Abstract
There is a well-established connection between smoking and depression, with depressed individuals over-represented among smokers and ex-smokers often experiencing increased depressive symptoms immediately after quitting. Nicotine in tobacco binds, activates and desensitizes nicotinic acetylcholine receptors (nAChRs), but it is not known whether activation or desensitization is more important for nicotine’s effects on depressive symptoms. In this article, we review the hypothesis that blockade rather than activation of neuronal nAChRs might be important for the effects of nicotinic agents on depressive symptoms based on clinical and preclinical studies of nicotinic drugs. The endogenous neurotransmitter for nAChRs is acetylcholine, and the effects of nicotine on depression-like behaviors support the idea that dysregulation of the cholinergic system might contribute to the etiology of major depressive disorder. Thus, pharmacological agents that limit acetylcholine signaling through neuronal nAChRs might be promising for the development of novel antidepressant medications.
Keywords: nicotinic acetylcholine receptors, smoking, major depressive disorder, antidepressant medications, mecamylamine, varenicline, cytisine
Smoking and Depression: self-medication or a vicious cycle?
The high co-morbidity between smoking and depression is more than just an anecdote. The connection between smoking and depression has been well established in the literature, and estimates of the prevalence of nicotine dependence in patients with major depression range from 50–60%, compared with about 25% in the general population 1. Furthermore, smokers with a history of major depression are 2–3 times more likely to have failed quit attempts compared with non-depressed smokers 2. Smoking cessation can lead to the onset of depressive symptoms in smokers with a history of depression 1, which suggests that some aspect of smoking, potentially nicotine (see Table 1 for structure) intake, has an effect on mood. Clinical studies have shown that a nicotine patch can reduce symptoms of depression, even in non-smoking, depressed patients 3, 4. Interestingly, chronic administration of low levels of nicotine (as delivered by the nicotine patch) is thought to desensitize, rather than activate, nicotinic acetylcholine receptors (nAChRs) 5, 6, providing a hint that blockade of nAChRs might be important for the effects of nicotinic agents on depressive symptoms. Animal studies have also demonstrated that nicotine can have antidepressant-like effects in rodent models of depression-like behavior such as the learned helplessness 7 and forced swim 8, 9 tests. Although it is possible that nicotine is activating nAChRs in these studies, the chronic regimens of nicotine administration used in those studies could also result in desensitization or inactivation of nAChRs 6, 10. Finally, antidepressants such as bupropion and nortriptyline have been used successfully for smoking cessation 11, 12, suggesting that medication of depressive symptoms aids quitting for some smokers, or that antidepressants might share common properties with other therapies used to treat smokers, such as the nicotine patch. Consistent with this possibility, comprehensive reviews on the subject have illustrated that many classes of clinically effective antidepressants can also act as non-competitive inhibitors of nAChRs 13. Because the endogenous neurotransmitter for nAChRs is acetylcholine, the effects of nicotine on depression-like behaviors provides evidence that dysregulation of the cholinergic system might contribute to the etiology of major depressive disorder 13.
Table 1.
structures of nicotinic agents that have been used successfully in rodent models of antidepressant efficacy.
