984200. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- Seth D, Rudolph J. Redox regulation of MAP kinase phosphatase 3. Biochemistry. 2006;45:8476–8487. doi: 10.1021/bi060157p. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem. Biophys. Res. Commun. 1993;196:7–11. doi: 10.1006/bbrc.1993.2208. [DOI] [PubMed] [Google Scholar]
- Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc. Natl. Acad Sci U. S. A. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatoyan A, Giulivi C. Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. J. Biol. Chem. 1998;273:11044–11048. doi: 10.1074/jbc.273.18.11044. [DOI] [PubMed] [Google Scholar]
- Tower GB, Coon CC, Benbow U, Vincenti MP, Brinckerhoff CE. Erk 1/2 differentially regulates the expression from the 1G/2G single nucleotide polymorphism in the MMP-1 promoter in melanoma cells. Biochim. Biophys. Acta. 2002;1586:265–274. doi: 10.1016/s0925-4439(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol. Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Kumar S, Borders S. Reduction-oxidation (redox) state regulation of extracellular matrix metalloproteinases and tissue inhibitors in cardiac normal and transformed fibroblast cells. J. Cell Biochem. 1996;61:139–151. doi: 10.1002/(sici)1097-4644(19960401)61:1<139::aid-jcb15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, White LA, Schroen DJ, Benbow U, Brinckerhoff CE. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): mechanisms that control enzyme activity, transcription, and mRNA stability. Crit Rev. Eukaryot. Gene Expr. 1996;6:391–411. doi: 10.1615/critreveukargeneexpr.v6.i4.40. [DOI] [PubMed] [Google Scholar]
- Warner RL, Bless NM, Lewis CS, Younkin E, Beltran L, Guo R, Johnson KJ, Varani J. Time-dependent inhibition of immune complex-induced lung injury by catalase: relationship to alterations in macrophage and neutrophil matrix metalloproteinase elaboration. Free Radic. Biol. Med. 2000;29:8–16. doi: 10.1016/s0891-5849(00)00282-3. [DOI] [PubMed] [Google Scholar]
- Wenk J, Brenneisen P, Wlaschek M, Poswig A, Briviba K, Oberley TD, Scharffetter-Kochanek K. Stable Overexpression of Manganese Superoxide Dismutase in Mitochondria Identifies Hydrogen Peroxide as a Major Oxidant in the AP-1-mediated Induction of Matrix-degrading Metalloprotease-1. J Biol Chem. 1999;274:25869–25876. doi: 10.1074/jbc.274.36.25869. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- Westermarck J, Seth A, Kahari VM. Differential regulation of interstitial collagenase (MMP-1) gene expression by ETS transcription factors. Oncogene. 1997;14:2651–2660. doi: 10.1038/sj.onc.1201111. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang H, Toh Y, Boyd DD. Repression of 92-kDa Type IV Collagenase Expression by MTA1 Is Mediated through Direct Interactions with the Promoter via a Mechanism, Which Is Both Dependent on and Independent of Histone Deacetylation. Journal of Biological Chemistry. 2003;278:2309–2316. doi: 10.1074/jbc.M210369200. [DOI] [PubMed] [Google Scholar]
- Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- Zaw KK, Yokoyama Y, Abe M, Ishikawa O. Catalase restores the altered mRNA expression of collagen and matrix metalloproteinases by dermal fibroblasts exposed to reactive oxygen species. Eur. J. Dermatol. 2006;16:375–379. [PubMed] [Google Scholar]
- Zhou JY, Liu Y, Wu GS. The role of mitogen-activated protein kinase phosphatase-1 in oxidative damage-induced cell death. Cancer Res. 2006;66:4888–4894. doi: 10.1158/0008-5472.CAN-05-4229. [DOI] [PubMed] [Google Scholar]