Abstract
Individuals with underlying inflammation present with a high prevalence of non-specific co-morbid symptoms including sleep disturbance and fatigue. However, the association between cellular expression of proinflammatory cytokines, alterations of sleep depth and daytime fatigue has not been concurrently examined. In healthy adults (24 – 61 years old), evening levels of monocyte intracellular proinflammatory cytokine production were assessed prior to evaluation of polysomonographic sleep and measures of fatigue the following day. Stimulated monocyte production of interleukin-6 (IL-6), but not tumor necrosis factor α (TNF- α), was negatively associated with slow wave sleep (ΔR2 =.17, p=.029). In contrast, stimulated monocyte production of IL-6 was positively associated with rapid-eye movement (REM) sleep duration during the first sleep cycle (ΔR2 = .26, p<.01). Moreover, evening stimulated production of IL-6 was associated with fatigue the following day (ΔR2 = .17, p=.05). Mediation analyses showed that slow wave sleep, but not REM sleep duration, mediated the relationship between evening levels of IL-6 production and daytime fatigue. These results indicate that increases in stimulated monocyte production of IL6 may be associated with changes in sleep architecture with decreases in slow wave sleep and increases in REM sleep duration. Relative loss of slow wave sleep may be one pathway through which cellular inflammation leads to daytime fatigue.
Keywords: proinflammatory cytokines, interleukin-6, cellular inflammation, fatigue, slow wave sleep, rapid eye movement sleep, sleep architecture
Introduction
Sleep disturbance is a common problem, affecting approximately one third of Americans. Research suggests that individuals who report some form of sleep disturbance may be at greater risk for a number of medical problems, including obesity, diabetes, heart disease, and depression (LeBlanc et al., 2007; Ohayon, 2005; Ohayon, 2008; Spiegel, 2008; Van Cauter et al., 2008). In addition, a number of studies have demonstrated that sleep problems take a major toll on health related quality of life, in which inadequate sleep is associated with worse self-reported general health, fatigue, and less vitality (Lee et al., 2009; Winkelman et al., 2009). Likewise, population based studies have shown that individuals with sleep problems have more physical health complaints and are more likely to miss work in the past 30 days (Bolge et al., 2009; Stein et al., 2008). Further, sleep disturbance is related to reduced quality of life across several dimensions, including declines in mental health, fatigue, and impairments in both physical and social functioning (Bolge et al., 2009; Byles et al., 2003; LeBlanc et al., 2007).
Proinflammatory cytokines are associated with a cluster of behavioral symptoms known as sickness behaviors. These symptoms, which include fatigue, sleep disturbance, depression, loss of appetite, and inability to concentrate (Dantzer, 2001; Dantzer, 2009), are common in individuals with medical conditions involving underlying inflammation (Collado-Hidalgo et al., 2008a; Lorton et al., 2008; Myers, 2008; Wood et al., 2006). Additionally, recent findings from population based studies reveal that markers of inflammation are associated with fatigue or reductions in vitality (Cho et al., 2009; Raison et al., 2009), and poor health related quality of life (Raison et al., 2009). Moreover, increases of the inflammatory marker, C-reactive protein (CRP), prospectively predict fatigue in community-dwelling adults (Cho et al., 2009). Taken together, these findings suggest that inflammation and alterations in sleep may contribute to fatigue and/or reductions in vitality.
Inflammation is thought to induce disturbances of sleep via alterations in the sleep cycle as defined by changes in sleep architecture. Indeed, prior studies have found that increases in circulating levels of IL-6 correlate with decreases of slow wave sleep (SWS) (Burgos et al., 2006; Hong et al., 2005), as well as increases in the amount and percentage of rapid eye movement (REM) sleep (Irwin et al., 2004) and REM sleep density (Motivala et al., 2005). In addition, stimulated production of IL-6 in mixed cell cultures is associated with increases in REM sleep amounts and percentage (Redwine et al., 2003). Moreover, in alcohol dependent persons who have high amounts of REM sleep, administration of a proinflammatory cytokine antagonist partially normalizes amounts of REM sleep, inducing lower levels of REM sleep comparable to levels found in controls (Irwin et al., 2009). Nevertheless, there are inconsistent results; one study found that IL-6 is associated with low amounts of REM sleep (Vgontzas et al., 2003).