| Common name | Systematic name (IUPAC) | Structure |
|---|---|---|
| Nicotine | 3-[(2S)-1-methylpyrrolidin-2-yl]pyridine | 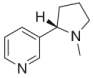 |
| Mecamylamine | (2R)-N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine | 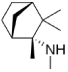 |
| Cytisine | (1R,5S)- 1,2,3,4,5,6- hexahydro- 1,5-methano-8H- pyrido[1,2a][1,5] diazocin-8-one |
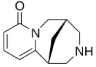 |
| 3-pyridylyl-cytisine | (1R,5S)-1,2,3,4,5,6-hexahydro-9-(3-pyridinyl)-1,5-methano-8H- pyrido[1,2-a][1,5]diazocin-8-one |
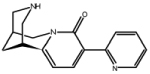 |
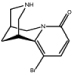
| 5-bromo-cytisine | (1R,5S)-11-bromo-1,2,3,4,5,6-hexahydro-1,5-methano-8H- pyrido[1,2-a][1,5]diazocin-8-one |
 |
| Varenicline | 7,8,9,10-tetrahydro- 6,10-methano- 6H–pyrazino (2,3-h)(3) benzazepine |
|
| Sazetidine-A | 6-[5-[(2S)-2-Azetidinylmethoxy]-3-pyridinyl]-5-hexyn-1-ol | 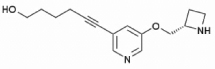 |
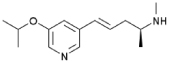
| Isopronicline | (2S,4E)-5-(5-isopropoxypyridin-3-yl)-N-methylpent-4-en-2- amine |
 |
| PNU-282987 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide | 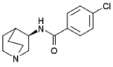 |
The hypercholinergic hypothesis of depression
The hypothesis that too much acetylcholine might lead to depression was put forward more than three decades ago by Janowsky and colleagues, who suggested that depression is associated with hyperactivation of the cholinergic system and decreased activity of the noradrenergic system 14. This hypothesis is consistent with early observations that organophosphate poisoning (which leads to profound inhibition of acetylcholinesterase (AChE) and therefore elevates acetylcholine levels throughout the brain and body) in humans leads to depression-like symptoms, and that orchardists who work with these compounds appeared to have higher rates of depression 15. Following up on these observations, Janowsky and colleagues showed that human subjects with an underlying affective disorder treated with the blood-brain penetrant AChE inhibitor physostigmine (but not the peripherally active AChE inhibitor neostigmine) showed decreased mania and increased depressive symptoms 16. Similarly, when physostigmine was infused at high doses in patients with no apparent neuropsychiatric illness, subjects reported symptoms of depression, although with great variability 17.
Preclinical studies provide further support for the idea that alterations of the cholinergic system can lead to depressive symptoms. The Flinders Sensitive Line (FSL) of rats was selectively bred for increased sensitivity to the centrally active AChE inhibitor diisopropylfluorophosphonate, resulting in increased acetylcholine levels in the brains of these animals 18. These rats also show increased numbers and function of high affinity nAChRs 19, 20. In addition, FSL rats exhibit several depression-like endophenotypes, including reduced locomotor activity, reduced body weight, increased REM sleep and cognitive deficits 21. By contrast, rats of the control Flinders Resistant Line (FRL) do not show these behavioral responses. FSL rats were tested in models of depression-like behavior and showed increased anhedonia in the chronic mild stress model of depression, and increased immobility in the forced swim model of antidepressant efficacy 22–24. These experiments illustrate that manipulations of the cholinergic system can lead to depression-like behavior. Other studies have shown the converse relationship as well: the induction of depression-like behavior by chronic inescapable footshock and swim stress in rodents induces hypersensitivity of the cholinergic system 25. Taken together, these observations in human subjects and in animal models strongly suggest that hyperactivity of the cholinergic system can contribute to the pathophysiology of depression. More recently, however, fewer studies have investigated the potential link between acetylcholine and mood disorders, probably because most efforts have focused on studies of monoamine neurotransmission, which has become the dominant hypothesis since the development of selective serotonin reuptake inhibitors (SSRIs) to treat depression. Though SSRIs are safe and are effective for a significant subset of depressed patients, up to 50% of patients are unresponsive to all available treatments 26 and new treatments are critically needed. Thus, it seems important to revisit the cholinergic hypothesis of depression.
Signaling through cholinergic receptors modulates affective state
The receptors for acetylcholine can be divided into two broad classes: nAChRs and muscarinic (mAChRs). If alterations of acetylcholine levels lead to depressive symptoms, a change in the activity of nAChRs and mAChRs is likely to be the mechanism transducing the altered acetylcholine signal. Authors have proposed that mAChRs are involved in mood control because scopolamine is able to induce rapid-onset antidepressant effects in clinical trials 27, 28. In addition, mAChRs were believed to be the crucial downstream effectors of AChE-induced depressive behavior 14. To date, however, only a few reports have explored the role of these receptors in mood regulation (reviewed in 29, 30) and further data are needed. We will focus in this review on the other major subclass of acetylcholine receptors: the nAChRs.
