Abstract
The latest technical improvements in the surgical armamentarium are remarkable. In particular, advancements in the urologic field are so exceptional that we could observe the flare-up of robot-assisted laparoscopic radical prostatectomy for prostate cancer and laser prostatectomy for benign prostatic hyperplasia (BPH). Photoselective vaporization of the prostate (PVP) and holmium laser prostatectomy are the most generalized options for laser surgery of BPH, and both modalities have shown good postoperative results. In comparison to transurethral prostatectomy (TURP), they showed similar efficacy and a much lower complication rate in randomized prospective clinical trials. Even in cases of large prostates, laser prostatectomy showed comparable efficacy and safety profiles compared to open prostatectomy. From a technical point of view, PVP is considered to be an easier technique for the urologist to master. Furthermore, patients can be safely followed up in an outpatient clinic. Holmium laser enucleation of the prostate (HoLEP) mimics open prostatectomy because the adenomatous tissue is peeled off the surgical capsule in both procedures. Therefore, HoLEP shows notable volume reduction of the prostate similar to open prostatectomy with fewer blood transfusions, shorter hospital stay, and cost reduction regardless of prostate size. Outcomes of laser prostatectomy for BPH are encouraging but sometimes are unbalanced because safety and feasibility studies were reported mainly for PVP, whereas long-term data are mostly available for HoLEP. We need longer-term randomized clinical data to identify the reoperation rate of PVP and to determine which procedure is the ideal alternative to TURP and open prostatectomy for each patient.
Keywords: Laser therapy, Prostatic hyperplasia, Solid-state lasers
INTRODUCTION
Technological improvements in modern medical science help us to treat patients much easier than before, as we have witnessed throughout the history of medicine. This is equally applicable to the surgical treatment of benign prostatic hyperplasia (BPH). The goal of the surgical management of BPH is to reduce the bulk of the prostate to relieve the obstruction of the urinary tract due to the enlarged prostate. The classical treatment is open prostatectomy or transurethral prostatectomy (TURP). Open prostatectomy is an invasive procedure, associated with significant morbidity, and requires a lower abdominal incision, with consequently longer hospitalization and recovery periods. Although TURP remains an effective treatment, 15% to 20% of patients develop significant complications, and 10% to 15% require a second intervention within 10 years [1]. To improve safety outcomes, a number of minimally invasive surgical techniques have been developed, such as needle ablation, electrovaporization, vaporization resection, holmium laser, ultrasound, and microwave therapy. These alternative surgical treatments have shown favorable outcomes to date [2,3]. Each laser used for prostatectomy has its unique wavelength and tissue interaction characteristic that make each wavelength act differently when applied to prostatic tissue [3]. In this article, we review the safety, efficacy, and durability of the various current laser treatments for BPH.
PHOTOSELECTIVE VAPORIZATION OF THE PROSTATE (PVP)
1. General aspects of PVP
With a potassium titanyl phosphate (KTP) crystal, the frequency of pulsed neodymium:yttrium aluminium garnet (Nd:YAG) laser energy is doubled and made a 532 nm wavelength. Because the KTP laser is strongly absorbed by hemoglobin and minimally absorbed in water, the KTP laser effectively enables vaporization rather than coagulation of prostatic tissue and has excellent hemostasis ability [2,4].
In 1998, Malek et al reported the first clinical trial with a 60 W KTP laser [5]. Later, the GreenLight company improved the power of the 520 nm laser up to 80 W KTP and 120 W high-performance system (HPS) with lithium triborate (LBO), which have led several urologists to use these new instruments for BPH treatment [4,6-9]. PVP is considered to be an easier technique for the urologist to master, and the operator can create a similar postoperative prostatic fossa resembling that of TURP [10,11]. However, no study to date has correctly assessed the learning curve for PVP in Korea [6,12-15].
The indication for PVP is almost the same as with TURP. With the use of the 80 W KTP laser instrument, prostates larger than 80 ml can be effectively treated [6,16,17]. As the 120 W HPS laser entered the urologic stage, treatment of large prostates became easier than before [6]. PVP can be used in patients with a high risk of treatment-related complications, in elderly aged 80 years or older, and even in anticoagulant users [6,18-20].
2. Surgical technique
1) Vaporization technique
Usually PVP can be performed in a smaller diameter cystoscopic sheath such as a 21 Fr than that of TURP. If possible, a video monitor needs to be placed right in front of the surgeon. The camera unit has to be protected from the laser light by a specific filter. The GreenLight laser has a green color as the name implies. Without the filter, the video monitor will be filled with green color whenever the laser firing is on. Normal saline is recommended as the irrigating fluid and can be drained continuously through the drainage system. After the cystoscopic instrument is inserted, the operator should check the intravesical environment and the position of the ureteral orifices. Laser firing should be under the guidance of the red guiding light. Vaporization can be started at the 6 o'clock position of the bladder neck or at one of the two lateral lobes of the prostate. Effective lasing makes many air bubbles in the cystoscopic field of vision. During vaporization, the position of the trigone, bladder neck, and ureteral orifices should be imprinted in the surgeon's mind for protection from unwanted damage. After making sufficient space in the prostatic fossa mimicking TURP, a Foley catheter needs to be placed for several to 24 hours before a voiding trial [21].
