Abstract
The counterregulatory response to hypoglycemia is a complex and well-coordinated process. As blood glucose concentration declines, peripheral and central glucose sensors relay this information to central integrative centers to coordinate neuroendocrine, autonomic, and behavioral responses and avert the progression of hypoglycemia. Diabetes, both type 1 and type 2, can perturb these counterregulatory responses. Moreover, defective counterregulation in the setting of diabetes can progress to hypoglycemia unawareness. While the mechanisms that underlie the development of hypoglycemia unawareness are not completely known, possible causes include altered sensing of hypoglycemia by the brain and/or impaired coordination of responses to hypoglycemia. Further study is needed to better understand the intricacies of the counterregulatory response and the mechanisms contributing to the development of hypoglycemia unawareness.
Keywords: Counterregulation, hypoglycemia, diabetes, hypoglycemia unawareness
Introduction
Iatrogenic hypoglycemia has been the main hindrance to the intensive treatment of diabetes mellitus. Insulin therapy aimed at maintaining close-to-normal glycemia can diminish the microvascular complications of type 1 and type 2 diabetes.1,2 However such tight glycemic control will also increase the frequency of hypoglycemia. For instance, episodes of hypoglycemia of such severity as to require the aid of a third party were approximately three times more frequent in subjects with type 1 diabetes randomized to the intensive treatment arm in the Diabetes Control and Complications Trials compared to those randomized to conventional treatment.1 One of the most worrisome aspects of hypoglycemia is that it can progress to an alteration in consciousness.1,3 Thus, the benefit of glycemic control must be balanced with avoidance of the detrimental effects of hypoglycemia. To achieve this balance, it is essential to understand the complex neuroendocrine response to hypoglycemia—and the alteration of this response in the setting of diabetes mellitus.
In this review, we will define hypoglycemia and outline the complex neuroendocrine response to it. We will discuss the changes that occur in the counterregulatory response to hypoglycemia in the setting of types 1 and 2 diabetes. Finally, we will discuss the syndrome of hypoglycemia unawareness and explore potential mechanisms for its development.
Definition of hypoglycemia
Plasma glucose concentrations are normally maintained within a narrow physiologic range via the intricate interplay of neural, hormonal and cellular factors. Hypoglycemia—when plasma glucose drops below the normal physiologic range—becomes clinically significant when accompanied by the signs and symptoms listed in Table 1.4,5 To ascertain that these signs and symptoms are due to hypoglycemia generally requires that they disappear following treatment with oral or parenteral glucose. This constellation of events was described by Whipple6 and has proven to be most helpful when hypoglycemia occurs in a non-diabetic person who is not on insulin or an insulin secretagogue, although it is frequently found in patients with diabetes as well. The biochemical definition of hypoglycemia is variable: some textbooks have defined hypoglycemia as a plasma glucose concentration of less than 45 to 55 mg/dL (2.5 – 3.1 mmol/L),7,8 whereas other authorities have defined hypoglycemia to occur at higher or lower plasma glucose levels. In 2004, the American Diabetes Association assembled a workgroup whose role, in part, was to determine how hypoglycemia should be defined. The workgroup formulated a more conservative biochemical definition for most episodes of hypoglycemia: a plasma glucose concentration at or below 70 mg/dL (3.9 mmol/L). The group further classified episodes of hypoglycemia into five distinct categories: severe hypoglycemia (an event causing such neurological changes as to require the aid of another person); documented symptomatic hypoglycemia (a plasma glucose at or less than 70 mg/dL accompanied by typical symptoms of hypoglycemia), asymptomatic hypoglycemia, probable symptomatic hypoglycemia, and relative hypoglycemia (symptoms suggestive of hypoglycemia with a measured plasma glucose concentration greater than 70 mg/dL).9 These definitions will be used in this review.
Table 1.
Symptoms and signs of hypoglycemia
| Symptoms | |
|---|---|
| Neurogenic | Neuroglycopenic |
| Shakiness | Warmth |
| Tremulousness | Weakness |
| Palpitations | Difficulty in thinking |
| Nervousness/anxiety | Difficulty speaking |
| Sweating | Confusion |
| Hunger | Tiredness |
| Tingling | Drowsiness |
| Stupor | |
| Seizures | |
| Coma | |
| Death | |
| Signs | |
| Autonomic activation | Neuroglycopenia |
| Pallor | Disorientation |
| Diaphoresis | Hypothermia |
| Increased heart rate | Focal neurological deficits |
| Increased systolic blood pressure |
Unresponsiveness |
Neuroendocrine responses to hypoglycemia
The counterregulatory factors
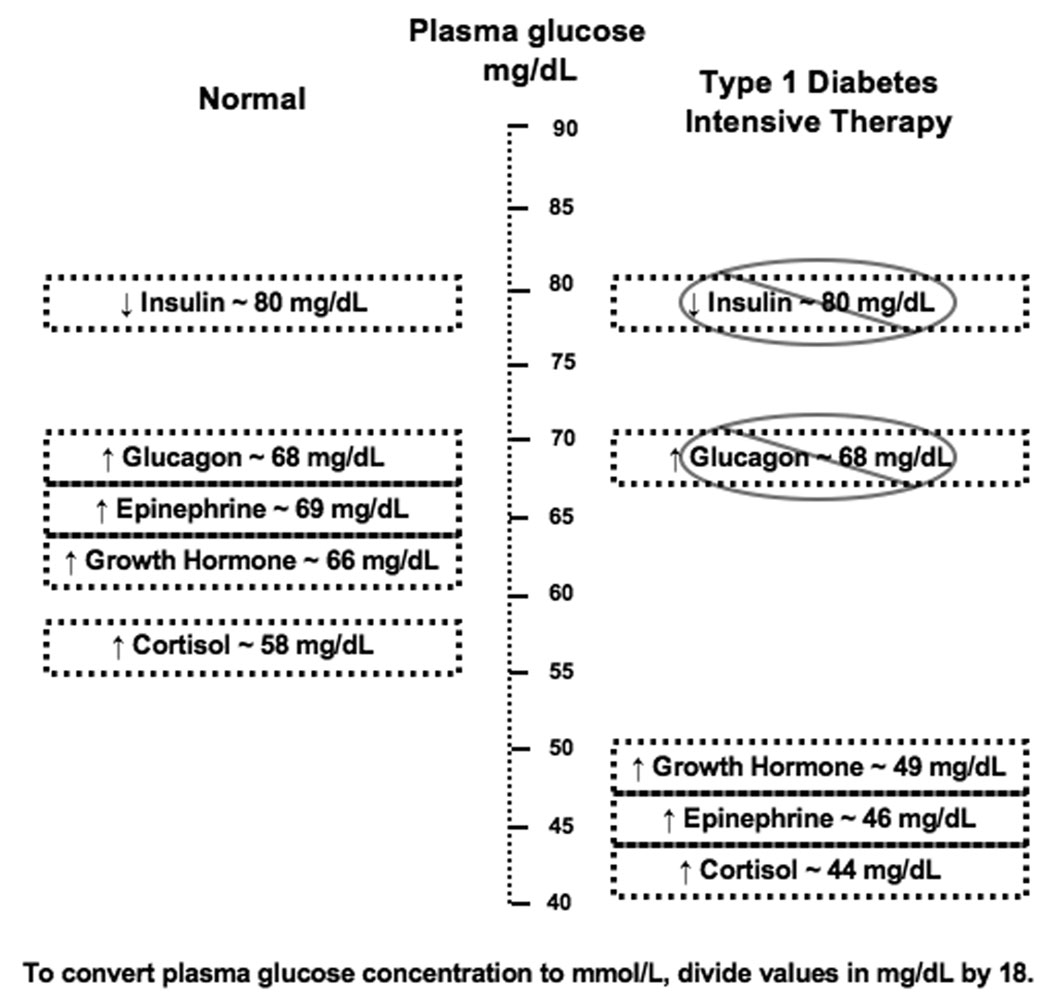
The central nervous system relies upon plasma glucose as its primary energy source. It therefore counteracts reduced blood glucose concentration with a well-orchestrated response to halt further progression of hypoglycemia and effect a prompt recovery in blood glucose concentration. In healthy humans, the initial response to prevent a decline in blood glucose concentration is a reduction in insulin secretion which begins while plasma glucose concentration is still in the physiologic range, at approximately 80 mg/dL (4.4 mmol/L).10 Glucagon and epinephrine are secreted as glucose levels fall slightly below the physiologic range, at approximately 68 mg/dL (3.8 mmol/L).10,11,12 Additionally, there is activation of the autonomic nervous system which increases the amounts of norepinephrine at the nerve terminals and epinephrine in the circulation. Beyond the dissipation of insulin, glucagon plays the primary role in the correction of hypoglycemia while epinephrine has a secondary role, as shown by experiments in which recovery from acute hypoglycemia was studied in healthy volunteers selectively rendered deficient of glucagon and/or epinephrine.13,14 Glucagon is secreted by pancreatic α-cells and acutely raises plasma glucose concentration by stimulating hepatic glucose production via glycogenolysis and gluconeogenesis. Epinephrine acts on alpha and beta adrenergic receptors at multiple end organs to effect a more sustained increase in plasma glucose concentration: epinephrine increases glycogenolysis and gluconeogenesis at the liver; reduces insulin secretion while increasing glucagon release from the pancreatic islets; reduces glucose uptake and utilization and increases glycolysis by muscle; and increases lipolysis in adipose tissue.15 Such redundancy in counterregulation, with the secretion of multiple factors within a narrow glycemic threshold, provides a failsafe system such that one mechanism of counterregulation—if it fails—can be supplanted by another.