As mentioned above, nAChRs are the primary target for smoked nicotine in the brain. A self-medication hypothesis has been proposed to explain the high co-morbidity between smoking and depression 31; however, smoked nicotine is not a highly effective antidepressant, because if it were, the prevalence of depressive symptoms would be expected to be lower in smokers than in non-smokers. Though smokers report that they are more likely to smoke when they experience depressive symptoms 32, immediate negative affect symptoms do not predict relapse during smoking cessation 33 and overall, smoking cessation leads to reduced stress 34. Similarly, a higher rate of suicide has been observed in smokers, with heavier smokers more likely to attempt suicide than lighter smokers 35–37. It has been proposed that withdrawal symptoms induce cycles of stress and negative affect that are alleviated by smoking a cigarette 34, and that reports of depression in recently abstinent smokers are due to withdrawal symptoms 38. Although it is possible that activation of nAChRs can be antidepressant, another possibility that is consistent with all these observations is that smokers self-medicate depressive symptoms by desensitizing their nAChRs. It has been reported that one puff of a cigarette is enough to saturate the high affinity nAChRs (those containing the β2 nAChR subunit) in the human brain 39 and it is known that after nicotine binding there is a long-term decrease in nAChR activity due to desensitization 10, 40. Thus, the brief activation caused by smoking a cigarette can lead to immediate increase in nAChR activity. Although it is still controversial, it is possible that this increase could lead to affective symptoms. Conversely, the long-term decrease in nAChR activity as a result of desensitization might result in the alleviation of depressive symptoms. Finally, nAChRs are upregulated as a result of smoking, and this increase in nAChR number is maintained for at least 2 weeks following smoking cessation 41 and may contribute to depressive symptoms following abstinence.
Consistent with the possibility that decreased activity of nAChRs might be an antidepressant in human smokers, knockout mice lacking the high affinity subclass of nAChRs (those lacking the β2 subunit) show decreased baseline immobility in the tail suspension and forced swims tests 42, suggesting that decreased signaling through nAChRs may result in an antidepressant-like phenotype. These studies have also shown that mice lacking high-affinity nAChRs are insensitive to the tricyclic antidepressant amitriptyline, suggesting that alterations of nicotinic signaling can play a role in the response to classical antidepressants. Interestingly, chronic nicotine administration, which increases the number of high-affinity (α4/β2) nAChRs results in increased response to classical antidepressants 43.
Nicotinic blockers and partial agonists have antidepressant-like effects in animal models
If smoking relieves depressive symptoms due to desensitization of nAChRs, then it should be possible to test this pharmacologically by determining whether drugs that decrease nAChR function or interfere with acetylcholine signaling through these receptors have antidepressant effects. In support of this hypothesis, preclinical studies have demonstrated that the non-selective, non-competitive nicotinic antagonist mecamylamine (see Table 1 for structure) has antidepressant-like effects in mice in several common tests of antidepressant efficacy, including the tail suspension and forced swim tests 42, 44, 45. The effects of mecamylamine depend on the expression of nAChRs because they are not observed in knockout mice lacking either the high-affinity (those lacking the β2 subunit) or the homomeric (those lacking the α7 subunit) nAChRs 44. In addition, antidepressant-like effects are also seen with a competitive antagonist of high-affinity nAChRs (dihydro-β-erythroidine), but are not seen with a competitive antagonist that does not cross the blood-brain barrier (hexamethonium) 44.
In addition to studies with full antagonists of nAChRs such as mecamylamine and dihydro-β-erythroidine, studies with partial agonists of nAChRs have also demonstrated antidepressant-like effects in mice. Partial agonists are complex because they increase activity of nAChRs submaximally on their own, but they limit the ability of acetylcholine to activate nAChRs. Thus, if elevated acetylcholine contributes to depression-like symptoms, the effect of a nicotinic partial agonist might be to act as an antidepressant in individuals with high acetylcholine levels but to increase depression-like symptoms in individuals with low acetylcholine levels.
