Abstract
Microbial biofilm architecture contains numerous protective features including extracellular polymeric material that render biofilms impermeable to conventional antimicrobial agents. This study evaluated the efficacy of antimicrobial photodynamic inactivation (aPDI) of Enterococcus faecalis biofilms. The ability of a cationic, phenothiazinium photosensitizer, methylene blue (MB) and an anionic, xanthene photosensitizer, rose bengal (RB) to inactivate biofilms of E. faecalis (OGIRF and FA 2-2) and disrupt the biofilm structure was evaluated. Bacterial cells were tested as planktonic suspensions, intact biofilms and biofilm-derived suspensions obtained by the mechanical disruption of biofilms. The role of a specific microbial efflux pump inhibitor (EPI), verapamil hydrochloride in the MB-mediated aPDI of E. faecalis biofilms was also investigated. The results showed that E. faecalis biofilms exhibited significantly higher resistance to aPDI when compared to E. faecalis in suspension (P < 0.001). aPDI with cationic MB produced superior inactivation of E. faecalis strains in a biofilm along with significant destruction of biofilm structure when compared to anionic RB (P < 0.05). The ability to inactivate biofilm bacteria was further enhanced when the EPI was used with M B (P < 0.001). These experiments demonstrated the advantage of a cationic phenothiazinium photosensitizer combined with an EPI to inactivate biofilm bacteria and disrupt biofilm structure.
INTRODUCTION
Photodynamic therapy (PDT) is based on the concept that a certain non-toxic photoactivatable compound or photosensitizer (PS) can be preferentially localized in certain tissues and subsequently activated by otherwise harmless light of the appropriate wavelength. The absorbed photons excite the dye to its reactive triplet state that will then generate reactive oxygen species (ROS), such as singlet oxygen and superoxide that are toxic to cells (1). PDT has been used successfully in the treatment of certain tumors and in ophthalmology for age-related macular degeneration (2, 3). Other applications of PDT at a less developed stage include treatments for psoriasis (4), arthritis (5), atherosclerosis (6) and restenosis (7) in both veins and arteries. In recent years, antimicrobial photodynamic inactivation (aPDI) has been proposed as an alternative treatment for localized infections in response to the ever-growing problem of antibiotic resistance (8–10).
A frequently employed class of antimicrobial PS are the blue dyes known as phenothiazinium salts, such as methylene blue (MB) (11), toluidine blue O (TBO) (12), and azure dyes (13). Phenothiazinium salts have phototoxic efficiency against a broad range of microorganisms (14–16). Presently, the only PS used clinically for antimicrobial treatments are phenothiazinium salts. The combination of MB or TBO together with red light is used to disinfect blood products, sterilize dental cavities and root canals and treat periodontitis (17). However, studies till date have not compared the role of cationic and anionic PS in the aPDI of bacterial biofilms.
Microbial efflux pumps (MEPs) have become broadly recognized as major components of microbial resistance to many classes of antibiotics (18). Some MEPs selectively extrude specific antibiotics while others, referred to as multidrug resistance pumps (MDRs) expel a variety of structurally diverse compounds with differing modes of action (19). Gram-positive species mainly have major facilitator type MEPs typified by NorA in Staphylococcus aureus. It has been suggested that amphipathic cations represent the existing natural substrates of MEPs (20) and these molecules have been frequently used to study MEP-mediated efflux. It has been established that disabling MEPs by employing either MEP mutants or synthetic efflux pump-inhibitors (EPIs) leads to a striking increase in the activity of a wide array of plant secondary metabolites including natural MEP substrates (21). We recently showed that phenothiazinium salts, which are structurally characterized as amphipathic cations, were substrates of MEPs in both gram-positive and gram-negative species (22) and the use of specific inhibitors of MEP could be used to potentiate aPDI of planktonic microbial cells (23).
Eradication of microbial biofilms with conventional treatments has been a challenging task due to the biological complexity of the biofilm structure and the variety of inanimate surfaces that microbial cells colonize (24, 25). There have been an increasing number of investigations using aPDI to inactivate biofilms (26–29). Enterococcus faecalis, a gram-positive bacterium, is a member of the commensal human flora and an opportunistic pathogen implicated as one of the leading causes of nosocomial infections (30, 31). E. faecalis has a predominant role in biofilms formed on biomedical devices (urinary catheters, central venous catheter, clogged biliary stents) (32) and is directly related to the emergence of multi-drug resistance (33). The majority of clinical isolates of E. faecalis have the ability to form mono-species biofilms in vitro (34, 35). Although the genetic basis of biofilm formation by E. faecalis is largely unknown, the enterococcal surface protein (Esp) (34, 36), the transcriptional regulator BopD, the quorum sensing locus fsr (37, 38), and gelatinase (GelE) have all been reported to be involved in promoting biofilm formation (35, 39). E. faecalis is frequently the only surviving bacteria in recurrent root canal infections (40–42).
This study aimed to evaluate the aPDI of two strains of E. faecalis biofilms (OG1RF and FA 2-2) using anionic RB and cationic MB (with and without EPI) and to assess their ability to inactivate the resident biofilm bacteria and disrupt the biofilm structure. To understand the response of biofilm bacteria to aPDI, E. faecalis was tested as planktonic/loose cells, as intact four-day-old biofilms and as biofilm-derived/loose cells obtained by the mechanical disruption of four-day old biofilms. Testing biofilm bacteria as intact biofilm and after mechanically disrupting from biofilm structure may help in understanding the role of biofilm matrix or extracellular polymeric substance (EPS) in aPDI. The role of a specific microbial EPI, verapamil hydrochloride in the MB-mediated aPDI of E. faecalis biofilms was also investigated in this study.
MATERIALS AND METHODS
Photosensitizers and light source
All chemicals used in the study were of analytical grade and were purchased from Sigma Aldrich (St. Louis, MO, USA) unless mentioned otherwise. Stock solutions of MB and RB were prepared in sterile deionized (DI) water at a concentration of 1 mM and stored for a maximum of two weeks at 4°C in the dark before use. From the stock solutions, 100 µM solutions of MB and RB were prepared. The EPI used in this study was verapamil hydrochloride which was used at a concentration of 100 µM (corresponding to 0.49 mg mL−1). Verapamil was dissolved in 10 mL of 100 µM MB solution. All three working PS solutions were stored at 4°C in the dark until further use. A non-coherent light source with interchangeable fiber bundles (LC122M, CA, USA) was employed. Thirty-nm band pass filters at ranges 540 ±15 nm for RB and 660 ±15 nm for MB were used. The total power output provided out of the fiber bundle ranged from 300–600 mW. Power measurements were quantified with a power meter (Newport, CA, USA).
