Abstract
The transient receptor potential A1 (TRPA1) channel contributes to nociceptive signaling in certain pain models. It has been suggested that Ca2+, which activates and modulates TRPA1, could play a critical regulatory role in this process. Since TRPA1 and TRPV1 channels are co-expressed and interact in neurons, we investigated whether activation and modulation of TRPA1 by Ca2+ is regulated by TRPV1. Cell-attached recordings showed that TRPA1 is activated by extracellular Ca2+ ([Ca2+]e) in concentration-response fashion. This activation, especially by 2mM [Ca2+]e was substantially suppressed by co-expression with TRPV1. Inside-out recordings demonstrated that intracellular Ca2+ ([Ca2+]i)-triggered activation of TRPA1 was attenuated by the presence of TRPV1 only at 2 mM [Ca2+]e, but not in Ca2+-free conditions. Further, depletion of internal Ca2+ stores by thapsigargin generated TRPA1-mediated currents, which is affected by TRPV1 in both Chinee hamster ovary cells and sensory neurons.
Since mustard oil current (IMO) is modulated by [Ca2+]e, we next examined whether alterations in the Ca2+-permeability of TRPV1 by mutating Y671 effect IMO properties. First it was demonstrated that the mutations in TRPV1 did not affect association of the TRPA1 and TRPV1 channels. However, these TRPV1 mutations, particularly Y671K, altered the following characteristics of TRPA1: magnitude of IMO in presence and absence of [Ca2+]e; the influence of [Ca2+]e on the voltage-dependency of IMO, and open probability of single-channel IMO. In summary, activation of TRPA1 by [Ca2+]e and [Ca2+]i is controlled by the TRPV1 channel, and characteristics of IMO depend on Ca2+ permeability of the TRPV1 channel.
Keywords: TRPA1, TRPV1, Pain
Introduction
Physiological studies using TRPA1-null-mutant mice (Bautista et al., 2006, Kwan et al., 2006, Kwan et al., 2009), antisense knock-down (Obata et al., 2005) and in vivo effects of TRPA1 antagonists (McNamara et al., 2007, Petrus et al., 2007) have demonstrated that TRPA1 controls the processing of nociceptive information in certain inflammatory and nerve injury pain models.
Mechanisms underlying information processing and stimulus integration by the TRPA1 channel in nociceptors have recently been vigorously studied. It was suggested that Ca2+ could play important role in these processes (Bautista et al., 2006, Zurborg et al., 2007). TRPA1 and TRPV1 can be activated by extracellular [Ca2+]e (Ahern et al., 2005, Cavanaugh et al., 2008) as well as intracellular Ca2+ ([Ca2+]i) (van der Stelt et al., 2005, Doerner et al., 2007, Zurborg et al., 2007). Activation of these channel by [Ca2+]e can result in a baseline supply of Ca2+ into cells (i.e. Ca2+ leak). This constant supply of Ca2+ may maintain a variety of basal Ca2+ dependent processes in nociceptors, including transcription regulation and phosporylation. On the other hand, activation of TRPA1 by [Ca2+]i could account for mechanisms of TRPA1 gating by inflammatory mediators (Bandell et al., 2004, Zurborg et al., 2007). Thus, inflammatory mediators can trigger an elevation in intracellular Ca2+ ([Ca2+]i) in sensory neurons via two possible pathways: depletion of internal Ca2+ stores via Gq/11-coupled pathways and/or activation of Ca2+-permeable channels (such as TRPV1, TRPA1 and TRPC3) on the plasma membrane (Bandell et al., 2004, Kim et al., 2004, Suh and Oh, 2005). Such elevation in [Ca2+]i can result in activation of a variety of channels, including the TRPA1, by inflammatory mediators (Liu et al., 2010). In addition, since [Ca2+]i can activate the TRPA1 channel in expression systems (Doerner et al., 2007, Zurborg et al., 2007), it has been proposed that [Ca2+]i could serve as a mediator providing a linkage between the TRPV1 and TRPA1 channels during acute inflammatory hyperalgesia (Bautista et al., 2006, McMahon and Wood, 2006).
Extracellular Ca2+ can also modulate TRPA1-meditaed responses. Thus, [Ca2+]e alters the magnitude (Jordt et al., 2004, Nagata et al., 2005), changes kinetics and regulates single-channel characteristics of mustard oil (MO)-gated responses (Nagata et al., 2005, Kim and Cavanaugh, 2007). Further, extracellular Ca2+-dependent properties of TRPA1-mediated responses are regulated by the TRPV1 channel in sensory neurons (Akopian et al., 2007, Salas et al., 2009, Staruschenko et al., 2010).
Despite this wealth of research, the potential roles of the TRPV1 channel in regulation of activation and modulation of TRPA1 by Ca2+ are poorly understood. To test this possibility, we have examined activation of TRPA1 by extracellular and intracellular Ca2+ in the presence and absence of the TRPV1 channel. We also investigated whether mutations in TRPV1 pore affecting Ca2+ permeability of the TRPV1 channel modify characteristics of MO-gated responses. These data could provide insight on Ca2+-dependent functional regulation of nociceptive processing by interacting TRPA1 and TRPV1 channels.
Experimental procedures
Animals and primary sensory neuron culture
All experiments on animals conformed to protocols approved by the University Texas Health Science Center at San Antonio (UTHSCSA) Animal Care and Use Committee (ACUC). We followed guidelines issued by the National Institutes of Health and the Society for Neuroscience to minimize the number of animals used and their suffering.