2) Vaporesection technique with GreenLight HPS (Seoul technique)
There are several limitations to the PVP technique. The PVP results in a bumpy surface of the prostate following vaporization, and pathologic evaluation of prostate tissue is not feasible. Also, it takes more laser fibers and a longer operative time for a larger prostate. The Seoul technique includes a planned vaporization-resection, not vaporization only. This technique involves vaporization of the prostate along the previously outlined margins and retrieval of the resulting wedge-shaped prostatic tissue [22]. The operation consists of the following 3 steps. First, the vaporization-resection of the median lobe is performed. One should dig into the prostate while vaporizing along the lateral margins of the median lobe. The proximal margin is the bladder neck, and the distal margin is the level of the verumontanum. Secondly, the vaporization-resection of both lateral lobes follows. Before the resection of the lateral lobes, 2 semicircular lines are placed on the distal part of both lateral lobes at the level of the verumontanum, the distal margin of the TUR-like cavity, maintaining a constant depth. The S-point is a concave point near the bladder neck made by the protruding lateral lobe and the roof of the prostatic urethra, the starting point for the resection of the lateral lobe. Finally, the operator must flatten the rough surface and make a complete cavity by additional vaporization. At the end of the procedure, one can remove the prostatic tissue by using the foreign body forceps. Son et al reported favorable results regarding improvement of voiding symptoms and urinary flow rate, with a very low complication rate [22].
3. Results
1) Short-term results
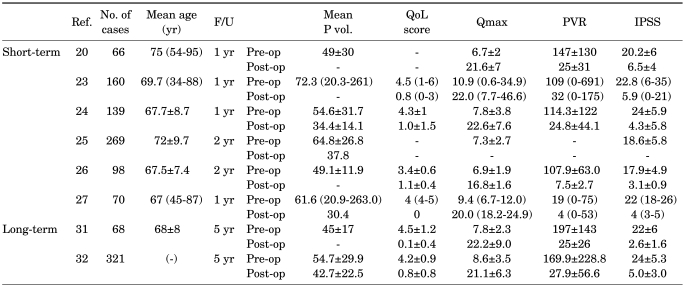
Araki et al prospectively evaluated 160 patients who underwent 80 W KTP-PVP [23]. After a maximum follow-up of 52 weeks, the mean International Prostate Symptom Score (IPSS) decreased significantly from 23 to 13, and the maximum urinary flow rate (Qmax) and postvoiding residual urine volume (PVR) also improved significantly (Table 1). Reich et al performed 80 W KTP-PVP on 66 patients with high cardiopulmonary risk (ASA score >=3) [20]. After a maximum follow-up of 12 months, there were no major complications. Also, the Qmax and IPSS score improved significantly. Among these 66 patients, 29 were taking anticoagulants. A multicenter prospective trial for KTP-PVP was reported by Te et al in 2004 [24]. This prospective clinical trial with 139 men showed statistically significant improvements in IPSS, quality of life (QoL) score, Qmax, and PVR after 12 months. The prostate volume measured by transrectal ultrasound was 54.6 ml at baseline and changed to 34.4 ml at 12 months. With direct comparison of traditional TURP and KTP-PVP, good clinical improvement was also achieved. In a retrospective study including 396 patients (KTP-PVP 269, TURP 127), Ruszat et al reported a lower complication rate and similar clinical efficacy except for Qmax in the KTP-PVP group [25]. Tugcu et al also compared TURP (n=98) and 80 W KTP-PVP (n=112) [26]. After 2 years of follow-up, both groups showed significant improvement of the voiding profile; however, the catheter indwelling period and hospital stay were shorter in the KTP-PVP group. In another 6-month retrospective report of HPS-PVP, Spaiviero et al reported significant improvement in the IPSS and Qmax in 70 patients [27]. From a series of studies on KTP-PVP in Korea, Kim et al and Ku et al and Lee et al showed that the 80 W KTP-PVP is safe and efficacious for up to 24 months of follow-up, regardless of prostate volume, although a larger prostate requires more time and energy delivery [28-30]. The complication rate was also lower and there was no de novo erectile dysfunction.
TABLE 1.
Short-term and long-term results of photoselective vaporization of the prostate
Ref.: reference number, F/U: follow-up period, Mean P vol.: mean prostate volume, QoL: quality of life, Qmax: maximum urinary flow rate, PVR: post void residual, IPSS: International Prostate Symptom Score, Pre-op: preoperation, Post-op: postoperation
2) Long-term results
Malek et al reported on results with a 5-year follow-up period in 2005 [31]. They reported excellent clinical outcomes and the symptomatic and urodynamic improvements were sustained with a minimum necessity for re-intervention. Complications were transient dysuria (6%), delayed hematuria (3%), bladder neck contracture (2%), and retention (1%). Hai evaluated the 5-year clinical outcomes and durability of KTP-PVP with available follow-up for 246 of the 321 patients and reported improvement in the IPSS (79%), QoL score (80%), Qmax (172%), TRUS (17%), and PVR (77%) [32].
3) Complications
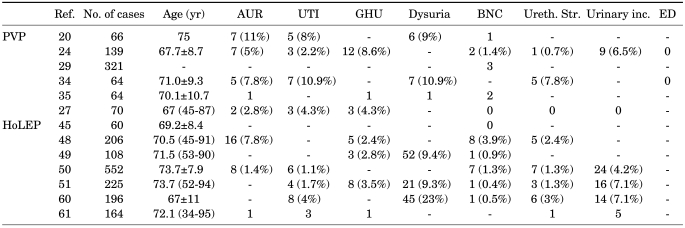
According to the prospective trial reported by Te et al, there was no significant blood loss or fluid absorption during or immediately after PVP after 1 year of follow-up [24]. Complications consisted of transient hematuria, dysuria, and urinary retention in 12 (8.6%), 13 (9.3%), and 7 (5%) patients, respectively. Spaliviero et al reported that perioperative complications after HPS-PVP included intraoperative bleeding (1.4%), postoperative clinically nonsignificant hematuria (78.5%), hematuria requiring clot evacuation (1.4%), urinary retention requiring recatheterization (2.8%), urinary tract infection (4.3%), and prostatitis (1.4%) [27]. No urethral stricture, bladder neck contracture, or urinary incontinence was noted. Also, other studies showed that the retention rate requiring intervention was 5% to 11% [30-35]. Both in a randomized trial and in a prospective study comparing PVP and TURP, the rate of urethral stricture or bladder neck contracture was low and in the same range as for TURP and HoLEP [34,36]. It is suggested that at least 10 to 20 procedures should be performed on small prostates (<40 ml) to avoid complications related to potential thermal damage before challenging large prostates [37]. Long-term follow-up also showed comparable results. After 5 years, complications were mild and rare, such as transient dysuria (6%), delayed hematuria (3%), and bladder neck contracture (2%); no incontinence or newly developed impotence was reported. However, up to 26% of the sexually active men experienced retrograde ejaculation [31].
