Abstract
Noninvasive monitoring of β-amyloid (Aβ) plaques, the neuropathological hallmarks of Alzheimer's disease (AD), is critical for AD diagnosis and prognosis. Current visualization of Aβ plaques in brains of live patients and animal models is limited in specificity and resolution. The retina as an extension of the brain portrays an appealing target for a live, noninvasive optical imaging of AD if disease pathology is manifested there. We identified retinal Aβ plaques in postmortem eyes from AD patients (n=8) and in suspected early stage cases (n=5), consistent with brain pathology and clinical reports; plaques were undetectable in age-matched non-AD individuals (n=5). In APPSWE/PS1ΔE9 transgenic mice (AD-Tg; n=18) and not in non-Tg wt mice (n=10), retinal Aβ plaques were detected following systemic administration of curcumin, a safe plaque-labeling fluorochrome. Moreover, retinal plaques were detectable earlier than in the brain and accumulated with disease progression. An immune-based therapy effective in reducing brain plaques, significantly reduced retinal Aβ plaque burden in immunized versus non-immunized AD mice (n=4 mice per group). In live AD-Tg mice (n=24), systemic administration of curcumin allowed noninvasive optical imaging of retinal Aβ plaques in vivo with high resolution and specificity; plaques were undetectable in non-Tg wt mice (n=11). Our discovery of Aβ specific plaques in retinas from AD patients, and the ability to noninvasively detect individual retinal plaques in live AD mice establish the basis for developing high resolution optical imaging for early AD diagnosis, prognosis assessment and response to therapies.
Keywords: human retina, Aβ deposit, Aβ plaque, Alzheimer’s disease, mild cognitive impairment, vaccination, curcumin, in vivo optical imaging, fluorescence, spectral classification
Introduction
Alzheimer’s disease (AD) is a common and devastating age-dependent neurodegenerative condition. At present, a definite diagnosis of AD is determined after brain autopsy by detecting accumulation of the hallmark proteolytic products of amyloid precursor protein (APP), β-amyloid peptides (Aβ), which form extracellular aggregates termed Aβ plaques, and by the presence of intracellular neurofibrillary tangles (Hardy and Selkoe, 2002; Sisodia and Price, 1995). Aβ plaques are believed to contribute to disrupted cellular activities and communication in the brain, leading to neurotoxic inflammation and neuronal death (McGeer and McGeer, 2002; Wyss-Coray, 2006). Major efforts have been invested in developing tools to enable noninvasive detection of amyloid plaques (e.g. through the skull) in living AD patients and animal models (Hintersteiner et al., 2005; Klunk et al., 2004; Nakada et al., 2008; Ng et al., 2007). However, such noninvasive monitoring of Aβ plaques is still clinically challenging, and is of limited resolution and availability (Klunk et al., 2005; Lockhart et al., 2007; Toyama et al., 2005). Optical detection constitutes a powerful, high-resolution and specific tool for in vivo imaging (Fujimoto and Farkas, 2009), as demonstrated using multiphoton microscopy to detect Aβ plaques in the mouse brain via an invasive cranial window (Meyer-Luehmann et al., 2008).
An alternative noninvasive approach to visualize amyloid plaques in AD patients at high resolution could be a direct optical imaging of the retina, provided that Aβ plaques are formed in patients’ retinas and share properties with those in the brain. Choosing the retina as a target for visualization of brain disease also relies on it being a direct extension of the brain, having the potential to faithfully reflect AD brain’s pathology. Retinal abnormalities in AD patients, some of which were detected at early stages, have been described in the past (Berisha et al., 2007; Blanks et al., 1996; Hinton et al., 1986; Katz and Rimmer, 1989; Sadun et al., 1987; Trick et al., 1989). These findings, however, mostly related to the NFL layer thickness reduction, seem to appear in additional pathologies of the eye and the brain including ocular hypertension, glaucoma, demyelinating optic neuritis, multiple sclerosis, and Parkinson’s disease (Jindahra et al., 2010; Parisi, 2003). Detection of ganglion cell death in retinas of live AD mouse models has been just recently documented (Cordeiro et al., 2010); however, this phenomenon is common to multiple neurodegenerative disorders including glaucoma and age-related macular degeneration. In addition, postmortem lenses from AD patients were reported to exhibit specific cytosolic nanometer-size Aβ aggregates and supranuclear cataracts in the peripheral lens (Goldstein et al., 2003). Encouraging results in APPSWE and Presenilin (PS) 1ΔE9 transgenic mice carrying the human mutated genes causing an early-onset familial AD, were recently reported: these mice found to develop human-Aβ deposits in their retinal tissues at advanced stages of the disease (Ning et al., 2008; Perez et al., 2009).
The existing reports emphasized the need for unequivocal identification of Aβ plaques hallmark pathology of Alzheimer’s disease in AD patients, especially at an early stage, for noninvasive in vivo detection of retinal plaques, and demonstration of their response to therapeutic intervention. We report here the presence of Aβ plaques in retinas of postmortem eyes from AD patients, and moreover, in retinas from those suspected as early stage cases. Using the APPSWE/PS1ΔE9 transgenic (AD-Tg) mice model, we provide evidence for the formation of Aβ plaques in the retina prior to their manifestation in the brain. Further, in these mice we found that an immune-based therapy, using a weak agonist of a myelin-derived peptide loaded onto dendritic cells (Koronyo-Hamaoui et al., 2009), was effective in reducing Aβ plaques in the retinas to an extent similar to that observed in the brain. Importantly, we were able to demonstrate that systemic injection of curcumin (diferuloylmethane), a natural and safe fluorochrome that binds and labels Aβ plaques (Garcia-Alloza et al., 2007; Yang et al., 2005), into live AD mice allows high-resolution and specific noninvasive in vivo visualization of retinal Aβ plaques.
Materials and methods
Mice
Double transgenic mice (females and males in equal numbers) harboring the chimeric mouse/human APP (APPSWE) and mutant human presenilin 1 (PS1ΔE9) genes, and their aged-matched wt littermates, were purchased from the Jackson Laboratories (Bar Harbor, ME, strain #4462). Both APPSWE and PS1ΔE9 mutations are associated with early-onset Alzheimer's disease, and their expression is directed to CNS neurons. The “humanized” APPSWE transgene allows the mice to produce and secrete a human Aβ peptide. All mice were bred and maintained in the Department of Comparative Medicine at Cedars-Sinai Medical Center. All experiments were approved and conducted according to the standards of the Cedars-Sinai Institutional Animal Care and Use Committee (IACUC).
Genotyping
Genomic DNA was extracted from 0.3 cm tail tip using a DNA extraction kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. Mice used in this study were genotyped for the presence of the transgenes by PCR, as previously described (Jankowsky et al., 2004b).
Immunization preparations
Mice were immunized with an altered myelin oligodendrocyte glycoprotein peptide [MOG45D; MEVGWYRSPFDRVVHLYRNGK (Ford and Evavold, 2004)], that was derived from the pMOG(35–55), in which aspartic acid is substituted for serine resulting in a non-encephalitogenic peptide. For immunizations, MOG45D (Invitrogen, Carlsbad, CA) was added to bone marrow-derived dendritic cells isolated from wt littermates. Preparation of dendritic cells for immunization was performed as previously described (Koronyo-Hamaoui et al., 2009).
Experimental design for immunization in mice
For immunization, seven-month old mice were randomly divided into three treatment groups (n=4 per group): AD-Tg mice injected with DC loaded with MOG45D peptide [0.5×106 cells/200 µl in phosphate-buffered saline (PBS) per animal], once a month for 3 months; AD-Tg mice injected with PBS in the same regimen; and non-treated non-Tg wt littermates. At the end of the study, mice were perfused under anesthesia with ice-cold PBS. Brains and eyes were collected for further quantitative analysis of Aβ-plaque load.
Histology preparation of eyes and brain from mice
Both PBS-perfused and non-perfused animals were used in this study. For whole-eye cryosections, eyes were enucleated and fixed immediately in 4% p-formaldehyde (PFA) overnight in 4°C. Whole eyes were then placed in 30% sucrose solution and 2.5% PFA for 2 hr, then washed three times for 15 min in PBS. The eyes were embedded in O.C.T. compound (Sakura Finetech, Torrance, CA), frozen gradually on dry ice, and sagittally cryosectioned (7 µm). For whole-mount retinas, the anterior eye portion was dissected out, eyecups were soaked for 10 min in hyaluronidase (type I-S) (0.07 mg/ml) (Sigma-Aldrich, St. Louis, MO) to liquefy and remove the vitreous residue,, and then washed three times for 10 min in PBS. Eyecups were then fixed with 2.5% PFA overnight in 4°C. Retinas were dissected free and whole-mounts were prepared flat on slides. For brain cryosections, brains were collected and fixed immediately in 2.5% PFA overnight at 4°C. Brains were then transferred into 30% sucrose in 2.5% PFA for 2 hr, washed three times for 15 min in PBS, embedded in O.C.T. and frozen gradually on dry ice, then coronally cryosectioned (30 µm).
Human subjects
Postmortem eyes and brains were obtained from the USC Alzheimer’s Disease Research Center (ADRC) Neuropathology Core (NIA AG05142), (IRB approval #042071), Department of Pathology, University of Southern California (Los Angeles, CA), and also purchased from National Disease Research Interchange (NDRI, Philadelphia, PA), under IRB protocols # 99491 and # 3201. NDRI maintains a human tissue collection protocol approved by a managerial committee and subject to National Institutes of Health oversight. Postmortem specimens were collected from patients with a definite AD diagnosis (n=8; average age 80 years, ranging from 48 to 94 years; with different disease severities, categorized based on their neuropathology reports), patients with a possible or probable AD diagnosis (n=5, average age 79 years, ranging from 65 to 92 years), and from age-matched normal controls (n=5; average age 76 years, ranging from 66 to 85 years, with absence of dementia and brain pathology; human donor eye records are summarized in Table S1). The groups did not differ significantly in age or years of education.
Assessment of human AD diagnosis and cognitive evaluation
Clinical and neuropathology reports, including neurological examination, neuropsychological testing, such as cognitive assessment, family history and medications, were provided by the USC ADRC Clinical Core (IRB protocol # 002003). Each participant had been tested individually by a trained psychometrist under the supervision of a licensed clinical neuropsychologist. Test scores from the evaluation closest to death were used in these analyses. Most cognitive evaluations were performed annually and in most cases, less than one year prior to death. Two global indicators of cognitive status were used for clinical assessment of patients, the Clinical Dementia Rating (CDR) and the Mini Mental Status Examination (MMSE: normal control=30-26; MCI=25-22; AD≤22). For neuropathologic diagnoses, modified CERAD (Mirra et al., 1991) were used as well as the NIA/Reagan protocols (NIA-Reagan Consensus, 1997). This includes Aβ burden and neurofibrillary pathology (NFT) assessments in multiple brain areas. Amyloid plaques and tangles in the brain were evaluated using the Thioflavin-S fluorescent and Gallyas silver stains in formalin-fixed, paraffin-embedded tissues. Scoring was performed by independent observations of three neuropathologists and an arbitrary score, reflecting amyloid or NFT burden as an average of all three readings (0=none, 1=sparse, 3=moderate, 5=abundant), was assigned to each individual. Braak scores were used to assess NFTs for disease progression.