Sprague-Dawley rats, 45-60-days old, were obtained from a commercial breeder (Charles River Laboratories, Inc., Wilmington, MA or Harlan, Indianapolis, IN, USA). B6.129S4 and B6.129S4-trpV1tml/jul (TRPV1 null-mutant) mice, 40-60-days old, were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). TRPA1 null-mutant mice were generated on the B6129P1/F2J background, and kindly provided by Dr. Kevin Kwan (Kwan et al., 2006).
Mice and rats were deeply anaesthetized with isoflurane (0.3 ml in 1 liter administered for 60-90 sec) and killed by decapitation. The trigeminal ganglia (TG) were quickly removed from the skull and placed in ice-cold Hank’s solution (Invitrogen, Carlsbad, California, USA). TG neurons were separated by treatment in a 1mg/ml collagenase-dispase (Roche, Indianapolis, IN) solution. Neurons were plated at low-density on poly-D-lysine/laminin coated coverslips (Clontech, Palo Alto, CA). Cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 100 ng/ml NGF-7.02S (Harlan, Indianapolis, IN). The use of dispase instead of trypsin during dissociation of neurons and NGF in the maintenance media was critical to achieve reproducible and consistent responses to mustard oil (MO) application. The experiments were performed 24-36 h after plating.
Constructs and expression in CHO cells
Expression plasmids of green fluorescent protein (GFP) in pEGFP-N1 (Clontech); TRPV1 (accession number - NM031982) in pcDNA3 (Invitrogen, Carlsbad, CA); TRPA1 (NM177781) in pcDNA5/FRT (Invitrogen); D646N TRPV1, Y671A TRPV1, Y671D TRPV1 and Y671K TRPV1 in pcDNA3 were used in the studies. These constructs were delivered into Chinese hamster ovary (CHO) cells using PolyFect (Qiagen, Valencia, CA) according to manufacturers’ protocols. Cells were used within 24-48 h after transfection.
Co-immunoprecipitation (Co-IP)
2-5×107 CHO cells were transfected with equimolar ratios of TRPV1 (or TRPV1 mutants) and myc-tagged TRPA1. Co-immunoprecipitation (Co-IP) and Western blotting was conducted as previously described (Jeske et al., 2006, Staruschenko et al., 2010) on 2-day transfected CHO cells. Anti-TRPV1 (Oncogene, San Diego, CA) and anti-myc (BD Biosciences) antibodies were utilized (Jeske et al., 2006).
Ca2+ imaging
Ca2+-imaging was performed as previously described (Akopian et al., 2007). Fluorescence images were analyzed by the MetaFluor Software (MetaMorph, Web Universal Imaging Corporation, Downingtown, PA). Ratiometric data were converted to [Ca+2]i (in μM) as previously described (Jeske et al., 2006). The net changes in Ca+2 influx were calculated by subtracting the basal [Ca+2]i (collected for 60 s) from the peak [Ca+2]i value. Increases in [Ca+2]i (Δ[Ca+2]i) above 30 nM were considered positive. This minimal threshold criterion was established by application of 0.1% DMSO (capsaicin was dissolved in 100% DMSO to concentration of 100mM) as a vehicle.
Electrophysiology
The whole-cell perforated patch and single-channel voltage-clamp recordings were performed at 22-24°C from the somata of TG neurons (15-45 pF) or CHO cells. Data were acquired and analyzed using an Axopatch200B amplifier and pCLAMP10 software (Axon Instruments, Union City, CA). Currents were filtered with an 8-pole, low pass Bessel filter at 0.1 kHz (cell-attached and inside-out) since dwelling time (τ) of the TRPA1 single-current was >0.5 sec (Cavanaugh et al., 2008); or 0.5 kHz (whole-cell and perforated patch) and sampled at 1 kHz or 2 kHz, respectively. Borosilicate pipettes (Sutter, Novato, CA) were polished to resistances of 5-7 MΩ (perforated patch) or 8-11 MΩ (cell-attached and inside-out) in corresponding pipette solutions. Access resistance (Rs) was compensated to achieve final Rs=12-15 MΩ (perforated-patch). Perforated patch recording were rejected when Rs changed >20% during recording, leak currents were >100pA, or input resistance was <200 MΩ. The amplitudes of the currents were analyzed when they were 5-fold larger than the noise (in root mean square).
Unitary current (i) was determined, as normal, from all-point amplitude histograms fitted with single- or multi-Gaussian curves using the standard 50% threshold criterion to differentiate between events. Events were inspected visually prior to acceptance. Channel activity, defined as NPo, was calculated using the following equation: NPo = (t1 + 2t2 + …+ntn), where N and Po are the number of TRP channels in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels. Po was calculated by dividing NPo by the number of active channels within a patch as defined by all-point amplitude histograms (Staruschenko et al., 2010). In order to increase accuracy in measurement of Po, only patches containing 5 channels or fewer were used.
I-V relations were obtained as previously described (Salas et al., 2009). Detailed protocols of solutions for electrophysiological recordings are standard and provided in (Akopian et al., 2008, Salas et al., 2009). Briefly, standard external solution (SES) contained 2 mM Ca2+. Ca2+-free solution contains 5 mM EGTA to buffer ambient Ca2+. Low concentrations (up to 1μM) of Ca2+ in solutions were established by mixing software calculated concentrations of EGTA and Ca2+ (see http://www.stanford.edu/~cpatton/webmaxc/webmaxcS.htm). The pipette solution for the perforated patch configurations included 250 μg/ml amphotericin B (Sigma). For single-channel recording in cell-attached and inside-out configuration, the bath solution (SES-SCh) consisted of (in mM): 100 K-gluconate, 4 KCl, 10 D-glucose, 10 Hepes (pH 7.3) and the indicated concentration of Ca2+. The pipette solution (SIS-SCh) was (mM): 100 Na-gluconate, 10 NaCl, 10 D-glucose, 10 Hepes (pH 7.3) and the indicated concentration of Ca2+. Drugs were applied using a fast, pressure-driven, computer controlled 8-channel system (ValveLink8; AutoMate Scientific, San Francisco, CA). Application times are stated in the legends to figures.