As glucose levels fall further, other counterregulatory factors are activated. Secretion of growth hormone occurs at a plasma glucose threshold of approximately 66 mg/dL (3.7 mmol/L) and secretion of cortisol at approximately 58 mg/dL (3.2 mmol/L).11,12 Growth hormone and cortisol induce changes in metabolic processes over longer periods of time (hours) by stimulating lipolysis in adipose tissue and ketogenesis and gluconeogenesis in the liver. These two hormones do not have an immediate role in the recovery from hypoglycemia. Rizza et al. studied the effects of pharmacologically induced deficiencies of glucagon, growth hormone, and catecholamines on the recovery from hypoglycemia in healthy subjects, while also isotopically determining the rates of glucose appearance and disappearance. Those who were rendered selectively growth hormone deficient during hypoglycemia had plasma glucose concentrations, glucose appearance rates, and glucose disappearance rates that were similar to those observed in control studies in which no hormone deficiencies were induced.14 Feldman et al. had similar findings in their study of 4 healthy subjects who were rendered pharmacologically cortisol and growth hormone deficient during hypoglycemia.16 While growth hormone and cortisol may not participate in the immediate recovery from acute hypoglycemia, these hormones have more prominent roles in the setting of prolonged hypoglycemia: they have been shown to limit the degree of hypoglycemia that occurs in healthy humans during prolonged intravenous insulin infusion.17,18 In fact, blockade of the growth hormone and cortisol responses to hypoglycemia has been shown to result in lower plasma glucose levels after prolonged hypoglycemia—despite a compensatory increase in epinephrine response.17,18 Boyle and Cryer, in their comparison of healthy controls to patients who were cortisol and growth hormone deficient due to hypopituitarism, also found that growth hormone and cortisol deficient subjects had significantly lower plasma glucose concentrations 12 hours after continuous insulin infusion to induce hypoglycemia. Interestingly, Boyle and Cryer found that both control subjects and those with hypopituitarism had comparable rates of glucose recovery from hypoglycemia after discontinuation of insulin infusions.19
Symptoms of hypoglycemia
Symptoms of hypoglycemia are typically categorized as neurogenic and neuroglycopenic (Table 1). Neurogenic symptoms stem from the physiological changes that result in activation of the autonomic nervous system during hypoglycemia. These symptoms are key to the perception of hypoglycemia and include increased sweating, hunger, tingling (mediated by activation of the cholinergic system) and the perception of shakiness/tremulousness, heart pounding, and nervousness/anxiety (mediated by activation of the adrenergic system). Neuroglycopenic symptoms result from the brain’s deprivation of glucose during hypoglycemia. Neuroglycopenic symptoms are more difficult to perceive and include warmth, weakness, difficulty thinking/confusion, and tiredness/drowsiness.4 The ultimate and most severe neuroglycopenic symptoms are coma and death. Mitrakou et al. studied the hierarchy of glycemic thresholds for the secretion of counterregulatory factors, development of symptoms, and development of cerebral dysfunction in healthy human subjects during hypoglycemia. They discovered that classically autonomic symptoms developed at a higher threshold [at a plasma glucose concentration of approximately 58 mg/dL(3.2 mmol/l)] while neuroglycopenic symptoms and decline in cognitive function tests developed at significantly lower plasma glucose thresholds, approximately 51 and 49 mg/dL (2.8 and 2.7 mmol/l), respectively.12
The role of the central nervous system in counterregulation
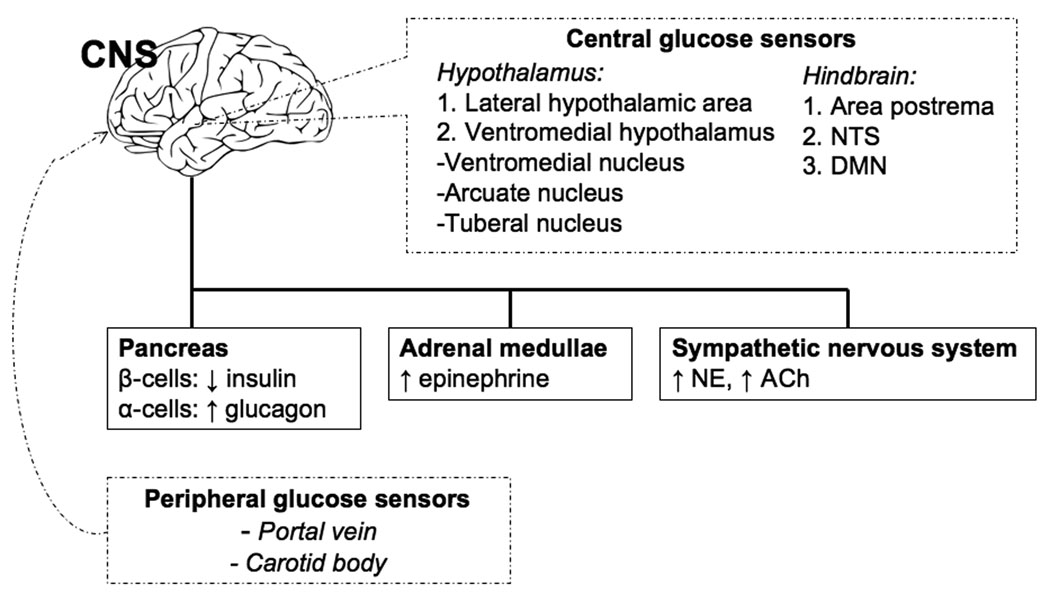
Animal studies have provided insight into the role of the CNS—itself dependent on glucose as its primary fuel—in the counterregulatory response. The CNS can be regarded as the great integrator of counterregulatory responses. It processes information on the state of local and peripheral glucose concentration and then coordinates the appropriate neuroendocrine, autonomic, and behavioral responses to hypoglycemia.20 The CNS detects fluxes in glucose concentration via glucose sensing neurons, which have been identified in the periphery and in the brain. Peripheral glucose sensors involved in the counterregulatory response are located in the hepatic portal-mesenteric vein and the carotid body.21,22 The central glucose sensing neurons are widely distributed in the brain but are most represented in areas involved in the regulation of neuroendocrine function, nutrient metabolism, and energy homeostasis: the hypothalamus (the lateral hypothalamic area and the ventromedial hypothalamus—which includes the ventromedial, arcuate and tuberal nuclei)23,24,25 and the hindbrain (the area postrema, the nucleus of the solitary tract, and the dorsal motor nucleus of the vagus).26,27,28 What makes these neurons glucose-sensing is that glucose, beyond serving as a fuel for these cells, also serves as a regulator of their activity. Thus, these neurons can be glucose-excited (GE), increasing their activity as ambient glucose levels rise, or glucose-inhibited (GI), decreasing their activity as ambient glucose levels rise. Multiple glucose sensing mechanisms have been proposed to exist in GE and GI neurons. These sensing mechanisms have been compared to those of β- and α-cells in the pancreatic islet since components of the islet glucose-sensing mechanism (GLUT 2 glucose transporter, glucokinase, and KATP channels) have all been identified in glucose-sensing neurons.29,30 Ultimately, glucose metabolism induces a change in the membrane potential of the neuron to stimulate neurotransmitter release and/or increase action potential frequency so that the signal can be relayed to downstream neurons. Multiple neurotransmitters have roles in relaying signals from glucose-sensing neurons. In animal studies, systemic hypoglycemia has been shown to increase extracellular concentrations of norepinephrine and γ-aminobutyric acid (GABA) in the ventromedial hypothalamus.31 Interestingly, pharmacologic antagonism of the GABA receptor in the ventromedial hypothalamus amplifies the counterregulatory response (specifically the glucagon and epinephrine responses), whereas pharmacologic agonism of the GABA receptor has the opposite effect.32 Perfusion of the ventromedial hypothalamus with glucose during systemic hypoglycemia prevents local increase in extracellular norepinephrine levels.33 Intracerebroventricular administration of the norepinephrine receptor agonist clonidine increases serum glucose concentrations in animals. Conversely, antagonism of the norepinephrine receptor (via yohimbine) blocks the hyperglycemic response to glucoprivation induced by 2-deoxyglucose.34 Other neurotransmitters such as glutamate35 and nitric oxide36,37 potentially also have roles in relaying signals from glucose sensing neurons. Eventually, the information on ambient glucose concentration that is derived from the widespread peripheral and central glucose sensing elements is integrated at the central level—primarily in networks located in the hypothalamus and hindbrain, as thoroughly reviewed by Watts and Donovan20—and then relayed to the motor neurons in the hypothalamus, hindbrain and autonomic ganglia that are actually responsible for effecting autonomic and neuroendocrine responses in the adrenal medulla, anterior pituitary and pancreatic islets (Figure 1).
Figure 1.
Neuroendocrine network coordinating the response to acute hypoglycemia. Dashed lines represent areas of glucose sensing. Dark lines represent areas involved in counterregulation. CNS, central nervous system; NTS, nucleus of the solitary tract; DMN, dorsal motor nucleus of the vagus; ↓, decreased; ↑, increased; NE, norepinephrine; ACh, acetylcholine.