Histology preparation of postmortem eyes and brain in human
Postmortem eyes were fixed and long-term stored in 10% neutral buffered formalin (NBF). In addition, four eyes were snap frozen and stored at −80°C. No apparent differences were observed in immunostaining between the two preparation methods, except for DAPI staining that was clearer in the snap frozen eyes without long-term 10% NBF fixation. For immunohistochemistry, fresh-frozen brains were cryosectioned, fixed with 2.5% PFA for 2 days and stored in PBS in 4°C. The fresh-frozen eyecups were hyaluronidase treated as above to liquefy and remove vitreous residues, washed for 5 min in PBS, and fixed in 2.5% PFA for 2 days. Retinas were dissected free, and whole-mounts were prepared. The long-term NBF-fixed eyecups were washed in PBS, retinas were dissected free, vitreous was cleaned manually, and whole-mounts were prepared.
Immunohistochemistry in mouse tissues
Mouse brain cryosections (30 µm), retinal cross-sections (7 µm) and whole-mount retinas were treated with a permeabilization/blocking solution containing 20% horse serum (Invitrogen) and 0.05% Triton X-100 (Sigma-Aldrich). Sections and whole-mounted retinas were stained overnight at 4°C with a specified combination of the following primary mAbs in PBS containing 10% blocking solution: mouse anti-human Aβ [against human amino acid (aa) residues 1–16, clone 6E10 (1:100; Millipore, Temecula, CA), and clone DE2b4 (1:100; AbD Serotec, Raleigh, NC), and against the aa 17–24, clone 4G8 (1:100; Covance, Princeton, NJ), and mAbs directed against C-terminus of human Aβ, aa 34–40, clone 11A5-B10, specific for isoforms ending at 40th aa (1:100; Millipore; does not cross-react with APP) and against C-terminus of human Aβ, aa 36–42, clone 12F4, specific for isoforms ending at 42nd aa (1:200; Covance; does not cross-react with APP)]; rabbit anti-mouse Aβ [against mouse aa 3–16, and reacting only with mouse or rat, Ab14220 (1:500; Abcam, Cambridge, MA)]. Next, sections were incubated for 1 hr at room temperature with secondary Abs in PBS (Cy5-conjugated donkey anti-mouse antibody or donkey anti-rabbit antibody (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA), washed three times with PBS, and a Vectashield mounting solution with or without 4',6-diamidino-2-phenylindole dihydrochloride (DAPI; Vector Laboratories, Burlingame, CA) was applied. Routine controls were processed using the same protocol with the omission of the primary antibody.
Immunohistochemistry in human tissues
Human brain cryosections (30 µm) and whole-mount retinas from AD patients and controls were treated with target retrieval solution (Dako, Carpinteria, CA) and with a permeabilization/blocking solution (as above). All human tissues were treated with Sudan Black B (SBB) to eliminate background fluorescence. Human tissues were stained with primary mAbs mentioned above, in PBS containing 10% blocking solution, for 48 hrs at 4°C. The samples were incubated for 1.5 hrs at room temperature with secondary Abs (see above) in PBS, and washed three times with PBS. Next, labeled samples were immersed for 10 min in 70% ethanol (v/v) supplemented with 0.3% (w/v) SBB, followed by three washes with 70% ethanol (v/v) and finally with PBS. After staining with SBB (Sigma-Aldrich; C26H24N4O, a lysochrome used for staining of neutral triglycerides and lipids), the same fluorescently labeled samples were stained with curcumin or ThioS (see separate sections below) and then mounted with or without DAPI. Alternatively, Aβ immunoreactivity was detected with 12F4 mAb and was visualized by nonfluorescent immunoperoxidase method using 3,3’-diaminobenzidine as the chromogen (DAB substrate, Sigma-Aldrich) and Vectastain ABC kit (Vector Laboratories) as specified by the manufacturer. Routine controls were processed using the same protocol with the omission of the primary antibody to assess nonspecific labeling.
Thioflavin-S staining
ThioS solution at 1% (w/v) was prepared by dissolving 1g Thioflavin-S (Sigma-Aldrich) in 70% ethanol (v/v). Mouse whole-mount retinas were stained for 12 min at room temperature, then dipped three times in 70% ethanol (v/v) and finally washed once in PBS. The samples were covered with mounting media without DAPI (Vector Laboratories). Human whole-mount retinas were first stained with Sudan Black B and washed three times with PBS for 5 min each wash. Samples were then stained with ThioS solution, dipped three times in 70% ethanol (v/v), finally washed once in PBS, and mounted as mentioned above.
Ex vivo curcumin staining
Curcumin solution was prepared by dissolving 2 mg curcumin (Sigma-Aldrich) in two drops of 0.5M NaOH (vigorous vortex mixing), following immediate dissolution and dilution in PBS to achieve a final concentration of 0.1 mg/ml (pH=7.9). Human whole-mounted retinas and brain sections were initially treated with 0.3% (w/v) Sudan Black B in 70% ethanol (v/v) for 10 min at room temperature. All mouse and human tissues (brain and retinal cryosections and/or whole-mounts) were stained with curcumin solution for 10 min at room temperature, and then washed three times with PBS for 15 min each. The samples were covered with ProLong Gold antifade mounting media with DAPI (Invitrogen) or Vectashield (Vector Laboratories) with or without DAPI.
Intravenous injections of curcumin for ex vivo plaque imaging
AD-Tg (n=18) and non-Tg (wt; n=10) mice were i.v. injected into the tail vein with curcumin in PBS (7.5 mg/kg/day) or with PBS alone, for five consecutive days. Subsequently, brains and eyes were excised, and brain cryosections and retinal whole-mounts were prepared for imaging.
Intravenous injections of curcumin for noninvasive in vivo retinal plaque imaging
Retinas of live AD-Tg (n=24) and wt (n=11) mice were imaged following systemic administration of curcumin (7.5 mg/kg/day), for five consecutive days or following a single i.v. injection two hours prior to imaging. Mice were anesthetized with 70 mg/kg Ketamine and 30 mg/kg Xylazine. Mouse pupils were dilated to about 2 mm in diameter with 0.5% phenylephrine hydrochloride ophthalmic solution (Bausch & Lomb, Rochester, NY) combined with 0.5% tropicamide ophthalmic solution (Mydral, Bausch & Lomb). Mouse eyes were covered with a drop of Gonak (Hypromellose ophthalmic demulcent solution, 2.5%; Akorn, Lake Forest, IL), which served as an optical coupling medium, and the retinas were imaged in vivo using the Micron II retinal imaging microscope (Phoenix Research Laboratories, San Ramon, CA). The Micron II is a retinal imaging microscope for rodents adjusted to visualize fluorescence signals at high resolution and is equipped with a 3-CCD camera (1000 to 1 dynamic range and 30 frames per sec output at XGA resolution), and specific set of filters suitable to detect curcumin fluorescence (as mentioned below for the Zeiss Axio Imager Z1). Images were repeatedly captured at several angles of the retina in order to visualize a larger field and eliminate non-specific reflection signals. Care was taken to keep anesthetized animals warm throughout the procedure.
Microscopy
Fluorescence and bright-field images were acquired using a Carl Zeiss Axio Imager Z1 fluorescence microscope equipped with ApoTome (Carl Zeiss MicroImaging, Inc.), and a Leica SP5 WLL double-spectral confocal microscope, using the same setting and exposure times for each experiment. For processing and analysis of the images, the AxioVision (Rel. 4.6.3) software (Carl Zeiss) was used. Fluorescence in the Zeiss Axio Imager Z1 was imaged using filter sets for excitation and emission at 365/50 and 445/50 nm for DAPI, 470/40 and 525/50 nm for ThioS, 550/25 and 605/70 nm for curcumin and 640/30 and 690/50 nm for Cy5. In the presented images, curcumin was assigned a green pseudocolor to differentiate from red Cy5.
Quantification
Images of stained tissues were obtained on an Axio Imager Z1 microscope (with motorized Z-drive) with AxioCam MRm monochrome camera ver. 3.0 (at a resolution of 1388 × 1040 pixels, 6.45 µm × 6.45 µm pixel size, dynamic range of >1:2200, that delivers low-noise images due to Peltier-cooled sensor). Quantitative analysis of Aβ plaque number and area (µm2) was performed from two whole-mounted retinas per mouse (n=4 mice per group). Each image, captured with 40x objectives with resolution of 0.25 µm, included an area of 0.04 mm2, and a total of 12 rectangle areas around the optic disc within scanning depth of 60 µm (multiple virtual section images at consecutive focal planes using a motorized scanning stage). Measurements of the average plaque radius (following curcumin staining) were completed for each animal group followed by calculation of the average plaque area in each animal group. For the acquisition, we used the same exposure times (approx. 75 ms) and the same gain values (0) for all images. The emission signals of Aβ plaques stained with curcumin were compared to the background signals in the retinal tissue, to determine signal to background ratio. The calculated signal-to-background noise ratio from the images was high and within the range of 3:1 to 10:1. Quantification of Aβ plaque number and area in the corresponding brains was performed using three coronal sections (two hemispheres each) per mouse in 450 µm intervals, over an area covering the hippocampus and cortex regions. Optical sections from each field of the specimen were imported into the NIH ImageJ program (National Institutes of Health). Conversion to grayscale was performed to distinguish between areas of immunoreactivity and background. Total area of immunoreactivity was determined using a standardized histogram-based threshold technique, and then subjected to particle analysis.
Spectral imaging analyses
Spectral imaging of mouse retinal tissues provided digital images of an object at a large, sequential number of wavelengths and generated precise optical signatures at every pixel. The fluorescence spectral signature of Aβ plaques labeled in vivo with curcumin was captured by our spectral imaging system using the following equipment: Nikon fluorescence microscopes (E800 and TE2000) with mercury and xenon arc lamps, a CCD camera, and an AOTF (acousto-optic tunable filters)-based spectral image acquisition system (~ 4 µm spectral resolution, commercialized by ChromoDynamics, Inc., Orlando, FL) (Wachman et al., 1997). Image acquisition was followed by image segmentation and classification using software that we previously developed (Burton, 2009). The final images provided a visual pseudo-color representation of the spectral signature extracted from the raw images, representing the size and location of the analyzed objects. Spectral analysis of individual Aβ plaques (regions of interest, ROI) as compared to background signals in human retinal tissues (single-labeled with curcumin, single labeled with Cy5-conjugated Abs, or double-labeled with both) was performed in quadruplicates using a Leica SP5 WLL double-spectral (excitation and emission) confocal microscope, with the same setting and exposure times for each ROI and background. Various excitation wavelengths and resulting emission spectra (at 5 nm intervals) were recorded, to determine the optimal wavelengths to excite and capture the fluorescence signal. GraphPad Prism 5 for Mac OS X version 5.0b was used to smooth curves by applying the fit spline/lowess method.
Statistical analysis
GraphPad Prism 5.0b (GraphPad Software, San Diego, CA) was used to analyze the data. Comparison of means between the three experimental groups (PBS-treated vs. immunized AD-Tg and non-Tg wt mice) was performed by one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparison post-test analysis to define specific p value of each group pair. Results are expressed as mean and ± SEM. P<0.05 was considered significant.