Data analyses
For detailed statistical analyses, GraphPad Prism 4.0 (GraphPad, San Diego, CA) was used. The data in Figures were given as mean ± standard error of the mean (SEM), with the value of n referring to the number of analyzed cells for each group. All experiments were performed at least in triplicate. The significant difference between groups was assessed by one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison post-hoc test. Two conditions were compared using unpaired t-tests. A difference was accepted as significant when p<0.05, <0.01 or <0.001 that were marked *, ** and ***, respectively.
Results
Activation of cells by extracellular Ca2+ is mutually regulated by TRPA1 and TRPV1 channel
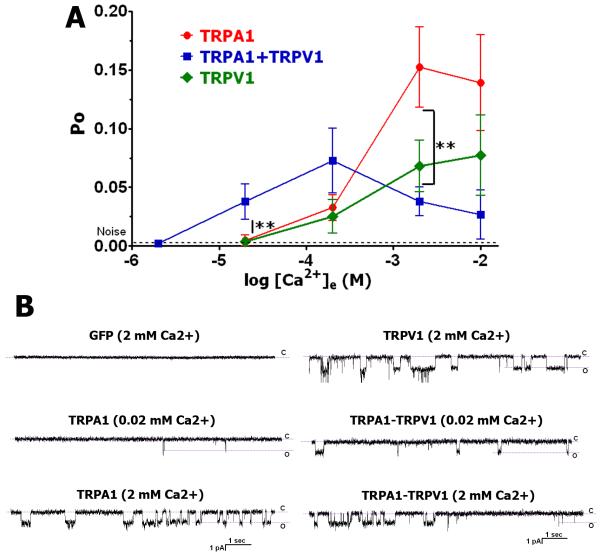
It has been reported that extracellular Ca2+ ([Ca2+]e; 1-3mM) is able to gate TRPA1 (Cavanaugh et al., 2008) as well as TRPV1 channels (Ahern et al., 2005). We examined whether this activity is influenced by co-expression of TRPA1 with the TRPV1 channel. 2mM [Ca2+]e did not activate GFP transfected CHO cells (Fig 1B; the upper left panel). However, [Ca2+]e at concentrations ranging from 20 μM to 10mM showed single-channel activity (cell-attached configuration; voltage-clamp Vh=−60mV) in CHO cells expressing the TRPV1 and TRPA1 channels (Fig 1A and Fig 1B). The responses were considered for analysis when TRPV1 and TRPA1 expressing CHO cells were activated by capsaicin (100 nM CAP) and mustard oil (10 μM MO), respectively. Figure 1A and corresponding traces (Fig 1B) also illustrate that co-expression of TRPV1 and TRPA1 (respond for both CAP and MO) affects [Ca2+]e–triggered single-channel activity of CHO cells. Thus, at a physiological concentration of [Ca2+]e (2 mM), TRPA1 and TRPV1 co-expressing cells exhibit less activity than cells expressing either TRPA1 or TRPV1 alone (Fig 1A). In contrast, at lower concentrations (<200 μM), TRPA1 and TRPV1 co-expressing cells exhibit higher activity (Fig 1A). One of the possible explanations for the biphasic effect in TRPA1-TRPV1 co-expressing cells is that at 2mM [Ca2+]e, co-expression of TRPV1 with TRPA1 leads to a reduction of TRPA1-mediated activities (Salas et al., 2009, Staruschenko et al., 2010).
Figure 1. Activation of TRPA1, TRPV1 and TRPA1-TRPV1 expressing CHO cells by extracellular Ca2+.
(A) Activation of TRPV1, TRPA1 and TRPA1-TRPV1 expressing CHO cells by a variety concentrations of extracellular Ca2+ ([Ca2+]e). Recordings were carried out in cell-attached configuration in voltage-clamp (Vh=−60mV) mode. Indicated concentrations of Ca2+ (X-axis) were included in the pipette solution. The bath solution contained 100nM Ca2+, which approximately corresponds to basal intracellular concentration of Ca2+. Recordings were made for 1 min. Open probability (Po) was measured for recordings between time points of 10 sec and 50 sec. Data analysis was by unpaired t-test. n=9-18. (B) Representative traces show single-channel activity during ≈10 sec stretch. Expression constructs and concentrations of Ca2+ in the pipette solution are indicated above the traces.
It was noted that at physiological concentrations of [Ca2+]e, TRPA1 exhibits basal activity (Bandell et al., 2004, Akopian et al., 2007, Macpherson et al., 2007). The basal activity of the TRPA1 and the TRPV1 channels could affect input resistance and leak values of whole-cell patched sensory neurons. Indeed, analyses of CAP (TRPV1-expressing), MO (TRPA1-expressing) and CAP and MO (TRPA1+TRPV1-co-expressing) positive neurons from trigeminal ganglia (TG) of rats, wild-type (WT) and TRPV1 null-mutant (TRPV1 KO) mice indicated a correlation (Salas et al., 2009). Thus, TG neurons from rat and WT mice responding for both CAP and MO showed 535±28 MΩ (n=115) input resistance and 32±11 pA (n=96) leak values, while only MO sensitive TG neurons from TRPV1 KO mice revealed lesser input resistance (247±54 MΩ, n=21) and higher leak values (75±26 pA, n=19). These differences are statistically significant (unpaired t-test, P<0.001 for leak values and input resistance).