The re-operation rate after traditional TURP due to recurrence of BPH was known to be less than 12% [38]. According to 5-year follow-up data, only 19 of 246 patients were treated with repeat PVP because of reobstruction due to large glands, and 3 underwent transurethral incision of the bladder neck [32]. Table 2 summarizes the studies of the complications after PVP.
TABLE 2.
Complication rates after PVP and HoLEP for BPH
PVP: photoselective vaporization of prostate, HoLEP: holmium laser enucleation of the prostate, BPH: benign prostatic hyperplasia, Ref.: reference number, AUR: acute urinary retention, UTI: urinary tract infection, GHU: gross hematuria, BNC: bladder neck contracture, Ureth. Str.: urethral stricture, Urinary inc.: urinary incontinence, ED: erectile dysfunction
HOLMIUM LASER PROSTATECTOMY
1. General aspects of the holmium laser
The holmium:yttrium aluminium garnet (Ho:YAG) laser has been investigated as an alternative to the Nd:YAG laser in the treatment of BPH because of its clear-cutting abilities, immediate relief of symptoms, and good level of hemostasis [39]. The Ho:YAG laser is a solid-state pulsed laser with a wavelength of 2,010 nm that is highly and rapidly absorbed by water, which constitutes 60% to 70% of the prostate. The laser penetrates only 0.4 mm of the tissue, predominantly causing vaporization. Dissipating heat causes simultaneous coagulation, with minimal evidence of coagulative tissue necrosis. The physical properties of this laser make it suitable for use in different tissues, including stones, due to the fact that water makes up a significant component of most calculi. The first combined holmium and Nd:YAG laser technique for prostatectomy was reported in 1995 by Chun et al [40] and Gilling and Fraundorfer [41]. The combination endoscopic laser ablation of the prostate technique used the Ho:YAG laser, delivered by a side-firing fiber to create a channel, as an adjunct to the Nd:YAG laser to improve on the outcome of visual laser ablation of the prostate (VLAP). However, the hemostatic properties of the Ho:YAG laser made the addition of the Nd:YAG laser largely redundant so that the improvement in patient outcomes was minimal [42]. This finding led to the development of other techniques that exclusively use the Ho:YAG laser to imitate either a transurethral incision of the prostate or TURP [43].
After holmium laser ablation of the prostate (HoLAP) with the 60 W laser was first introduced in 1995 by Gilling et al [41], the high-powered (>60 W) holmium laser was introduced and was shown to have the ideal physical properties to achieve accurate hemostasis in prostatic tissue with shorter operative times than before. To increase the efficacy of HoLAP, holmium laser resection of the prostate (HoLRP) and subsequently holmium laser enucleation of the prostate (HoLEP) were developed by Gilling and Fraundorfer [44]. The excellent hemostatic features of the holmium wavelength and the use of iso-osmotic saline solution as the irrigating fluid enable operations to be performed on prostates of any size. In a series of reports, HoLEP has been demonstrated as a true endourologic alternative to open prostatectomy in large prostates, and glands of larger than 300 g have been successfully enucleated [45-51]. A retrospective analysis of 225 patients with a mean prostate volume of 126 g (range, 80-351 g, median, 111 g) demonstrated safe and effective results [51]. At 3 years postoperatively, Qmax increased from 8.1 to 28.5 ml/s and PVR decreased from 325 to 46 ml. IPSS improved from 18.7 to 3.7, and the QoL score improved from 3.7 to 0.7.
Nevertheless, uptake of the procedure has been limited partly because of a perception of a steep learning curve. Certainly, HoLEP is such a challenging technique that if a surgeon starts this procedure without supervision of experts, at least 50 patients are estimated to be sufficient to complete the initial learning curve [52]. Adequate mentoring by an experienced urologist, however, can possibly shorten this learning curve [51].
2. Surgical technique
In HoLRP, the laser fiber cuts the prostatic lobes into pieces small enough to be evacuated through the resectoscope sheath, while dissecting the adenomatous tissue down to the prostatic capsule to create a TUR-like cavity. Later, the development of a trasurethral mechanical tissue morcellator enabled a true enucleating technique: the HoLEP. Shelling out the adenoma, the laser fiber moves in exactly the same plane as the surgeon's index finger does when performing open prostatectomy. Gilling, who had first introduced the holmium laser prostatectomy, elucidated his surgical technique as follows [53]. After preliminary cystoscopic evaluation, bilateral bladder neck incisions are made, extending from the ureteric orifices to the verumontanum. These are deepened down to the level of the surgical capsule. Throughout the procedure, hemostasis is obtained by using the defocused laser as each of the bleeding vessels is encountered. Then the median lobe is enucleated starting at the verumontanum. The incisions are connected just above the verumontanum and the fibers connecting the median lobe to the capsule are divided. The lobe is disconnected at the bladder neck and placed in the bladder for later morcellation. The lateral lobes are each enucleated in a retrograde fashion starting at the lower margin of the lobe at the apex. The bladder neck is incised at the 12 o'clock position and the incision extended laterally and distally to the level of the verumontanum. The lobe is then peeled down off the capsule and this is progressively extended distally over the length of the lobe. The upper and lower incisions are connected at the apex and the lateral lobe is enucleated in the capsular plane, working from the upper to the lower incisions. Once each of the lobes has been placed in the bladder, the prostatic fossa is further inspected for hemostasis. Prostate tissues in the bladder are fragmented and aspirated with the morcellator. Because of the excellent hemostatic ability of the holmium wave-length and the use of iso-osmotic saline solution as the irrigating fluid, the transfusion rate is minimal, and TUR syndrome cannot happen. Studies on several hundreds of patients have demonstrated that HoLEP is a true endourologic alternative to open prostatectomy in large prostates [45,46,48-51,54].