Results
Curcumin binds to retinal Aβ plaques in a mouse model of Alzheimer’s disease
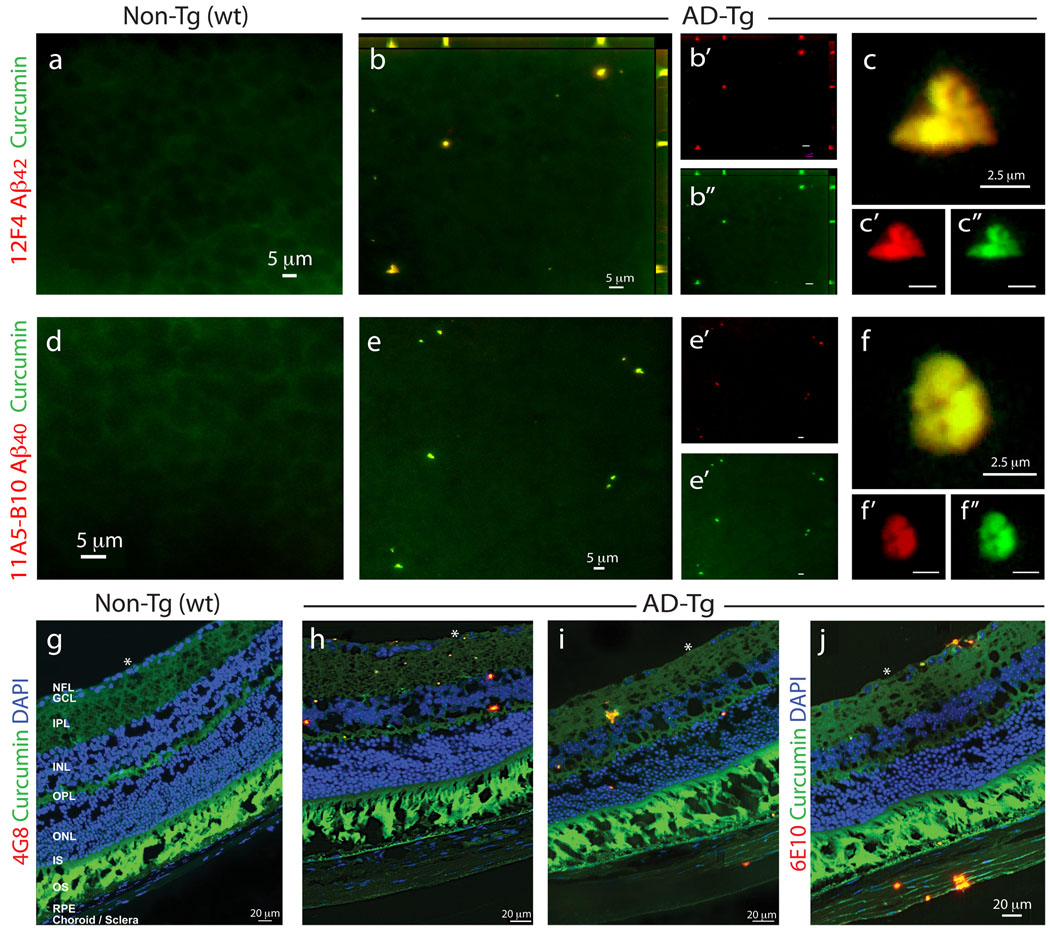
We first assessed the potential of using curcumin for the development of an in vivo noninvasive method to detect retinal Aβ plaques. To this end, we first verified that curcumin could specifically ex vivo label Aβ plaques in the retinas of AD-Tg mice. Retinal whole-mounts and cross-sections from AD-Tg and non-Tg (wt) mice were co-stained with curcumin and four different anti-Aβ monoclonal antibodies (mAbs) (Fig. 1). Retinal Aβ plaques, co-labeled with curcumin and 11A5-B10 or 12F4 mAbs [recognizing the C-terminal amino acid sequence of Aβ40 and Aβ42 isoforms, respectively], were virtually absent in non-Tg (wt) littermates (Fig. 1a,d), whereas they were clearly detected in AD-Tg mice (Figs. 1b,c and 1e,f). Staining with anti-Aβ42 mAb resulted in stronger fluorescence signal than with the anti-Aβ40 mAb (Figs. 1c’ vs. 1f’), in agreement with the reported brain Aβ40:42 ratio of 1:2 in this mouse model (Jankowsky et al., 2004a). Additional staining with ThioS that labels mature/fibrillar Aβ plaques (Fig. S1a–c), demonstrated a staining pattern and size of retinal Aβ plaques very similar to curcumin labeling; both dyes were reported to bind the β-pleated sheet structures of Aβ plaques (Garcia-Alloza et al., 2007; Goldstein et al., 2003). The specificity of curcumin-labeled Aβ plaques in the mouse retina was further confirmed with 4G8 and 6E10 antibodies in cross-sections (Fig. 1g–j). In line with previous data using ThioS/10D5 antibody (Ning et al., 2008; Perez et al., 2009), curcumin-positive retinal Aβ plaques were found in AD-Tg mice in various locations including the nerve fiber layer (NFL), retinal ganglion cell layer (RGC), inner (IPL) and outer (OPL) plexiform layers, and inner nuclear layer (INL); some plaques were also seen in the sclera (Fig. 1i,j). Relatively high background fluorescence was observed in the photoreceptor outer segment (OS) layer of the retina, as this layer in the mouse displays an endogenous fluorescence; however, curcumin- and immuno-labeling of plaques appeared above the autofluorescence levels and were easily distinguishable from these diffused background signals.
Figure 1.
Detection of retinal Aβ plaques in AD-Tg mice by ex vivo curcumin labeling. Whole-mount retinas and whole-eye sagittal cryosections from 10-month-old non-Tg (wt) and AD-Tg mice stained with various anti-human Aβ mAbs (12F4, 11A5-B10, 4G8 and 6E10; secondary Ab-Cy5 conjugate; red color) and curcumin (green color). Double-labeled Aβ plaques appear in yellow color. (a–c) Representative images of whole-mount retinas double-stained with curcumin and Aβ42 mAb (12F4; specific to the C-terminal sequence ending at aa 42). (a) No retinal Aβ plaques were detected in wt mice, whereas (b) they were found in AD-Tg mice; here and below, whenever z-axis projection images are presented, the axes ZY and ZX are shown on the top and right side of the image. (c) Specific staining patterns of Aβ42-containing retinal plaques at a higher magnification; (b’–c”) Separate channels for each staining; (d–f) Whole-mount retinas double-stained with anti-human Aβ40 mAb (11A5-B10; specific to the C-terminal sequence ending at aa 40) and curcumin. (d) No detection of Aβ plaques in wt mice. (e) Specific Aβ plaques were found in AD-Tg mice. (f) Higher magnification image demonstrating Aβ40-containing plaques and their specific staining pattern. (e’–f”) separate channels. (g–j) Whole-eye cross-sections stained with anti-Aβ mAbs (4G8 or 6E10), curcumin and DAPI nuclei staining. (g) No evidence for double-positive curcumin and anti-human Aβ plaques in wt mice. (h–j) Curcumin-positive Aβ plaques co-labeled with 4G8 or 6E10 were identified in various retinal layers, including the GCL-ganglion cell layer, IPL-inner plexiform layer, INL-inner plexiform layer, OPL-outer plexiform layer and ONL-outer Nuclear Layer. (i,j) Aβ plaques were also detected in the sclera. White Asterisks indicate DAPI nuclei staining in the GCL: undamaged in wt retinas and deficient in retinas from AD-Tg mice.
Specific curcumin in vivo-labeling of Aβ plaques in AD mice and early plaque detection in the retina
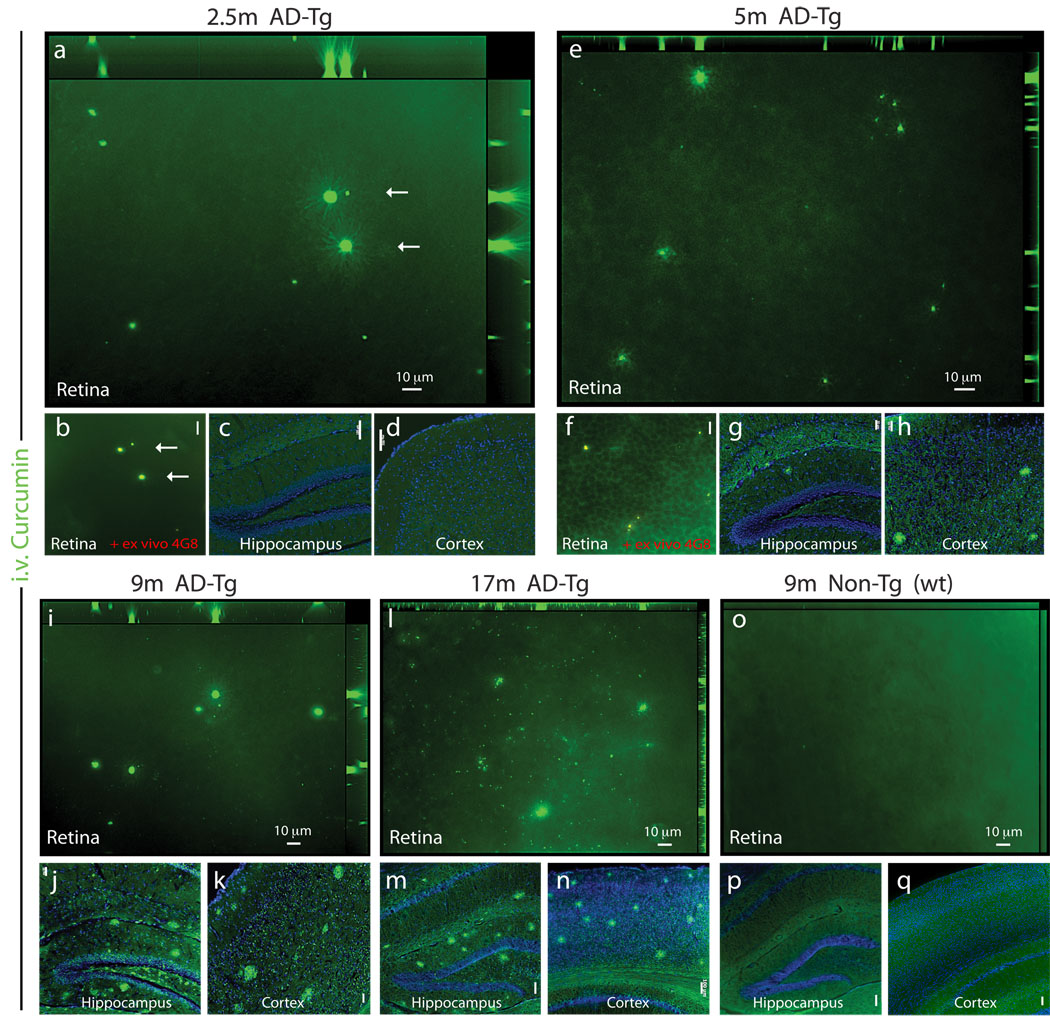
To establish the use of curcumin for in vivo imaging of plaques in the retina, we tested its bioavailability to the eye when injected systemically. Labeled plaques following systemic administration of curcumin were detected in the retinas and brains of AD-Tg mice, but not of the wt controls (Fig. 2). These findings confirmed that curcumin crosses the blood-brain barrier (Garcia-Alloza et al., 2007) and the blood-retina barrier and indicated its high affinity for Aβ plaques in vivo. Imaging of retina, brain cortex, and hippocampus of AD-Tg mice at the ages of 2.5, 5, 9, and 17 months demonstrated a qualitative age-dependent correlation between plaque deposition in the retina and the brain, and increased accumulation over the course of disease progression (Fig. 2a–n). Importantly, plaques were detected in the retina (Fig. 2a,b), but not in the brain (Fig. 2c,d), as early as at 2.5 months of age in AD-Tg mice, suggesting that Aβ plaques in the retina precede brain plaques. We further confirmed that these curcumin-labeled plaques were co-localized ex vivo with mAb 4G8 in the same tissue location (Fig. 2b). Aβ plaques were first detectable in the brain at the age of 5 months (Fig. 2g,h), in line with previous descriptions of disease initiation and progression in this strain of AD-Tg mice (Garcia-Alloza et al., 2006). Retinal Aβ plaques, similar to plaques in the brain, were more frequently found in older AD-Tg mice, at the age of 9 and 17 months (Fig. 2i–n). As expected, plaques could not be detected in the retinas and brains of 9 month-old wt mice (Fig. 2o–q).