Activation of the cells by intracellular Ca2+ is mutually modulated by TRPA1 and TRPV1
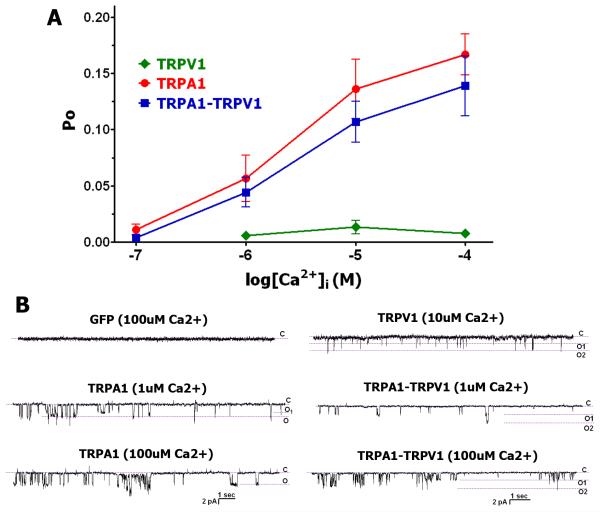
Activation of TRPA1 by intracellular Ca2+ ([Ca2+]i; 0.1-100 uM) with a Ca2+-free extracellular solution (i.e. pipette solution) has been reported (Doerner et al., 2007, Zurborg et al., 2007). We examined whether the TRPV1 channel alters [Ca2+]i-gated activation. Recordings were conducted in inside-out configuration with a Ca2+-free pipette solution. CAP, MO and CAP+MO-sensitive CHO cells were considered TRPV1, TRPA1 and TRPA1-TRPV1 positive, respectively. Figure 2A and corresponding representative traces in Fig 2B demonstrate that with a Ca2+-free extracellular solution, TRPV1 does not significantly influence activation of TRPA1 by 0.1-100uM [Ca2+]i. Further, TRPV1 itself is slightly activated by 1-100uM [Ca2+]i (Fig 2A and 2B).
Figure 2. Activation of TRPA1, TRPV1 and TRPA1-TRPV1 expressing CHO cells by intracellular Ca2+ in Ca2+-free extracellular solution.
(A) Activation of TRPV1, TRPA1 and TRPA1-TRPV1 expressing CHO cells by a variety concentrations of intracellular Ca2+ ([Ca2+]i). Recordings were carried out in inside-out configuration in voltage-clamp (Vh=−60mV) mode. Indicated concentrations of Ca2+ (X-axis) were applied to the patches for 1 min. The pipette solution is Ca2+-free. Open probability (Po) was measured for recordings between time points of 10 sec and 50 sec. Data analysis was by unpaired t-test. n=10-18. (B) Representative traces show single-channel activity during ≈10 sec stretch. Expression constructs and concentrations of applied Ca2+ (i.e. [Ca2+]i) are indicated above the traces.
Since TRPA1-mediated responses are regulated by TRPV1 in the presence of 2mM [Ca2+]e (Salas et al., 2009) and 2mM [Ca2+]e activates both TRPA1 and TRPV1 channels, we investigated the role of the TRPV1 channel in the regulation of TRPA1 activation by [Ca2+]i in the presence of physiological concentrations of [Ca2+]e (i.e. 2mM). Under these conditions, TRPV1 significantly suppressed TRPA1-mediated responses to 1 and 10 uM [Ca2+]i (Fig 3A and 3B). Altogether, TRPV1 influences TRPA1-mediated responses to [Ca2+]i only at 2 mM pipette (i.e. extracellular) solution. This behavior is similar to those observed for other TRPA1 agonists, such as MO and certain cannabinoids (Salas et al., 2009).
Figure 3. Activation of TRPA1 and TRPA1-TRPV1 expressing CHO cells by intracellular Ca2+ in 2 mM Ca2+ extracellular solution.
(A) Activation of TRPA1 and TRPA1-TRPV1 expressing CHO cells by 1μM and 10 μM of [Ca2+]i. Recordings were carried out in inside-out configuration in voltage-clamp (Vh=−60mV) mode. Indicated concentrations of Ca2+ (under X-axis) were applied to the patches for 1 min. Pipette solutions contain 2 mM Ca2+. Open probability (Po) was measured for recordings between time points of 10 sec and 50 sec. Data analysis was by unpaired t-test. n=10-15. (B) Representative traces show single-channel activity during ≈10 sec stretch. Expression constructs and concentrations of applied Ca2+ (i.e. [Ca2+]i) are indicated above the traces.
An elevation in [Ca2+]i of up to 1uM can be achieved by treating cells with 2uM thapsigargin. Elevation in [Ca2+]i can trigger whole-cell currents in TRPA1, TRPV1 and TRPA1-TRPV1 expressing cells (van der Stelt et al., 2005). Accordingly, we evaluated thapsigargin-gated currents (ITHAP) in CHO cells sensitive to CAP, MO and both CAP and MO, which are expressing TRPV1, TRPA1 and TRPA1-TRPV1, respectively. To maintain physiological conditions, recordings were carried out in perforated patch configuration with 2 mM extracellular Ca2+. GFP expressing cells were not significantly activated by 2 uM thapsigargin (5.9±1.4 pA, n=6; Fig 4A and 4B). In accordance with results presented in Figure 3, ITHAP was substantially smaller in TRPA1-TRPV1 compared to TRPA1 expressing cells (Fig 4A and 4B). In addition, TRPV1 positive cells generated small currents to thapsigargin application (Fig 4A and 4B) in agreement with a previous report (van der Stelt et al., 2005).