Cytisine (see Table 1 for structure) is a nicotinic partial agonist that has low efficacy at high-affinity (α4/β2) nAChRs but which is a full agonist at ganglionic (α3/β4) and homomeric (α7) nAChRs 46, 47. Cytisine has been used in Eastern Europe as a smoking cessation aid since the 1960s with limited but significant effects 48. Similarly, varenicline (Chantix ®; see Table 1 for structure) is a partial agonist at high-affinity (α4/β2) nAChRs with higher efficacy than cytisine 49, and also an agonist at ganglionic (α3/β4) and homomeric (α7) 50 nAChRs that is currently used for smoking cessation in the US and several other countries. Both medications are thought to aid in smoking cessation by activating nAChRs enough to prevent withdrawal while limiting the rewarding effect of nicotine 51. Both cytisine 52 and varenicline 53 have antidepressant-like effects in mice that are similar to the effects of the full nicotinic antagonist mecamylamine. Because both cytisine and varenicline are agonists of ganglionic (α3/β4) and homomeric (α7) nAChRs, it is possible that these agonist properties are involved in the antidepressant-like properties of these compounds; however, novel derivatives of cytisine such as 3-pyridylyl cytisine and 5-bromo-cytisine (see Table 1 for structures) that are low-efficacy partial agonists of high-affinity (α4/β2) nAChRs but which have little or no agonist effects at ganglionic (α3/β4) and homomeric (α7) nAChRs also have antidepressant-like effects in mice 54. Similarly, sazetidine-A, a partial agonist of α4β2 nAChRs with high affinity for non-resting conformations of the receptor which can potently desensitize the α4β2 nAChR subtype 55, and the nicotinic partial agonist isopronicline 56 (see Table 1 for structures), have antidepressant-like properties in mice 57, suggesting that it may be the ability of these compounds to interfere with acetylcholine signaling, rather than their ability to act as agonists at nAChRs, that is most important for their antidepressant properties.
Antidepressant effects of nicotinic blockers and partial agonists in human subjects
Mecamylamine (Inversine®) is approved for use in human subjects and was originally used to treat hypertension. Interest in mecamylamine as a potential therapy for affective disorders was first raised when it was used in subjects with Tourette’s disorder and showed significant ability to decrease depression-like symptoms in these patients 58, 59. There are now several published clinical trials of nicotinic blockers or partial agonists in human subjects with major depressive disorder. In Tourette’s patients with co-morbid bipolar disorder or major depressive disorder, mecamylamine alone was effective in reducing depressive symptoms 60, 61. The other clinical trials have involved patients already on an SSRI and have added mecamylamine 62–64; http://clinicaltrials.gov/ct2/show/NCT00593879 ClinicalTrials.gov Identifier NCT00593879; http://www.targacept.com/wt/page/tc_5214) or varenicline 65 as a second medication. These trials have shown that nicotinic compounds are effective antidepressants in patients who have been unresponsive to an SSRI. Thus, the evidence suggests that limiting acetylcholine signaling through nAChRs is a reasonable strategy for developing novel antidepressant medications.
Varenicline and suicidality
Numerous recent reports have suggested that some smokers taking varenicline experience an increase in suicidal ideation. This has lead to a black box warning by the FDA and has resulted in considerable concern by patients and physicians. This seems paradoxical if varenicline also has antidepressant effects 65. A recent report on patients in primary care in the UK suggests that there was no increase in suicidal ideation in a cohort of more than 80,000 smokers taking varenicline as compared with smokers using nicotine replacement or other medications to quit smoking 66; however, this study was not sufficiently powered to detect a change of twofold, so it could easily miss individuals with particular sensitivity to cholinergic modulation who experience an increase in suicidal ideation. Indeed, this study found that subjects taking varenicline actually had a decrease in antidepressants prescribed during the follow up period, which might suggest that there was an overall decrease in depression symptoms in this sample. It has also been suggested that smoking cessation itself could cause the increase in suicidal ideation, which would be consistent with findings that quitting smoking can provoke an episode of depression 67. Further, several studies have suggested that common genetic factors link smoking and depression 68, which would result in more depressed individuals using smoking cessation aids in comparison to non-smokers, resulting in a false correlation between medication use and suicidality. Alternatively, genetic factors could make smokers prone to pro-depressant effects of varenicline. Because varenicline is a high-efficacy partial agonist, if increased nAChR signaling can result in depressive symptoms, a subset of individuals might achieve sufficient agonism of nAChRs on the medication to result in the type of depressive symptoms observed in individuals given the AChE antagonist physostigmine. As noted above, the results of physostigmine treatment are variable and are most obvious in patients with affective disorders 16; therefore, patients with sensitivity to cholinergic modulation might experience depressive symptoms from taking varenicline due to its agonist properties. Another possibility is that the ability of varenicline to activate a number of nAChRs results in increased psychiatric symptoms. For example, agonism of ganglionic (α3β4) nAChRs could lead to increased anxiety 69 as has been seen with cytisine 52. Clinical trials are underway in populations with psychiatric disorders that will clarify the relationship between varenicline and affective disorders. Future clinical trials should use a well-validated and treatment sensitive measure of suicide tracking to provide accurate data to assess the effects of novel nicotinic drugs on suicidality 70, 71.
