Abstract
Theranostics, the fusion of therapy and diagnostics for optimizing efficacy and safety of therapeutic regimes, is a growing field that is paving the way towards the goal of personalized medicine for the benefit of patients. The use of light as a remote-activation mechanism for drug delivery has received increased attention due to its advantages in highly specific spatial and temporal control of compound release. Photo-triggered theranostic constructs could facilitate an entirely new category of clinical solutions which permit early recognition of the disease by enhancing contrast in various imaging modalities followed by the tailored guidance of therapy. Finally, such theranostic agents could aid imaging modalities in monitoring response to therapy. This article reviews recent developments in the use of light-triggered theranostic agents for simultaneous imaging and photoactivation of therapeutic agents. Specifically, we discuss recent developments in the use of theranostic agents for photodynamic-, photothermal- or photo-triggered chemo-therapy for several diseases.
Keywords: Photodynamic Therapy, Nanotechnology, Photothermal Therapy, Cancer, Infections, Imaging, Diagnostics, Targeting, Multifunctional, Drug Delivery
1. Introduction
Global healthcare costs have been rising steeply over the last decade [1]. However there hasn’t been a dramatic reduction in disease related deaths to warrant such a drastic rise in costs [2]. During this time there has been a paradigm shift in disease management and clinicians are gradually moving from the traditional “one drug fits all” approach towards the idea of personalized medicine – ‘the right drug for the right person administered at the right time’ [3–4]. Although significant awareness has been created about personalized medicine, its full potential has yet to be tapped [5–6]. The field of theranostics has sprung from the recognition that heterogeneous diseases require more personalized solutions [7]. Theranostics refers to the fusion of therapy and diagnostics, with the purpose of optimizing efficacy and safety, as well as streamlining the process of drug development and as a field is still in its infancy. The convergences of a number of scientific breakthroughs have made the development of theranostics possible [8]. In the field of biology, the human genome project and the development of biomarker initiatives, among others, have enhanced the understanding of disease progression. Technologies such as genotyping or gene expression profiling make it possible to transfer this newly acquired biological knowledge into the development of diagnostic tests [8]. Theranostics empower physicians with high-medical value testing for science-driven treatment decisions; improve patient outcomes and patient safety by identifying patients who won’t respond to a drug or who are likely to experience an adverse event; increase the efficiency of drug development, helping pharmaceutical companies by pinpointing those patients most likely to benefit from the new drug; and positively impact health economics, thus helping physicians select optimal and cost effective therapy. Although there is broad agreement that this nascent field has much potential in improving healthcare, there are a number of challenges that need to be overcome before it translates into routine use in the clinic [8]. Chief among these hurdles is the availability and use of a single platform for diagnosis and therapy.
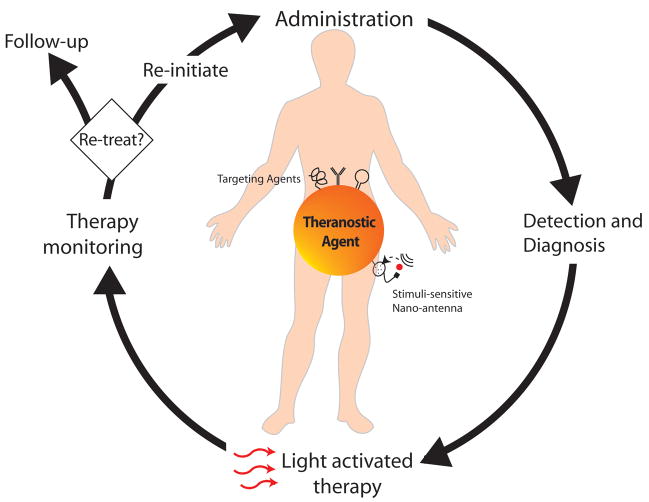
A tool that may help in overcoming this hurdle by successfully integrating therapeutic and diagnostic agents is nanotechnology [9–12]. Application of nanotechnology to medical science has been emerging as a new field of interdisciplinary research among medicine, biology, toxicology, pharmacology, chemistry, material science, engineering, and mathematics, and is expected to bring a major breakthrough to address several unsolved medical issues [9–12]. Nanomedicine – the use of nanotechnology for medicine is starting to make an impact in areas like disease imaging and diagnosis, drug delivery and as reporters of therapeutic efficacy and of disease pathogenesis [9–12]. Many multifunctional nanoparticle (NP) technologies, capable of performing one or more of the above duties, are now in various stages of preclinical and clinical development [9–12]. Theranostic nanomedicine refers to such an integrated nano-platform which can diagnose, deliver targeted therapy and monitor response to therapy. A scheme illustrating the potential role of theranostic agents at various stages of disease management is shown in Figure 1.
Figure 1.
Role of photo-triggered theranostic agents at various stages of disease management. Following administration of a single, integrated theranostic agent, a clinician can diagnose disease, detect location of disease, deliver light at the disease locations for activating targeted therapy and following treatment, monitor response to therapy. At this stage, by monitoring the patients response to the therapy, the clinician can decide to either re-initiate treatment or if sufficient regression or cure of disease is observed,call the patient for a follow up visit.