Figure 2.
Bioavailability of systemically administered curcumin to the mouse eye: accumulation of curcumin-labeled retinal Aβ plaques with disease progression and detection at early pre-symptomatic stage. (a–q) Representative z-axis projection images of whole-mount retinas and brain coronal cryosections from AD-Tg and non-Tg (wt) mice at various ages, following i.v. curcumin injections for 5 days: In 2.5-month-old AD-Tg mouse (a,b) retinal curcumin-labeled in vivo of Aβ plaques (green) were visible, with further (b) validation of Aβ plaque identity in the same retinal location (arrows) by staining ex vivo with anti-human Aβ mAb 4G8 (secondary Ab-Cy5 conjugate). Co-localization of curcumin and Aβ antibody in yellow pseudocolor). Scale bar = 10 µm. (c,d) No plaques were detected in the brain hippocampus and cortex of 2.5-month-old AD-Tg mice. Scale bars = 100 µm. (e–h) 5-month-old AD-Tg mouse: (e) Presence of curcumin-labeled plaques in the retina and (f) co-labeling following 4G8 mAb staining ex vivo. Scale bar = 20 µm; (g,h) detection of plaques in the brain. Scale bars = 50 µm. (i–k) 9-month-old AD-Tg mouse: (i) Multiple plaques in the retina and (j,k) in the brain. Scale bars = 50 µm. (l–n) 17-month-old AD-Tg mouse: (l) Numerous plaques in the retina and (m,n) in the brain. Scale bars =100 µm. (o–q) 9-month-old non-Tg (wt) mouse: (o) No Aβ plaques in the retina nor (p,q) in the brain. Scale bars =100 µm.
Reduction of retinal Aβ plaque burden in AD mice following an immune-based therapy
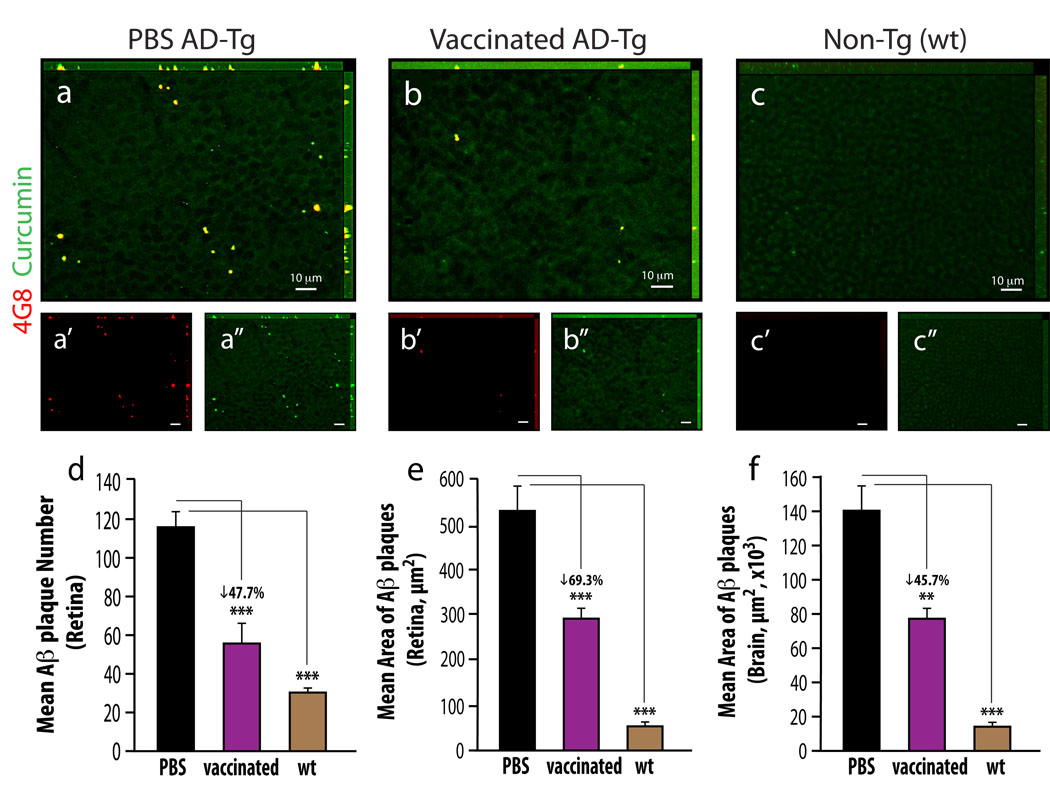
To strengthen our contention of the retina as a viable, functional target for imaging AD, we investigated whether retinal plaques behaved similarly to brain plaques in response to the same therapy. We previously showed that immunization with an altered myelin-derived peptide [MOG45D, derived from pMOG35–55 (Ziv et al., 2006)] loaded on dendritic cells (DCs), effectively restricted Aβ plaque burden in brains of AD-Tg mice (Koronyo-Hamaoui et al., 2009). We therefore used the same immunization to assess its effect on retinal plaques, while establishing curcumin as a potential fluorochrome to monitor plaque changes. Quantitative analysis of curcumin-labeled Aβ plaque number and area was performed on brain sections and whole-mounted retinas isolated from three experimental groups: 10-months old MOG45D-immunized or PBS-treated AD-Tg mice and age-matched untreated wt controls (Fig. 3). A substantial reduction of retinal Aβ plaque burden by mean number and area was found in immunized AD-Tg mice compared to PBS-treated controls (representative images: Fig. 3b vs. 3a; quantitative analyses: 3d and 3e, P<0.0001). Notably, a significant decrease in total plaque area, relative to PBS-treated mice, was also observed in brain hippocampi and cortices of the same immunized AD-Tg mice (Fig. 3f; P<0.0001). No Aβ plaques (double labeled with curcumin and anti-human Aβ antibody) were detected in the wt mice, as they do not possess the human transgene (Fig. 3c). Whereas human Aβ-containing plaques were obviously absent in the wt mice, occasional small and sparse curcumin-positive plaques (≤1 µm; anti-human Aβ negative) were detected at higher magnification. These small plaques, which were detected using curcumin in all three experimental groups, were co-labeled with the specific anti-mouse Aβ antibody (Fig. S2a–e), confirming their identity as endogenously formed mouse Aβ deposits.
Figure 3.
Reduced Aβ-plaque burden in retinas from AD-Tg mice following MOG45D-loaded dendritic cells immunization. (a–c) Representative z-axis projection images of whole-mount retinas from (a) PBS-treated control and (b) MOG45D-immunized AD-Tg mice, and from (c) non-Tg (wt) mouse, stained ex vivo with curcumin and anti-human Aβ mAb (4G8; followed by secondary Ab-Cy5 conjugate). (d,e) Following MOG45D-loaded DCs immunization, a significant reduction in mean curcumin-positive plaque number and area was observed in retinas from immunized AD-Tg mice as compared to PBS-treated controls and non-Tg (wt) mice. (f) A significant decrease in total area covered by plaques was detected in brain hippocampus and cortex from the same mice following the immunotherapy. Curcumin staining revealed the same decrease in plaque burden following immunization in the retina and in the brain, while Aβ mAbs confirmed its specificity to Aβ, suggesting that curcumin is a suitable dye for monitoring Aβ plaques. Error bars represent SEM. Asterisks indicate statistical significance: *** P<0.0001; ** P<0.005, analyzed by one-way ANOVA followed by Bonferroni multiple comparison post-test.
Noninvasive imaging of curcumin-labeled retinal plaques in live AD mice
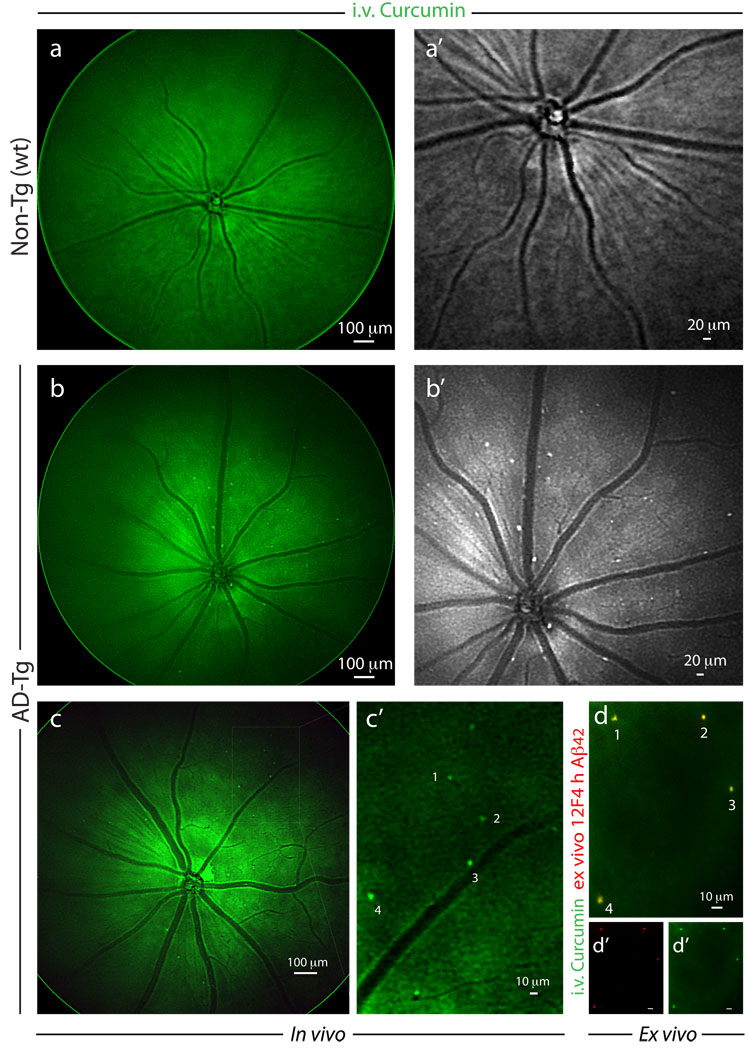
The results summarized above encouraged us to proceed to imaging plaques in live animals (Fig. 4). Following systemic administration of curcumin, in vivo imaging of the retina was performed in live mice utilizing Micron II rodent retinal imaging microscope. Retinal Aβ plaques were absent in curcumin-injected non-Tg (wt) mice (Fig. 4a,a’) and in PBS-injected AD-Tg mice (not shown); whereas they were clearly identified in live AD-Tg mice (Fig. 4b,b’). This in vivo optical imaging modality enabled us to identify individual plaques or plaque clusters at high resolution (Fig. 4b’). We confirmed the specificity of the signals captured in live mice by performing a new experiment in which we excised retinas following in vivo imaging of curcumin-labeled Aβ plaques (Fig. 4c,c’), and determined their identity ex vivo by staining with specific mAb 12F4 (Fig 4d). Another ex vivo labeling with mAb 4G8 of an additional whole-mounted AD-Tg mouse retina, demonstrated the Aβ specificity of curcumin staining and that Aβ plaques could be found inside blood vessels (intraluminal) and especially in the abluminal position (Fig. S3a). Overall, average plaque size detected in vivo was very similar to that observed by immunostaining ex vivo.