Augmentation strategies and future clinical practice
Both rodent 42, 53 and human 62, 65 studies show that mecamylamine and varenicline can induce antidepressant-like effects in subjects administered an ineffective dose of a classical antidepressant such as an SSRI. Indeed, in a review in 1986, Dilsaver noted that the cholinergic hypothesis should more accurately be termed the cholinergic-monoaminergic interaction theory, because any manipulation of one neurotransmitter system in the brain has extensive consequences on a large number of other systems as well 72. Therefore, the future of antidepressant treatment might be the use of augmentation strategies in patients who are non-responsive to monotherapy. These data further suggest that mood and antidepressant modulation by nicotinic compounds is not simply unidirectional, that both inhibition and activation of nAChRs may result in antidepressant effects under different circumstances, and that the effects of nicotinic compounds on different receptors, brain areas and neurotransmitter systems will result in differing outcomes in individuals with different levels of stress or depressive symptoms63.
As noted above, both preclinical 42, 53 and clinical 62–64 studies have shown that the antidepressant effects of subthreshold or ineffective doses of SSRIs can be enhanced by nicotinic antagonists or partial agonists; however the nature of the interaction between SSRIs and nicotinic drugs is still not entirely clear and several non-exclusive mechanisms for this interaction are conceivable. First, as mentioned previously, most antidepressants have been shown to act as nicotinic antagonists in the nanomolar range 13 and therefore the interaction between classical antidepressants and nicotinic drugs could occur at the receptor level as a result of both compounds blocking nAChR. This might also explain why several classes of antidepressants can aid in smoking cessation and can potentiate the effects of other smoking cessation therapies 73. Another interaction probably also occurs at the system level, because both mecamylamine and nicotine can potentiate serotonin neurotransmission in the hippocampus, and therefore might improve the efficacy of SSRIs 74. Further clinical trials are necessary to determine whether nicotinic agents will be useful antidepressant compounds on their own, or whether combination with SSRIs or other monoamine-based therapies will be more clinically efficacious.
Activation or desensitization? An ongoing controversy in modulation of mood by smoking
Although the primary hypothesis explored in this review is that decreasing endogenous acetylcholine signaling through nAChRs can be antidepressant, there is still controversy as to whether inhibition or activation (or both) of nAChRs results in antidepressant effects. For example, it should be noted that mecamylamine does not show antidepressant-like properties in FSL rats and that it can block the antidepressant-like effects of nicotine in the forced swim test in rats 19. In addition, mecamylamine is used to induce withdrawal following chronic nicotine exposure and the resulting withdrawal syndrome is characterized by an elevated reward thresholds in rats 75. With respect to interactions with monoamine systems, in one study nicotine, but not mecamylamine, could potentiate the effects of citalopram and reboxetine, two monoamine-reuptake inhibitor drugs acting on serotonin and norepinephrine, respectively 76. Furthermore, because partial agonists can, by definition, partially activate nAChRs directly, as well as decreasing signaling of acetylcholine in vivo, studies showing antidepressant-like effects of nicotinic partial agonists could result from either activation or inhibition of nicotinic signaling.
Although it is not yet possible to reconcile these disparate findings, it is possible that rats, like human subjects without affective disorders 16, are not as sensitive, or have a highly variable response, to manipulations using cholinergic agents. In addition, it is possible that the depression-like symptoms experienced during acute abstinence from nicotine and measured with the intracranial self-stimulation paradigm are not the same as those measured in tests of antidepressant-efficacy such as the forced swim and tail suspension tests. Further, both activation and inhibition of nAChRs may have antidepressant effects through distinct mechanisms 77 or through disctinct nAChR subtypes. We have focused in this review on the high affinity α4β2 nAChRs, but the nicotinic partial agonist varenicline is also a full agonist at α7 nAChRs 50, the α7 agonist PNU-282987 (see Table 1 for structure) has antidepressant-like properties 45 and α7 knockout mice are not responsive to the antidepressant-like effects of mecamylamine 44. Thus, activation of α7 nAChRs but inhibition of α4β2 nAChRs may contribute to the antidepressant effects of nicotine. Further, varenicline is also a full agonist at 5-HT3 receptors 78, which are implicated in mood regulation 79, so the effects of varenicline in human depressed patients are likely to be complex.