In this study, we sought to clarify the functional basis for altered inflammation in association with sleep by measuring the production of proinflammatory cytokines by monocytes following ligation of the Toll-like receptor 4 with lipopolysaccharide (LPS). We have previously found that sleep loss induces marked increases in monocyte production of IL-6 and TNF-α (Irwin et al., 2010; Irwin et al., 2006b), and such increases in the cellular expression of proinflammatory cytokines is associated with fatigue in breast cancer survivors (Collado-Hidalgo et al., 2008b). In addition, Toll-like receptors mediate innate immune responses to common pathogens, and aberrant increases of Toll-like receptor activity have been linked to inflammatory diseases such as rheumatoid arthritis (Andreakos, 2004), Crohn’s disease (Andreakos, 2004), and heart failure (Satoh et al., 2006).
Given the associations between circulating levels of proinflammatory cytokines and measures of sleep architecture (i.e., SWS and REM sleep ) (Burgos et al., 2006; Hong et al., 2005; Irwin et al., 2004; Motivala et al., 2005; Redwine et al., 2003) and evidence that such activation might drive increases in REM sleep (Irwin et al., 2009), we examined the impact of evening stimulated monocyte production of inflammatory cytokines on measures of sleep architecture, hypothesizing that greater evening expression of cellular markers of inflammation would be associated with less sleep depth (i.e., lower amounts of SWS) and greater amounts of REM sleep. Finally, given evidence that cellular markers of inflammation are associated with fatigue (Collado-Hidalgo et al., 2006; Collado-Hidalgo et al., 2008b), as well as findings that decreases in sleep depth correlate with daytime fatigue (Irwin et al., 2006a), we evaluated the association of activation of cellular markers of inflammation and daytime fatigue, and explored whether alterations in sleep depth mediated this relationship.
Methods
Participants
Participants included 31 men (N= 16) and women (N=15) who were recruited via referrals and advertisements seeking medically healthy adults to participate in a sleep study between October 2006 and June 2008. All gave consent to participate in this University of California, Los Angeles (UCLA) institutional review board approved research study. Participants were between the ages of 24 and 61 (Mean=37.16, SEM=1.59), had body mass indices (calculated as weight in kilograms divided by the square of height in meters) less than 30, were nonsmokers, and regularly slept between 10:30 PM and 7:30 AM as confirmed by 2-week sleep diaries. All participants were medically healthy, as determined by medical history, physical examination, and laboratory testing. None of them had a history of mental illness as determined by Structured Clinical Interview for Diagnostic and Statistical Manual—IV (SCID) and no participants had sleep disorders. None of the participants fulfilled criteria for primary substance dependence or had used substances including alcohol in the last 2 weeks as confirmed by random urine substance screenings. Further, no participants were taking medications known to alter sleep-wake activity (e.g., beta blockers, psychotropic medications) within 2 weeks of the sleep protocol.
Procedures
Participants spent 3 consecutive days (24-hour periods) in the National Institutes of Health General Clinical Research Center, which included an initial screening/adaptation night and two nights of sleep testing; only results from the first night of sleep testing are included as that night preceded the evaluation of fatigue. After adaptation to the sleep laboratory with screening for sleep apnea and nocturnal myoclonus, participants underwent testing with polysomnography, with ambient light dimmer than 50 lux. Participants adhered to their normal sleep schedules, with lights out between 11 PM and 7 AM. During the nocturnal period, a bedside urinal was used if participants needed to urinate during the night. Participants were awakened in the morning by turning on a dim light and calling the participant’s name. All participants remained on the GCRC during with day until the sleep protocol was completed.
Sleep monitoring was conducted with placement of electrodes for EEG (C3 or C4), electrooculography, and submental electromyography recordings. EEG sleep records were visually scored according to the criteria of Rechtschaffen and Kales (Rechtschaffen and Kales, 1968) as previously described (Irwin et al., 2002). For purposes of this study, calculated sleep variables included total sleep time (TST), sleep efficiency ( % of TST/time in bed), time to sleep onset, Stage 1 sleep, Stage 2 sleep, SWS (comprised of Stage 3 and Stage 4 sleep), REM latency, REM density, and REM duration during the first sleep cycle. TST was considered the total number of minutes from sleep onset to final wake-up time and included time spent in Stages 1 through 4 of sleep and REM sleep. There were no differences in the polysomnographic measures of sleep continuity and sleep architecture between the adaptation/screening night and the testing night.