The precise roles of peripheral and central glucose sensors in the initiation of the counterregulatory response are yet to be completely elucidated. Animal studies have shown that blockade (by various methods) at any one site of glucose sensing can blunt at least part of the counterregulatory response, suggesting there is no exclusivity of glucosensing and initiation of counterregulation to a particular glucosensing area. Central glucose sensors play a prominent role in the eliciting the counterregulatory response. The region of the brain that has garnered the most interest has been the ventromedial nucleus of the hypothalamus. Bilateral ventromedial hypothalamic destruction by local ibotenic acid injection38 and localized ventromedial hypothalamus perfusion with D-glucose39 have been shown to cause a marked reduction in the secretion of glucagon, epinephrine, and norepinephrine in response to insulin-induced hypoglycemia. Moreover, producing localized cellular glucopenia in the ventromedial hypothalamus via infusion of 2-deoxyglucose has been shown to produce a counterregulatory hormonal response similar to that seen with systemic hypoglycemia.40 There may be other brain glucose sensing areas that are also involved in mounting the counterregulatory response. Biggers et al. maintained cerebral euglycemia (by glucose infusion into both the carotid and vertebral arteries) in the setting of peripheral insulin-induced hypoglycemia and found that glucagon release and endogenous glucose production are blunted.41 However, selectively maintaining euglycemia in either the carotid or the vertebral arteries during peripheral insulin-induced hypoglycemia only minimally attenuated the counterregulatory response, suggesting that multiple brain areas and redundant central pathways are involved in counterregulation.42 Indeed, insulin-induced hypoglycemia and cellular glucose deprivation (by 2-deoxyglucose infusion) induce Fos protein immunoreactivity, which is an indicator of neuronal activation, in both the hypothalamus and the hindbrain.43,44 The hindbrain appears to have a role in glucosensing and the coordination of the counterregulatory response. DiRocco et al. have demonstrated that cellular glucose deprivation (induced by 2-deoxyglucose infusion in the brainstem) can produce sympathoadrenal activation in decerebrate rats. This suggests that a hindbrain-mediated reflexive counterregulatory response that occurs without hypothalamic contribution may exist.45
While most insights into glucose sensing and the coordination of counterregulation are derived from animal studies, human studies have also advanced our knowledge in this area. Human studies of brain activation during hypoglycemia have shown a fairly consistent core network of brain areas that are activated during hypoglycemia, including the hypothalamus, brainstem, anterior cingulate cortex, uncus, putamen, medial frontal gyrus and posterior cingulate (areas involved in glucosensing, initiation of counterregulatory response and cognitive functioning during hypoglycemia). Page et al. reported that in healthy humans, cerebral blood flow to the hypothalamus increased during an episode of hypoglycemia before there was a significant elevation in counterregulatory hormones levels.46 Musen et al., using functional magnetic resonance imaging, noted that in healthy humans, hypothalamic activation during hypoglycemia occurred at a mean glucose level of 68 mg/dL (3.8 mmol/L), which is the level that is typically associated with counterregulatory hormone release.47 Teves et al., who induced more pronounced hypoglycemia [mean blood glucose of 54 mg/dL (3.0 mmol/L)] than Musen et al., also reported thalamic activation along with activation in the medial prefrontal cortex using positron emission tomography to quantify cerebral blood flow, an index of brain activation.48
The peripheral glucose sensors may also contribute to counterregulation. In animal studies, normalizing glucose levels in the portal-mesenteric vein during graded insulin-induced hypoglycemia49 or ablation of portal vein afferents50,51 have been shown to blunt the sympathoadrenal counterregulatory response. Koyama et al. found that dogs with carotid body resections had attenuated glucagon and cortisol secretion and decreased endogenous glucose production after insulin-induced hypoglycemia.52 Saberi et al. found that glucose sensors in the portal-mesenteric vein play a more significant role in initiating sympathoadrenal responses to slow-onset hypoglycemia rather than fast-onset hypoglycemia.53 However, experiments designed to examine the role of peripheral glucose sensing in counterregulation in humans have yielded inconsistent findings. While some studies have shown that oral glucose loading (to elevate portal glucose concentration only) during a hyperinsulinemic-hypoglycemic clamp can suppress54 or augment55 the counterregulatory response, others have shown that increasing portal vein glucose concentrations during hypoglycemia has no effect on counterregulatory or symptomatic responses to hypoglycemia.56 Some of these inconsistencies may be related to subtle methodologic differences in protocols.57 Beyond their function in glucose sensing in the post-prandial state, the role of peripheral glucose sensors in the initiation of the counterregulatory response during hypoglycemia is yet to be clarified.
Factors that affect normal counterregulation
Multiple factors contribute to the intensity of the counterregulatory response in healthy humans. The recurrence of hypoglycemia itself has been shown to affect counterregulation. One episode of hypoglycemia is sufficient to blunt the counterregulatory response to subsequent hypoglycemia.58,59,60 Studies have also shown that antecedent exercise can blunt the neuroendocrine counterregulatory responses to subsequent hypoglycemia.61 Augmentation of the hypothalamic-pituitary-adrenal axis (via infusions of exogenous cortisol62 and adrenocorticotrophic hormone63) and stimulation of both type 1 (with fludrocortisone) and type 2 (with dexamethasone) glucocorticoid receptors64 has been shown to attenuate the counterregulatory response to subsequent hypoglycemia in healthy humans. Thus, it can be hypothesized that activation of the hypothalamic-pituitary-adrenal axis may be the common mechanism by which some of the aforementioned factors (whether exercise or antecedent hypoglycemia) exert effects on the counterregulatory response. Age appears to blunt some facets of the counterregulatory response, with Meneilly et al. demonstrating that healthy elderly subjects had impaired glucagon and epinephrine responses and reduced awareness of autonomic symptoms during insulin-induced hypoglycemia when compared to younger healthy subjects.65 The counterregulatory response appears to vary by gender. Fanelli et al. observed that healthy females subjected to insulin-induced hypoglycemia had lower responses (but similar glycemic thresholds) in glucagon, epinephrine and growth hormone secretion than their male counterparts.10 The time of day appears to influence several components of the neuroendocrine response to hypoglycemia, with the most notable being an enhancement in epinephrine and cortisol responses during early nighttime hypoglycemia.66 Finally, hyperinsulinemia itself can suppress the counterregulatory response to hypoglycemia, an important point to bear in mind as we discuss changes in counterregulation related to diabetes.67
Neuroendocrine responses to hypoglycemia in type 1 diabetes
Type 1 diabetes alters the normal counterregulatory response to hypoglycemia in a variety of ways (Figure 2). Firstly, due to exogenous insulin therapy, patients with type 1 diabetes cannot reduce systemic insulin levels as blood glucose concentrations begin to decline. Thus, the initial decrement in insulin that normally occurs as a response to prevent further decline in blood glucose concentration is impaired in type 1 diabetes. Secondly, the expected increment in glucagon secretion in response to hypoglycemia is compromised, even though glucagon responses to stimuli other than hypoglycemia remain generally intact.68,69 The loss of the paracrine interaction between α- and β-cells in the pancreatic islet has been posited as a cause of the loss of hypoglycemia-induced glucagon secretion in type 1 diabetes. Several studies have demonstrated that α-cell secretion of glucagon is under tonic inhibition by insulin produced from nearby β-cells and that a reduction in intraislet insulin levels during hypoglycemia reverses this tonic inhibition and stimulates glucagon secretion.70,71 These findings suggest that, as β-cell mass declines over the course of diabetes, loss of insulin secretion and loss of insulin flux dampens the paracrine stimulation of glucagon secretion in insulin-deficient patients with type 1 diabetes. Finally, the response of epinephrine to a given level of hypoglycemia is blunted and the glycemic threshold for its secretion is shifted to lower plasma glucose concentrations in well-controlled type 1 diabetes,69,72,73 creating the situation where the adrenergic symptoms of hypoglycemia fail to appear until the blood glucose drops to the lowered threshold that elicits the epinephrine response. This change in epinephrine response does not appear to be related to a structural abnormality of the adrenal medullae74 since patients with defective epinephrine response to hypoglycemia have normal epinephrine responses to exercise, standing or a meal.75,76 The change in epinephrine response is most likely the result of preceding hypoglycemia,77,73 which shifts glycemic thresholds for initiation of counterregulation. This hypothesis is further supported by the following two findings: even healthy individuals have been shown to have a decreased epinephrine response to subsequent hypoglycemia less than a day after an episode of antecedent hypoglycemia;59 and some of the blunting of the epinephrine response in type 1 diabetes is ameliorated by avoidance of hypoglycemia,78 suggesting that adrenal medullary function is intact. And while blunted epinephrine response is demonstrable in patients with type 1 diabetes who otherwise do not have signs, symptoms, or cardiovascular reflex abnormalities suggestive of autonomic dysfunction,77 there does appear to be an additional effect of autonomic neuropathy in the diminution of epinephrine response to hypoglycemia.77,79,80 In summary, the first, second and third defenses against hypoglycemia—namely, the dissipation of insulin and increments in glucagon and epinephrine—are impaired in type 1 diabetes, causing the clinical syndrome of defective glucose counterregulation.74,81 This has significant clinical implications for affected patients: patients with type 1 diabetes, with combined deficiencies of the glucagon and epinephrine responses can have a 25-fold increase in risk for severe iatrogenic hypoglycemia during intensive insulin therapy when compared to patients with type 1 diabetes who have deficient glucagon but normal epinephrine responses to hypoglycemia.74
Figure 2.
Counterregulatory factors and glucose threshold for their secretion in the normal setting and in type 1 diabetes
These differences in the neurohormonal response to hypoglycemia in patients with type 1 diabetes are accompanied by changes in activation of brain regions associated with glucosensing and initiation of the counterregulatory response. Dunn et al. studied 13 men with type 1 diabetes (6 with hypoglycemia awareness and 7 with hypoglycemia unawareness). They measured subjects’ regional brain metabolism by [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) during euglycemia and hypoglycemia. During hypoglycemia, subjects with type 1 diabetes and hypoglycemia awareness had increased FDG uptake (and therefore increased brain metabolism and neuronal activation) in multiple areas that may be involved in mounting the behavioral responses to hypoglycemia, including the left amygdala, bilateral ventral striatum, occipital cortex, brainstem and bilateral orbifrontal cortex.82 Musen et al., in their comparison of brain activation during hypoglycemia in patients with type 1 diabetes and controls, found that while there was activation of a core network of brain regions in both groups, subjects with type 1 diabetes showed increased activation in the superior temporal gyrus and insula relative to control subjects.47 These differences in areas of activation are of uncertain significance. However it is also notable that Musen et al. demonstrated that subjects with diabetes and higher hemoglobin A1c (HbA1c) levels had significantly more hypothalamic activation than those with lower HbA1c levels, implying that stricter glycemic control (which has been shown to alter the neurohormonal response to hypoglycemia) may also alter activation of the hypothalamus during hypoglycemia.