It seems probable that interfering with acetylcholine signaling will have multiple effects on brain systems involved in affective behavior, with blockade or desensitization of nAChRs on neurons in some pathways improving mood, while activation of nAChRs on different subsets of neurons contributes to mood regulation, adding complexity to the cholinergic hypothesis of depression. Thus, it is clearly simplistic to state that inhibition or activation of nAChRs is antidepressant in all circumstances, and further work is necessary to determine which patients might benefit from treatment with nicotinic antagonists or very low efficacy partial agonists, and whether other patients might benefit from nicotinic agonists.
Conclusion
Numerous clinical and preclinical studies have now established that decreasing acetylcholine transmission at specific nAChRs can positively affect mood, although the data also suggest that a fine balance between receptor activation and desensitization is required to yield relevant antidepressant-like effects 10. Though the exact neurobiological basis for the antidepressant-like effects of nicotinic agents remains to be clarified, alteration of nAChR function alone or in combination with other monoamine-based antidepressants represents a new avenue for treatment of affective disorders. Nicotinic antagonists and partial agonists have already been developed with pharmacological profiles that make them potential candidates to improve depression-like symptoms, and some are already used for other indications including smoking cessation. If the side effects are acceptable, the results from preclinical studies and current ongoing clinical trials further suggest that currently approved nicotinic compounds could provide new alternatives in patients showing poor response to more classical antidepressants.
ACKNOWLEDGEMENTS
This work was supported by the State of Connecticut, Department of Mental Health and Addiction Services and NIH grants MH77681 and DA00436.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report that Yale University and the University of Bonn have a patent on novel cytisine derivatives for use as potential antidepressant medications.
REFERENCES
- 1.Glassman AH, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- 2.Covey LS, et al. Cigarette smoking and major depression. J Addictive Diseases. 1998;17:35–46. doi: 10.1300/J069v17n01_04. [DOI] [PubMed] [Google Scholar]
- 3.Salin-Pascual RJ, et al. Effects of transdermal nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology. 1995;121:476–479. doi: 10.1007/BF02246496. [DOI] [PubMed] [Google Scholar]
- 4.Salin-Pascual RJ, et al. Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression. J Clin Psychiatry. 1996;57:387–389. [PubMed] [Google Scholar]
- 5.Reitstetter R, et al. Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther. 1999;289:656–660. [PubMed] [Google Scholar]
- 6.Pidoplichko VI, et al. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 7.Semba J, et al. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- 8.Djuric VJ, et al. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav. 1999;67:533–537. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- 9.Tizabi Y, et al. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacologia. 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- 10.Picciotto M, et al. It is not "either/or": Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall SM, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 12.Hurt RD, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 13.Shytle RD, et al. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 14.Janowsky DS, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 15.Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet. 1961;1:1371–1374. doi: 10.1016/s0140-6736(61)92004-9. [DOI] [PubMed] [Google Scholar]
- 16.Janowsky DS, et al. Acetylcholine and depression. Psychosomatic Medicine. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Risch SC, et al. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1:186–192. doi: 10.1097/00004714-198107000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Overstreet DH, Russell RW. Selective breeding for diisopropyl fluorophosphate-sensitivity: behavioural effects of cholinergic agonists and antagonists. Psychopharmacology. 1982;78:150–155. doi: 10.1007/BF00432254. [DOI] [PubMed] [Google Scholar]
- 19.Tizabi Y, et al. Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000;66:73–77. doi: 10.1016/s0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 20.Auta J, et al. Expression and function of striatal nAChRs differ in the flinders sensitive (FSL) and resistant (FRL) rat lines. Neuropharmacology. 2000;39:2624–2631. doi: 10.1016/s0028-3908(00)00082-4. [DOI] [PubMed] [Google Scholar]