Blood sampling was conducted at 11 PM (evening) prior to sleep testing and again at 8 AM (morning) after sleep, with collection of samples via an indwelling venous forearm. Samples were analyzed for expression of intracellular proinflammatory cytokines in monocyte populations.
Fatigue was measured in the evening following sleep testing using the vitality subscale of the Short Form 36 Health Survey Questionnaire (SF-36; (Ware and Sherbourne, 1992). Items that make up the vitality subscale assess whether respondents are full of pep, tired, worn out, or have energy. High scores on this scale represent more vitality, whereas low scores represent fatigue.
Monocyte Cellular Expression of Cytokines
Monocyte intracellular expression of IL-6 and TNF-α was assessed by flow cytometry (see Collado-Hidalgo et al., 2006 for a complete description of these procedures). Heparin treated blood was mixed with 100pg/mL lipopolysaccharide (LPS; Sigma, St Louis, Mo) and 10 μg/mL brefeldin A (Sigma) and incubated for 4 hours at 37° C in a platform mixer followed by an overnight incubation at 4° C. We have previously found in dose response profile studies that 100 pg/ml of LPS for 4 hours yields a threshold response with detection of stimulated production of IL-6 and TNF-α combined. This is greater than levels found in unstimulated control samples, yet routinely less than the submaximal response, yielding combined levels of IL-6 and TNF-α response between 50% to 75% (Irwin et al., 2010; Irwin et al., 2006b). Doses of LPS at 50 pg/ml fail to yield a threshold response in many persons, whereas doses in 200 pg/ml yield a clustering of responses in the maximal range between 80% to 90%. After cells were permeabilized in fluorescence-activated cell sorting permeabilizing buffer (BD Biosciences, San Jose, CA) and fluorescence-conjugated antibodies were added, about 12 000 CD14+ events were counted to determine the net stimulated percentage of cytokine secreting monocytes, with quadrant coordinates set based on unstimulated cells. To determine percentage of stimulated cells expressing TNF-α or IL-6, unstimulated cytokine-positive event percentages were subtracted from stimulated percentages to obtain net stimulated cytokine-positive event percentages.
Statistical Analyses
Monocyte Cellular Cytokine Expression and Polysomnographic Sleep
Data were analyzed using SPSS 16.0 software. Prior to conducting the analyses, SWS and REM sleep measures were log transformed to control for skewness. Correlation analyses were conducted as an initial examination between evening- and morning stimulated proinflammatory cytokine expression and sleep variables. Based on these results, hierarchical linear regression analyses were then conducted to examine whether cellular expression of IL-6 and TNF-α in the evening were associated with SWS and REM sleep measures during the night after controlling for age and BMI. In these analyses, SWS and REM sleep measures were entered as outcome variables. Based on previous findings that age, BMI, gender, and ethnicity are associated with inflammation and sleep (Hong et al., 2005; Lim et al., 2005; O’Connor et al., 2009; O’Connor et al., 2007), these variables were entered as covariates on Block 1 of the model. Cellular expression of IL-6 and TNF-α were entered as predictor variables on Block 2 of the model.
Secondary Mediation Analyses: Cytokine Expression, Sleep, and Fatigue
For inflammatory markers that were significant predictors of REM sleep and SWS, we conducted mediation analyses to test whether the altered REM sleep and SWS observed in individuals with greater monocyte cellular cytokine production leads to fatigue the following day. These analyses were conducted in a series of hierarchical linear regression analyses using the method outlined by Baron and Kenny (Baron and Kenny, 1986), with REM sleep measures and SWS entered as mediators of relationships between monocyte cellular cytokine production and fatigue. To establish mediation, we then tested whether the following four conditions were met. First, the mediator (SWS, REM sleep measures) must be related to the outcome variable (fatigue). Second, the predictor variable (stimulated IL-6, TNF-α) must be related to the mediator (SWS, REM sleep measures). Third, the predictor variable must be related to the outcome variable (fatigue). Finally, when the outcome variable is regressed onto both the mediator and the predictor variables simultaneously, there should be a stronger association between the mediator and the outcome variables than between the predictor and the outcome variables. We tested these conditions in three steps using a series of hierarchical regression analyses. Age, BMI, gender, and ethnicity were entered as covariates on Block 1 of the analyses.