Figure 4. Activation of TRPA1, TRPV1 and TRPA1-TRPV1 expressing CHO cells and sensory neurons by thapsigargin.
(A) Activation of TRPV1, TRPA1 and TRPA1-TRPV1 expressing CHO cells by 2μM thapsigargin. Recordings were carried out in perforated patch whole-cell configuration in voltage-clamp mode. Magnitude of ITHAP at Vh=−60mV is presented. Thapsigargin was applied for 1 min. Expression plasmids are indicated below X-axis. Numbers of analyzed cells are noted. (B) Representative current-voltage (I-V) traces at maximal responses to 2uM thapsigargin are presented for CHO cells expressing TRPV1, TRPA1 and TRPA1-TRPV1. I-V was generated by voltage ramp (from −100 mV to +60 mV applied for 450 msec). The ramp was applied to cells every 3 sec. (C) Activation of sensory neurons from trigeminal ganglia (TG) of wild-type (WT), TRPV1 (TRPV1 KO) and TRPA1 (TRPA1 KO) null-mutant mice by 2μM thapsigargin. Recordings were carried out in perforated patch whole-cell configuration in voltage-clamp mode (Vh=−60mV). Thapsigargin was applied for 1 min. Mouse lines are indicated below the X-axis. Numbers of analyzed cells are noted. (D) Representative whole-cell voltage clamp ITHAP traces recorded from sensory neurons of WT and TRPV1 KO TG. Application times of 2uM thapsigargin are indicated by horizontal bars.
Unlike in CHO cells, the absence of the TRPV1 channel in sensory neurons leads to a reduction of TRPA1-currents triggered by MO and WIN55,212-2 (Salas et al., 2009). Therefore, we also measured ITHAP in TG sensory neurons from WT, TRPA1 KO and TRPV1 KO mice. Thapsigargin activated TG sensory neurons from these animals, and often displayed biphasic current (Fig 4D). Figure 4C and representative traces (Fig 4D) demonstrate that ITHAP is significantly reduced in sensory neurons lacking either TRPV1 or TRPA1. The presence of TRPV1 and TRPA1 channels in sensory neurons were confirmed by application of CAP and MO, respectively. Altogether, these data indicate that both TRPA1 and TRPV1 contribute to ITHAP in sensory neurons. However, other channels such as TRPC3, TRPC6 and Ca2+-dependent Cl--channels expressed in nociceptors may influence the magnitude of ITHAP (Chen et al., 2006, Liu et al., 2010). Thus, small ITHAP (12.1±4.2 pA, n=10) was recorded from capsaicin and mustard oil insensitive sensory neurons. This current is still statistically significantly smaller than ITHAP recorded from capsaicin-sensitive (TRPA1-KO animals) and mustard oil-sensitive (TRPV1-KO) sensory neurons.
The Magnitude of MO-activated responses depend on TRPV1 Ca2+ permeability
One of the major differences in the properties of TRPA1-mediated currents when comparing TRPA1- versus TRPA1-TRPV1-expressing systems is a voltage-dependent blockade of channel activity that is influenced by extracellular Ca2+ (Salas et al., 2009). Removal of [Ca2+]i for inside-out, single-channel recording, and the chelation of intracellular Ca2+ with EGTA or BAPTA, did not reverse this effect (Salas et al., 2009, Staruschenko et al., 2010). Therefore, we sought to examine whether mutations in the pore of the TRPV1 channel affecting Ca2+ permeability could alter features of IMO in TRPA1-TRPV1 co-expressing CHO cells. In this regard, there are several appropriate candidate mutations of TRPV1. The first is the D646N mutation (Garcia-Martinez et al., 2000). This mutation corresponds to the D682N mutation in the TRPV4 channel which is strongly linked to voltage-dependent pore blockage of TRPV4 by Ca2+ (Voets et al., 2002). Secondly, the Y671K mutation was reported to drastically reduce Ca2+-permeability of the TRPV1 channel (Mohapatra et al., 2003). In contrast, the Y671D mutation significantly increases Ca2+ permeability of the TRPV1 channel, whereas the Y671A mutation has no reported effect on Ca2+ permeability (Mohapatra et al., 2003). Accordingly, we evaluated whether the D646N, Y671K, Y671D and Y671A mutations of TRPV1 altered the magnitudes of MO responses in TRPA1-TRPV1 co-expressing CHO cells.