- 21.Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 22.Overstreet DH. Selective breeding for increased cholinergic function: development of a new animal model of depression. Biological Psychiatry. 1986;21:49–58. doi: 10.1016/0006-3223(86)90007-7. [DOI] [PubMed] [Google Scholar]
- 23.Overstreet DH, et al. Impaired active avoidance responding in rats selectively bred for increased cholinergic function. Physiology & Behavior. 1990;47:787–788. doi: 10.1016/0031-9384(90)90097-n. [DOI] [PubMed] [Google Scholar]
- 24.Pucilowski O, et al. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiology & Behavior. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- 25.Dilsaver SC, et al. Stress induces supersensitivity of a cholinergic system in rats. Biological Psychiatry. 1986;21:1093–1096. doi: 10.1016/0006-3223(86)90294-5. [DOI] [PubMed] [Google Scholar]
- 26.Ruhe HG, et al. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry. 2006;67:1836–1855. doi: 10.4088/jcp.v67n1203. [DOI] [PubMed] [Google Scholar]
- 27.Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bymaster FP, et al. Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. J Pharm Exp Therap. 1993;267:16–24. [PubMed] [Google Scholar]
- 30.Howland RH. The antidepressant effects of anticholinergic drugs. J Psychosocial Nursing Mental Health Serv. 2009;47:17–20. doi: 10.3928/02793695-20090508-01. [DOI] [PubMed] [Google Scholar]
- 31.Stefanis C, Kokkevi A. Depression and drug use. Psychopathology. 1986;19:124–131. doi: 10.1159/000285143. [DOI] [PubMed] [Google Scholar]
- 32.Schleicher HE, et al. The role of depression and negative affect regulation expectancies in tobacco smoking among college students. Journal of American College Health. 2009;57:507–512. doi: 10.3200/JACH.57.5.507-512. [DOI] [PubMed] [Google Scholar]
- 33.Shiffman S, et al. Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology. 2006;184:608–618. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- 34.Parrott AC. Smoking cessation leads to reduced stress, but why? Int J Addict. 1995;30:1509–1516. doi: 10.3109/10826089509055846. [DOI] [PubMed] [Google Scholar]
- 35.Tanskanen A, et al. Smoking and suicidality among psychiatric patients. Am J Psychiatry. 1998;155:129–130. doi: 10.1176/ajp.155.1.129. [DOI] [PubMed] [Google Scholar]
- 36.Hemenway D, et al. Smoking and suicide among nurses. Am J Public Health. 1993;83:249–251. doi: 10.2105/ajph.83.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller M, et al. Cigarette smoking and suicide: a prospective study of 300,000 male active-duty Army soldiers. Am J Epidemiology. 2000;151:1060–1063. doi: 10.1093/oxfordjournals.aje.a010148. [DOI] [PubMed] [Google Scholar]
- 38.Markou A, et al. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 39.Brody AL, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lester RAJ. Activation and desensitization of heteromeric neuronal nicotinic receptors: implications for non-synaptic transmission. Bioorganic & Medicinal Chemistry Letters. 2004;14:1897–1900. doi: 10.1016/j.bmcl.2004.02.081. [DOI] [PubMed] [Google Scholar]
- 41.Staley JK, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caldarone BJ, et al. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Andreasen JT, et al. Chronic oral nicotine increases brain [3H]epibatidine binding and responsiveness to antidepressant drugs, but not nicotine, in the mouse forced swim test. Psychopharmacology. 2009;205:517–528. doi: 10.1007/s00213-009-1560-1. [DOI] [PubMed] [Google Scholar]
- 44.Rabenstein RL, et al. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild type but not β2 or α7 nicotinic acetylcholine receptor knockout mice. Psychopharmacology. 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- 45.Andreasen JT, et al. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacology. 2009;23:797–804. doi: 10.1177/0269881108091587. [DOI] [PubMed] [Google Scholar]
- 46.Papke RL, Heinemann SF. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol Pharmacol. 1994;45:142–149. [PubMed] [Google Scholar]
- 47.Papke RL, Porter Papke JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Etter J-F. Cytisine for smoking cessation: a literature review and a meta-analysis. Arch Int Med. 2006;166:1553–1559. doi: 10.1001/archinte.166.15.1553. [DOI] [PubMed] [Google Scholar]