Results
Sample Characteristics
Sample characteristics are listed in Table 1. The ethnic group breakdown of the sample was 34.3% Caucasian American, 31.4% African American, 14.3% Asian American, and 11.4% Hispanic. Approximately half of the sample had an annual income of $30,000 or less and the average years of education was 15.37 (SEM=.30). Immune and sleep data are included in Table 2.
Table 1.
Sample Characteristics (N=31)
| Variables | M±SEM |
|---|---|
| Age | 37.16± 1.59 |
| BMI | 24.60 ± .74 |
| Years of Education | 15.55 ± .31 |
| SF-36 Vitality/Fatigue | 72.41 ± 2.423 |
| Ethnicity | N (%) |
| African American | 10 (32.3) |
| Asian American | 3 (9.7) |
| Caucasian American | 11 (35.5) |
| Hispanic | 2 (6.5) |
| Income | N (%) |
| <$30,0000 | 15 (48.4) |
| $30,000–49,999 | 8 (25.8) |
| ≥$50,000 | 7 (22.6) |
Table 2.
Sleep Characteristics and Proinflammatory Cytokine Activity on Both Nights (N=31)
| Variables | Mean±SEM | |
|---|---|---|
| Night 1 | Night 2 | |
| Evening IL-6 Production (%) | 10.21 ± 1.17 | 7.96 ± 1.14 |
| Evening TNF-a Production (%) | 23.99 ± 2.39 | 20.28 ± 1.62 |
| Total Sleep Time (TST; minutes) | 391.12. ± 11.07 | 414.05 ± 14.46 |
| Time to Sleep Onset (minutes) | 32.13 ± 7.45 | 24.32 ± 5.14 |
| Sleep Efficiency (TST/Time in Bed × 100; %) | 80.89 ± 2.22 | 82.94 ± 2.30 |
| Stage 1 (% TST) | 4.34 ± 0.43 | 5.46 ± 0.56 |
| Stage 2 (% TST) | 65.04 ± 1.51 | 62.22 ± 2.06 |
| SWS (% TST) | 7.05 ± 1.21 | 9.29 ± 1.43 |
| REM Duration (minutes) | 17.48 ± 2.29 | 16.22 ± 1.91 |
| REM Density (minutes) | 1.37 ± 0.14 | 1.59 ± 0.18 |
| REM Latency (minutes) | 64.25 ± 5.68 | 62.16 ± 4.61 |
| REM (% TST) | 22.63 ± 1.43 | 23.03 ± 1.29 |
Bivariate correlations examined relationships between stimulated monocyte production of IL-6 and TNF-α, sleep variables, and fatigue. Consistent with our hypotheses, evening stimulated monocyte production of IL-6 was associated with less SWS (r=−.517, p = .01) and a longer REM duration (r= .553, p = .008). Production of IL-6 was unrelated to stages 1 or 2 sleep, TST, sleep efficiency, time to sleep onset, REM sleep, REM latency or REM density. Evening production of TNF-α was unrelated to sleep stages, total sleep time, sleep efficiency, or time to sleep onset. Neither morning production of IL-6 nor TNF-α were related to any of the sleep variables.