3. Results
1) Short-term results
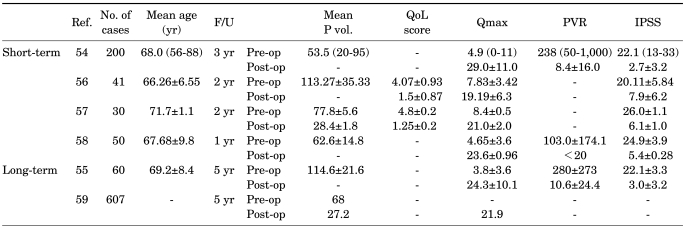
In each of the studies reported, HoLEP proved to be at least equal to both TURP and open prostatectomy in terms of relief from bladder outlet obstruction while providing all of the advantages of a minimally invasive approach, including reduced morbidity, short catheterization time, and short hospital stay (Table 3) [54-57]. In a randomized study, Gupta et al prospectively compared HoLEP to TURP and to transurethral vaporesection of the prostate to compare HoLEP with other minimally invasive ablative procedures [58]. Fifty patients were enrolled per arm, and blood loss, catheter and nursing time, and recatheterization were found to be significantly lower in the HoLEP group.
TABLE 3.
Short-term and long-term results of holmium laser prostatectomy for BPH
Ref.: reference number, F/U: follow-up period, Mean P vol.: mean prostate volume, QoL: quality of life, Qmax: maximum urinary flow rate, PVR: post void residual, IPSS: International Prostate Symptom Score, Pre-op: preoperation, Post-op: postoperation
2) Long-term results
In a meta-analysis of data regarding medium- and long-term urinary functional results, a total of 607 patients at a mean follow-up of 43.5 months were studied [58]. Functional results proved durable, with a mean Qmax of 21.9 ml/s and a mean reoperation rate of 4.3% (range, 0-14.1%). A significant drop in serum PSA levels from baseline (mean, 6.3 ng/dl to 1.63 ng/dl, postoperatively) and in prostate volume at transrectal ultrasound (mean: from 68 ml to 27.2 ml, postoperatively) demonstrated an effective anatomic relief of obstruction. So far as the surgeon achieves complete enucleation, especially at the apex, the durability of the results seems to persist. Cautious pre- and postoperative urodynmic assessment has confirmed that not only is the relief of obstruction with HoLEP superior, or at least equivalent to TURP, but that HoLEP is equivalent urodynamically to open prostatectomy [55]. This result could also explain the relatively mild storage symptoms that are generally present in approximately 30% of the patients at 1 month after surgery and that persist in 10% of patients at 3 months. These symptoms, however, are generally self-limiting and can be successfully treated with medications such as non-steroidal anti-inflammatory drugs and anticholinergics.
4. Complications
The pooled results of large case series revealed low complication rates of recatheterization (2.9%), urinary tract infection (2.3%), urethral stricture/bladder-neck contracture (3.2%), and reoperation (2.8%) [45,48-51,60-62]. The perioperative mortality rate was 0.05% (1 of 1,847 patients). The operation time was significantly longer in the HoLEP group, but the perioperative morbidity was significantly lower. The blood loss was significantly less, and no blood transfusions were required. Shah et al reported a distribution of complications stratified evenly among all sizes of prostates analyzed, with a higher percentage (4.8%) of urethral strictures in prostates larger than 100 g [62].
OTHER LASERS
1. Thulium laser
In 2005, the thulium laser entered clinical practice and has become the most innovative and universally accepted laser equipment in urology after the introduction of the holmium laser [63]. The thulium laser is a high-performance laser using a similar 2,000 nm wavelength to the holmium laser but is delivered as a continuous wave (CW) rather than pulsed. Rapid absorption in water, short penetration depth, and incisional and hemostatic features are similar to those of the holmium laser, but the cutting is much smoother owing to the CW mode. The thulium laser is not only suitable for transurethral vaporization, bladder neck incision [64], or vaporesection [65,66], but is also suitable for vapoenucleation of the prostate [67-69]. Mattioli et al reported data on 99 patients with small prostates (<35 g), showing clinically efficient vaporization in this group of patients [70]. The term vaporesection was introduced to point out the physical characteristics of the 2,000 nm CW Tm:TAG laser system with increased vaporization capacity [64]. With vaporesection, tissue ablation is not only achieved by resection of TUR-like tissue chips, but also by simultaneous vaporization. Meanwhile, using the vapoenucleation technique, the prostatic tissue is enucleated in a three-lobe technique (median lobe, lateral lobes) as described in HoLEP, but again, the vaporizing capacities of the Tm:YAG laser improve the tissue ablation by concurrent vaporization. There is one randomized clinical trial comparing thulium with holmium laser enucleation [69]. In both groups, catheter removal was undertaken in an average of 18 hours with 95% of patients voiding successfully. Blood loss was minimal, and improvements in symptom scores, QoL scores, and peak urinary flow rates were similar at 1 year postoperatively. No significant adverse events occurred. However, large series and long-term results are missing.