Neuroendocrine responses to hypoglycemia in type 2 diabetes
There have been fewer studies on the neuroendocrine responses to hypoglycemia in patients with type 2 diabetes. Some investigations have shown impaired counterregulatory responses to hypoglycemia in patients with type 2 diabetes while others have not. For instance, Bolli et al. reported attenuated glucagon, growth hormone, and cortisol responses (but no change in epinephrine response and increased norepinephrine release) during subcutaneous insulin-induced hypoglycemia in patients with type 2 diabetes who had no clinical evidence of autonomic neuropathy.83 Other studies have reported reduced glucagon but increased epinephrine responses to hypoglycemia in type 2 diabetes, with variable effects on the remaining counterregulatory hormones.84,85 In contrast, Boden et al. observed that although basal glucagon concentrations were higher in patients with type 2 diabetes than in controls, there was no difference in the glucagon and epinephrine responses to hypoglycemia between the two groups.86 Similarly, Polonsky et al. and Heller et al. reported normal responses of glucagon, epinephrine, cortisol and growth hormone in patients with type 2 diabetes subjected to intravenous insulin-induced hypoglycemia when compared to controls.87,88 The most we can conclude from these disparate findings is that in the setting of hypoglycemia, glucagon response may be normal or blunted, while epinephrine response remains intact, if not augmented in patients with type 2 diabetes. The variability in these findings may in part be related to the variable methods of hypoglycemia induction, the recruitment of patients with autonomic neuropathy in some studies, and other factors (including age, glycemic control, and gender) that could influence counterregulation. The variation of glucagon response may also be related to variation in subjects’ residual β-cell function, which can decline with longer duration of type 2 diabetes. Israelian et al. found that even moderately insulin-deficient subjects with type 2 diabetes (as defined by homeostasis model assessment of β-cell function) had a slower decline in insulin secretion and reduced glucagon and growth hormone responses to hypoglycemia when compared to healthy controls—although the responses of epinephrine, norepinephrine, and cortisol were similar in both groups.89 To further test the hypothesis that the glucagon response to hypoglycemia may be reduced in subjects with more pronounced insulin-deficiency, Segel et al. studied the counterregulatory response to hypoglycemia in patients with type 2 diabetes treated with oral hypoglycemics and “insulin-deficient” patients with type 2 diabetes treated with insulin (who had low C-peptide levels) and controls. The insulin-deficient subjects with type 2 diabetes had a near-absence of the glucagon response to hypoglycemia when compared to controls and those with type 2 diabetes treated with oral hypoglycemics.90
As previously discussed, antecedent hypoglycemia itself has been shown to blunt the counterregulatory response to subsequent hypoglycemia in healthy individuals and in subjects with type 1 diabetes. However, the findings in patients with type 2 diabetes have been variable. While some researchers have found no effect of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia,91 others have reported a shift in the glycemic thresholds for onset of counterregulatory responses and hypoglycemic symptoms in patients with type 2 diabetes subjected to subsequent hypoglycemia.90 As in type 1 diabetes, tightening glycemic control in individuals with type 2 diabetes shifts the threshold for counterregulatory hormone release to lower plasma glucose concentrations during hypoglycemia. Davis et al. committed subjects with type 2 diabetes to 6 months of intensive therapy to lower mean HbA1c from 10.2 ± 0.5 to 6.7 ± 0.3%. After intensive therapy, patients had reduced symptomatic and epinephrine responses to insulin-induced hypoglycemia.92 Similarly, earlier studies have shown that tightening of glycemic control in type 2 diabetes can shift the glycemic threshold for the epinephrine and symptomatic response to lower plasma glucose concentrations, reduce the degree of epinephrine response to hypoglycemia,93,94 and blunt cortisol response to hypoglycemia.93 There appears to be a positive correlation between HbA1c and the glucose level required for epinephrine and norepinephrine secretion.95
Patients with type 2 diabetes are exposed to a broad variety of therapies for glycemic management and investigators have studied the counterregulatory responses to hypoglycemia in patients taking different therapies for type 2 diabetes. Bolli et al. found similar counterregulatory responses to hypoglycemia in their study of type 2 patients who were insulin-treated and non-insulin treated.83 Conversely, Landstedt-Hallin et al. reported that type 2 patients treated with glibenclamide (a sulfonylurea) and insulin had a blunting of the glucagon response (but not the epinephrine response) to hypoglycemia when compared to type 2 patients given insulin treatment only.96 This effect may be related to the aforementioned hypothesis that high intraislet insulin concentrations—in this case related to sulfonylurea therapy—may inhibit glucagon release in response to low blood glucose. However, Choudhary et al. did not have similar findings in the sulfonylurea-treated patients with type 2 diabetes they studied. In their study, although the glycemic threshold for development of symptoms of hypoglycemia shifted to higher plasma glucose concentrations in the sulfonylurea-treated type 2 patients compared to matched insulin-treated type 2 patients or healthy controls, the glycemic threshold for the release—and the magnitude of peak responses—of counterregulatory hormones was not different between all these groups.97
Hypoglycemia unawareness
Clinical description
As discussed above, recent antecedent hypoglycemia can cause defective glucose counterregulation in healthy individuals and in patients with both type 1 and type 2 diabetes.98,60,59,77,73,90 With exposure to antecedent hypoglycemia, the glycemic threshold for counterregulation shifts to lower plasma glucose concentrations, as does the threshold for development of the symptoms of hypoglycemia and the development of cognitive dysfunction.99 This phenomenon—termed hypoglycemia-associated autonomic failure—manifests primarily as reduced epinephrine in the setting of an absent or attenuated glucagon and reduced symptomatic responses to recurrent iatrogenic hypoglycemia, with glycemic thresholds for initiation of these responses shifting to lower plasma glucose concentrations.81 In such patients, persistent exposure to iatrogenic hypoglycemia can lead to progression to hypoglycemia unawareness. In the absence of neurogenic symptoms which previously alerted the patient with diabetes to the need to correct progressing hypoglycemia, neuroglycopenic symptoms (i.e. the alteration in cognition, alteration in mental status, seizure and eventual coma) become the first manifestations of hypoglycemia, increasing the risk of morbidity associated with hypoglycemia.
Potential mechanisms
The etiology of hypoglycemia unawareness is not yet clearly elucidated and has been an area of great research interest. Hypoglycemia unawareness may stem from altered sensing of hypoglycemia by the brain. There may be a functional change in the brain’s glucose sensing neurons or an alteration in neurotransmission that results from antecedent hypoglycemia. Additionally, the brain may not sense glucose deprivation if its metabolism is supported by extra fuel during hypoglycemia, whether the fuel is in the form of excess glucose, glycogen, lactate or ketones. Alternately, the development of hypoglycemia unawareness may be related to impaired coordination of the counterregulatory response. In this case, although the brain may sense hypoglycemia, it may not be able to mount an appropriate neuroendocrine response. Likely, there are multiple mechanisms contributing to the development of hypoglycemia unawareness. We shall discuss these potential mechanisms in detail below.
As mentioned above, the development of hypoglycemia unawareness may in part be related to impaired coordination of the counterregulatory response. It is important to note that hypoglycemia unawareness is distinct from diabetic autonomic neuropathy. Unlike autonomic neuropathy, the defect in counterregulation seen in hypoglycemia unawareness is dynamic. It can be induced by recurrent antecedent hypoglycemia and improved (and to a certain extent reversed) by avoidance of hypoglycemia.78,100,101 Although, interestingly, the improvement in clinical hypoglycemia unawareness (i.e. the restoration of neurogenic symptoms of hypoglycemia) is not consistently related to restoration of the epinephrine response to hypoglycemia.102
Regional changes in brain glucose sensing
Regional changes in glucose sensing areas during hypoglycemia have been demonstrated in studies of subjects with hypoglycemia unawareness. Cranston et al. compared regional brain uptake of the labeled glucose analog [18F]fluorodeoxyglucose (FDG) using positron emission tomography (PET) in hypoglycemia unaware and hypoglycemia aware subjects with type 1 diabetes at euglycemia and during hypoglycemia. They found that the hypoglycemia unaware group had reduced FDG uptake in the subthalamic brain region—an area implicated in glucosensing—in response to hypoglycemia.103 The same group, in their study of brain activation in type 1 diabetes with and without hypoglycemia unawareness found that hypoglycemia unaware subjects had attenuation of FDG uptake in the amygdala, occipital cortex, cerebellum, and brainstem during hypoglycemia.82 Arbelaez et al. demonstrated increase in regional cerebral blood flow, as measured by [15O]water and positron emission tomography, in the dorsal midline thalamus during hypoglycemia in healthy individuals exposed to recurrent hypoglycemia compared to controls.104 The authors conclude that, in the setting of recurrent hypoglycemia, activation of the dorsal midline thalamus inhibits the counterregulatory response to subsequent hypoglycemia. These changes seen on imaging studies may reflect a change in neuronal function in response to recurrent hypoglycemia. In a rodent study, cells in the arcuate nucleus show an apoptotic response to single and recurrent episodes of hypoglycemia, with an associated reduction in the expression of neuropeptide Y (NPY) and proopiomelanocortin (POMC) mRNA.105 These neuronal changes may also be accompanied by changes in neurotransmission. Rodent studies have shown that the activity of hypothalamic tyrosine hydroxylase—which serves as an index of acute noradrenergic activation—is significantly blunted after recurrent hypoglycemia.106 Further, studies in a rodent model of recurrent hypoglycemia have shown that GABA levels in the ventromedial hypothalamus are significantly higher at baseline and decrease to a lesser degree in recurrently hypoglycemic rodents than in controls during a bout of hypoglycemia.107 Hence, focal impairment in central glucosensing and neurotransmission may contribute to hypoglycemia unawareness.