Monocyte Cytokine Production and Sleep Architecture
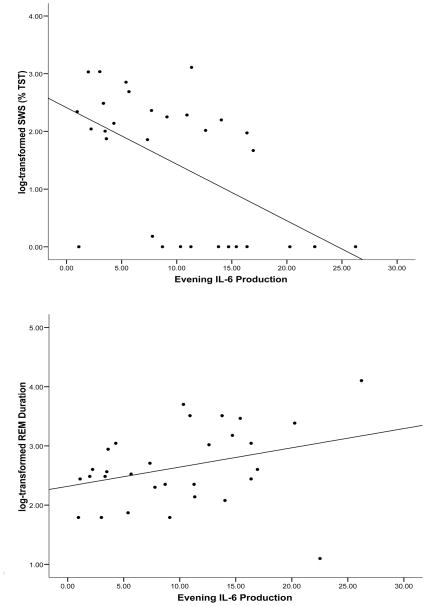
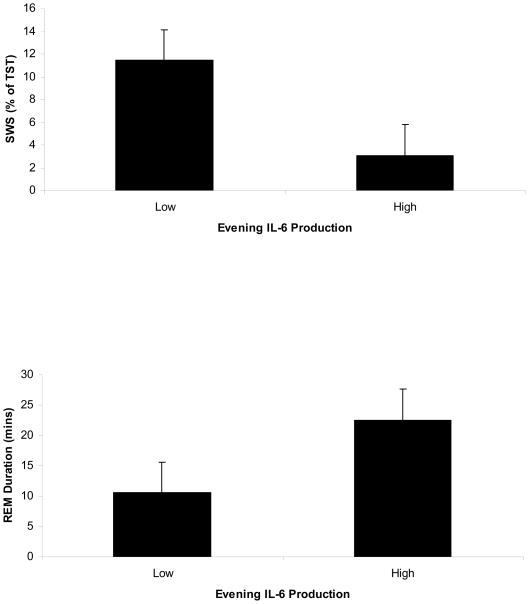
Given the bivariate associations between evening levels of monocyte cellular expression of IL-6 and measures of SWS and REM duration, hierarchical linear regression analyses were conducted with this predictor variable. Age, BMI, gender, and ethnicity explained 8.3% of the variance in SWS. After controlling for these covariates, greater evening stimulated monocyte production of IL-6 was associated with less SWS (ΔR2 = .17, p=.029) (see Figure 1). As can be seen in Figure 2, participants in the lowest quartile of IL-6 production spent 11.3% of TST in SWS, compared to 2.5% of TST among those in the highest quartile of IL-6 production.
Figure 1.
Relationships between evening stimulated monocyte production of IL-6, log transformed SWS, and log transformed REM duration. Greater production of IL-6 was associated with shorter SWS and longer REM duration.
Figure 2.
Figure depicting SWS and REM duration in participants with the highest and lowest quartile evening stimulated monocyte production of IL-6.
The covariates explained 12.5% of the variance in REM duration. As can be seen in Figure 1, greater evening stimulated monocyte production of IL-6 was associated with a longer REM duration (ΔR2 = .18, p=.017). Figure 2 illustrates that REM duration was 10.6 minutes among participants in the lowest quartile of IL-6 production, compared to 22.5 minutes among those in the highest quartile of IL-6 production. TNF-α was unrelated to SWS or REM duration.
Mediation Analyses: Evening Cytokine Expression, Sleep Architecture, and Fatigue
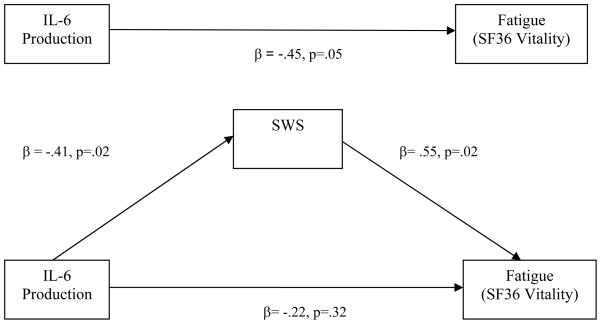
Mediation analyses were conducted to test our hypothesis that SWS and REM duration would mediate relationships between evening proinflammatory cytokine expression and fatigue. Age, BMI, gender, and ethnicity accounted for 10% of the variance in fatigue. After controlling for these covariates, less SWS was associated with fatigue the subsequent day (ΔR2 = .15, p =.03; Condition 1 of the Mediation Model). Condition 2 of the Mediation Model was satisfied by our finding that greater evening stimulated monocyte production of IL-6 was associated with less SWS (see previous section). Evening monocyte production of IL-6 was associated with fatigue (ΔR2 = .17, p=.05; Condition 3). Table 3 lists the final hierarchical linear regression analysis testing Condition 4 of the Mediation Model. As can be seen in Table 3, when both production of IL-6 and SWS were examined simultaneously as predictors of fatigue, only SWS was significantly associated with fatigue the subsequent day (β= .55, p=.02). In other words, increased evening production of IL-6 was associated with fatigue, and this relationship was mediated by shorter SWS (see Figure 3). Thus, Condition 4 of the Mediation Model was satisfied and there was support for our hypothesis that associations between proinflammatory cytokine expression and fatigue would be mediated by altered SWS.