2. Diode laser
Various types of diode lasers performing at wavelengths of 940, 980, or 1,470 nm are available for diode-laser prostatectomy. To date, only a few studies have reported clinical applications of these lasers with a maximum follow-up of 1 year. Seitz et al reported that the high-power, 980 nm wavelength diode laser is a new promising alternative with a more rapid ablation rate and excellent hemostatic properties in ex vivo and in vivo animal models [71]. There are only few clinical pilot studies for the vaporization of the prostate using diode lasers. Two series used 980 nm diode lasers of different manufacturers [71,72] and one used a 1,470 nm diode laser prototype [71]. Complication rates were low and the authors reported low or no perioperative bleeding. In a single nonrandomized clinical series comparing the diode laser treatment at 980 nm with the LBO laser [47], the authors described excellent hemostatic properties of the diode laser. Even though most of the patients continued anticoagulation treatment, only 2 patients (4%) needed irrigation postoperatively vs. 25 patients (40%) in the LBO laser arm. However, as a result of the increased tissue necrosis induced by the diode laser compared to the LBO laser, irritative symptoms as short-term complications such as prolonged dysuria or transient urge incontinence occurred more often in the diode laser group. During the follow-up, retreatment rates and incontinence rates were also higher in the diode laser arm [47]. In a recent comparative clinical study followed-up for 1 year using the GreenLight HPS laser (532 nm, 120 W) and the Diolas LFD diode laser (980 nm, 200 W), Chiang et al demonstrated that the diode laser showed superior hemostatic properties compared with the GreenLight HPS laser [73]. Postoperative incontinence and postoperative irritative symptoms were more noticeable after diode laser prostatectomy. Higher incidence of dysuria with sloughing tissues and epididymitis was noted after diode laser prostatectomy. Other complications were comparable for both procedures.
CONCLUSIONS
Until now, outcomes of laser prostatectomy for BPH are very encouraging. However, more clinical data are warranted for laser prostatectomy to replace the status of TURP as a gold standard of surgical treatment for BPH.
Footnotes
The authors have nothing to disclose.
References
- 1.Mebust WK, Holtgrewe HL, Cockett AT, Peters PC. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 1989;141:243–247. doi: 10.1016/s0022-5347(17)40731-2. [DOI] [PubMed] [Google Scholar]
- 2.Reich O, Bachmann A, Schneede P, Zaak D, Sulser T, Hofstetter A. Experimental comparison of high power (80 W) potassium titanyl phosphate laser vaporization and transurethral resection of the prostate. J Urol. 2004;171:2502–2504. doi: 10.1097/01.ju.0000128803.04158.76. [DOI] [PubMed] [Google Scholar]
- 3.Kuntz RM. Laser treatment of benign prostatic hyperplasia. World J Urol. 2007;25:241–247. doi: 10.1007/s00345-007-0170-y. [DOI] [PubMed] [Google Scholar]
- 4.Kang HW, Jebens D, Malek RS, Mitchell G, Koullick E. Laser vaporization of bovine prostate: a quantitative comparison of potassium-titanyl-phosphate and lithium triborate lasers. J Urol. 2008;180:2675–2680. doi: 10.1016/j.juro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Malek RS, Barrett DM, Kuntzman RS. High-power potassium-titanyl-phosphate (KTP/532) laser vaporization prostatectomy: 24 hours later. Urol. 1998;51:254–256. doi: 10.1016/s0090-4295(97)00613-4. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Son H, Paick JS. Comparative analysis of the efficacy and safety of photoselective vaporization of the prostate for treatment of benign prostatic hyperplasia according to prostate size. Korean J Urol. 2010;51:115–121. doi: 10.4111/kju.2010.51.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauffman EC, Kang HW, Choi BB. The effect of laser-fiber sweeping speed on the efficiency of photoselective vaporization of the prostate in an ex vivo bovine model. J Endourol. 2009;23:1429–1435. doi: 10.1089/end.2009.0400. [DOI] [PubMed] [Google Scholar]
- 8.Hoekstra RJ, van Melick HH, Kok ET, Ruud Bosch JL. A 10-year follow-up after transurethral resection of the prostate, contact laser prostatectomy and electrovaporization in men with benign prostatic hyperplasia; long-term results of a randomized controlled trial. BJU Int. 2010;106:822–826. doi: 10.1111/j.1464-410X.2010.09229.x. [DOI] [PubMed] [Google Scholar]
- 9.Ku JH, Cho JY, Cho SY, Kim SW, Paick JS. The one year outcome after KTP laser vaporization of the prostate according to the calculated vaporized volume. J Korean Med Sci. 2009;24:1187–1191. doi: 10.3346/jkms.2009.24.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alivizatos G, Skolarikos A. Greenlight laser in benign prostatic hyperplasia: turning green into gold. Curr Opin Urol. 2008;18:46–49. doi: 10.1097/MOU.0b013e3282f0d63b. [DOI] [PubMed] [Google Scholar]
- 11.Sulser T, Reich O, Wyler S, Ruszat R, Casella R, Hofstetter A, et al. Photoselective KTP laser vaporization of the prostate: first experiences with 65 procedures. J Endourol. 2004;18:976–981. doi: 10.1089/end.2004.18.976. [DOI] [PubMed] [Google Scholar]
- 12.Choo SH, Han DH, Lee SW. The efficacy and safety of KTP photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: the 2-year results. Korean J Urol. 2008;49:831–836. [Google Scholar]