Bacterial strains and culture conditions
E. faecalis strains OG1RF and FA 2-2 having different biofilm forming capacities were used in the study (gifted by Dr. Mylonakis, Massachusetts General Hospital, MA, USA). Bacterial cells were cultured in brain heart infusion (BHI) broth with aeration at 37°C under shaking conditions in an orbital incubator (100 rpm). Cell growth was assessed with a spectrophotometer (Shimadzu, Mini 1240, Japan) at 600 nm (OD600). Cells were used for experiments in the logarithmic growth phase (OD600 ≈ 0.7 or 107 cells mL−1).
Biofilm quantification assay
The biofilm quantification assay was conducted based on a protocol by Mohamed et al. with modifications (43). E. faecalis (OG1RF and FA 2-2) that had been grown overnight were diluted 1:100 in trypticase soy broth (TSB) medium and 200 µL of each cell suspension was dispensed into sterile flat-bottomed 96-well polystyrene plates (Thermo Fisher Scientific, Denmark). After stationary aerobic incubation at 37°C for 24 hrs, the broth was carefully aspirated, wells were washed three times with 200 µL phosphate buffered saline (PBS) and air dried for 30 min at room temperature. The biofilms were then stained with 200 µL of 1% crystal violet for 30 min, washed again with PBS and the bound crystal violet solubilized in 200 µL of ethanol-acetone (80:20, vol/vol). Thereafter, the optical density (OD) was measured at 570 nm (OD570) by using a microtiter plate reader (Bio-Tek ELx800, VT, USA). Each assay was performed in quadruplicate on three occasions for a total of 12 readings for each strain. Wells containing uninoculated medium served as negative controls to determine the background OD. After subtraction of the mean background OD570 readings, the 12 readings per strain were averaged to obtain the mean OD570 reading for either strain.
aPDI of biofilms
Four-day old biofilms of E. faecalis were developed in sterile flat-bottomed 24-well polystyrene plates (Thermo Fisher Scientific, Denmark) using BHI broth as the growth medium. The biofilms were kept constantly hydrated with the medium changed once in 48 hrs. The growth medium was aspirated from the wells prior to the experiment. Visual examination revealed biofilm growth on the walls of the wells. The biofilms were then incubated with either 1 mL of MB, MB + EPI or RB at a concentration of 100 µM at 37°C in the dark for 15 min. The excess PS was removed using a micropipette. Wells were irradiated with the non-coherent light source to deliver a fluence range of 10 J/cm2 to 40 J/cm2 on the surface of the biofilm. During irradiation, the tip of the optical fiber was placed at the level of the outer edge of the well. Controls included biofilms incubated with PBS without calcium and magnesium chloride in the dark (untreated), biofilms incubated with PS or PS with EPI in the dark (dark toxicity) and biofilms subjected to illumination in the absence of PS (light alone).
After aPDI treatment, fresh BHI medium was added to the wells and vigorously shaken. The medium was flushed using a micropipette for mechanically disrupting the biofilms on the walls of the wells. The multi-well plates were incubated at 37°C for 15 min to allow for the enrichment of the biofilm bacteria. Samples were serially diluted 10-fold in PBS to give dilutions of 10−1 to 10−6 times the original concentrations and were streaked horizontally on square BHI agar plates as described by Jett et al. (44). This allowed a maximum of 7 logs of killing to be measured. Plates were incubated at 37°C overnight to enumerate the colony forming units (CFU) mL−1. Survival fractions were expressed as ratios of CFU of bacteria treated with light and PS to CFU of bacteria treated with neither light or PS (untreated controls).
aPDI of bacterial suspensions
Towards comparison, experiments were also conducted on planktonic and biofilm-derived cells of E. faecalis (OG1RF and FA 2-2). For planktonic experiments, 500 µL of bacterial suspensions were taken in sterile eppendorf tubes, the cells were harvested by centrifugation for 4 min at 4600 g at 4°C and washed twice in sterile PBS. The cell pellet was harvested by centrifugation and according to the experimental group, 500 µL of MB or RB (100 µM concentration) or PBS was added to the samples and incubated at 37°C in the dark for 15 min. The samples were centrifuged again, the supernatant aspirated and the cell pellet was irradiated at fluence ranging from 2 to 15 J/cm2. Control conditions included incubation with PBS in the dark, incubation with PS in the dark and illumination in the absence of PS. Following irradiation, serial dilutions and streaking of aliquots on agar plates was carried out as described earlier.
For experiments on biofilm-derived cells, four-day old biofilms of each of the two strains were grown as described previously. The growth medium was aspirated and the wells containing biofilms were gently washed twice with PBS to remove the planktonic cells. Sterile PBS was added and the biofilm was disrupted by mechanical flushing. The OD of the suspension was adjusted to 0.7 at 600 nm that corresponded to 107 cells mL−1. The bacterial suspensions were taken in sterile eppendorf tubes and the aPDI experiments using MB and MB + EPI as the PS were conducted in a method similar to the planktonic experiments.
Laser Confocal Scanning Microscopy (LCSM) of biofilms subjected to aPDI
LCSM (Leica Microscopy, Wetzlar, Germany) was used to examine the structure of the E. faecalis strain OG1RF biofilm before and after aPDI. For better appreciation of the three-dimensional structure of the biofilm, two-week old biofilms grown on a glass coverslip that was fixed covering a groove (6 mm diameter) at the bottom of a petri dish using BHI medium were used. The two-week old biofilms were produced in a similar manner to the four-day old biofilms used in the earlier experiments with medium changed every 48 hrs for up to two weeks. Biofilms were incubated with MB or RB at a concentration of 100 µM at 37°C in the dark for 15 min and then illuminated with a non-coherent light source (fluence of 40 J/cm2) as described previously. Following irradiation, the specimens were gently rinsed once in sterile PBS. The LIVE/DEAD BacLight Viability kit L-7007 (Molecular probes, Eugene, OR, USA) contains separate vials of two fluorescent nucleic acid stains - SYTO 9 and propidium iodide. The dyes were mixed (3 µL) each in 1 mL of DI water according to the manufacturer’s instructions. LCSM images of the biofilms were obtained using a Kr/Ar laser as the source of illumination (488 nm excitation wavelength) fitted with a long-pass 500–523 nm and 622–722 nm emission filter settings for green and red signals, respectively. Eight randomly chosen areas from each of the three samples per group were imaged using 60× water-immersion lenses. The optical sections of the biofilm structure were recorded and analyzed by using IMARIS 6.3 software (Bitplane Scientific, Zurich, Switzerland). The three-dimensional images and cross sections through the biofilm were generated by the software. The software package allowed visualization, segmentation and interpretation of three-dimensional microscopy datasets. Measurement of the biofilm thickness and distribution of live (green) and dead (red) cells were assessed by the frame and new spots option respectively of the IMARIS software package.