Table 3.
Final Hierarchical Regression Analysis Demonstrating that SWS Mediates the Relationship Between Evening IL-6 Production and Fatigue (N=31)
| Variable | B | SEB | β | R2 | ΔR2 |
|---|---|---|---|---|---|
| Block 1 | 0.10 | 0.10 | |||
| BMI | −0.32 | 0.57 | −0.10 | ||
| Age | 0.25 | 0.24 | 0.182 | ||
| Gender | −6.48 | 4.67 | −0.25 | ||
| Ethnicity | 0.24 | 1.39 | 0.03 | ||
| Block 2 | 0.32 | 0.22* | |||
| IL-6 Production | −.59 | 0.45 | −0.22 | ||
| SWS | 6.38 | 2.44 | 0.55 |
Figure 3.
Path analysis demonstrating that log transformed SWS mediates the relationship between evening stimulated monocyte production of IL-6 and fatigue. When both IL6 and SWS are entered into the regression model, SWS, but not IL-6, remains a significant predictor of fatigue.
Mediation analyses were also conducted to determine whether alterations in REM duration mediated relationships between IL-6 and fatigue. After controlling for age and BMI, REM duration was unrelated to fatigue. Thus, there was no evidence that increased REM duration mediated relationships between production of IL-6 and fatigue.
Discussion
This study examined relationships between proinflammatory cytokine expression, sleep architecture, and fatigue. There was support for our hypothesis that increased proinflammatory cytokine expression would be associated with alterations in sleep architecture. Specifically, greater evening stimulated monocyte production of IL-6 was associated with less SWS. This supports the results of research demonstrating that individuals with high circulating IL-6 have less SWS (Burgos et al., 2006; Hong et al., 2005), and suggests that immune cells (i.e., monocytes) may be a source for changes in circulating levels of IL-6 in association with SWS.
Increased evening production of IL-6 was also associated with a longer REM duration during the first sleep cycle. We have previously found that both circulating and stimulated production of IL-6 correlate with increases in REM sleep amounts (Irwin et al., 2004; Motivala et al., 2005; Redwine et al., 2003). However, in the present study, the strongest correlation between stimulated IL-6 and REM sleep was with REM duration, which is defined as time in REM sleep during the first sleep cycle. Given that the first sleep cycle is generally dominated by SWS in normal sleepers, our findings may reflect a shift away from SWS toward more REM sleep in participants who have high proinflammatory cytokine expression. Thus, as depicted in Figure 2, this alteration in sleep stages significantly reduces the overall percentage of SWS that individuals obtain during their total sleep time.
It is interesting that TNF-α was unrelated to sleep architecture in the current study. Based on research finding that increased TNF-α is associated with longer REM sleep in alcoholics (Irwin and Rinetti, 2004), we expected that heightened production of TNF-α would have a deleterious effect on sleep architecture. However, these factors were unrelated in the current study. It is possible that IL-6, as opposed to TNF-α may play a unique role in regulating SWS and REM sleep in healthy humans. Whereas we have found that a TNF antagonist partially normalizes REM sleep (Irwin et al., 2009), this medication has biologic effects on in vivo levels of both TNF-α and IL-6.
Psychological stress may be a key factor predicting increases in IL-6 production and alterations in sleep architecture. It has been well documented that chronic stress is associated with increased SNS activity (Thomas et al., 2004) as well as a greater production of proinflammatory cytokines (Kiecolt-Glaser et al., 2002; Kiecolt-Glaser et al., 2003). Additionally, studies have found that psychological stress is associated with a reduction in SWS and an increase in REM sleep (Cheeta et al., 1997; Kim and Dimsdale, 2007). Given that psychological stress triggers SNS activity (Grippo and Johnson, 2009), it is interesting that we observed a similar pattern concerning the relationship between IL-6 and sleep stages as has been shown between SNS and sleep stages. Specifically, increased nocturnal SNS activity is associated with a reduction in SWS and an increase in REM sleep (Plante, 2006; Rasch et al., 2007). Taken together, these findings suggest that heightened nocturnal SNS activity, possibly due to stress, may lead to increased production of proinflammatory cytokines, which may alter sleep stages. However, we did not examine whether psychological stress and SNS output are driving increased proinflammatory cytokine production in the current study. Future research should be conducted to examine relationships between proinflammatory cytokines, sleep architecture, and vitality in individuals who are experiencing stressful life events.