- 13.Lee J, Kang SH, Kim JJ. Evaluation of the quality of life and the efficacy of treatment after high power potassium-titanyl-phosphate (KTP) laser vaporization for patients with a prostate volume greater than 40cc. Korean J Urol. 2007;48:956–964. [Google Scholar]
- 14.Hwang CH, Cho CK, Lee YK, Hong SJ. Comparative analysis of short-term efficacy and complication of photoselective vaporization for benign prostatic hyperplasia which was classified by prostate size. Korean J Urol. 2007;48:826–831. [Google Scholar]
- 15.Hwang EC, Joo JS, Min KD, Oh BR, Kang TW, Kwon DD, et al. A short-term comparative study on efficacy and safety of standard transurethral resection and high power (80W) potassium-titanyl-phosphate laser vaporization of the prostate. Korean J Urol. 2005;46:1251–1255. [Google Scholar]
- 16.Rajbabu K, Chandrasekara SK, Barber NJ, Walsh K, Muir GH. Photoselective vaporization of the prostate with the potassium-titanyl-phosphate laser in men with prostates of >100 mL. BJU Int. 2007;100:593–598. doi: 10.1111/j.1464-410X.2007.06985.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfitzenmaier J, Gilfrich C, Pritsch M, Herrmann D, Buse S, Haferkamp A, et al. Vaporization of prostates of > or =80 mL using a potassium-titanyl-phosphate laser: midterm-results and comparison with prostates of <80 mL. BJU Int. 2008;102:322–327. doi: 10.1111/j.1464-410X.2008.07563.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandhu JS, Ng CK, Gonzalez RR, Kaplan SA, Te AE. Photoselective laser vaporization prostatectomy in men receiving anticoagulants. J Endourol. 2005;19:1196–1198. doi: 10.1089/end.2005.19.1196. [DOI] [PubMed] [Google Scholar]
- 19.Fu WJ, Hong BF, Wang XX, Yang Y, Cai W, Gao JP, et al. Evaluation of greenlight photoselective vaporization of the prostate for the treatment of high-risk patients with benign prostatic hyperplasia. Asian J Androl. 2006;8:367–371. doi: 10.1111/j.1745-7262.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- 20.Reich O, Bachmann A, Siebels M, Hofstetter A, Stief CG, Sulser T. High power (80 W) potassium-titanyl-phosphate laser vaporization of the prostate in 66 high risk patients. J Urol. 2005;173:158–160. doi: 10.1097/01.ju.0000146631.14200.d4. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich E, Schiefelbein F, Schoen G. Technique and short-term outcome of green light laser (KTP, 80W) vaporization of the prostate. Eur Urol. 2007;52:1632–1637. doi: 10.1016/j.eururo.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Son H, Ro YK, Min SH, Choo MS, Kim JK, Lee CJ. Modified vaporization-resection for photoselective vaporization of the prostate using a greenlight™ high performance system 120W laser : the Seoul Technique. Urology. 2010 doi: 10.1016/j.urology.2010.06.034. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Araki M, Lam PN, Wong C. High-power potassium-titanyl-phosphate laser photoselective vaporization prostatectomy for symptomatic benign prostatic hyperplasia. J Endourol. 2008;22:1311–1314. doi: 10.1089/end.2008.0140. [DOI] [PubMed] [Google Scholar]
- 24.Te AE, Malloy TR, Stein BS, Ulchaker JC, Nseyo UO, Hai MA, et al. Photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: 12-month results from the first United States multicenter prospective trial. J Urol. 2004;172:1404–1408. doi: 10.1097/01.ju.0000139541.68542.f6. [DOI] [PubMed] [Google Scholar]
- 25.Ruszat R, Wyler SF, Seitz M, Lehmann K, Abe C, Bonkat G, et al. Comparison of potassium-titanyl-phosphate laser vaporization of the prostate and transurethral resection of the prostate: update of a prospective non-randomized two-centre study. BJU Int. 2008;102:1432–1438. doi: 10.1111/j.1464-410X.2008.07905.x. [DOI] [PubMed] [Google Scholar]
- 26.Tugcu V, Tasci AI, Sahin S, Zorluoglu F. Comparison of photoselective vaporization of the prostate and transurethral resection of the prostate: a prospective nonrandomized bicenter trial with 2-year follow-up. J Endourol. 2008;22:1519–1525. doi: 10.1089/end.2007.0321. [DOI] [PubMed] [Google Scholar]
- 27.Spaliviero M, Araki M, Culkin DJ, Wong C. Incidence, management, and prevention of perioperative complications of GreenLight HPS laser photoselective vaporization prostatectomy: experience in the first 70 patients. J Endourol. 2009;23:495–502. doi: 10.1089/end.2008.0299. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Cho MC, Ku JH, Kim SW, Paick JS. The efficacy and safety of photoselective vaporization of the prostate with a potassium-titanyl-phosphate laser for symptomatic benign prostatic hyperplasia according to prostate size: 2-year surgical outcomes. Korean J Urol. 2010;51:330–336. doi: 10.4111/kju.2010.51.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ku JH, Kim SW, Paick JS. Impact of prostate volume on the efficacy of high-power potassium-titanyl-phosphate photoselective vaporization of the prostate: a retrospective, short-term follow-up study on evaluating feasibility and safety. Yonsei Med J. 2010;51:877–882. doi: 10.3349/ymj.2010.51.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CJ, Cho MC, Ku JH, Kim SW, Paick JS. Changes in nocturia after photoselective vaporization of the prostate for patients with benign prostatic hyperplasia. Korean J Urol. 2010;51:531–536. doi: 10.4111/kju.2010.51.8.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malek RS, Kuntzman RS, Barrett DM. Photoselective potassium-titanyl-phosphate laser vaporization of the benign obstructive prostate: observations on long-term outcomes. J Urol. 2005;174:1344–1348. doi: 10.1097/01.ju.0000173913.41401.67. [DOI] [PubMed] [Google Scholar]