Statistical analyses
The aPDI experiments on bacterial suspensions and biofilms were performed in triplicate. Data points are given as mean ± standard error of the mean (SEM). Statistical tests were performed using SPSS software (Version 9.0; SPSS Inc., IL, USA). Data were analyzed using Kruskal-Wallis one-way analysis of variance and Mann-Whitney U-tests. The level of statistical significance was set at P < 0.05.
RESULTS
Biofilm quantification assay
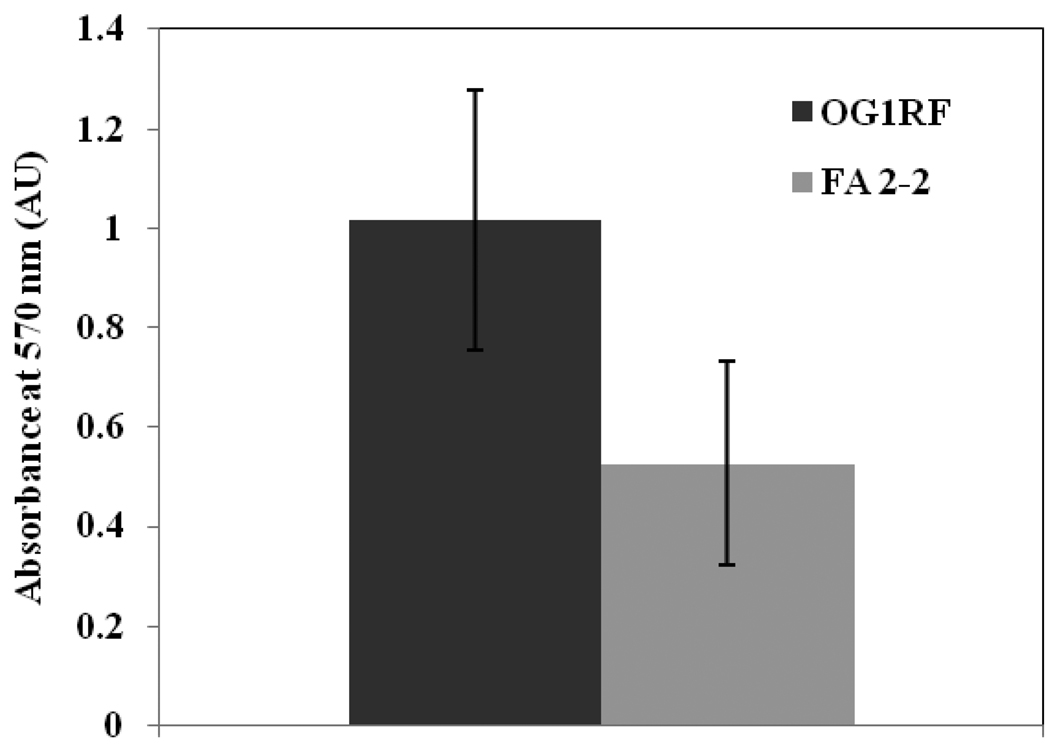
The results of the biofilm quantification assay using crystal violet are shown in Figure 1. Both strains of E. faecalis (OG1RF and FA 2-2) had the ability to form biofilm under the experimental conditions. The two E. faecalis stains were categorized based on the approach of others (34) as ‘medium biofilm former’ (OD570 1 – 2; OG1RF) and ‘weak biofilm former’ (OD570 greater than 0.5 but less than 1; FA 2-2). It was observed that between the two tested strains of E. faecalis, the strain OG1RF formed approximately twice as much biofilm compared to FA 2-2.
Figure 1.
Average absorbance values obtained with the crystal violet biofilm quantification assay of two strains of E. faecalis biofilms. Data represent mean ± SD of twelve readings.
aPDI of biofilms
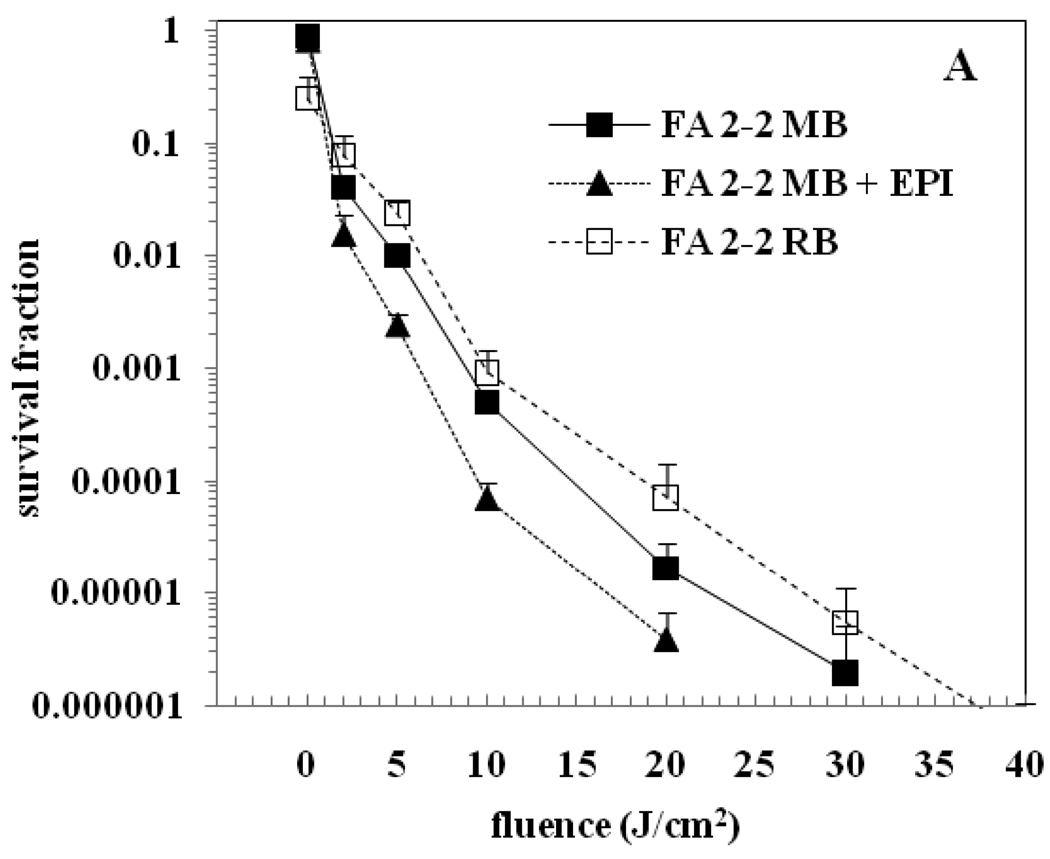
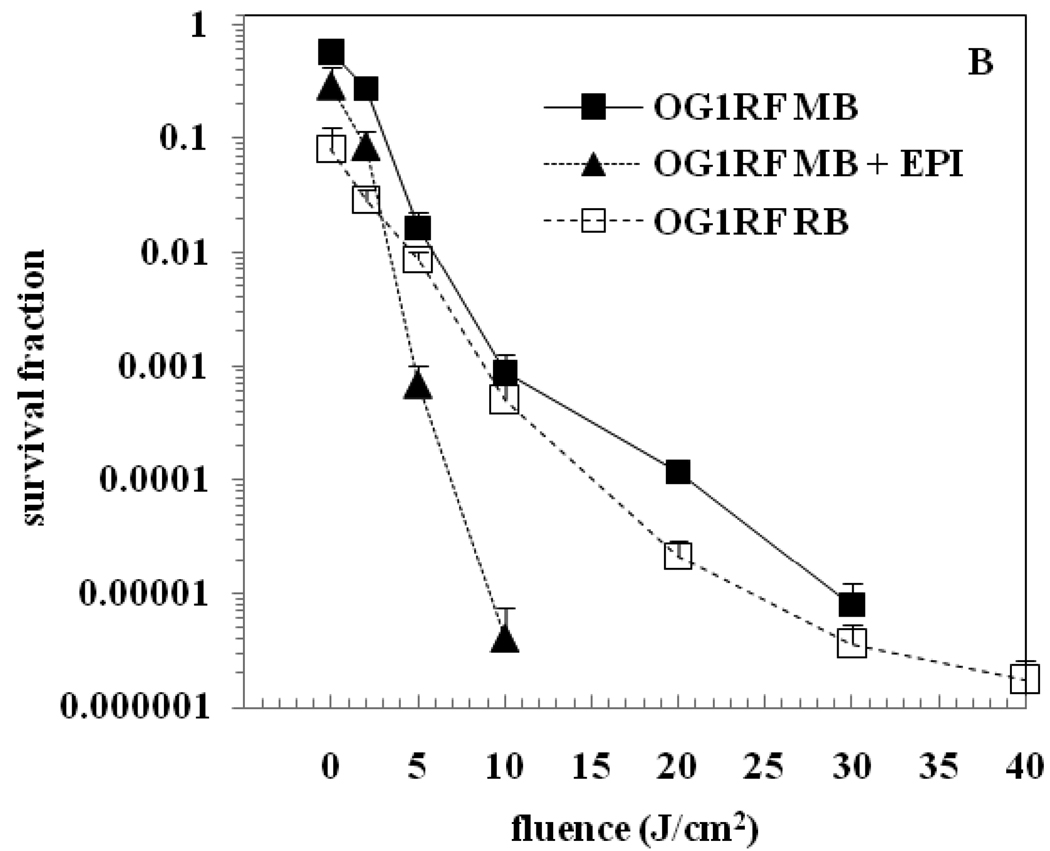
Figure 2 (A & B) shows surviving bacteria following aPDI of E. faecalis FA 2-2 and E. faecalis OG1RF biofilms respectively, using MB, MB + EPI and RB as the PS. The survival fraction at 0 J/cm2 represents the dark toxicity. The dark toxicity of RB was observed to be higher when compared to MB. There was no significant reduction in the bacterial viability after exposure to light alone even up to a fluence of 40 J/cm2 (data not shown). All