Our findings extend research on proinflammatory cytokines and sleep architecture beyond circulating cytokine levels to examine relationships between sleep architecture and cellular cytokine expression. Given that circulating cytokine levels can come from sources other than immune cells and may not necessarily be indicative of immune dysregulation, our findings provide further evidence that increased proinflammatory cytokine activity resulting from immune dysregulation plays an important role in regulating sleep stages.
Within the current study, increased production of IL-6 was associated with fatigue. It is noteworthy that SWS, but not REM sleep, mediated this relationship. Specifically, shorter SWS in individuals with increased proinflammatory cytokine activity explained reports of fatigue. Our previous research has shown that monocyte production of IL-6, as measured following ligation of the Toll-4 receptor, is increased following sleep deprivation (Irwin et al., 2010; Irwin et al., 2006b), and is uniquely related to fatigue in breast cancer survivors (Collado-Hidalgo et al., 2006; Collado-Hidalgo et al., 2008a). Moreover, a cytokine gene polymorphism that leads to the over-expression of IL-6 (Collado-Hidalgo et al., 2008b) correlates with increases in monocyte production of IL-6, which together are associated with fatigue in breast cancer survivors (Collado-Hidalgo et al., 2006).
These findings are also consistent with Cho and colleagues (Cho et al., 2009) in which circulating markers of inflammation (i.e., C-reactive protein) was linked to greater fatigue among healthy individuals who participated in the CARDIA population based study (Cho et al., 2009). Additionally, the current findings suggest that a reduction in SWS may be one pathway through which inflammation leads to a reduction in general health and fatigue in healthy individuals. It is interesting that in the current study as well as in the study conducted by Cho and colleagues (Cho et al., 2009), participants were generally young and had no major medical conditions. Given that inflammation was associated with fatigue in both studies, these findings highlight the deleterious effects of immune dysregulation on general health and well being in otherwise healthy individuals. It is unknown whether associations between proinflammatory cytokines, sleep architecture, and fatigue are the same in individuals who have already developed medical conditions. Future research should examine the extent to which these findings extend to these individuals.
There are several limitations in the current study that should be considered when interpreting these findings. First, this study was conducted on a small convenience sample of community dwelling adults and should be replicated in larger samples. It should also be noted that screening for sleep apnea and myoclonus was conducted during the adaptation night. This could have impacted participants’ ability to adapt to the sleep laboratory, resulting in “sleep rebound” during the first night of testing, although there were no differences in sleep parameters between the screening/adaption night and the testing night. Ideally, participants’ sleep would be assessed in their home environment to obtain a more reliable measure of the relationship between proinflammatory cytokines and sleep parameters. However, this was not feasible in the current study.
This is the first published study to examine associations between stimulated IL-6 and sleep architecture. Additionally, given the correlational nature of this study, we cannot infer that a causal relationship exists between proinflammatory cytokines, sleep architecture, and fatigue. Thus, it should be cautioned that these findings are preliminary and need to be replicated in future research. Nonetheless, the current results have important implications on relationships between immune activity, sleep, and general health. Since it is believed that SWS serves a restorative function (Tasali et al., 2008), disruption of this sleep stage may lead to daytime fatigue.