Increased glucose transport to the brain
Following an episode of hypoglycemia there appears to be an upregulation of glucose transport into the brain. This phenomenon may increase the availability of glucose during subsequent episodes of hypoglycemia. Higher-than-normal levels of brain glucose may sustain cerebral metabolism during subsequent hypoglycemia and may contribute to the development of hypoglycemia unawareness. While rodent studies of shorter-term (up to 3 days) hypoglycemia have shown decreased glucose concentration in the ventromedial hypothalamus,108 rodent studies of longer-standing hypoglycemia (up to 2 weeks) have shown increased brain glucose uptake and blood-brain barrier GLUT-1—which mediates passage of glucose across the blood-brain barrier—mRNA and protein expression.109 Other rodent studies have confirmed that, while acute hypoglycemia has no effect on brain glucose transport, chronic hypoglycemia increases brain glucose transport110 and GLUT-1 mRNA and protein expression.111,112 Human studies have shown that brain glucose uptake is maintained at normal, if not augmented levels, despite relative glucose deprivation due to recurrent antecedent hypoglycemia. In their study of brain glucose uptake in healthy humans subjected to 24 hours of between-meal hypoglycemia, Segel et al. found that (when compared to controls) the rate of blood-to-brain glucose transport and cerebral glucose metabolism were unchanged in the group exposed to recurrent antecedent hypoglycemia, despite a marked reduction in glucose availability.113 Boyle et al. studied brain glucose uptake (based on cerebral blood flow and brain arteriovenous glucose difference) in healthy humans before and after 56 hours of between-meal hypoglycemia. They found that brain glucose uptake and cerebral function were maintained at normal levels even after the prolonged hypoglycemia.114 The same group studied brain glucose uptake in well-controlled (and presumably recurrently hypoglycemic) patients with type 1 diabetes and found that this group had normal brain glucose uptake before and during hypoglycemia. Conversely, less tightly controlled patients with type 1 diabetes and normal controls had a decrement in brain glucose uptake during hypoglycemia.115 Further, our group has found that brain glucose concentrations (as measured by in vivo 1H nuclear magnetic resonance (NMR) spectroscopy) during a hyperglycemic clamp were significantly higher in subjects with type 1 diabetes and hypoglycemia unawareness than in healthy controls, again suggesting that changes in brain glucose transport may arise as a result of recurrent hypoglycemia.116
Utilization of alternate fuels
Hypoglycemia unawareness may be related to the brain’s utilization of alternate fuels during hypoglycemia, allowing it to maintain normal metabolism in the face of low plasma glucose concentration. These alternate fuels may include glycogen, lactate and ketones. Glycogen is the major energy reserve in brain. Although it is localized almost exclusively to astrocytes, glycogen can be metabolized to lactate and then exported to neurons for energy such that—in in vitro studies—astrocyte stores of glycogen can improve neuronal survival during glucose deprivation.117,118,119 It has been hypothesized that supercompensation of brain glycogen content following an episode of hypoglycemia may provide the brain with additional fuel stores during subsequent hypoglycemia. Our group has demonstrated that brain glycogen content is higher following hypoglycemia than it is following euglycemia in both rats and humans.120,121 However, Herzog et al. did not find brain glycogen content to be different in animals subjected to recurrent hypoglycemia than in animals maintained at euglycemia, although they did find that animals subjected to recurrent hypoglycemia displayed faster recovery of brain glucose and glycogen levels after an episode of hypoglycemia than did animals not exposed to previous hypoglycemia.122
The brain has been shown to upregulate acetate transport during hypoglycemia in humans with well-controlled type 1 diabetes.123 Since acetate transport occurs via monocarboxylic acid transporters, which are also used for lactate and ketones, it has been hypothesized that lactate and ketone levels in the brain may also be increased in the setting of recurrent hypoglycemia, thereby providing alternative fuels to be used during subsequent periods of glucose deprivation. In rodents, the perfusion of the ventromedial hypothalamus with L-lactate markedly decreased counterregulatory hormone responses to hypoglycemia.124 In healthy humans, infusion of lactate during hypoglycemia has been shown to diminish the neuroendocrine responses to hypoglycemia, lower the glucose concentration at which these responses begin, and lower the glucose concentration at which brain function deteriorates.125 Similarly, ketones have been proposed as an alternate energy source during hypoglycemia. Amiel et al. studied healthy subjects who were infused with saline or beta-hydroxybutyrate. They found that the glycemic threshold for the epinephrine response occurred at a lower plasma glucose concentration and peak epinephrine, norepinephrine, cortisol and growth hormone responses were significantly lower during ketone infusion.126 Veneman et al. noted similar changes in the magnitude of—and the glycemic threshold for the development of—counterregulatory hormone responses, symptoms and cognitive dysfunction during hypoglycemia with beta-hydroxybutyrate infusion.127 It is notable that the presence of endogenous hyperketonemia after an episode of hypoglycemia (without exogenous ketone infusion) has not been shown to produce changes in the counterregulatory response when compared to blockade of ketone production (by acipimox, an inhibitor of lipolysis).128
Augmentation of the hypothalamic-pituitary-adrenal axis
It has been hypothesized that elevated cortisol levels—along with elevated levels of adrenocorticotrophic hormone (ACTH) and corticotrophin-releasing hormone (CRH)—in response to antecedent hypoglycemia may contribute to defective counterregulation and the development of hypoglycemia unawareness. In animal studies, rats pre-treated with CRH, ACTH or corticosterone show blunting in the counterregulatory response to subsequent hypoglycemia to a comparable degree as animals exposed to antecedent hypoglycemia. This action might be mediated in large part by CRH, since pharmacologic antagonism of the CRH receptor 1 in the same animal models reversed the blunting effect of CRH pre-treatment on the counterregulatory response.129 Yet, other animal studies have had contrary findings: Jacobson et al. have found that CRH knockout (hence CRH and glucocorticoid deficient) mice exposed to recurrent hypoglycemia do not show improvement in the blunting of the counterregulatory response during subsequent hypoglycemia when compared to control animals;130 Inouye et al. have reported that, while an episode of hypoglycemia results in increased CRH mRNA expression in insulin-treated diabetic animals exposed to an episode of hypoglycemia, exposure to antecedent hypoglycemia actually results in a less pronounced rise in CRH mRNA.131 Exogenous cortisol infusion62 and ACTH infusion63 in healthy humans have been shown to attenuate the counterregulatory response to subsequent hypoglycemia. Similarly, antecedent stimulation of both type 1 (with fludrocortisone) and type 2 (with dexamethasone) glucocorticoid receptors in healthy humans has been shown to blunt the levels of epinephrine, norepinephrine, glucagon and growth hormone response to subsequent hypoglycemia.64 Further, while normal controls exposed to antecedent hypoglycemia experience blunting of the counterregulatory response during subsequent hypoglycemia, patients with primary adrenocortical failure (and therefore an absence of a more pronounced cortisol response) exposed to antecedent hypoglycemia display no change in counterregulatory hormone response to subsequent hypoglycemia.132 Yet, infusing cortisol to achieve levels comparable to those that occur during hypoglycemia did not reduce the adrenomedullary (epinephrine) response of the neurogenic symptoms to subsequent hypoglycemia in healthy humans.133 Moreover, Goldberg et al. have found that that blockade of endogenous cortisol production during antecedent hypoglycemia does not enhance the impaired counterregulatory responses to subsequent hypoglycemia.134 Thus, the role of cortisol and the hypothalamic-pituitary-adrenal axis in defective counterregulation is yet to be fully elucidated.
The opioid signaling system
The opioid signaling system has also been hypothesized to contribute to the development of hypoglycemia unawareness by attenuating the counterregulatory response. Studies have shown that β-endorphin levels are increased after hypoglycemic stress in humans.135 Subsequent human studies of insulin-induced hypoglycemia with and without blockade of opiates by naloxone in well-controlled subjects with type 1 diabetes with defective counterregulation and in healthy controls showed augmentation in the counterregulatory response with use of naloxone—with naloxone resulting in increased epinephrine and cortisol response in controls and increased epinephrine, growth hormone, and cortisol response in the subjects with diabetes during hypoglycemia.136 Most recently, Leu et al. reported that opioid receptor blockade with naloxone during antecedent hypoglycemia prevented blunting of the counterregulatory response to subsequent hypoglycemia, suggesting that the opioid signaling system may indeed play a role in defective counterregulation.137
In summary, the potential mechanisms for the development of hypoglycemia unawareness are numerous. Impaired sensing of hypoglycemia by the brain is likely the primary contributor to hypoglycemia unawareness. This may occur as a result of structural changes in glucose sensing neurons, alterations in neurotransmission or the availability of alternate fuels for brain metabolism. Defective coordination of counterregulation may also have a role in the development of hypoglycemia unawareness. To date, cortisol and the opioid signaling system have been studied as possible causes of defective counterregulation that could contribute to hypoglycemia unawareness. Future research is needed to understand the complex sequence of events that is necessary to develop hypoglycemia-induced autonomic failure.
Conclusions
In summary, the physiologic response to hypoglycemia is a complex and well-coordinated process. In healthy humans, there is an ordered, failsafe response system that begins with a reduction in insulin secretion while blood glucose concentration is still in the physiologic range. As blood glucose concentration declines further, peripheral and central glucose sensors relay this information to central integrative centers to coordinate the secretion of counterregulatory hormones (glucagon, epinephrine, norepinephrine, growth hormone and cortisol, respectively) and avert the progression of hypoglycemia. Type 1 diabetes perturbs these counterregulatory responses: circulating insulin levels cannot be reduced (due to exogenous insulin therapy); glucagon secretion is blunted or absent; and epinephrine secretion is blunted and shifted to a lower plasma glucose concentration. Moreover, type 1 diabetes may affect central glucose sensing. Counterregulation has also been shown to be impaired in type 2 diabetes. In this setting, the glucagon response to hypoglycemia may be normal or blunted, while epinephrine response remains intact, if not augmented. Patients affected by type 1 and type 2 diabetes can develop the syndrome of hypoglycemia unawareness. Hypoglycemia unawareness develops as recurrent iatrogenic hypoglycemia shifts the glycemic threshold for counterregulation and development of hypoglycemic symptoms to lower plasma glucose concentrations. In this setting, neurogenic symptoms—which are usually the initial warning symptoms of hypoglycemia—are blunted and the first manifestation of hypoglycemia becomes neuroglycopenia. The mechanisms underlying the development of hypoglycemia unawareness may be related to both altered central sensing of hypoglycemia and impaired coordination of responses to hypoglycemia. Many facets of the coordination of the counterregulatory response and the development of hypoglycemia unawareness remain unknown and will require further study in the future.