three PS when combined with light irradiation produced a dose-dependent significant killing of E. faecalis FA 2-2 and E. faecalis OG1RF biofilms relative to the untreated controls (P < 0.05). Figure 2(A) shows that aPDI with MB produced roughly 5 logs reduction of E. faecalis FA 2-2 biofilm at 30 J/cm2. aPDI with MB + EPI produced the same degree of bacterial killing at 20 J/cm2 while aPDI with RB required 35 J/cm2 to produce the same result. Figure 2(B) shows that aPDI with MB produced over 5 logs reduction of E. faecalis OG1RF biofilm at 30 J/cm2 while aPDI with MB + EPI produced the same degree of bacterial killing at 10 J/cm2. There was no conspicuous difference in the aPDI of E. faecalis OG1RF biofilm between RB and MB. Addition of an EPI with MB significantly increased the aPDI of OG1RF biofilms compared with the use of MB alone (P < 0.001). Overall, among the three PS groups tested, aPDI using MB + EPI produced the maximum bacterial reduction in four-day old E. faecalis biofilms.
Figure 2.
aPDI of biofilms of E. faecalis. (A) Strain FA 2-2 (B) Strain OG1RF. Biofilms were incubated with 100 µM concentration of the PS for 15 min followed by light irradiation. The survival fraction at 0 J/cm2 indicates the dark toxicity.
aPDI of bacterial suspensions
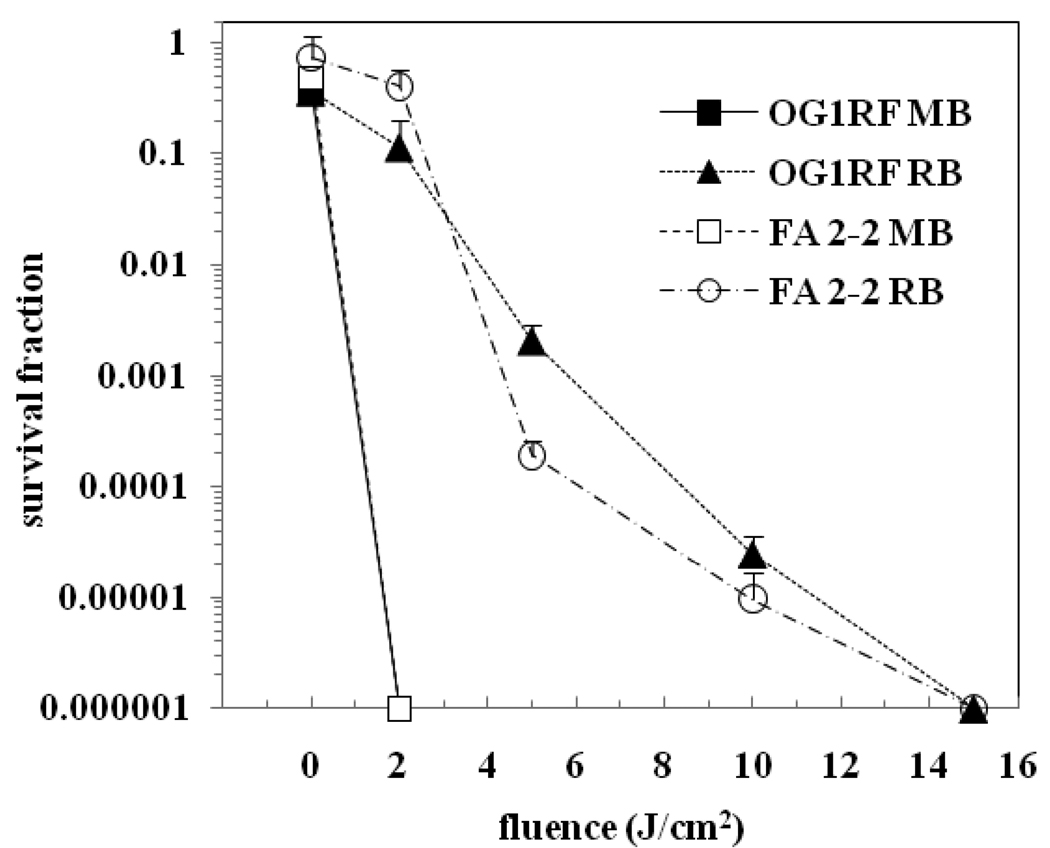
The results of aPDI experiments comparing MB and RB on E. faecalis OG1RF and E. faecalis FA 2-2 planktonic cells are shown in Figure 3. MB was found to be very effective in inactivating planktonic cells with 100% killing seen at the lowest tested fluence of 2 J/cm2 relative to the untreated controls (P < 0.001). RB on the other hand, required a fluence of 15 J/cm2 for complete killing. The effect of light alone on bacterial viability was found to be insignificant.