Acknowledgments
This work was supported in part by grants T32-MH19925, HL 079955, AG 026364, CA 10014152, CA116778, RR00827, P30-AG028748, General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreakos E. Common and uncommon features of rheumatoid arthritis and chronic obstructive pulmonary disease: clues to a future therapy. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4:85–92. doi: 10.2174/1568008043339910. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bolge SC, Doan JF, Kannan H, Baran RW. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res. 2009;18:415–422. doi: 10.1007/s11136-009-9462-6. [DOI] [PubMed] [Google Scholar]
- Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–253. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Byles JE, Mishra GD, Harris MA, Nair K. The problems of sleep for older women: changes in health outcomes. Age Ageing. 2003;32:154–163. doi: 10.1093/ageing/32.2.154. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Ruigt G, van Proosdij J, Willner P. Changes in sleep architecture following chronic mild stress. Biol Psychiatry. 1997;41:419–427. doi: 10.1016/S0006-3223(96)00058-3. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Seeman TE, Bower JE, Kiefe CI, Irwin MR. Prospective Association Between C-Reactive Protein and Fatigue in the Coronary Artery Risk Development in Young Adults Study. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behav Immun. 2008a doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behav Immun. 2008b;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19:165–172. doi: 10.1016/j.bbi.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–641. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66:191–195. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Rinetti G. Disordered sleep, nocturnal cytokines, and immunity: interactions between alcohol dependence and African-American ethnicity. Alcohol. 2004;32:53–61. doi: 10.1016/j.alcohol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Valladares EM, Motivala S, Thayer JF, Ehlers CL. Association between nocturnal vagal tone and sleep depth, sleep quality, and fatigue in alcohol dependence. Psychosom Med. 2006a;68:159–166. doi: 10.1097/01.psy.0000195743.60952.00. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006b;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Dimsdale JE. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med. 2007;5:256–278. doi: 10.1080/15402000701557383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc M, Beaulieu-Bonneau S, Merette C, Savard J, Ivers H, Morin CM. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007;63:157–166. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lee M, Choh AC, Demerath EW, Knutson KL, Duren DL, Sherwood RJ, Sun SS, Chumlea WM, Towne B, Siervogel RM, Czerwinski SA. Sleep disturbance in relation to health-related quality of life in adults: the Fels longitudinal study. J Nutr Health Aging. 2009;13:576–583. doi: 10.1007/s12603-009-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Hong S, Nelesen R, Dimsdale JE. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch Intern Med. 2005;165:910–915. doi: 10.1001/archinte.165.8.910. [DOI] [PubMed] [Google Scholar]
- Lorton D, Lubahn CL, Zautra AJ, Bellinger DL. Proinflammatory cytokines and sickness behavior in rheumatic diseases. Curr Pharm Des. 2008;14:1242–1260. doi: 10.2174/138161208799316375. [DOI] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35:802–807. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2007;293:R145–151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med. 2005;165:35–41. doi: 10.1001/archinte.165.1.35. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Nocturnal awakenings and comorbid disorders in the American general population. J Psychiatr Res. 2008;43:48–54. doi: 10.1016/j.jpsychires.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Plante GE. Sleep and vascular disorders. Metabolism. 2006;55:S45–49. doi: 10.1016/j.metabol.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Raison CL, Lin JM, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327–337. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Rasch B, Dodt C, Molle M, Born J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology. 2007;32:884–891. doi: 10.1016/j.psyneuen.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda: 1968. [DOI] [PubMed] [Google Scholar]
- Redwine L, Dang J, Hall M, Irwin M. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med. 2003;65:75–85. doi: 10.1097/01.psy.0000038943.33335.d2. [DOI] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Maesawa C, Akatsu T, Ishikawa Y, Minami Y, Hiramori K, Nakamura M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int J Cardiol. 2006;109:226–234. doi: 10.1016/j.ijcard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Spiegel K. Sleep loss as a risk factor for obesity and diabetes. Int J Pediatr Obes. 2008;3(Suppl 2):27–28. doi: 10.1080/17477160802404681. [DOI] [PubMed] [Google Scholar]
- Stein MB, Belik SL, Jacobi F, Sareen J. Impairment associated with sleep problems in the community: relationship to physical and mental health comorbidity. Psychosom Med. 2008;70:913–919. doi: 10.1097/PSY.0b013e3181871405. [DOI] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KS, Nelesen RA, Ziegler MG, Bardwell WA, Dimsdale JE. Job strain, ethnicity, and sympathetic nervous system activity. Hypertension. 2004;44:891–896. doi: 10.1161/01.HYP.0000148499.54730.0d. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Bixler EO, Lin HM, Prolo P, Vela-Bueno A, Kales A, Chrousos GP. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–2095. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30:473–483. [PubMed] [Google Scholar]
- Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32:772–778. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006;33:535–542. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]