We initially examined CAP responses in cells co-expressing TRPA1 and one of the TRPV1 previously mentioned TRPV1 mutants. In agreement with the previous report (Mohapatra et al., 2003), CAP (50 nM)-induced Ca2+ influx detected by Ca2+-imaging was significantly increased or decreased in cells co-expressing TRPA1 and Y671D or Y671K mutations, respectively (Fig 5A). We next characterized MO (10 μM)-induced Ca2+ influx in TRPA1/mutant TRPV1 co-expressing cells. Similar to CAP responses, the co-expression of TRPA1 with Y671D or Y671K TRPV1, but not with D646N and Y671A TRPV1, led to significantly increased or reduced MO-triggered Ca2+ influx, respectively (Fig 5A). It is possible that the increased Ca2+-permeability of the Y671D TRPV1 channel could contribute to activation of TRPA1 by [Ca2+]i accumulation in transfected CHO cells (Fig 3; (Doerner et al., 2007, Zurborg et al., 2007). In addition, an increase in [Ca2+]i could stimulate anandamide production that activates TRPV1 in concentration-response manner (van der Stelt et al., 2005). To evaluate the possibility that mutations in TRPV1 could affect association with TRPA1, co-immunoprecipitation (Co-IP) of myc tagged TRPA1 with wild-type (wt) and TRPV1 mutants was carried out. Figure 5B illustrates that mutations to TRPV1 did not ablate TRPA1-TRPV1 interactions. Altogether, mutations in TRPV1 affecting Ca2+ permeability influenced magnitudes of MO-evoked Ca2+-influx, but did not disrupt TRPA1-TRPV1 interaction
Figure 5. TRPV1 mutations affect magnitude of MO responses in TRPA1-TRPV1 cells, but do not ablate TRPA1-TRPV1 co-immunoprecipitation (Co-IP).
(A) MO- or CAP-induced accumulation of [Ca2+]i in CHO cells co-transfected with TRPA1 and wild-type (WT) TRPV1 or indicated TRPV1 mutants. Numbers of responding CHO cells are indicated. (B) N-terminally myc-tagged TRPA1 was co-expressed with TRPV1 (wt) or TRPV1 mutants (D646N, Y671A, Y671D and Y671K; marked DN, YA, YD and YK respectively) in CHO cells. Anti-TRPV1 and anti-myc immunoreactivity was detected by WB which are noted at the right side of the panels. WBs were performed after co-IP with either TRPV1 or myc antibodies (noted above panels). Myc-TRPA1 (upper panel) and TRPV1 (lower panel) corresponding bands are denoted by arrows.
MO-gated current (IMO) properties are influenced by TRPV1 Ca2+ permeability
We next examined whether mutations affecting TRPV1 Ca2+ permeability alter properties of IMO in TRPA1-TRPV1 expressing CHO cells. The magnitude of IMO depends on extracellular Ca2+ in TRPA1-TRPV1, but not cells expressing TRPA1 alone (Salas et al., 2009). Therefore, we first investigated the effects of the TRPV1 mutations on IMO peaks. In the presence of Ca2+, IMO (Vh=−60 mV) in TRPA1-TRPV1 expressing cells was substantially smaller than in cells expressing TRPA1 and either Y671D or Y671K TRPV1, but not TRPA1 with Y671A or D646N TRPV1 (Fig 6A and 6B). Further, IMO was not affected by removal of external Ca2+ in TRPA1-Y671D TRPV1 or TRPA1-Y671K TRPV1 cells (Fig 6A and 6B). In contrast, IMO recorded from TRPA1-WT, TRPA1-D646N or TRPA1-Y671A TRPV1 cells were significantly larger in Ca2+-free solution and comparable with IMO from TRPA1-Y671D or TRPA1-Y671K TRPV1 cells (Fig 6B). Altogether, the alteration of TRPV1 Ca2+-permeability by mutations in position Y671 leads to IMO characteristics in TRPA1-TRPV1 co-expressing cells similar to those displayed in cells expressing TRPA1 alone (Salas et al., 2009).
Figure 6. The Y671 TRPV1 mutation effects on properties of IMO in TRPA1/TRPV1 cells.
Recordings were carried out in perforated patch voltage clamp (Vh=−60 mV) configuration. (A) Representative traces of IMO in TRPA1-TRPV1 (marked WT) and TRPA1-Y671K TRPV1 (marked Y671K) CHO cells bathed in extracellular solution containing 2 mM or 0 mM Ca2+. Durations of drug applications are denoted. (B) Magnitude of IMO depends on mutations in TRPV1 and extracellular Ca2+. Cells were bathed in SES (2mM Ca2+) or 0Ca-ES (Ca2+-free). External solutions and mutations of TRPV1 are indicated. Numbers of recorded cells are marked within bars. (C) Representative IMO current-voltage (I-V) traces in TRPA1-TRPV1 (marked WT), TRPA1-Y671K TRPV1 (marked Y671K) TRPA1-Y671D TRPV1 (marked Y671D) and TRPA1-Y671A TRPV1 (marked Y671A) CHO cells bathed in extracellular solution containing 2 mM Ca2+ or Ca2+-free solution. (D) Rectification ratios of I-V IMO are differentially affected by mutations in TRPV1. n=6-14.
Since Ca2+ influences the voltage-dependency of IMO (Salas et al., 2009), we sought to determine whether the TRPV1 mutations altered the rectification ratios of the IMO current-voltage (I-V) curves. In this respect, D646N and Y671A mutations were not different from WT TRPV1 (Fig 6C and 6D). Removal of external Ca2+ also resulted in a dramatic reduction of pronounced outward rectification in I-V IMO recorded from TRPA1-Y671A TRPV1 and TRPA1-TRPV1 expressing cells (Fig 6C and 6D). In contrast, in TRPA1-Y671D and TRPA1-Y671K TRPV1 cells, the IMO I-V curves exhibited no substantial outward rectification. Moreover, the presence of external Ca2+ has no effect on IMO I-V rectification in these cells (Fig 6C and 6D). Altogether, alterations in TRPV1 Ca2+ permeability abolishes the influence of extracellular Ca2+ on the voltage-dependency of IMO, resulting in IMO properties similar to those displayed in TRPA1-expressing cells (Salas et al., 2009).