- 32.Hai MA. Photoselective vaporization of prostate: five-year outcomes of entire clinic patient population. Urology. 2009;73:807–810. doi: 10.1016/j.urology.2008.08.502. [DOI] [PubMed] [Google Scholar]
- 33.Reich O, Bachmann A, Siebels M, Hofstetter A, Stief CG, Sulser T. High power (80 W) potassium-titanyl-phosphate laser vaporization of the prostate in 66 high risk patients. J Urol. 2005;173:158–160. doi: 10.1097/01.ju.0000146631.14200.d4. [DOI] [PubMed] [Google Scholar]
- 34.Bachmann A, Schürch L, Ruszat R, Wyler SF, Seifert HH, Müller A, et al. Photoselective vaporization (PVP) versus transurethral resection of the prostate (TURP): a prospective bi-centre study of perioperative morbidity and early functional outcome. Eur Urol. 2005;48:965–971. doi: 10.1016/j.eururo.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu JS, Ng C, Vanderbrink BA, Egan C, Kaplan SA, Te AE. High-power potassium-titanyl-phosphate photoselective laser vaporization of prostate for treatment of benign prostatic hyperplasia in men with large prostates. Urology. 2004;64:1155–1159. doi: 10.1016/j.urology.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Bouchier-Hayes DM, Anderson P, Van Appledorn S, Bugeja P, Costello AJ. KTP laser versus transurethral resection: early results of a randomized trial. J Endourol. 2006;20:580–585. doi: 10.1089/end.2006.20.580. [DOI] [PubMed] [Google Scholar]
- 37.Chandrasekera S, Muir G. Potassium titanyl phosphate laser prostatectomy: a review. Curr Opin Urol. 2007;17:22–26. doi: 10.1097/MOU.0b013e32801145fc. [DOI] [PubMed] [Google Scholar]
- 38.Madersbacher S, Lackner J, Brössner C, Röhlich M, Stancik I, Willinger M, et al. Reoperation, myocardial infarction, and mortality after transurethral and open prostatectomy: a nation-wide, long term analysis of 23,123 cases. Eur Urol. 2005;47:499–504. doi: 10.1016/j.eururo.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Dixon CM. Lasers for the treatment of benign prostatic hyperplasia. Urol clin North Am. 1995;22:413–422. [PubMed] [Google Scholar]
- 40.Chun SS, Razvi HA, Denstedt JD. Laser prostatectomy with the holmium:YAG laser. Tech Urol. 1995;1:217–221. [PubMed] [Google Scholar]
- 41.Gilling PJ, Cass CB, Malcolm AR, Fraundorfer MR. Combination holmium and Nd:YAG laser ablation of the prostate: initial clinical experience. J Endourol. 1995;9:151–153. doi: 10.1089/end.1995.9.151. [DOI] [PubMed] [Google Scholar]
- 42.Wollin TA, Denstedt JD. The holmium laser in urology. J Clin Laser Med Surg. 1998;16:13–20. doi: 10.1089/clm.1998.16.13. [DOI] [PubMed] [Google Scholar]
- 43.Larizgoitia I, Pons JM. A systematic review of the clinical efficacy and effectiveness of the holmium:YAG laser in urology. BJU Int. 1999;84:1–9. doi: 10.1046/j.1464-410x.1999.00096.x. [DOI] [PubMed] [Google Scholar]
- 44.Gilling PJ, Fraundorfer MR. Holmium laser prostatectomy: a technique in evolution. Curr Opin Urol. 1998;8:11–15. doi: 10.1097/00042307-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Kuntz RM, Lehrich K, Ahyai S. Transurethral holmium laser enucleation of the prostate compared with transvesical open prostatectomy: 18-month follow-up of a randomized trial. J Endourol. 2004;18:189–191. doi: 10.1089/089277904322959851. [DOI] [PubMed] [Google Scholar]
- 46.Moody J, Lingeman JE. Holmium laser enucleation for prostate adenoma greater than 100 gm.: comparison to open prostatectomy. J Urol. 2001;165:459–462. doi: 10.1097/00005392-200102000-00025. [DOI] [PubMed] [Google Scholar]
- 47.Ruszat R, Seitz M, Wyler SF, Müller G, Rieken M, Bonkat G, et al. Prospective single-centre comparison of 120-W diode-pumped solid-state high-intensity system laser vaporization of the prostate and 200-W high-intensive diode-laser ablation of the prostate for treating benign prostatic hyperplasia. BJU Int. 2009;104:820–825. doi: 10.1111/j.1464-410X.2009.08452.x. [DOI] [PubMed] [Google Scholar]
- 48.Kuo RL, Paterson RF, Siqueira TM, Jr, Watkins SL, Simmons GR, Steele RE, et al. Holmium laser enucleation of the prostate: morbidity in a series of 206 patients. Urology. 2003;62:59–63. doi: 10.1016/s0090-4295(03)00124-9. [DOI] [PubMed] [Google Scholar]
- 49.Kuo RL, Kim SC, Lingeman JE, Paterson RF, Watkins SL, Simmons GR, et al. Holmium laser enucleation of the prostate (HoLEP): the Methodist hospital experience with greater than 75 gram enucleations. J Urol. 2003;170:149–152. doi: 10.1097/01.ju.0000070686.56806.a1. [DOI] [PubMed] [Google Scholar]
- 50.Elzayat EA, Habib EI, Elhilali MM. Holmium laser enucleation of the prostate: a size-independent new "gold standard". Urology. 2005;66(5 Suppl):108–113. doi: 10.1016/j.urology.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Elzayat EA, Elhilali MM. Holmium laser enucleation of the prostate (HoLEP): the endourological alternative to open prostatectomy. Eur Urol. 2006;49:87–91. doi: 10.1016/j.eururo.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Du C, Jin X, Bai F, Qiu Y. Holmium laser enucleation of the prostate: the safety, efficacy, and learning experience in China. J Endourol. 2008;22:1031–1036. doi: 10.1089/end.2007.0262. [DOI] [PubMed] [Google Scholar]