Figure 3.
aPDI of planktonic suspensions of E. faecalis strains OG1RF and FA 2-2. Cells were incubated with 100 µM concentration of the PS for 15 min followed by light irradiation. The survival fraction at 0 J/cm2 indicates the dark toxicity.
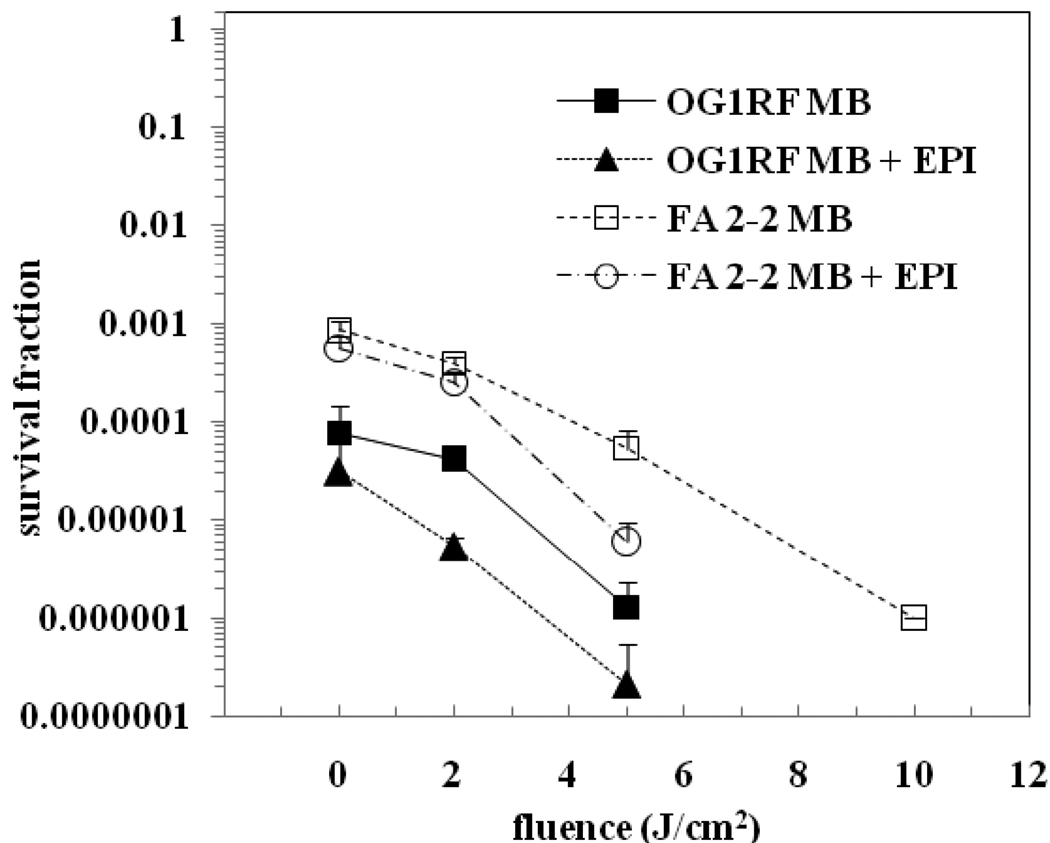
The results of the aPDI experiments on biofilm-derived cells comparing MB and a combination of MB + EPI are shown in Figure 4. It was seen that the dark toxicity of MB whether or not combined with EPI was high on these biofilm-derived cells (survival fraction at 0 J/cm2). Four logs of dark toxicity were seen for E. faecalis OG1RF biofilm-derived cells and three logs of dark toxicity for E. faecalis FA 2-2 biofilm-derived cells. Photosensitization with MB + EPI produced about 3 logs further reduction of OG1RF cells at a fluence of 5 J/cm2, while MB alone produced around 2 logs further reduction in OG1RF cells at the same fluence. Photosensitization with MB + EPI resulted in approximately 2 logs further reduction of FA 2-2 cells at 5 J/cm2, while the same fluence produced only around 1 log further reduction in FA 2-2 cells with MB. As seen with planktonic cells, the effect of light alone on bacterial viability was found to be insignificant. Interestingly, the light-mediated killing in the presence of the PS was intermediate between that seen for planktonic cells and intact biofilms. There was potentiation of bacterial killing in the presence of the EPI and it appeared that this potentiation was somewhat greater for the strain OG1RF than it was for the strain FA 2-2.
Figure 4.
aPDI of biofilm-derived cells of E. faecalis strains OG1RF and FA 2-2. Cells were incubated with 100 µM concentration of the PS for 15 min followed by light irradiation. The survival fraction at 0 J/cm2 indicates the dark toxicity.