Single-channel IMO is regulated by TRPV1 Ca2+ permeability
To further evaluate properties of IMO in TRPA1-Y671K TRPV1 co-expressing cells, we examined single-channel IMO in cells co-expressing TRPA1 with Y671K TRPV1 channels. In the presence of extracellular Ca2+, IMO in TRPA1-Y671K TRPV1 cells responded similarly to TRPA1-expressing cells (Fig 7; (Staruschenko et al., 2010)). Thus, single-channel conductance did not undergo substantial rectification (Fig 7; G60=66.3 vs G−60=44.9), as for TRPA1 expressing CHO cells (Staruschenko et al., 2010). In TRPA1-TRPV1 co-expressing cells, the open probability (Po) was significantly larger at positive holding potentials (Staruschenko et al., 2010). In contrast, Po of IMO in TRPA1-Y671K TRPV1 CHO cells displayed similar values at −60 mV and 60 mV (Fig 7; Po60=0.67 vs Po-60=0.63). In summary, alteration of Ca2+ permeability of the TRPV1 channels leads not only to whole-cell properties of IMO in TRPA1-TRPV1 containing cells similar to those recorded from TRPA1-expressing cells (Salas et al., 2009), but also to single-channel characteristics observed in TRPA1-expressing cells (Staruschenko et al., 2010).
Figure 7. Single-channel IMO in cells expressing TRPA1/Y671K TRPV1.
Recordings were performed in cell-attached configuration in voltage-clamp mode from TRPA1/Y671K TRPV1 co-transfected CHO cells. (A) Representative traces of single-channel IMO (25 μM) were recorded at different indicated holding potentials. c indicates the closed state of the channel (B) Averaged current-voltage relationship for single-channel IMO (25 μM), n=8-10. (C) Summary graphs of open probability (Po) for IMO at +60 and −60 mV, n=8-10.
Discussion
Behavioral tests in TRPA1 and TRPV1 null-mutant mouse lines and pharmacological studies indicate that these channels play a critical role in pain transduction during pathological conditions triggered by tissue damage and inflammation (Caterina et al., 2000, Davis et al., 2000, Bolcskei et al., 2005, Bautista et al., 2006, Kwan et al., 2006, Petrus et al., 2007). It has been suggested that activation and regulation of TRPA1 and TRPV1 by inflammatory mediators can underlie mechanisms for inflammatory hyperalgesia (Caterina et al., 2000, Bandell et al., 2004, Jordt et al., 2004, Bautista et al., 2006, Kwan et al., 2006). An elevation in [Ca2+]i could play critical roles in both activation (Zurborg et al., 2007) and regulation (Schmidt et al., 2009) of the TRPA1 channel, as well as functional interaction between TRPA1 and TRPV1 (Bautista et al., 2006, McMahon and Wood, 2006). In addition, it was shown that [Ca2+]e can modulate activity of the TRPA1 channel (Salas et al., 2009, Staruschenko et al., 2010).
Since TRPA1 and TRPV1 are extensively co-expressed in sensory ganglia (Story et al., 2003, Bandell et al., 2004, Jordt et al., 2004, Obata et al., 2005, Diogenes et al., 2007) and undergo functional interaction, we investigated how TRPV1 influences TRPA1 activation and modulation by Ca2+. Our data demonstrate that: co-expression of TRPA1 and TRPV1 leads to significant suppression of [Ca2+]e–gated activity at a physiological concentration of extracellular Ca2+ (2 mM); TRPV1 attenuates TRPA1-mediated [Ca2+]i-gated activity at 2 mM of extracellular Ca2+; both TRPV1 and TRPA1 contributes to thapsigargin-gated currents in the expression system and sensory neurons; Ca2+ permeability of TRPV1 underlies the mechanism accounting for Ca2+-dependent properties of TRPA1-mediated responses in TRPA1-TRPV1 co-expressing cells. It is also worth noting that with accordance with previous report (Ahern et al., 2005), TRPV1 can be activated by extracellular Ca2+, and small currents are also detected at elevated levels of [Ca2+]i. Altogether, responses of Ca2+-gated currents in TRPA1 and TRPA1-TRPV1-expressing cells are similar to currents generated by other TRPA1 agonists such as MO and WIN55,212-2 (Salas et al., 2009, Staruschenko et al., 2010). Most feasible explanation for these events is direct functional interaction between the TRPA1 and TRPV1 channels. However, some aspects of TRPA1-TRPV1 interaction could still remain indirect.
Responses to [Ca2+]e are mutually modulated by the TRPV1 and TRPA1 channels. Interestingly, this modulation is biphasic (Fig 1A). Thus, at low concentrations of [Ca2+]e (< 1 mM), it appears that activity in TRPA1-TRPV1 co-expressing cells is a sum of activities in TRPA1 and TRPV1 homomer expressing cells. However, at physiological concentrations of [Ca2+]e (> 2mM), [Ca2+]e –gated activity is suppressed in TRPA1-TRPV1 co-expressing cells (Fig 1A). One possible explanation for such a biphasic effect is that TRPV1 inhibits TRPA1-mediated responses only at presence of 2 mM [Ca2+]e (Akopian et al., 2007, Salas et al., 2009, Staruschenko et al., 2010). This inhibition is noted only at negative potentials, because 2 mM of [Ca2+]e generates strong voltage-dependence in cells co-expressing TRPA1 and TRPV1 (Salas et al., 2009).