Acknowledgements
This work was supported by NIH Grant 2 T32 DK7203-33 (to N.T.).
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.Pramming S, Thorsteinsson B, Bendtson I, Binder C. Symptomatic hypoglycaemia in 411 type 1 diabetic patients. Diabet. Med. 1991;8:217–222. doi: 10.1111/j.1464-5491.1991.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 4.Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes. 1993;42:1791–1798. doi: 10.2337/diab.42.12.1791. [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE, Davis SN, Shamoon HS. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 6.Whipple AO. The surgical therapy of hyperinsulinism. J. Int. Chir. 1938;3:237–276. [Google Scholar]
- 7.Cryer PE. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 2008. Hypoglycemia. [Google Scholar]
- 8.Masharani U, Gitelman SE. Basic and Clinical Endocrinology. East Norwalk, CT: Appleton & Lange; 2007. Hypoglycemic disorders. [Google Scholar]
- 9.American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 10.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, Di Vincenzo A, Annibale B, Lepore M, Lalli C, Sindaco P, Brunetti P, Bolli G. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia. 1994;37:797–807. doi: 10.1007/BF00404337. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J. Clin. Invest. 1987;79:777–781. doi: 10.1172/JCI112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am. J. Physiol. 1991;260:E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 13.Gerich J, Davis J, Lorenzi M, Rizza R, Bohannon N, Karam J, Lewis S, Kaplan R, Schultz T, Cryer P. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am. J. Physiol. Endocrinol. Metab. 1979;236:E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- 14.Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. J. Clin. Invest. 1979;64:62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cryer PE. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43:1378–1389. doi: 10.2337/diab.43.11.1378. [DOI] [PubMed] [Google Scholar]
- 16.Feldman J, Plonk J, Bivens C. The role of cortisol and growth hormone in the counterregulation of insulin-induced hypoglycemia. Horm. Metab. Res. 1975;7:378–381. doi: 10.1055/s-0028-1093731. [DOI] [PubMed] [Google Scholar]
- 17.DeFeo P, Perriello G, Torlone E, Ventura MM, Santeusanio F, Brunetti P, Gerich JE, Bolli GB. Demonstration of a role for growth hormone in glucose counterregulation. Am. J. Physiol. 1989;256:E835–E843. doi: 10.1152/ajpendo.1989.256.6.E835. [DOI] [PubMed] [Google Scholar]
- 18.De Feo P, Perriello G, Torlone E, Ventura MM, Fanelli C, Santeusanio F, Brunetti P, Gerich JE, Bolli GB. Contribution of cortisol to glucose counterregulation in humans. Am. J. Physiol. 1989;257:E35–E42. doi: 10.1152/ajpendo.1989.257.1.E35. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PJ, Cryer PE. Growth hormone, cortisol, or both are involved in defense against, but are not critical to recovery from, hypoglycemia. Am. J. Physiol. 1991;260:E395–E402. doi: 10.1152/ajpendo.1991.260.3.E395. [DOI] [PubMed] [Google Scholar]
- 20.Watts AG, Donovan CM. Sweet talk in the brain: Glucosensing, neural networks, and hypoglycemic counterregulation. Front. Neuroendocrinol. 2010;31:32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes. 1997;46:1521–1525. doi: 10.2337/diab.46.9.1521. [DOI] [PubMed] [Google Scholar]
- 22.Pardal R, López-Barneo J. Low glucose-sensing cells in the carotid body. Nat. Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- 23.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and Osmosensitive Neurones of the Rat Hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 24.Ono T, Nishino H, Fukuda M, Sasaki K, Muramoto K, Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res. 1982;232:494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- 25.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- 26.Funahashia M, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: In vitro study on the isolated slice preparation. Brain Res. Bull. 1993;32:531–535. doi: 10.1016/0361-9230(93)90303-s. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984;307:109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- 28.Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J. Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Routh VH, Song Z, Liu X. The role of glucosensing neurons in the detection of hypoglycemia. Diabetes. Technol. Ther. 2004;6:413–421. doi: 10.1089/152091504774198133. [DOI] [PubMed] [Google Scholar]
- 30.McCrimmon R. Glucose sensing during hypoglycemia: lessons from the lab. Diabetes Care. 2009;32:1357–1363. doi: 10.2337/dc09-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beverly JL, de Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am. J. Physiol. 2001;280:R563–R569. doi: 10.1152/ajpregu.2001.280.2.R563. [DOI] [PubMed] [Google Scholar]
- 32.Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes. 2006;55:1080–1087. doi: 10.2337/diabetes.55.04.06.db05-0958. [DOI] [PubMed] [Google Scholar]
- 33.de Vries MG, Lawson MA, Beverly JL. Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. Am. J. Physiol. 2005;289:R977–R981. doi: 10.1152/ajpregu.00403.2005. [DOI] [PubMed] [Google Scholar]
- 34.Smythe GA, Edwards SR. A role for central postsynaptic alpha 2-adrenoceptors in glucoregulation. Brain Res. 1991;562:225–229. doi: 10.1016/0006-8993(91)90625-6. [DOI] [PubMed] [Google Scholar]
- 35.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am. J. Physiol. 2007;292:R1418–R2148. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 37.Fioramonti X, Marsollier N, Song Z, Fakira KA, Patel RM, Brown S, Duparc T, Pica-Mendez A, Sanders NM, Knauf C, Valet P, McCrimmon RJ, Beuve A, Magnan C, Routh VH. Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes. 2010;59:519–528. doi: 10.2337/db09-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J. Clin. Invest. 1994;93:1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J. Clin. Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 41.Biggers DW, Myers SR, Neal D, Stinson R, Cooper NB, Jaspan JB, Williams PE, Cherrington AD, Frizzell RT. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes. 1989;38:7–16. doi: 10.2337/diab.38.1.7. [DOI] [PubMed] [Google Scholar]
- 42.Frizzell RT, Jones EM, Davis SN, Biggers DW, Myers SR, Connolly CC, Neal DW, Jaspan JB, Cherrington AD. Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes. 1993;42:1253–1261. doi: 10.2337/diab.42.9.1253. [DOI] [PubMed] [Google Scholar]
- 43.Yuan PQ, Yang H. Neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am. J. Physiol. 2002;283:E436–E448. doi: 10.1152/ajpendo.00538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res. 1998;805:41–54. doi: 10.1016/s0006-8993(98)00655-6. [DOI] [PubMed] [Google Scholar]
- 45.DiRocco RJ, Grill HJ. The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science. 1979;204:1112–1114. doi: 10.1126/science.451558. [DOI] [PubMed] [Google Scholar]
- 46.Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes. 2009;58:448–452. doi: 10.2337/db08-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musen G, Simonson DC, Bolo NR, Driscoll A, Weinger K, Raji A, Theberge J, Renshaw PF, Jacobson AM. Regional brain activation during hypoglycemia in type 1 diabetes. J. Clin. Endocrinol. Metab. 2008;93:1450–1457. doi: 10.1210/jc.2007-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teves D, Videen TO, Cryer PE, Powers WJ. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc. Natl. Acad. Sci. 2004;101:6217–6221. doi: 10.1073/pnas.0307048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donovan CM, Hamilton-Wessler M, Halter JB, Bergman RN. Primacy of liver glucosensors in the sympathetic response to progressive hypoglycemia. Proc. Natl. Acad. Sci. 1994;91:2863–2867. doi: 10.1073/pnas.91.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neuronsEndocrinol. Am. J. Physiol. Metab. 2007;293:E96–E101. doi: 10.1152/ajpendo.00415.2006. [DOI] [PubMed] [Google Scholar]
- 51.Hevener AL, Bergman RN, Donovan CM. Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes. 2000;49:8–12. doi: 10.2337/diabetes.49.1.8. [DOI] [PubMed] [Google Scholar]
- 52.Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- 53.Saberi M, Bohland M, Donovan CM. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes. 2008;57:1380–1386. doi: 10.2337/db07-1528. [DOI] [PubMed] [Google Scholar]
- 54.Smith D, Pernet A, Reid H, Bingham E, Rosenthal J, Macdonald I, Umpleby A, Amiel SA. The role of hepatic portal glucose sensing in modulating responses to hypoglycemia in man. Diabetologia. 2002;45:1416–1424. doi: 10.1007/s00125-002-0909-3. [DOI] [PubMed] [Google Scholar]
- 55.Heptulla RA, Tamborlane WV, Ma TY-Z, Rife F, Sherwin RS. Oral glucose augments the counterregulatory hormone response during insulin-induced hypoglycemia in humans. J. Clin. Endocrinol. Metabol. 2001;86:645–648. doi: 10.1210/jcem.86.2.7198. [DOI] [PubMed] [Google Scholar]