Figure 4.
Noninvasive in vivo optical imaging of curcumin-labeled Aβ plaques in AD-Tg mice retina. (a–c) Following i.v. administration of curcumin (7.5 mg/kg) for 5 consecutive days, retinas of 8–12 month-old AD-Tg and wt mice were in vivo fluorescently imaged using Micron II rodent retinal microscope. Representative in vivo mouse fundus images: (a) no plaques could be visualized in wt mouse; (a’) higher magnification in grayscale. (b) Retinal curcumin-labeled plaques (green spots) were visible in live AD-Tg mouse; (b’) higher magnification in grayscale. (c,c’) Representative images demonstrate in vivo detection of curcumin-labeled plaques in the retinas of 8 month-old AD-Tg mice, following a single i.v. injection of curcumin (7.5 mg/kg) two hours prior to imaging. (c’) Enlarged image of the selected area from (c) image, demonstrates that this imaging modality enables the identification of individual plaques or plaque clusters at a high spatial resolution. Note that blood vessels appear unstained (dark), possibly due to blood flow in the live mouse. (d) Whole-mount retina prepared from the same in vivo imaged mouse eye as in (c), following perfusion. An additional ex vivo staining with anti-human Aβ42 mAb (12F4; secondary Ab-Cy5 conjugate) further confirmed the specificity of curcumin signals (captured by Micron II) to Aβ plaques. (d’,d”) separate channels for each staining. A similar pattern of curcumin-positive plaques imaged in vivo was identified ex vivo following antibody labeling (indicated by the numbers 1–4).
We next identified the specific optical signature of retinal Aβ plaques in curcumin-injected non-perfused mice in order to further verify curcumin-labeled Aβ plaque detection and to reduce the possibility of monitoring non-specific signals emerging from non-plaque regions or background noise. For this purpose, we monitored plaques in the vicinity of blood vessels using a spectral optical imaging technology (Burton, 2009; Wachman et al., 1997). We detected both curcumin-labeled plaques and blood vessels in a single wavelength channel specific to curcumin (Fig. S3b). By applying band-sequential spectral image acquisition and spectral signature-based image segmentation (using software that translated output into pseudocolor-classified digital images), “true” (unmixed) signals from curcumin-labeled Aβ plaques were easily distinguishable from those generated by the blood vessels (Fig. S3c). These results indicated that the detected curcumin fluorescence signal was specific to labeled Aβ plaques and not due to non-specific background emission.
Identification of Aβ plaques in retinal samples from AD patients
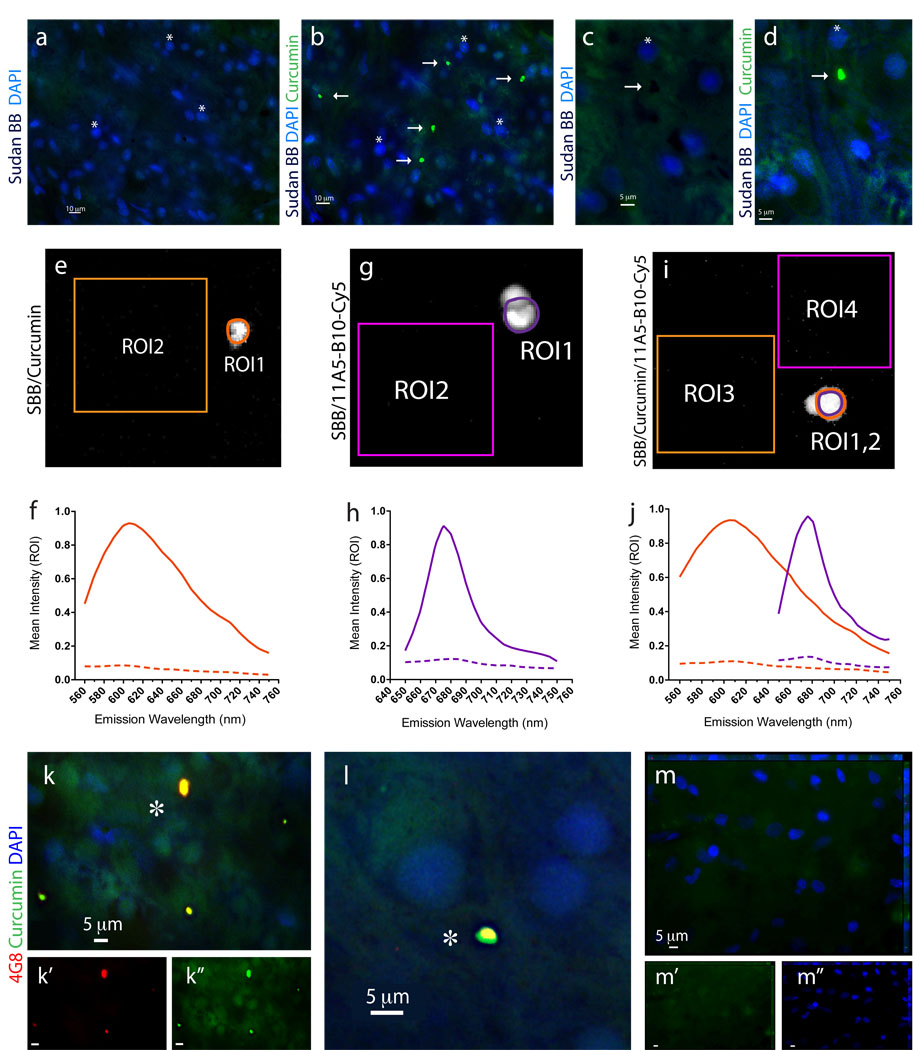
The in vivo detection of retinal Aβ plaques via curcumin in live AD-Tg mice, prompted us to look for their existence in human retinas from AD patients. To this end, postmortem eyes from patients with definite diagnosis of AD and age-matched non-AD controls were used (human donors are summarized in Table 1). To overcome challenges of identifying retinal Aβ plaques it was essential to (1) remove the vitreous, (2) eliminate autofluorescence and non-specific signals, observed in the excitation range of 360–710 nm and associated with lipofuscin/lipid deposits and long-term formalin fixation (Baschong et al., 2001; Schnell et al., 1999) by Sudan Black B (SBB) treatment, and (3) use a high-resolution optical imaging (Figs. 5–7). Following SBB treatment and curcumin staining, plaques were identified in whole-mounted retinas from AD patients (Fig. 5b,d), whereas none were detected at the same location in the absence of curcumin (Fig. 5a,c).
Figure 5.
Identification of Aβ plaques in the human retina of AD patients via a specific curcumin labeling. All stainings of human whole-mount retinas included a Sudan Black B (SBB) pretreatment to eliminate non-specific autofluorescence signals. (a–d) Whole-mount retinas from human AD patients were first immersed with SBB and subsequently stained with curcumin and DAPI; (a,c) no plaques were observed after staining with SBB, whereas (b,d) subsequent staining of the same human retinas with curcumin revealed the presence of Aβ plaques (indicated by arrows; asterisks mark the nuclei of the same tissue location). (c,d) At higher magnification, dark spots of SBB staining are evident, and following curcumin staining a specific Aβ plaque signal is detected in the same retinal location. (e–j) Signal specificity of individual retinal Aβ plaques, single-labeled with curcumin or with anti-Aβ40 (11A5-B10; secondary Ab-Cy5 conjugate), or double-labeled with both, was confirmed by spectral image analysis performed in quadruplicates in a Leica SP5 WLL double-spectral confocal microscope. Regions of interest (ROI) were marked and their corresponding signal intensity was recorded at increasing emission wavelengths from 560 nm to 750 nm to create the spectral curves for Aβ plaques versus background. (e) Representative image of a single curcumin-labeled Aβ plaque in a retinal whole-mount (ROI1) and tissue background (ROI2) captured at excitation/emission wavelengths of 550/605 nm. (f) Spectral analysis curves of individual curcumin-labeled Aβ plaque, at excitation wavelength of 550 nm, as compared to tissue background (dashed line). (g) Representative image of single retinal Aβ plaque (ROI1) and background (ROI2) after staining with Cy5-antibody 11A5-B10 conjugate captured at excitation/emission wavelengths of 640/675 nm. (h) Spectral analysis curves of individual Cy5-antibody-labeled Aβ plaque, at excitation wavelength of 640 nm, as compared to tissue background (dashed line). (i.j) Representative image and spectra curves of retinal Aβ plaque double-labeled with curcumin (ROI1; orange line) and Cy5-antibody 11A5-B10 conjugate (ROI2; purple line), and corresponding background areas (ROI3 and ROI4; dashed lines), at excitation wavelengths of 550 nm (for curcumin spectra) and 640 nm (for Ab-Cy5 conjugate). Peak wavelengths for curcumin and Cy5-antibody captured in the same individual Aβ plaque are distinct and separable; they remain the same as after single stainings. (k–m) Whole-mount retinas from AD patients and normal control stained with curcumin and 4G8; (k,l) Aβ plaques indicated by asterisks show a single-globular compacted morphology. DAPI stains nuclei. (l) Higher magnification image of an extracellular Aβ plaque with compacted morphology. (m) No Aβ plaques were detected in retinas from normal controls.