What could be the physiological relevance in regulation of the activation of TRPA1-containg cells by 2mM [Ca2+]e? Such activation could result in a basal Ca2+ leak into TRPA1-expressing cells. The basal Ca2+ leak may be necessary to maintain the required Ca2+-dependent cell processes such as transcription, translation and/or protein trafficking. However, high levels of Ca2+-influx could lead to chemo-taxis and eventual death for sensory neurons. Thus, almost no sensory neurons from TRPV1 KO TG displayed large (>300 pA) MO-gated currents (Salas et al., 2009). One possible explanation for this finding is that TRPV1 suppresses the Ca2+ leak into sensory neurons and protects them from cell-death. In the absence of TRPV1, highly expressing TRPA1-positive cells (≈8% of MO-sensitive sensory neurons; (Salas et al., 2009) either do not survive during development or suffer down-regulation in TRPA1 expression. Further, it was observed that many TRPA1-expressing CHO cells do not appear healthy in the absence of the TRPV1 channel (Bandell et al., 2004, Akopian et al., 2007, Salas et al., 2009). Altogether, TRPV1 may play certain protective roles for sensory neurons with high expression levels of TRPA1.
What could be the physiological relevance in the regulation of activation of TRPA1-containg cells by [Ca2+]i? In a TRPA1-TRPV1 expression system, TRPV1 attenuates [Ca2+]i-gated and thapsigargin-gated activity. However, in sensory neurons, the absence of TRPV1 produces significantly smaller thapsigargin-gated currents. This behavior resembles responses generated by other TRPA1 agonists such as MO and WIN 55,212-2 (Salas et al., 2009). Single-channel analysis of IMO in CHO cells and sensory neurons showed that intrinsic properties of IMO are independent on the type of cells (i.e. CHO or sensory neurons) co-expressing TRPA1-TRPV1 (Staruschenko et al., 2010). This implies that the density of functional TRPA1 on the plasma membrane of sensory neurons may be reduced in cells lacking TRPV1 (Staruschenko et al., 2010). Considering these findings, it could be proposed that TRPA1-TRPV1 co-expression on the plasma membrane of sensory neurons is necessary to generate large responses to the elevation in [Ca2+]i that can be triggered by inflammatory mediators such as bradykinin, histamine and 5-HT. Thus, in sensory neuron culture, it appear that particular conditions (i.e. NGF, absence of trypsin treatment) likely promote TRPA1 expression on the plasma membrane (Salas et al., 2009). In summary, in sensory neurons, TRPV1 could promote expression of functional TRPA1 on the plasma membrane; this in turn may be necessary to generate [Ca2+]i–dependent responses to certain inflammatory mediators.
TRPA1 agonist such as MO, certain cannabinoids and [Ca2+]i activate TRPA1 through different pathways (Kim and Cavanaugh, 2007, Macpherson et al., 2007, Zurborg et al., 2007, Cavanaugh et al., 2008). However, TRPV1 regulation of TRPA1 activities is independent of agonist nature (Salas et al., 2009). Regulation of TRPA1 activities by the TRPV1 channel happens only in the presence of 2 mM Ca2+ (Staruschenko et al., 2010). It appears that extracellular Ca2+ inhibits TRPA1-mediated responses in TRPA1-TRPV1, but not in TRPA1-expressing cells. Our data imply that the Y671 amino acid located in the trans-membrane domain 6 of the TRPV1 channel and responsible for Ca2+ permeability of TRPV1 (Mohapatra et al., 2003), is resonsible for the blocking effects of external Ca2+ in TRPA1-TRPV1 expressing cells. Interestingly, mutation of TRPV1 in the Y671 position does not alter co-IP of the channels. This finding provides additional evidence on functional interaction of TRPA1 and TRPV1, and regulation of TRPA1 activities by the TRPV1 channel. This suggests that the main modification to TRPA1 in TRPA1-TRPV1 co-expressing cells happens in or near pore. Although, it still remains a possibility that TRPV1 presence will alter the interaction of certain agonists with the TRPA1 channel. Thus, it is possible that a binding position for drugs could modulate TRPA1-responses by controlling Ca2+ permeability of TRPV1. Altogether, the co-expression of TRPA1 and TRPV1 channels that influence the activation of cells by Ca2+ may sub-serve regulatory control of peripheral mechanisms of inflammatory hyperalgesia.
Acknowledgements
We would like to thank Dr. David Julius (UCSF, San Francisco, CA) for kindly gifting rTRPV1 cDNA, Dr. Ardem Patapoutian (The Scripps Research Institute, San Diego, CA) for kindly gifting TRPA1 cDNA and Dr. Carla Nau (University Erlangen-Nuremberg, Erlangen, Germany) for providing Y671 TRPV1 cDNA. Research was supported by grants NS061884 (to NAJ) and DE019311 and DE017696 (to ANA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ahern GP, Brooks IM, Miyares RL, Wang XB. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci. 2005;25:5109–5116. doi: 10.1523/JNEUROSCI.0237-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci. 2008;28:1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, Almasi R, Pinter E, Petho G, Szolcsanyi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154:1467–1476. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 Regulates TRPV1 Phosphorylation in Sensory Neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci. 2007;27:6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell. 2006;124:1123–1125. doi: 10.1016/j.cell.2006.03.006. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Wang SY, Wang GK, Nau C. A tyrosine residue in TM6 of the Vanilloid Receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol Cell Neurosci. 2003;23:314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single-channel properties of the TRPA1 channel. J Biol Chem. 2010 doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des. 2005;11:2687–2698. doi: 10.2174/1381612054546789. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Trevisani M, Vellani V, De Petrocellis L, Moriello A Schiano, Campi B, McNaughton P, Geppetti P, Di Marzo V. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. Embo J. 2005;24:3026–3037. doi: 10.1038/sj.emboj.7600784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]