- 53.Gilling P. Holmium laser enucleation of the prostate (HoLEP) BJU Int. 2008;101:131–142. doi: 10.1111/j.1464-410X.2007.07341.x. [DOI] [PubMed] [Google Scholar]
- 54.Ahyai SA, Lehrich K, Kuntz RM. Holmium laser enucleation versus transurethral resection of the prostate: 3-year follow-up results of a randomized clinical trial. Eur Urol. 2007;52:1456–1463. doi: 10.1016/j.eururo.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 55.Kuntz RM, Lehrich K, Ahyai SA. Holmium laser enucleation of the prostate versus open prostatectomy for prostates greater than 100 grams: 5-year follow-up results of a randomized clinical trial. Eur Urol. 2008;53:160–166. doi: 10.1016/j.eururo.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 56.Naspro R, Suardi N, Salonia A, Scattoni V, Guazzoni G, Colombo R, et al. Holmium laser enucleation of the prostate versus open prostatectomy for prostates >70 g: 24-month follow-up. Eur Urol. 2006;50:563–568. doi: 10.1016/j.eururo.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Wilson LC, Gilling PJ, Williams A, Kennett KM, Frampton CM, Westenberg AM, et al. A randomized trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: results at 2 years. Eur Urol. 2006;50:569–573. doi: 10.1016/j.eururo.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Gupta N, Sivaramakrishna, Kumar R, Dogra PN, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40g. BJU Int. 2006;97:85–89. doi: 10.1111/j.1464-410X.2006.05862.x. [DOI] [PubMed] [Google Scholar]
- 59.Naspro R, Bachmann A, Gilling P, Kuntz R, Madersbacher S, Montorsi F, et al. A review of the recent evidence (2006-2008) for 532-nm photoselective laser vaporization and holmium laser enucleation of the prostate. Eur Urol. 2009;55:1345–1357. doi: 10.1016/j.eururo.2009.03.070. [DOI] [PubMed] [Google Scholar]
- 60.Vavassori I, Hurle R, Vismara A, Manzetti A, Valenti S. Holmium laser enucleation of the prosate combined with mechanical morcellation: two years of experience with 196 patients. J Endourol. 2004;18:109–112. doi: 10.1089/089277904322836767. [DOI] [PubMed] [Google Scholar]
- 61.Peterson MR, Matlaga BR, Kim SC, Kuo RL, Soergel TM, Watkins SL, et al. Holmium laser enucleation of the prostate for men with urinary retention. J Urol. 2005;174:998–1001. doi: 10.1097/01.ju.0000170230.26743.e4. [DOI] [PubMed] [Google Scholar]
- 62.Shah HN, Kausik V, Hegde S, Shah JN, Bansal MB. Evaluation of fluid absorption during holmium laser enucleation of prostate by breath ethanol technique. J Urol. 2006;175:537–540. doi: 10.1016/S0022-5347(05)00239-9. [DOI] [PubMed] [Google Scholar]
- 63.Fried NM, Murray KE. High-power thulium fiber laser ablation of urinary tissues at 1.94 microm. J Endourol. 2005;19:25–31. doi: 10.1089/end.2005.19.25. [DOI] [PubMed] [Google Scholar]
- 64.Bach T, Herrmann TR, Ganzer R, Burchardt M, Gross AJ. RevoLix vaporesection of the prostate: initial results of 54 patients with an 1-year follow-up. World J Urol. 2007;25:257–262. doi: 10.1007/s00345-007-0171-x. [DOI] [PubMed] [Google Scholar]
- 65.Bach T, Herrmann TR, Ganzer R, Blana A, Burchardt M, Gross AJ. Thulium:YAG vaporesection of the prostate: first results. Urologe A. 2009;48:529–534. doi: 10.1007/s00120-008-1931-y. [DOI] [PubMed] [Google Scholar]
- 66.Bach T, Wendt-Nordahl G, Michel MS, Herrmann TR, Gross AJ. Feasibility and efficacy of Thulium: YAG laser enucleation (VapoEnucleation) of the prostate. World J Urol. 2009;27:541–545. doi: 10.1007/s00345-008-0370-0. [DOI] [PubMed] [Google Scholar]
- 67.Bach T, Herrmann TR, Haecker A, Michel MS, Gross A. Thulium:yttrium-aluminium-garnet laser prostatectomy in men with refractory urinary retention. BJU Int. 2009;104:361–364. doi: 10.1111/j.1464-410X.2009.08412.x. [DOI] [PubMed] [Google Scholar]
- 68.Bach T, Netsch CH, Haecker A, Michel MS, Herrmann TR, Gross AJ. Thulium:YAG laser enucleation (VapoEnucleation) of the prostate: safety and durability during intermediate-term follow-up. World J Urol. 2010;28:39–43. doi: 10.1007/s00345-009-0461-6. [DOI] [PubMed] [Google Scholar]
- 69.Gordon S, Watson G. Thulium laser enucleation of the prostate. Eur Urol. 2006;5(Suppl):310. [Google Scholar]
- 70.Mattioli S, Munoz R, Recasens R, Berbegal C, Cortada J, Urmeneta JM, et al. Treatment of benign prostatic hyperplasia with the Revolix laser. Arch Esp Urol. 2008;61:1037–1043. [PubMed] [Google Scholar]
- 71.Seitz M, Ruszat R, Bayer T, Tilki D, Bachmann A, Stief C, et al. Ex vivo and in vivo investigations of the novel 1,470 nm diode laser for potential treatment of benign prostatic enlargement. Lasers Med Sci. 2009;24:419–424. doi: 10.1007/s10103-008-0591-x. [DOI] [PubMed] [Google Scholar]
- 72.Leonardi R. Preliminary results on selective light vaporization with the side-firing 980 nm diode laser in benign prostatic hyperplasia: an ejaculation sparing technique. Prostate Cancer Prostatic Dis. 2009;12:277–280. doi: 10.1038/pcan.2009.5. [DOI] [PubMed] [Google Scholar]
- 73.Chiang PH, Chen CH, Kang CH, Chuang YC. GreenLight HPS laser 120-W versus diode laser 200-W vaporization of the prostate: comparative clinical experience. Lasers Surg Med. 2010;42:624–629. doi: 10.1002/lsm.20940. [DOI] [PubMed] [Google Scholar]