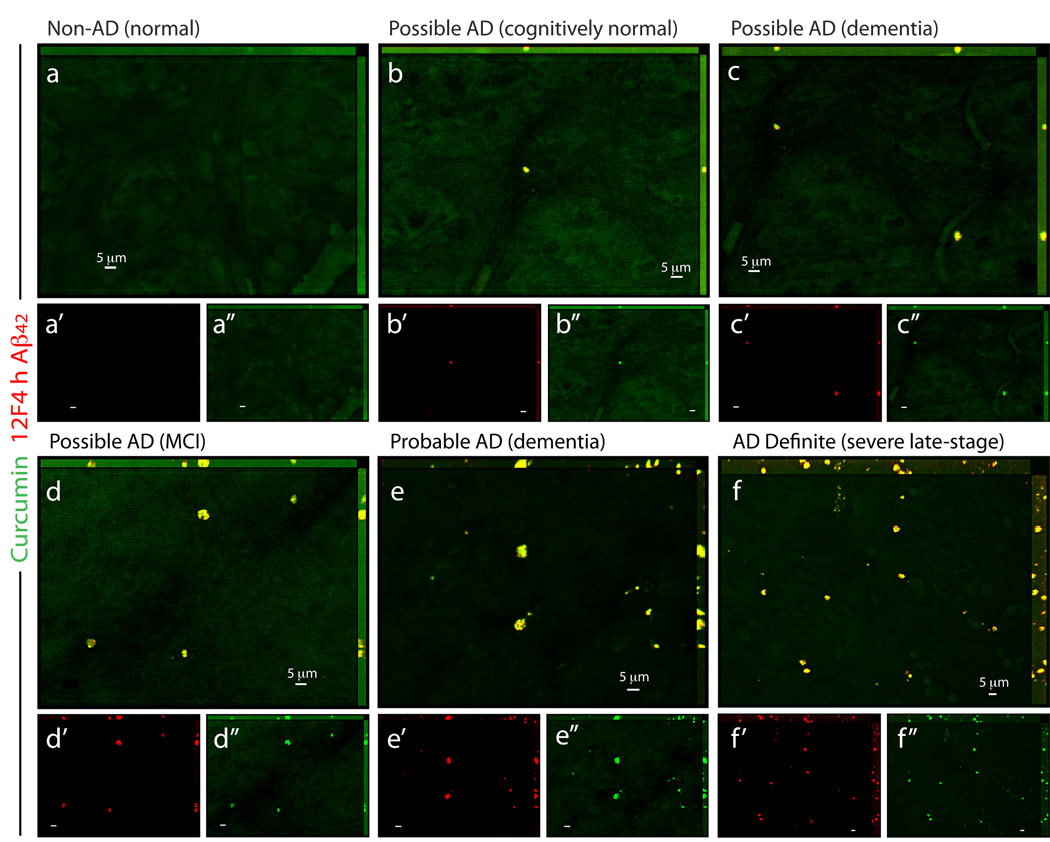
Figure 7.
Detection of retinal Aβ plaques in suspected early AD patients. (a–f) Representative whole-mount retinas from normal individuals (Non-AD) and from possible/probable AD patients (based on the combined clinical diagnosis and postmortem brain pathology; Table S1), stained with curcumin and anti-Aβ42 mAb (12F4; secondary Ab-Cy5 conjugate). (a) In the retina from cognitively normal individual with no AD pathology, Aβ plaques could not be detected. (b) In cognitively normal individual with mild Aβ-plaque brain pathology (mainly diffused plaques), sparse retinal Aβ plaques were identified. (c,d) In patients with possible AD diagnosis, clusters of Aβ plaques were observed, and (e) in retinas from patients with several years of dementia and postmortem diagnosis of probable AD, Aβ plaques were more common throughout the retina. (f) Abundant Aβ plaques were observed in the retina from severe-stage AD definite patient (based on combined clinical diagnosis and postmortem brain pathology; Table S1). (a’–f”) separate channels for each staining.
To ensure that each fluorochrome, when bound to Aβ plaques, had its distinct emission characteristic properly captured, we conducted spectral analyses of individual plaques when single-stained with curcumin (Fig. 5e) or with anti-Aβ40 antibody (with secondary-Cy5; Fig. 5g), and when co-stained with both (Fig. 5i). Regions of interest (ROI; plaques or background) were marked, and their corresponding signal intensity was measured at increasing emission wavelengths (560–750 nm) to determine the optimal wavelength range for the excitation and capture of the distinct emission signal of each fluorochrome compared to the background (Fig. 5f,h,j). The optimal excitation for curcumin bound to Aβ plaques was at 550 nm, with emission peaking at 605–610 nm (Fig. 5f). The optimal excitation for Cy5-Abs bound to anti-Aβ mAbs attached to Aβ plaques was 640 nm, with emission peaking at 675 nm (Fig. 5h). DAPI staining also had a distinct spectral pattern, as expected, given its optimal excitation/emission wavelengths of 365/445 nm. Spectral analysis of double-labeled individual plaques revealed separate emission peaks for each fluorochrome (similar to the single labeling) that could be distinctly captured by our selected filter sets (Fig. 5j; see Material and methods).
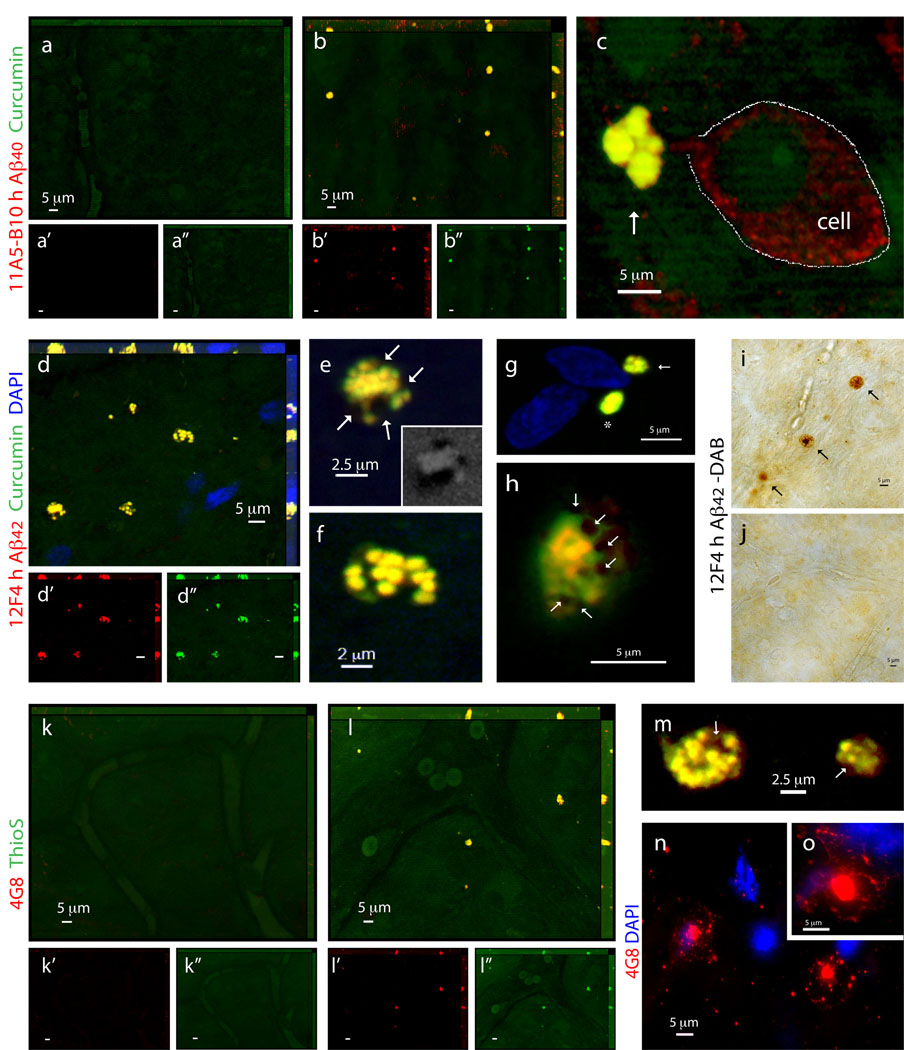
Having confirmed the ability to spectrally resolve the signals from curcumin and Cy5-labeled antibodies, we performed double staining with curcumin and several antibodies recognizing diverse epitopes within Aβ peptide in retinas from definite AD patients and non-AD controls (Figs. 5k-m and 6). Aβ plaques, positive for both curcumin and 4G8 mAb, were clearly apparent in all AD patients (Fig. 5k, and 5l seen extracellular at higher magnification), ranging from 1 to 10 µm in diameter (typically around 5 µm). In the retinas from control individuals stained with curcumin and 4G8, we could not detect Aβ plaques (Fig. 5m). To further verify the specificity of curcumin-labeled Aβ plaques in the human retina, we used 11A5-B10 and 12F4 mAbs recognizing the C-terminal Aβ40 and Aβ42 forms, respectively, found to be elevated in brains of AD patients (Selkoe, 2008). Aβ plaques could not be detected in normal control retinas stained with curcumin and anti-Aβ40 (Fig. 6a). In contrast, in the retinas from AD patients, multiple Aβ40-containing curcumin-labeled plaques were found (Fig. 6b,c). Importantly, labeling with anti-Aβ40 revealed the presence of intracellular Aβ40 (probably its soluble form) as well as extracellular Aβ plaques (co-labeled with curcumin; Fig. 6c at higher magnification). Further analysis of human retinas with curcumin and anti-Aβ42 mAbs detecting the more aggregated form of Aβ, revealed the presence of Aβ42-containing plaques in all samples from AD patients (Fig. 6d–h) but not from the controls (Fig. 7a). The presence of Aβ plaques in retinas from AD patients but not from non-AD controls was also confirmed by non-fluorescent immunoperoxidase method using anti-Aβ42 mAb and DAB as a chromogen (Fig. 6i,j).
Figure 6.
Characterization of retinal Aβ plaques identified in postmortem retinas of definite AD patients. (a–c) Representative z-axis projection images of whole-mount retinas of (a) normal individuals compared to (b,c) AD patients following curcumin and anti-human Aβ40 mAb 11A5-B10 stainings. (a) No Aβ plaques could be detected in normal control retinas, whereas (b) clearly found in retinas from AD patients. (a’–b”) Separate channels for each staining. (c) At higher magnification, extracellular Aβ plaque with compacted large cluster is indicated by an arrow (intracellular Aβ40 is demarcated by a dotted line). (d–h) Whole-mount retinas from AD patients stained with curcumin and anti-human Aβ42 mAb 12F4. (d’,d”) Separate channels. Note co-localization of curcumin and antibody. (e,f) Higher magnification images of Aβ plaques demonstrated their compacted morphology, consisting of multiple small dense cores connected in larger cluster. (e) Aβ plaques containing lipid deposits indicated by arrows; right bottom image captured in DAPI channel shows dark spots of SBB staining representing lipid deposits associated with retinal Aβ plaque. (g) Aβ plaques stained with curcumin and 12F4 mAb, display either compacted single-globular (asterisk) or cluster (arrow) morphology, both lack notable lipid-associated deposits. (h) Aβ plaque with compacted morphology and associated dark SBB staining spots suggesting the presence of lipid deposits (arrows). (i,j) Immunoperoxidase staining of Aβ plaques labeled with primary mAb 12F4 (plaques are indicated by black arrows) in retinal whole-mount from (i) AD patient and (j) non-AD control. DAB was used as a chromogen. (k–m) Representative retinas from (k) normal individual compared to (l) AD patient stained with ThioS and anti-Aβ mAb 4G8. ThioS- and 4G8 double-positive parenchymal Aβ plaques are found in AD patients’ retinas but not in normal controls. (k’–l”) Separate channels. (m) Higher magnification demonstrating compacted morphology of Thio-S-positive retinal Aβ plaques in AD patients’ retinas. (n,o) Single-labeled Aβ plaques using 4G8 mAb (secondary Ab-Cy5 conjugate) in the retinal innermost layers: Aβ plaques have classical morphology consisting of a central dense-core and radiating fibrillar arms.