- 56.Rossetti P, Porcellati F, Lucidi P, Busciantella Ricci N, Candeloro P, Cioli P, Santeusanio F, Bolli GB, Fannelli CG. Portal vein glucose sensors do not play a major role in modulating physiological responses to insulin-induced hypoglycemia in humans. Diabetes. 2009;58:194–202. doi: 10.2337/db08-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donovan CM, Bohland MA. Hypoglycemic detection at the portal vein absent in humans or yet to be elucidated? Diabetes. 2009;58:21–23. doi: 10.2337/db08-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis SN, Shavers C, Mosqueda-Garcia R, Costa F. Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes. 1997;46:1328–1335. doi: 10.2337/diab.46.8.1328. [DOI] [PubMed] [Google Scholar]
- 59.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after one episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 60.Davis MR, Shamoon H. Counterregulatory adaptation to recurrent hypoglycemia in normal humans. J. Clin. Endocrinol. Metab. 1991;73:995–1001. doi: 10.1210/jcem-73-5-995. [DOI] [PubMed] [Google Scholar]
- 61.Galassetti P, Mann S, Tate D, Neill RA, Costa F, Wasserman DH, Davis SN. Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 2001;280:E908–E917. doi: 10.1152/ajpendo.2001.280.6.E908. [DOI] [PubMed] [Google Scholar]
- 62.Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J. Clin. Invest. 1996;98:680–691. doi: 10.1172/JCI118839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGregor VP, Banarer S, Cryer PE. Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. Am. J .Physiol. 2002;282:E770–E777. doi: 10.1152/ajpendo.00447.2001. [DOI] [PubMed] [Google Scholar]
- 64.Gustavson SM, Sandoval DA, Ertl AC, Bao S, Raj SR, Davis SN. Stimulation of both type I and type II corticosteroid receptors blunts counterregulatory responses to subsequent hypoglycemia in healthy man. Am. J. Physiol. Endocrinol. Metab. 2008;294:E506–E512. doi: 10.1152/ajpendo.00589.2007. [DOI] [PubMed] [Google Scholar]
- 65.Meneilly G, Cheung E, Tuokko H. Altered responses to hypoglycemia of healthy elderly people. J. Clin. Endocrinol. Metab. 1994;78:1341–1348. doi: 10.1210/jcem.78.6.8200936. [DOI] [PubMed] [Google Scholar]
- 66.Merl V, Kern W, Peters A, Oltmanns KM, Gais S, Born J, Fehm HL, Schultes B. Differences between nighttime and daytime hypoglycemia counterregulation in healthy humans. Metabolism. 2004;53:894–898. doi: 10.1016/j.metabol.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 67.Diamond MP, Hallarman L, Starick-Zych K, Jones TW, Connolly-Howard M, Tamborlane WV, Sherwin RS. Suppression of counterregulatory hormone response to hypoglycemia by insulin per se. J. Clin. Endocrinol. Metab. 1991;72:1388–1390. doi: 10.1210/jcem-72-6-1388. [DOI] [PubMed] [Google Scholar]
- 68.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 69.Bolli G, De Feo P, Compagnucci P, Cartechini MG, Angeletti G, Santeusanio F, Brunetti P, Gerich JE. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus: interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983;32:134–141. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- 70.Peacey SR, Rostami-Hodjegan A, George E, Tucker GT, Heller SR. The use of tolbutamide-induced hypoglycemia to examine the intraislet role of insulin in mediating glucagon release in normal humans. J. Clin. Endocrinol. Metab. 1997;82:1458–1461. doi: 10.1210/jcem.82.5.3910. [DOI] [PubMed] [Google Scholar]
- 71.Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. 2002;51:958–965. doi: 10.2337/diabetes.51.4.958. [DOI] [PubMed] [Google Scholar]
- 72.Bolli GB, Dimitriadis GD, Pehling GB, Baker BA, Haymond MW, Cryer PE, Gerich JE. Abnormal glucose counterregulation after subcutaneous insulin in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1984;310:1706–1711. doi: 10.1056/NEJM198406283102605. [DOI] [PubMed] [Google Scholar]
- 73.Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes. 1988;37:901–907. doi: 10.2337/diab.37.7.901. [DOI] [PubMed] [Google Scholar]
- 74.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II Diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 75.Hirsch BR, Shamoon H. Defective epinephrine and growth hormone responses in type I diabetes are stimulus specific. Diabetes. 1987;36:20–26. doi: 10.2337/diab.36.1.20. [DOI] [PubMed] [Google Scholar]
- 76.Rattarasarn C, Dagogo-Jack S, Zachwieja JJ, Cryer PE. Hypoglycemia-induced autonomic failure in IDDM is specific for stimulus of hypoglycemia and is not attributable to prior autonomic activation. Diabetes. 1994;43:809–818. doi: 10.2337/diab.43.6.809. [DOI] [PubMed] [Google Scholar]
- 77.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin dependent diabetes mellitus: recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J. Clin. Invest. 1993;91:819–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fanelli C, Epifano L, Rambotti AM, Pampanelli S, Di Vincenzo A, Modarelli F, Lepore M, Annibale B, Ciofetta M, Bottini P, Porcellati F, Scionti L, Santeusanio F, Brunetti P, Bolli GB. Meticulous prevention of hypoglycemia (near-)normalizes magnitude and glycemic thresholds of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with IDDM of short duration. Diabetes. 1993;42:1683–1689. doi: 10.2337/diab.42.11.1683. [DOI] [PubMed] [Google Scholar]
- 79.Bottini P, Boschetti E, Pampanelli S, Ciofetta M, Del Sindaco P, Scionti L, Brunetti P, Bolli GB. Contribution of autonomic neuropathy to reduced plasma adrenaline responses to hypoglycemia in IDDM: evidence for a nonselective defect. Diabetes. 1997;46:814–823. doi: 10.2337/diab.46.5.814. [DOI] [PubMed] [Google Scholar]
- 80.Meyer C, Grobman R, Mitrakou A, Mahler R, Veneman T, Gerich J, Bretzel RG. Effects of autonomic neuropathy on counterregulation and awareness of hypoglycemia in type 1 diabetic patients. Diabetes Care. 1998;21:1960–1966. doi: 10.2337/diacare.21.11.1960. [DOI] [PubMed] [Google Scholar]
- 81.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 82.Dunn JT, Cranston I, Marsden PK, Amiel SA, Reed LJ. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: a new player in hypoglycemia unawareness? Diabetes. 2007;56:2766–2773. doi: 10.2337/db07-0666. [DOI] [PubMed] [Google Scholar]
- 83.Bolli G, Tsalikian E, Haymond M, Cryer P, Gerich J. Defective glucose counterregulation after subcutaneous insulin in non-insulin dependent diabetes mellitus. J. Clin. Invest. 1984;73:1532–1541. doi: 10.1172/JCI111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shamoon H, Friedman S, Canton C, Zacharowicz L, Hu M, Rosetti L. Increased epinephrine and skeletal muscle responses to hypoglycemia in noninsulin dependent diabetes mellitus. J. Clin. Invest. 1994;93:2562–2571. doi: 10.1172/JCI117267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meneilly G, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes. 1994;43:403–410. doi: 10.2337/diab.43.3.403. [DOI] [PubMed] [Google Scholar]
- 86.Boden G, Soriano M, Hoeldtke R, Owen O. Counterregulatory hormone response, and glucose recovery after hypoglycemia in non-insulin dependent diabetic patients. Diabetes. 1983;32:1055–1059. doi: 10.2337/diab.32.11.1055. [DOI] [PubMed] [Google Scholar]
- 87.Polonsky K, Herold K, Gilden J, Bergenstal RM, Fang VS, Moossa AR, Jaspan JB. Glucose counterregulation in patients after pancreatectomy: comparison with other clinical forms of diabetes. Diabetes. 1984;33:1112–1119. doi: 10.2337/diab.33.11.1112. [DOI] [PubMed] [Google Scholar]
- 88.Heller S, MacDonald I, Tattersall R. Counterregulation in type 2 (noninsulin dependent) diabetes mellitus: normal endocrine and glycemic responses, up to 10 years after diagnosis. Diabetologia. 1987;30:924–929. doi: 10.1007/BF00295875. [DOI] [PubMed] [Google Scholar]
- 89.Israelian Z, Szoke E, Woerle J, Bokhari S, Shorr M, Schwenke D, Cryer PE, Gerich J, Meyer C. Multiple defects in counterregulation of hypoglycemia in modestly advanced type 2 diabetes mellitus. Metabolism. 2006;55:593–598. doi: 10.1016/j.metabol.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 91.Peacey S, Bedford C, Marlow S. Antecedent hypoglycemia does not alter the physiological response to subsequent hypoglycemia in NIDDM [Abstract] Diabetes. 1996;45:56A–199A. [Google Scholar]
- 92.Davis SN, Mann S, Briscoe VJ, Ertl AC, Tate DB. Effects of intensive therapy and antecedent hypoglycemia on counterregulatory responses to hypoglycemia in type 2 diabetes. Diabetes. 2009;58:701–709. doi: 10.2337/db08-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Korzon-Burakowska A, Hopkins D, Matyka K, Lomas J, Pernet A, Macdonald I, Amiel S. Effects of glycemic control on protective responses against hypoglycemia in type 2 diabetes. Diabetes Care. 1998;21:283–290. doi: 10.2337/diacare.21.2.283. [DOI] [PubMed] [Google Scholar]
- 94.Burge MR, Sobhy TA, Qualls CR, Schade DS. Effect of short-term glucose control on glycemic thresholds for epinephrine and hypoglycemic symptoms. J. Clin. Endocrinol. Metabol. 2001;86:5471–5478. doi: 10.1210/jcem.86.11.7993. [DOI] [PubMed] [Google Scholar]
- 95.Levy CJ, Kinsley BT, Bajaj A, Simonson DC. Effect of glycemic control on glucose counterregulation during hypoglycemia in NIDDM. Diabetes Care. 1998;21:1330–1338. doi: 10.2337/diacare.21.8.1330. [DOI] [PubMed] [Google Scholar]
- 96.Landstedt-Hallin L, Adamson U, Lins PE. Oral glibenclamide suppresses glucagon secretion during insulin-induced hypoglycemia in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 1999;84:3140–3145. doi: 10.1210/jcem.84.9.6002. [DOI] [PubMed] [Google Scholar]