- 49.Rollema H, et al. Pharmacological profile of the alpha(4)beta(2) nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Mihalak KB, et al. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 51.Coe JW, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 52.Mineur YS, et al. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rollema H, et al. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. Eur J Pharmacol. 2009;605:114–116. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mineur YS, et al. Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Therap. 2009;329:377–386. doi: 10.1124/jpet.108.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao Y, et al. Sazetidine-A, a novel ligand that desensitizes alpha4 beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- 56.Gatto GJ, et al. TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects. CNS Drug Reviews. 2004;10:147–166. doi: 10.1111/j.1527-3458.2004.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner JR, et al. Nicotinic partial agonists varenicline and sazetidine-a have differential effects on affective behavior. J Pharm Exp Ther. 2010;334:665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silver AA, et al. Multicenter, double-blind, placebo-controlled study of mecamylamine monotherapy for Tourette's disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:1103–1110. doi: 10.1097/00004583-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 59.Silver AA, et al. Mecamylamine in Tourette's syndrome: a two-year retrospective case study. J Child Adol Psychopharmacol. 2000;10:59–68. doi: 10.1089/cap.2000.10.59. [DOI] [PubMed] [Google Scholar]
- 60.Shytle RD, et al. Neuronal nicotinic receptor inhibition for treating mood disorders: preliminary controlled evidence with mecamylamine. Depression & Anxiety. 2002;16:89–92. doi: 10.1002/da.10035. [DOI] [PubMed] [Google Scholar]
- 61.Shytle RD, et al. Comorbid bipolar disorder in Tourette's syndrome responds to the nicotinic receptor antagonist mecamylamine (Inversine) Biological Psychiatry. 2000;48:1028–1031. doi: 10.1016/s0006-3223(00)00945-8. [DOI] [PubMed] [Google Scholar]
- 62.George TP, et al. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: A preliminary study. J Clin Psychopharm. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- 63.Dunbar GC, et al. Mecamylamine in the treatment of depressed patients who were inadequate responders to citalopram first-line therapy; a double-blind placebo controlled add-on study. J Psychopharmacology. 2007;21:A40. [Google Scholar]
- 64.Dunbar G. Positive effects of the nicotinic channel blocker TC-5214 as augmentation treatment in patients with major depressive disorder who are inadequate responders to a first-line SSRI; nAChR2009 satellite meeting of the 39th annual meeting of the Society for Neuroscience; 2009. http://www.faqs.org/sec-filings/091016/TARGACEPT-INC_8-K/dex991.htm. [Google Scholar]
- 65.Philip NS, et al. Varenicline Augmentation in Depressed Smokers: An 8-Week, Open-Label Study. J Clin Psychiatry. 2009;70:1026–1031. doi: 10.4088/jcp.08m04441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunnell D, et al. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ. 2009;339:b3805. doi: 10.1136/bmj.b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Covey LS, et al. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990;31:350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- 68.Kendler KS, et al. Smoking and major depression. A causal analysis. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 69.Salas R, et al. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coric V, et al. Sheehan Suicidality Tracking Scale (Sheehan-STS): Preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry. 2009;6:26–31. [PMC free article] [PubMed] [Google Scholar]
- 71.Meyer R, et al. Suicidality and risk of suicide--definition, drug safety concerns, and a necessary target for drug development: a brief report. J Clin Psychiatry. 2010;71:1040–1046. doi: 10.4088/JCP.10cs06070ablu. [DOI] [PubMed] [Google Scholar]
- 72.Dilsaver SC. Cholinergic-monoaminergic interaction in the pathophysiology of the affective disorders? Int Clin Psychopharmacol. 1986;1:181–198. doi: 10.1097/00004850-198607000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Hughes JR, et al. Antidepressants for smoking cessation. [update of Cochrane Database Syst Rev. 2002;(2): CD000031] Cochrane Database of Systematic Reviews [computer file] 2000 doi: 10.1002/14651858.CD000031. CD000031. [DOI] [PubMed] [Google Scholar]
- 74.Kenny PJ, et al. Evidence for a complex influence of nicotinic acetylcholine receptors on hippocampal serotonin release. J Neurochem. 2000;75:2409–2414. doi: 10.1046/j.1471-4159.2000.0752409.x. [DOI] [PubMed] [Google Scholar]
- 75.Epping-Jordan MP, et al. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 76.Andreasen JT, Redrobe JP. Nicotine, but not mecamylamine, enhances antidepressant-like effects of citalopram and reboxetine in the mouse forced swim and tail suspension tests. Behav Brain Res. 2009;197:150–156. doi: 10.1016/j.bbr.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacology. 2009;20:286–295. doi: 10.1097/FBP.0b013e32832c713e. [DOI] [PubMed] [Google Scholar]
- 78.Rollema H, et al. Varenicline overdose in a teenager--a clinical pharmacology perspective. Clin Toxicol. 2009;47:605. doi: 10.1080/15563650902970689. [DOI] [PubMed] [Google Scholar]
- 79.Modica MN, et al. Serotonin 5-HT3 and 5-HT4 ligands: an update of medicinal chemistry research in the last few years. Curr Med Chem. 2010;17:334–362. doi: 10.2174/092986710790192730. [DOI] [PubMed] [Google Scholar]