Staining of human retinas with ThioS and anti-Aβ mAbs confirmed the absence of Aβ plaques in non-AD control samples (Fig. 6k), whereas abluminal plaques in AD patients’ samples were revealed (Fig. 6l, plaques were mostly located outside the blood vessels; Fig. 6m, separate image demonstrating ThioS-positive plaques at a higher magnification). Overall, labeling patterns of retinal Aβ plaques by curcumin or ThioS and anti-Aβ mAbs, were similar in samples from AD patients and the mouse model. A diversity of Aβ plaque morphology was observed in retinas from AD patients: we found with a lower frequency the “classical/neuritic” plaque structure, possessing a central dense core and radiating fibrillar arms consisting of Aβ deposits (Fig. 6n,o), and with a higher frequency the “compacted” (“burned-out”) plaques composed of a dense-core globular amyloid deposit with no apparent radiating fibrils. Compacted plaques had either a single core (Figs. 5k,l and 6g) or clusters: consisting of few dense cores in a cluster (Fig. 6b,c,g) or multiple small dense cores connected to each other in a relatively large core (Fig. 6d-f,l-m). Some Aβ plaques included lipid deposits stained in dark color by SBB (Fig. 6e,h,m), which may entail their pathological impact based on recent report demonstrating the neurotoxicity of the lipid-containing plaques (Martins et al., 2008). It should be noted that classical plaque structure seemed more common in the brains from AD patients than in their respective retinas, and that the fibrillary arms are clearly positive for anti-Aβ Abs whereas less intensely stained with curcumin (Fig. S4a–c). Very similar plaque structures were observed in AD-Tg mouse brains stained with curcumin or ThioS and co-labeled with Aβ mAbs, with the same distinctive patterns for each staining method as seen in human brains; a reflection of the recognized epitopes (Fig. S4d–f). Curcumin, similar to ThioS, binds to Aβ plaque structure rather than a specific sequence, and intensely stains the central dense core. It is also suggested that the dense-core Aβ plaques seen in AD patients’ retinas are formed at early stages of the disease, considering the “life pathogenesis” hypothesis for development of different plaque types (Armstrong, 1998).
Detection of Aβ plaques in retinal samples from suspected early stage AD patients
Aβ plaques, positively stained with curcumin, were further detected in postmortem retinas from suspected AD patients that were possibly at early disease stages (Fig. 7). These included a group of individuals that could be identified as early stage AD (Morris and Price, 2001), based on their combined clinical diagnosis and postmortem brain neuropathology: patients with few years of dementia suspected for possible or probable AD diagnosis, a patient with mild cognitive impairment (MCI) that had higher probability to develop AD (Petersen et al., 1999), and a preclinical individual with postmortem detection of initial Aβ plaque pathology in the brain (Table S1, patients #9–13). We found a qualitative correlation between the severities of the clinical diagnosis verified by postmortem neuropathology and retinal Aβ plaque burden (Fig. 7b–f). Overall, we were able to identify Aβ plaques, which were specifically detected with curcumin, in the retinas from definite and suspected early AD patients.
Discussion
The present study demonstrates that a noninvasive in vivo monitoring of AD hallmark pathology via optical imaging with high specificity and resolution is feasible through the retina. Two key issues towards translation of this approach for the detection of Alzheimer’s in humans have been accomplished here: in vivo imaging of Aβ plaques in the retina of live AD animals, and identification of amyloid plaques in retinas from human Alzheimer’s disease patients, even before clinical symptoms allow disease diagnosis with certainty, thereby creating the basis for developing an early diagnostic marker specific to Alzheimer’s disease.
The detection of retinal Aβ plaques in suspected patients is important, as previous reports indicated that a considerable percentage of these patients suffering from mild dementia and/or MCI (exhibiting memory impairments but not fully demented) will develop AD (Morris and Price, 2001; Petersen et al., 1999). Moreover, we were able to identify Aβ plaques in the retina from a cognitively normal individual who had sparsely diffused plaques in the hippocampus discovered only after brain autopsy.
Neuropathological abnormalities associated with AD, especially Aβ alterations (Jack et al., 2010), may occur during the prodromal phase, as early as decades before the clinical phase of full disease manifestation. Therefore, there is a need for early diagnosis allowing early intervention, in order to achieve an efficient response to therapy (Holmes et al., 2008). In this respect, our data from AD mouse model on the detection of Aβ plaques via curcumin in the retina before they appeared in the brain, and plaque burden correlation with the progression of brain pathology, appear encouraging. Moreover, the significant reduction of Aβ plaques observed in retinas from AD mice following immunization with myelin-derived peptide supports our contention that retinal plaque pathology faithfully represent the brain disease and encourage the development of retinal imaging via curcumin for monitoring AD plaques and assessment response to therapies.
Retinal Aβ plaques in our postmortem samples from AD patients were detected mostly within the inner layers, which makes live imaging/screening of potential patients’ retinas a feasible strategy, most likely through improvement of available ophthalmoscopy tools, by the addition of adaptive optics (Carroll et al., 2008) and spectral imaging. Although the current human study has a relatively limited sample size, our results provide, for the first time, a proof for the existence of amyloid plaque pathology in the retina that is specific to Alzheimer’s disease. In addition, these data may form the basis for a more quantitative clinical trial. Importantly, the bioavailability of curcumin to the mouse eye following its systemic injection and its high affinity to Aβ plaques enabled the detection of these plaques in live animals. In terms of safety, Phase I and II trials using curcumin in patients with cancer have proven its low toxicity in humans even at high doses (12 g/day), and when given over extended periods of time (Dhillon et al., 2008). Translation of curcumin doses given intravenously or orally from mice to humans (<1 g) for retinal plaque visualization is expected to remain within the determined safety levels. Furthermore, recent studies have reported various approaches to significantly increase curcumin stability and bioavailability in humans (Anand et al., 2007).
Aβ plaques in retinas from AD patients appear to be a specific diagnostic marker as compared to the previously described early visual dysfunctions (Katz and Rimmer, 1989; Sadun et al., 1987) and retinal abnormalities, especially atrophy of the NFL (Berisha et al., 2007; Blanks et al., 1996; Hinton et al., 1986; Trick et al., 1989), which were also evident in other eye disorders and neurodegenerative conditions (Jindahra et al., 2010; Parisi, 2003). Moreover, based on their unique size, signature and distribution within the retinas, Aβ plaques observed in AD patients could be eventually used for differential diagnosis. For instance, plaques detected in age-related macular degeneration are locally restricted to retinal pigment epithelium within drusen and appear smaller in size (Anderson et al., 2004). In glaucoma, NFL and GCL are altered, and increased intracellular expression of Aβ was found in RGCs in a rat model (Guo et al., 2007), but no Aβ plaques were detected in glaucoma patients or animal models.
Conclusions
This study provides the first demonstration of Aβ plaques in postmortem retinas from suspected and definite AD patients that reflected AD brain pathology. In addition, systemic administration of curcumin to AD mice resulted in specific in vivo labeling of retinal Aβ plaques. This novel approach enabled noninvasive and high-resolution monitoring of individual retinal Aβ plaques in live AD mice. Finally, curcumin-visualized retinal plaques were shown to decrease in number and size following immunization with altered myelin-derived peptide, with dynamics and extent similar to their brain counterparts in an animal model. The reported data thus provide the basis for direct noninvasive optical imaging of AD plaque pathology through the retina with high resolution and sensitivity. Future studies are required to demonstrate the ability to detect Aβ plaques in the retinas of live AD patients for disease diagnosis and monitoring.
Supplementary Material
Supplementary Figure 1 Aβ deposition in whole-mount retina of AD-Tg mice visualized by ThioS ex vivo. Examination of mice whole-mount retina stained with ThioS and anti-human Aβ mAb (4G8). (a) No plaques detected in Non-Tg mouse retinas, whereas (b,c) ThioS-positive Aβ plaques, co-labeled with 4G8, were clearly visible in retinas from AD-Tg mice. (b’,b”) separate channels for each staining. (c) Higher magnification image demonstrating Aβ plaques and their specific staining pattern by ThioS and 4G8.
Supplementary Figure 2 Endogenous mouse Aβ deposition in non-Tg mouse retina. (a–d) Representative images of 10-month-old Non-Tg (wt) mouse whole-mount retina stained with anti-mouse Aβ Ab (secondary Ab-Cy5 conjugate), curcumin and DAPI. (a–c) separate channels for each staining. (d) Merged image demonstrating sparse and small mouse Aβ deposits that are occasionally observed in the wt mouse retina. (e) Higher magnification image demonstrating endogenous mouse-Aβ plaques (≤1 µm) found in wt mouse.
Supplementary Figure 3 Optical signature of mouse retinal Aβ plaques labeled in vivo with curcumin. (a) Representative confocal z-axis projection image of sagittal/coronal virtual section of whole-mount retina, demonstrating retinal Aβ plaques (red) stained ex vivo with anti-human Aβ mAb (4G8; secondary Ab-Cy5 conjugate); plaques were found in the parenchyma (asterisks) and inside the blood vessels (white arrows). (b,c) Images from whole-mount retina of AD-Tg mouse were captured using a fluorescence microscope equipped with AOTF-based spectral imaging system, and analyzed and visualized by segmentation and classification software. (b) Aβ plaques (in white) and blood vessels (indicated by red arrows), were visible with curcumin-labeling in vivo and imaged at a single channel (excitation 562/40 nm; emission 624/40 nm). (c) Spectral imaging provides the specific optical signature (OS) of Aβ plaques labeled with curcumin (red pseudocolor) in the same retinal region (white arrows indicate the same plaques in this tissue location), thus allowing elimination of background and non-specific signals via spectral unmixing (Burton, 2009).
Supplementary Figure 4 Curcumin labeling patterns of Aβ plaques in brains from AD patients and the mouse model. (a) Aβ plaques were visualized by curcumin in brain cryosections from AD patients and co-localized with 6E10 mAb. (b) Higher magnification image demonstrating the fine structure of Aβ plaque and its specific staining pattern by curcumin and 6E10 mAb. Curcumin stains mostly the central dense core and less the surrounding fibrillary arms that are strongly positive for anti-Aβ Abs. (a’,a” and b’,b”) Separate channels for each staining. (c) Aβ plaques are not seen in a brain from non-AD control subject. (d–f) Curcumin and ThioS ex vivo labeling patterns of Aβ plaques in brain cryosections from AD-Tg mice, preferential staining of central core of brain Aβ plaques with both curcumin and ThioS: (d) Aβ plaques visualized by curcumin and Aβ antibody, but (e) not found in brains from non-Tg (wt) littermates. (d’,d”) Separate channels. (f) ThioS label Aβ plaques in brain of AD-Tg mice and co-stained with Aβ antibody. DAPI stained nuclei. (f’,f”) Separate channels.