LCSM of biofilms subjected to aPDI
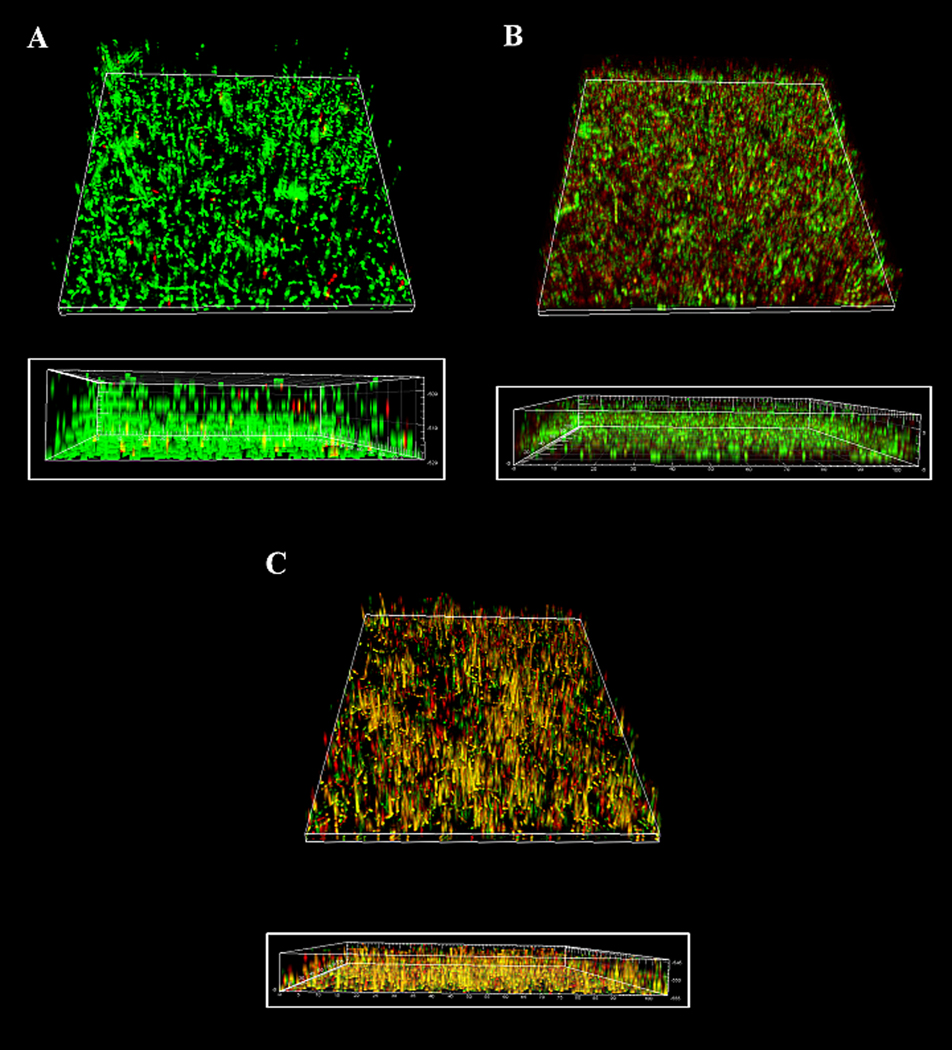
The crystal violet based quantitative assay was used to re-confirm the biofilm forming capacity of E. faecalis strains OG1RF and FA 2-2. It was evident from this assay that E. faecalis strain OG1RF formed greater amount of biofilm when compared to E. faecalis strain FA 2-2 (Figure 1). Hence, in order to observe the effect of aPDI on the biofilm structure, experiments were conducted on the strain OG1RF. The multilayered E. faecalis biofilm structure formed by OGR1F strains on the petri dishes is shown in Figure 5 A. The viable cells are indicated by green, the dead cells by red and the intermediate areas by yellow. There were both viable and dead cells distributed within the biofilm structure, with generally more viable cells on the outer surface of the biofilm, and more dead cells in the inner aspects of the biofilm. The average thickness of the control (untreated) biofilm was 22 µm. PS alone and light irradiation alone did not bring about significant inactivation of the resident bacteria or disruption of the biofilm structure (not shown). aPDI with RB as the PS did not produce substantial destruction of the biofilm structure (average thickness 15 µm), nor did it result in a considerable reduction in the number of viable cells in the biofilm (Figure 5 B). On the other hand, aPDI with MB as the PS produced a greater destruction of the biofilm structure (average thickness of 11 µm) along with increased killing of resident bacterial cells (Figure 5 C). The viable to dead cell ratio of bacteria determined from the LCSM images showed significant differences between the control group and aPDI with MB group (P < 0.001). There was also a significant difference between the aPDI with RB group and aPDI with MB group (P < 0.05). The control group showed the greatest number of viable cells while aPDI with MB resulted in the least number of viable cells along with a maximum increase in dead cells.
Figure 5.
The three-dimensional LCSM reconstruction of E. faecalis biofilms subjected to aPDI (inset shows the sagittal section). (A) The untreated biofilm (B) The biofilm incubated with 100 µM RB followed by irradiation at 40 J/cm2 (C) The biofilm incubated with 100 µM MB followed by irradiation at 40 J/cm2. (The colors represent: green = viable, red = dead, yellow = intermediate).
DISCUSSION
Present-day treatments of bacterial infections are based on compounds that aim to kill or inhibit the growth of bacteria. Two problems are recognized to be intrinsically associated with this approach: (A) the frequently observed development of resistance to antimicrobial compounds and (B) the fact that all therapeutics are considerably less effective on bacteria growing as biofilm structures when compared with free-floating planktonic cells. The use of planktonic cultures for antimicrobial testing usually gives highly effective kills that do not correlate with clinical findings (45). Compared to bacteria in suspension, the behavior of bacteria in biofilms is notably different and should be accounted for in laboratory tests (46). Bacteria in biofilms are also reported to respond differently depending on their growth phase, the concentration and the frequency of exposure to the antimicrobial agent (47), which may be more clinically relevant. This is of particular importance as evidence has accumulated over the past few years that most chronic and recalcitrant bacterial infections in endodontic infections involve biofilms. Earlier studies have highlighted the inability of antibiotic combinations at larger than minimal inhibitory concentration (MIC) or minimum bactericidal concentration (MBC) for extended period did not eradicate biofilm structure (48). Inability to completely eradicate biofilm structures in proximity to host immune cells will result in persistent infection and/or subsequent re-establishment of infection, with probably more treatment-resistant resident bacteria.
aPDI relies on the formation of ROS, mostly singlet oxygen, to kill bacteria. However, the ability of ROS generated during aPDI to disrupt biofilm structures has not been studied in detail. The process of disrupting biofilm structure by aPDI can be expected to depend upon the interaction of PS with the resident bacteria and biofilm structure. This is generally influenced by the physico-chemical characteristics of the biofilm structure, resident bacteria and PS. Consequently, experiments in this study were conducted not only to evaluate the ability of a cationic phenothiazinium dye MB (with and without the EPI) and an anionic xanthene dye RB to kill biofilm bacteria but also to disrupt biofilm structure. The comparative experiments on planktonic and biofilm-derived cells showed that the order of susceptibility to aPDI was of the order: planktonic cells > biofilm-derived cells > biofilm. It was observed from this study that aPDI with MB as the PS produced superior ability to inactivate E. faecalis strains in a biofilm state than RB.