- 97.Choudhary P, Mills K, Emery C, MacLeod K, Amiel S, Heller S. Patients with type 2 diabetes treated with sulphonylureas develop symptoms of hypoglycaemia but not adrenergic or cognitive responses, at higher glucose levels than insulin treated patients. Diabetologia. 2005;48:A39–A40. [Google Scholar]
- 98.Veneman T, Mitrakou M, Mokan M, Cryer P, Gerich J. Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes. 1993;42:1233–1237. doi: 10.2337/diab.42.9.1233. [DOI] [PubMed] [Google Scholar]
- 99.Fanelli CG, Paramore DS, Hershey T, Terkamp C, Ovalle F, Craft S, Cryer PE. Impact of nocturnal hypoglycemia on hypoglycemic cognitive dysfunction in type 1 diabetes. Diabetes. 1998;47:1920–1927. doi: 10.2337/diabetes.47.12.1920. [DOI] [PubMed] [Google Scholar]
- 100.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Di Vincenzo A, Modarelli F, Ciofetta M, Lepore M, Annibale B, Torlone E, Perriello G, De Feo P, Santeusanio F, Brunetti P, Bolli GB. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of a rationale, intensive insulin therapy in IDDM. Diabetologia. 1994;37:1265–1276. doi: 10.1007/BF00399801. [DOI] [PubMed] [Google Scholar]
- 101.Fanelli C, Pampanelli S, Lalli C, Del Sindaco P, Ciofetta M, Lepore M, Porcellati F, Bottini P, Di Vincenzo A, Bottini P, Brunetti P, Bolli GB. Long-term intensive therapy of IDDM diabetic patients with clinically overt autonomic neuropathy: effects on awareness of, and counterregulation to hypoglycemia. Diabetes. 1997;46:1172–1181. doi: 10.2337/diab.46.7.1172. [DOI] [PubMed] [Google Scholar]
- 102.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426–1434. doi: 10.2337/diab.43.12.1426. [DOI] [PubMed] [Google Scholar]
- 103.Cranston I, Reed LJ, Marsden PK, Amiel SA. Changes in regional brain 18F–fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counterregulatory failure. Diabetes. 2001;50:2329–2336. doi: 10.2337/diabetes.50.10.2329. [DOI] [PubMed] [Google Scholar]
- 104.Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470–475. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tkacs NC, Dunn-Meynell AA, Levin BE. Presumed apoptosis and reduced arcuate nucleus neuropeptide Y and proopiomelanocortin mRNA in non-coma hypoglycemia. Diabetes. 2000;49:820–826. doi: 10.2337/diabetes.49.5.820. [DOI] [PubMed] [Google Scholar]
- 106.Figlewicz DP, van Dijk G, Wilkinson CW, Gronbeck P, Higgins M, Zavosh A. Effects of repetitive hypoglycemia on neuroendocrine response and brain tyrosine hydroxylase activity in the rat. Stress. 2002;5:217–226. doi: 10.1080/1025389021000010558. [DOI] [PubMed] [Google Scholar]
- 107.Chan O, Chen HY, Herzog R, Czyzyk D, Zhu WL, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent Hypoglycemia. Diabetes. 2008;57:1363–1370. doi: 10.2337/db07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- 109.Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J. Neurochem. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- 110.McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB. Chronic hypoglycemia increases brain glucose transport. Am. J. Physiol. 1986;251:E442–E447. doi: 10.1152/ajpendo.1986.251.4.E442. [DOI] [PubMed] [Google Scholar]
- 111.Koranyi L, Bourey RE, James D, Mueckler M, Fiedorek FT, Jr, Permutt MA. Glucose transporter gene expression in rat brain: pre-translational changes associated with chronic insulin-induced hypoglycemia, fasting and diabetes. Mol. Cell. Neurosci. 1991;2:244–252. doi: 10.1016/1044-7431(91)90051-o. [DOI] [PubMed] [Google Scholar]
- 112.Kumagai AK, Kang YS, Boado RJ, Partridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–1404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- 113.Segel SA, Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, Powers WJ, Cryer PE. Blood-to-brain glucose transport, cerebral glucose metabolism and cerebral blood flow are not increased after hypoglycemia. Diabetes. 2001;50:1911–1917. doi: 10.2337/diabetes.50.8.1911. [DOI] [PubMed] [Google Scholar]
- 114.Boyle PJ, Nagy RJ, O’Connor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc. Natl. Acad. Sci. 1994;91:9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boyle PJ, Kempers SF, O’Connor AM, Nagy RJ. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995;333:1726–1731. doi: 10.1056/NEJM199512283332602. [DOI] [PubMed] [Google Scholar]
- 116.Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J. Neurosci. Res. 2005;79:42–47. doi: 10.1002/jnr.20296. [DOI] [PubMed] [Google Scholar]
- 117.Swanson RA, Choi DW. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J. Cereb. Blood Flow Metab. 1993;13:162–169. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- 118.Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- 119.Brown AM, Tekkök SB, Ransom BR. Glycogen regulation and functional role in mouse white matter. J. Physiol. 2003;549:501–512. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J. Neurosci. Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER. Human brain glycogen metabolism during and after hypoglycemia. Diabetes. 2009;58:1978–1985. doi: 10.2337/db09-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herzog RI, Chan O, Yu S, Dziura J, McNay EC, Sherwin RS. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology. 2008;149:1499–1504. doi: 10.1210/en.2007-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes. 2006;55:929–934. doi: 10.2337/diabetes.55.04.06.db05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Borg MA, Tamborlane WV, Shulman GI, Sherwin RS. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes. 2003;52:663–666. doi: 10.2337/diabetes.52.3.663. [DOI] [PubMed] [Google Scholar]
- 125.Maran A, Cranston I, Lomas J, Macdonald I, Amiel S. Protection by lactate of cerebral function during hypoglycaemia. Lancet. 1994;343:16–20. doi: 10.1016/s0140-6736(94)90876-1. [DOI] [PubMed] [Google Scholar]
- 126.Amiel S, Archibald H, Chusney G, Williams A, Gale E. Ketone infusion lowers hormonal responses to hypoglycaemia: evidence for acute cerebral utilization of a non-glucose fuel. Clin. Sci. 1991;81:189–194. doi: 10.1042/cs0810189. [DOI] [PubMed] [Google Scholar]
- 127.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Effect of hyperketonemia and hyperlactacidemia on symptoms, cognitive dysfunction and counterregulatory hormone responses during hypoglycemia in normal humans. Diabetes. 1994;43:1311–1317. doi: 10.2337/diab.43.11.1311. [DOI] [PubMed] [Google Scholar]
- 128.Fanelli C, Di Vincenzo A, Modarelli F, Lepore M, Ciofetta M, Epifano L, Pampanelli S, Brunetti P, Bolli GB. Post-hypoglycaemic hyperketonaemia does not contribute to brain metabolism during insulin-induced neuroglycopenia in humans. Diabetologia. 1993;36:1191–1197. doi: 10.1007/BF00401065. [DOI] [PubMed] [Google Scholar]
- 129.Flanagan DE, Keshavarz T, Evans ML, Flanagan S, Fan XN, Jacob RJ, Sherwin RS. Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes. 2003;52:605–613. doi: 10.2337/diabetes.52.3.605. [DOI] [PubMed] [Google Scholar]
- 130.Jacobson L, Pacak K. Combined corticotropin-releasing hormone and glucocorticoid deficiency does not enhance counterregulatory responses after recurrent hypoglycemia in mice. Metabolism. 2005;54:1259–1265. doi: 10.1016/j.metabol.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inouye KE, Chan O, Yue JT, Andrews M, Li Q, Matthews SG, Vranic M. The effect of long-term insulin treatment with and without antecedent hypoglycemia on neuropeptide and corticosteroid receptor expression in the brains of diabetic rats. Brain Res. Bull. 2008;77:149–157. doi: 10.1016/j.brainresbull.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 132.Davis SN, Shavers C, Davis B, Costa F. Prevention of an increase in plasma cortisol during hypoglycemia preserves subsequent counterregulatory responses. J. Clin. Invest. 1997;100:429–438. doi: 10.1172/JCI119550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raju B, McGregor VP, Cryer PE. Cortisol elevations comparable to those that occur during hypoglycemia do not cause hypoglycemia-associated autonomic failure. Diabetes. 2003;52:2083–2089. doi: 10.2337/diabetes.52.8.2083. [DOI] [PubMed] [Google Scholar]
- 134.Goldberg PA, Weiss R, McCrimmon RJ, Hintz EV, Dziura JD, Sherwin RS. Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure. Diabetes. 2006;55:1121–1126. doi: 10.2337/diabetes.55.04.06.db05-1169. [DOI] [PubMed] [Google Scholar]
- 135.Nakao K, Nakai Y, Jingami H, Oki S, Fukata J, Imura H. Substantial rise of plasma β-endorphin levels after insulin-induced hypoglycemia in human subjects. J. Clin. Endocrinol. Metab. 1979;49:838–841. doi: 10.1210/jcem-49-6-838. [DOI] [PubMed] [Google Scholar]
- 136.Caprio S, Gerety G, Tamborlane WV, Jones T, Diamond M, Jacob R, Sherwin RS. Opiate blockade enhances hypoglycemic counterregulation in normal and insulin-dependent diabetic subjects. Am. J. Physiol. 1991;260:E852–E858. doi: 10.1152/ajpendo.1991.260.6.E852. [DOI] [PubMed] [Google Scholar]
- 137.Leu J, Cui MH, Shamoon H, Gabriely I. Hypoglycemia-associated autonomic failure Is prevented by opioid receptor blockade. J. Clin. Endocrinol. Metab. 94:3372–3380. doi: 10.1210/jc.2009-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]