Acknowledgments
We thank Drs. J.Y. Hwang, Y. Kohanzadeh, A.G. Nowatzyk, K.V. Ramanujan and K. Wawrowsky for useful discussions and imaging collaboration. M.S. holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology at The Weizmann Institute of Science, Israel. This work was supported in part by the Marciano Family Foundation, the US Navy Bureau of Medicine and Surgery, R01 EY13431 and M01 RR00425, the Winnick Family Foundation, and by the University of Southern California Alzheimer's Disease Research Center NIA AG005142.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement
The authors declare that no conflict of interest exists.
References
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of β amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. β-Amyloid plaques: stages in life history or independent origin? Dement Geriatr Cogn Disord. 1998;9:227–238. doi: 10.1159/000017051. [DOI] [PubMed] [Google Scholar]
- Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49:1565–1572. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer's disease. Invest Ophthalmol Vis Sci. 2007;48:2285–2289. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–384. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- Burton K, Jeong J, Wachsmann-Hogiu S, Farkas DL. Spectral optical imaging in biology and medicine in Biomedical Optical Imaging. Oxford University Press; 2009. (ISBN: 978-0- 19-515044-5) [Google Scholar]
- Carroll J, Choi SS, Williams DR. In vivo imaging of the photoreceptor mosaic of a rod monochromat. Vision Res. 2008;48:2564–2568. doi: 10.1016/j.visres.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MF, Guo L, Coxon KM, Duggan J, Nizari S, Normando EM, Sensi SL, Sillito AM, Fitzke FW, Salt TE, Moss SE. Imaging multiple phases of neurodegeneration: a novel approach to assessing cell death in vivo. Cell Death and Disease. 2010;1:1–11. doi: 10.1038/cddis.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Ford ML, Evavold BD. An MHC anchor-substituted analog of myelin oligodendrocyte glycoprotein 35–55 induces IFN-gamma and autoantibodies in the absence of experimental autoimmune encephalomyelitis and optic neuritis. Eur J Immunol. 2004;34:388–397. doi: 10.1002/eji.200324502. [DOI] [PubMed] [Google Scholar]
- Fujimoto JG, Farkas DL, editors. Biomedical Optical Imaging. Oxford University Press; 2009. (ISBN: 978-0-19-515044-5) [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA, Faget KY, Fitch KA, Masters CL, Tanzi RE, Chylack LT, Jr, Bush AI. Cytosolic β-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet. 2003;361:1258–1265. doi: 10.1016/S0140-6736(03)12981-9. [DOI] [PubMed] [Google Scholar]
- Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW, Cordeiro MF. Targeting amyloid-β in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–13449. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hintersteiner M, Enz A, Frey P, Jaton AL, Kinzy W, Kneuer R, Neumann U, Rudin M, Staufenbiel M, Stoeckli M, Wiederhold KH, Gremlich HU. In vivo detection of amyloid-β deposits by near-infrared imaging using an oxazine-derivative probe. Nat Biotechnol. 2005;23:577–583. doi: 10.1038/nbt1085. [DOI] [PubMed] [Google Scholar]
- Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum Mol Genet. 2004a;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Gonzales V, Jenkins NA, Copeland NG, Borchelt DR. APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1. Neurobiol Aging. 2004b;25:885–892. doi: 10.1016/j.neurobiolaging.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Jindahra P, Hedges TR, Mendoza-Santiesteban CE, Plant GT. Optical coherence tomography of the retina: applications in neurology. Curr Opin Neurol. 2010;23:16–23. doi: 10.1097/WCO.0b013e328334e99b. [DOI] [PubMed] [Google Scholar]
- Katz B, Rimmer S. Ophthalmologic manifestations of Alzheimer's disease. Surv Ophthalmol. 1989;34:31–43. doi: 10.1016/0039-6257(89)90127-6. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, Debnath ML, Holt DP, Huang GF, Shao L, DeKosky ST, Price JC, Mathis CA. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-β in Alzheimer's disease brain but not in transgenic mouse brain. J Neurosci. 2005;25:10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronyo-Hamaoui M, Ko MK, Koronyo Y, Azoulay D, Seksenyan A, Kunis G, Pham M, Bakhsheshian J, Rogeri P, Black KL, Farkas DL, Schwartz M. Attenuation of AD-like neuropathology by harnessing peripheral immune cells: local elevation of IL-10 and MMP-9. J Neurochem. Lockhart, 2009 doi: 10.1111/j.1471-4159.2009.06402.x. [DOI] [PubMed] [Google Scholar]
- Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG. PIB is a non-specific imaging marker of amyloid-beta (Aβ) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- Martins IC, Kuperstein I, Wilkinson H, Maes E, Vanbrabant M, Jonckheere W, Van Gelder P, Hartmann D, D'Hooge R, De Strooper B, Schymkowitz J, Rousseau F. Lipids revert inert Aβ amyloid fibrils to neurotoxic protofibrils that affect learning in mice. EMBO J. 2008;27:224–233. doi: 10.1038/sj.emboj.7601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Local neuroinflammation and the progression of Alzheimer's disease. J Neurovirol. 2002;8:529–538. doi: 10.1080/13550280290100969. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignonz A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Nakada T, Matsuzawa H, Igarashi H, Fujii Y, Kwee IL. In vivo visualization of senile-plaque-like pathology in Alzheimer's disease patients by MR microscopy on a 7T system. J Neuroimaging. 2008;18:125–129. doi: 10.1111/j.1552-6569.2007.00179.x. [DOI] [PubMed] [Google Scholar]
- Ng S, Villemagne VL, Berlangieri S, Lee ST, Cherk M, Gong SJ, Ackermann U, Saunder T, Tochon-Danguy H, Jones G, Smith C, O'Keefe G, Masters CL, Rowe CC. Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer's disease. J Nucl Med. 2007;48:547–552. doi: 10.2967/jnumed.106.037762. [DOI] [PubMed] [Google Scholar]
- Ning A, Cui JZ, To E, Hsiao Ashe K, Matsubara JA. Amyloid-β deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer's disease. Semin Ophthalmol. 2003;18:50–57. doi: 10.1076/soph.18.2.50.15855. [DOI] [PubMed] [Google Scholar]
- Perez SE, Lumayag S, Kovacs B, Mufson EJ, Xu S. β-Amyloid deposition and functional impairment in the retina of the APPswe/PS1ΔE9 transgenic mouse model of Alzheimer's disease. Invest Ophthalmol Vis Sci. 2009;50:793–800. doi: 10.1167/iovs.08-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Sadun AA, Borchert M, DeVita E, Hinton DR, Bassi CJ. Assessment of visual impairment in patients with Alzheimer's disease. Am J Ophthalmol. 1987;104:113–120. doi: 10.1016/0002-9394(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, Price DL. Role of the β-amyloid protein in Alzheimer's disease. FASEB J. 1995;9:366–370. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- Toyama H, Ye D, Ichise M, Liow JS, Cai L, Jacobowitz D, Musachio JL, Hong J, Crescenzo M, Tipre D, Lu JQ, Zoghbi S, Vines DC, Seidel J, Katada K, Green MV, Pike VW, Cohen RM, Innis RB. PET imaging of brain with the β-amyloid probe, [11C]6-OH-BTA-1, in a transgenic mouse model of Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2005;32:593–600. doi: 10.1007/s00259-005-1780-5. [DOI] [PubMed] [Google Scholar]
- Trick GL, Barris MC, Bickler-Bluth M. Abnormal pattern electroretinograms in patients with senile dementia of the Alzheimer type. Ann Neurol. 1989;26:226–231. doi: 10.1002/ana.410260208. [DOI] [PubMed] [Google Scholar]
- Wachman ES, Niu W, Farkas DL. AOTF microscope for imaging with increased speed and spectral versatility. Biophys J. 1997;73:1215–1222. doi: 10.1016/S0006-3495(97)78154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci U S A. 2006;103:13174–13179. doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Aβ deposition in whole-mount retina of AD-Tg mice visualized by ThioS ex vivo. Examination of mice whole-mount retina stained with ThioS and anti-human Aβ mAb (4G8). (a) No plaques detected in Non-Tg mouse retinas, whereas (b,c) ThioS-positive Aβ plaques, co-labeled with 4G8, were clearly visible in retinas from AD-Tg mice. (b’,b”) separate channels for each staining. (c) Higher magnification image demonstrating Aβ plaques and their specific staining pattern by ThioS and 4G8.
Supplementary Figure 2 Endogenous mouse Aβ deposition in non-Tg mouse retina. (a–d) Representative images of 10-month-old Non-Tg (wt) mouse whole-mount retina stained with anti-mouse Aβ Ab (secondary Ab-Cy5 conjugate), curcumin and DAPI. (a–c) separate channels for each staining. (d) Merged image demonstrating sparse and small mouse Aβ deposits that are occasionally observed in the wt mouse retina. (e) Higher magnification image demonstrating endogenous mouse-Aβ plaques (≤1 µm) found in wt mouse.
Supplementary Figure 3 Optical signature of mouse retinal Aβ plaques labeled in vivo with curcumin. (a) Representative confocal z-axis projection image of sagittal/coronal virtual section of whole-mount retina, demonstrating retinal Aβ plaques (red) stained ex vivo with anti-human Aβ mAb (4G8; secondary Ab-Cy5 conjugate); plaques were found in the parenchyma (asterisks) and inside the blood vessels (white arrows). (b,c) Images from whole-mount retina of AD-Tg mouse were captured using a fluorescence microscope equipped with AOTF-based spectral imaging system, and analyzed and visualized by segmentation and classification software. (b) Aβ plaques (in white) and blood vessels (indicated by red arrows), were visible with curcumin-labeling in vivo and imaged at a single channel (excitation 562/40 nm; emission 624/40 nm). (c) Spectral imaging provides the specific optical signature (OS) of Aβ plaques labeled with curcumin (red pseudocolor) in the same retinal region (white arrows indicate the same plaques in this tissue location), thus allowing elimination of background and non-specific signals via spectral unmixing (Burton, 2009).
Supplementary Figure 4 Curcumin labeling patterns of Aβ plaques in brains from AD patients and the mouse model. (a) Aβ plaques were visualized by curcumin in brain cryosections from AD patients and co-localized with 6E10 mAb. (b) Higher magnification image demonstrating the fine structure of Aβ plaque and its specific staining pattern by curcumin and 6E10 mAb. Curcumin stains mostly the central dense core and less the surrounding fibrillary arms that are strongly positive for anti-Aβ Abs. (a’,a” and b’,b”) Separate channels for each staining. (c) Aβ plaques are not seen in a brain from non-AD control subject. (d–f) Curcumin and ThioS ex vivo labeling patterns of Aβ plaques in brain cryosections from AD-Tg mice, preferential staining of central core of brain Aβ plaques with both curcumin and ThioS: (d) Aβ plaques visualized by curcumin and Aβ antibody, but (e) not found in brains from non-Tg (wt) littermates. (d’,d”) Separate channels. (f) ThioS label Aβ plaques in brain of AD-Tg mice and co-stained with Aβ antibody. DAPI stained nuclei. (f’,f”) Separate channels.