Further, we observed that the ability to inactivate biofilm bacteria was significantly enhanced when an EPI was used along with MB. Kvist et al. reported that efflux pumps are highly active in bacterial biofilms, thus making efflux pumps attractive targets for anti-biofilm measures (49). Zhang and Mah also reported that efflux pumps are more highly expressed in biofilm cells than their planktonic counterparts (50). We have previously reported that phenothiazinium PS such as MB are substrates of efflux pumps in gram-positive species such as S. aureus, as well as gram-negative species such as Escherichia coli and Pseudomonas aeruginosa (22). Studies have shown that S. aureus principally expresses major facilitator type efflux pumps such as NorA and QacA (51). More recently, we have also shown that aPDI of S. aureus with MB could be potentiated by combination with known inhibitors of NorA such as the diphenyl urea IN271, reserpine and 5’methoxyhydnocarpin (23). E. faecalis however has been reported to express efflux pumps of the ATP-binding cassette (ABC) class rather than major facilitator class efflux pumps (52). Verapamil is a calcium channel blocker, which has been shown to also act as an inhibitor of P-glycoprotein and related multi-drug efflux pumps in cancer cells. Cancer associated efflux pumps are members of the ABC class, and verapamil has been reported to inhibit ABC efflux pumps in several species of bacteria (53).
Analysis of bacterial biofilms by LCSM yielded stacks of digital images that can be combined to give a three-dimensional view of the biofilm. The LSCM analysis showed the presence of multilayered mono-species biofilms formed by E. faecalis prior to and following aPDI with MB and RB. There was a conspicuous difference in the bacterial viability following aPDI with MB and RB compared to the untreated biofilm. It was found that aPDI with RB did not produce a substantial destruction of biofilm structure, while aPDI with MB was able to damage the biofilm structure to a greater extent. It should be noted that the CFU based aPDI experiments that were conducted on four-day old biofilms, demonstrated a significant bacterial killing with RB. However, it is important to realize that though RB can produce significant killing of E. faecalis in planktonic suspensions and four-day old biofilms, the ability to kill E. faecalis in two-week old biofilms was noticeably lesser than that of MB. The reason for this could be directly related to the EPS associated with biofilms. The basis for this explanation is a study by Gad et al. who reported that the characteristics of extracellular slime plays a significant role in determining the binding and intracellular penetration of PS that vary in charge (11). They showed that the uptake of an anionic PS was higher in slime producing strains of gram-positive bacteria than the mutants while the killing was much less. It has been suggested that the EPS associated with the biofilm could increase the binding of cationic or anionic PS molecules but at the same time could hinder their penetration to more sensitive intracellular locations. The inferior performance of anionic RB during aPDI of E. faecalis biofilms may be explained by the EPS trapping the PS on the outside of the cell due to ionic or hydrophobic interactions. In addition to this, the teichoic acids give the gram-positive cell wall an overall negative charge due to the presence of phosphodiester bonds between teichoic acid monomers and this could promote electrostatic repulsion with the negatively charged RB. Both these factors could thereby reduce the amount of PS penetrating to the plasma membrane which is known to be a major site for the damage attributed to aPDI. Therefore, although anionic RB may accumulate well within the biofilm structure, it lacks the positive charge needed to gain deeper penetration in order to produce a high magnitude bacterial killing and structural damage to the biofilm. Another reason could be that the PS trapped within the EPS could act as an optical shield by absorbing the photons and generating harmless ROS outside the cell while reducing the photons reaching the PS within the cell (11).
The present findings showed that E. faecalis biofilms exhibited significantly higher resistance to aPDI when compared to E. faecalis in suspension. The biofilms of the E. faecalis strains subjected to aPDI with cationic MB as the PS produced superior inactivation of bacteria along with significant destruction of biofilm structure when compared to anionic RB. Furthermore, the addition of EPI to MB enhanced the MB uptake by the bacterial cells, which further enhanced the efficacy of aPDI with MB to eliminate E. faecalis biofilms. These experiments highlighted the advantage of cationic phenothiazinium PS with EPI to inactivate biofilm bacteria and disrupt biofilm structure.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Disease (grant AI050875 to MRH). The authors are grateful to Eleutherios Mylonakis (Massachusetts General Hospital, Boston, MA), Frank Stermitz (Colorado State University, Fort Collins Co), Fred Ausebel (Massachusetts General Hospital, Boston, MA). The support from the Department of Restorative Dentistry, National University of Singapore and the funding from the National University of Singapore ARF Grant number R-224-000-024-112 is gratefully acknowledged.
REFERENCES
- 1.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one - photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henney JE. From the Food and Drug Administration. JAMA. 2000;283:2779. [PubMed] [Google Scholar]
- 3.Bressler NM, Bressler SB. Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciences. Invest. Ophthalmol. Vis Sci. 2000;41:624–628. [PubMed] [Google Scholar]
- 4.Boehncke WH, Elshorst-Schmidt T, Kaufmann R. Systemic photodynamic therapy is a safe and effective treatment for psoriasis. Arch Dermatol. 2000;136:271–272. doi: 10.1001/archderm.136.2.271. [DOI] [PubMed] [Google Scholar]
- 5.Trauner KB, Hasan T. Photodynamic treatment of rheumatoid and inflammatory arthritis. Photochem. Photobiol. 1996;64:740–750. doi: 10.1111/j.1751-1097.1996.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 6.Rockson SG, Lorenz DP, Cheong WF, Woodburn KW. Photoangioplasty: An emerging clinical cardiovascular role for photodynamic therapy. Circulation. 2000;102:591–596. doi: 10.1161/01.cir.102.5.591. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins MP, Buonaccorsi GA, Raphael M, Nyamekye I, McEwan JR, Bown SG, Bishop CC. Clinical study of adjuvant photodynamic therapy to reduce restenosis following femoral angioplasty. Br. J. Surg. 1999;86:1258–1263. doi: 10.1046/j.1365-2168.1999.01247.x. [DOI] [PubMed] [Google Scholar]
- 8.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J. Antimicrob. Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 9.Malik Z, Hanania J, Nitzan Y. Bactericidal effects of photoactivated porphyrins – An alternative approach to antimicrobial drugs. J. Photochem. Photobiol. B, Biol. 1990;5:281–293. doi: 10.1016/1011-1344(90)85044-w. [DOI] [PubMed] [Google Scholar]
- 10.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gad F, Zahra T, Hasan T, Hamblin MR. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 2004;48:2173–2178. doi: 10.1128/AAC.48.6.2173-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatti MA, MacRobert, Meghji S, Henderson B, Wilson M. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem. Photobiol. 1998;68:370–376. [PubMed] [Google Scholar]
- 13.Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol. Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 14.Usacheva MN, Teichert MC, Beil MA. Comparison of the Methylene Blue and Toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 2001;29:165–173. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- 15.Zeina B, Greenman J, Purcell WM, Das B. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 2001;144:274–278. doi: 10.1046/j.1365-2133.2001.04013.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill J, Wilson M, Wainwright M. Comparative antistreptococcal activity of photobactericidal agents. J. Chemother. 2003;15:329–334. doi: 10.1179/joc.2003.15.4.329. [DOI] [PubMed] [Google Scholar]
- 17.Wainwright M. The use of dyes in modern biomedicine. Biotech. Histochem. 2003;78:147–155. doi: 10.1080/10520290310001602404. [DOI] [PubMed] [Google Scholar]
- 18.Poole K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005;56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 19.Borges-Walmsley MI, Walmsley AR. The structure and function of drug pumps. Trends Microbiol. 2001;9:71–79. doi: 10.1016/s0966-842x(00)01920-x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis K. Versatile drug sensors of bacterial cells. Curr Biol. 1999;9:403–407. doi: 10.1016/s0960-9822(99)80254-1. [DOI] [PubMed] [Google Scholar]
- 21.Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002;46:3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tegos GP, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob. Agents Chemother. 2008;52:3202–3209. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prince AS. Biofilm, antimicrobial resistance, and airway infection. N. Engl. J. Med. 2002;347:1110–1111. doi: 10.1056/NEJMcibr021776. [DOI] [PubMed] [Google Scholar]
- 25.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella Pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000;44:1818–1824. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George S, Kishen A. Augmenting the Antibiofilm efficacy of Advanced Noninvasive Light activated Disinfection with emulsified oxidizer and oxygen carrier. J. Endod. 2008;34:1119–1123. doi: 10.1016/j.joen.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Fimple JL, Fontana CR, Foschi F, Ruggiero K, Song X, Pagonis TC, Tanner AC, Kent R, Doukas AG, Stashenko PP, Soukos NS. Photodynamic treatment of endodontic polymicrobial infection in vitro. J. Endod. 2008;34:728–734. doi: 10.1016/j.joen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S, Foschi F, Doucette S, Bammann LL, Fontana CR, Doukas AG, Stashenko PP. Photodynamic therapy for endodontic disinfection. J. Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Garcez AS, Ribeiro MS, Tegos GP, Núñez SC, Jorge AO, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers. Surg. Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Guerrero ML, Herrero L, Bellver M, Gadea I, Roblas RF, de Górgolas M. Nosocomial enterococcal endocarditis: a serious hazard for hospitalized patients with enterococcal bacteraemia. J. Intern. Med. 2002;252:510–515. doi: 10.1046/j.1365-2796.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- 31.Karmarkar MG, Gershom ES, Mehta PR. Enterococcal infections with special reference to phenotypic characterization & drug resistance. Indian. J. Med. Res. 2004;119:22–25. [PubMed] [Google Scholar]
- 32.Sandoe JA, Witherden IR, Cove JH, Heritage J, Wilcox MH. Correlation between enterococcal biofilm formation in vitro and medical-device-related infection potential in vivo. J. Med. Microbiol. 2003;52:547–550. doi: 10.1099/jmm.0.05201-0. [DOI] [PubMed] [Google Scholar]
- 33.Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo-Arana A, Valle J, Solano C, Arrizubieta MJ, Cucarella C, Lamata M, Amorena B, Leiva J, Penadés JR, Lasa I. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 2001;67:4538–4545. doi: 10.1128/AEM.67.10.4538-4545.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 2004;72:3658–3663. doi: 10.1128/IAI.72.6.3658-3663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004;72:6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hancock LE, Perego M. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 2004;186:5629–5639. doi: 10.1128/JB.186.17.5629-5639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillai SK, Sakoulas G, Eliopoulos GM, Moellering RC, Jr, Murray BE, Inouye RT. Effects of glucose on fsr-mediated biofilm formation in Enterococcus faecalis. J. Infect. Dis. 2004;190:967–970. doi: 10.1086/423139. [DOI] [PubMed] [Google Scholar]
- 39.Kristich CJ, Li YH, Cvitkovitch DG, Dunny GM. Esp-independent biofilm formation by Enterococcus faecalis. J. Bacteriol. 2004;186:154–163. doi: 10.1128/JB.186.1.154-163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molander A, Reit C, Dahlen G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 1998;31:1–7. [PubMed] [Google Scholar]
- 41.Dahlen G, Samuelsson W, Molander A, Reit C. Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral. Microbiol. Immunol. 2000;15:309–312. doi: 10.1034/j.1399-302x.2000.150507.x. [DOI] [PubMed] [Google Scholar]
- 42.Portenier I, Tuomos MT, Waltimo T, Haapasalo M. Enterococcus faecalis- the root canal survivor and 'star' in post-treatment disease. Endodontic Topics. 2003;6:135. [Google Scholar]
- 43.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 2004;72:3658–3663. doi: 10.1128/IAI.72.6.3658-3663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 45.Thrower Y, Pinney RJ, Wilson M. Susceptibilities of Actinobacillus actinomycetemcomitans biofilms to oral antiseptics. J. Med. Microbiol. 1997;46:425–429. doi: 10.1099/00222615-46-5-425. [DOI] [PubMed] [Google Scholar]
- 46.Wilson M. Susceptibility of oral bacterial biofilms to antimicrobial agents. J. Med. Microbiol. 1996;44:79–87. doi: 10.1099/00222615-44-2-79. [DOI] [PubMed] [Google Scholar]
- 47.Desai M, Bühler T, Weller PH, Brown MR. Increasing resistance of planktonic and biofilm cultures of Burkholderia cepacia to ciprofloxacin and ceftazidime during exponential growth. J. Antimicrob. Chemother. 1998;42:153–160. doi: 10.1093/jac/42.2.153. [DOI] [PubMed] [Google Scholar]
- 48.Dunne WM, Jr, Mason EO, Jr, Kaplan SL. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother. 1993;37:2522–2526. doi: 10.1128/aac.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kvist M, Hancock V, Klemm P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl. Environ. Microbiol. 2008;74:7376–7382. doi: 10.1128/AEM.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Mah TF. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 2008;190:4447–4452. doi: 10.1128/JB.01655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonas BM, Murray BE, Weinstock GM. Characterization of emeA, a NorA homolog and multidrug resistance efflux pump, in Enterococcus faecalis. Antimicrob. Agents Chemother. 45:3574–3579. doi: 10.1128/AAC.45.12.3574-3579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higgins CF. ABC transporters: from microorganisms to man. Annu. Rev. Cell. Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 53.Safa AR. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]