The selectivity and specificity for disease destruction can be enhanced by using externally activatable theranostic agents to produce localized cytotoxicity with little collateral damage. The ability to control drug dosing in terms of quantity, location, and time is a key goal for drug delivery science, as improved control maximizes therapeutic effect while minimizing side effects. Systems responsive to a stimulus such as temperature, pH, applied magnetic or electrical field, ultrasound, light, or enzymatic action have been proposed as triggered delivery systems [13]. Light-triggered theranostics are attracting increasing attention over the past few years due to its advantages in spatial and temporal control of compound release [14]. Recently, light has been used to release therapeutic agents from delivery systems or to activate agents that produce cytotoxic species. In fact, amongst the approved nano-constructs listed by the food and drug administration (FDA), is a light-activatable agent (Visudyne), widely used for the treatment of age-related macular degeneration (AMD) which is the major cause of blindness amongst the elderly in the developed world. Technological advances in fiber optic fluorescence imaging, a modality which allows investigators to reach into body cavities via minimally invasive endoscopes, have considerably broadened the applications of in vivo optical imaging [15]. This article reviews recent developments in the use of light-triggered theranostic agents for simultaneous imaging and photoactivation of therapeutic agents. The use of lasers and minimally invasive fiber-optic tools, along with the development of new agents that respond to NIR wavelengths for better tissue penetration, make direct targeting of deep tissues possible and thus enabling treatment of several pathologies.
Cancer is one of the most pressing public health concerns of the 21st century. The statistics are daunting; it was projected that 550,000 people would die of cancer and that another 1.4 million would be diagnosed with the disease in 2009 in the United States alone [16]. Another major cause of death, especially in the developing world, are infectious diseases which are making a come-back owing to the problems of drug-resistance and lack of sensitive diagnostic tests [10]. Infectious diseases, caused by bacteria, viruses, fungi and other parasites are major causes of death, disability, and social and economic disruption for millions of people. Over 9.5 million people die each year due to infectious diseases – nearly all live in developing countries [10]. Despite the existence of safe and effective interventions, many people lack access to needed preventive and treatment care [10]. Cardiovascular diseases (e.g. atherosclerosis) continue to be the biggest cause of death in the developed world [9]. Taken together, there is a vital un-met need for agents that can be used for simultaneous detection, diagnosis and remotely triggered therapy for selective destruction of diseases tissue.
Photo-triggered theranostic constructs could enable an entirely new category of clinical solutions, which permit early recognition of the disease through the use of contrast agents combined with existing imaging modalities (MRI, optical imaging, ultrasound) followed by the tailored release of the therapeutic agent. Here, we will discuss recent developments in the use of theranostic agents for photodynamic-, photothermal- or photo-triggered chemo-therapy for several diseases including cancer and infectious diseases. Sections 2–4 of this review are focused on the application of light-triggered theranostic agents for cancer while section 5 discusses their use for non-cancer pathologies. Generally, this kind of multifunctional agents will provide information on location of disease; targeted and on demand drug release that will lead to more effective therapies, eliminating the potential for both under and overdosing; the need for fewer administrations; optimal use of the drug in question; and increased patient compliance.
2. Photodynamic therapy and imaging for cancer
Photodynamic therapy (PDT) is an emerging, externally activatable, treatment modality for various diseases [17]. PDT can be defined as the administration of a non-toxic drug or dye known as a photosensitizer (PS) either systemically, locally, or topically to a patient bearing a lesion, which is frequently, but not always cancer [17]. After a sufficient incubation period with the PS, this lesion is then selectively illuminated with light of appropriate wavelength, which, in the presence of oxygen, leads to the generation of cytotoxic species and consequently to cell death and tissue destruction. PDT is clinically approved for treatment of several diseases including cancer and offers several advantages over conventional chemotherapy by providing additional selectivity through the spatial confinement of light used for PS activation [17]. A wide range of PSs have been evaluated so far and only a few of them have successfully transitioned from bench to bedside applications [17]. PS molecules are inherently fluorescent and this can be used for imaging and locating disease, photodiagnosis, often referred to, somewhat incorrectly, as photodynamic diagnosis (PDD). This approach is becoming of increasing interest for oncological applications. It is based on a higher accumulation of PS in tumors compared to normal tissue and is now being routinely used for diagnosis in bladder cancer [17] and fluorescence-guided resection in surgical procedures [17]. The use of PDT as a cancer therapy is particularly attractive because of its fundamental specificity and selectivity [17]. This is due to the fact that the PS concentrates specifically within the malignant tissue so when the light is directly focused on the lesion, it causes PDT reactive oxygen species (ROS) to be generated resulting in cellular destruction at the region of interest. For this very reason, in recent years, PDT has become the subject of intense investigation as a possible treatment modality for various forms of cancer. Similar to chemotherapy, PDT still requires agents which exhibit selectivity for the target cells. Similar to radiotherapy, the mode of action with PDT involves the use of electromagnetic radiation in order to generate radical species in situ. However, PDT is a much milder approach for cancer treatment than either. The reason for this lies in the combination of the mode of action of the PSs employed and their activation in situ by relatively long wavelength, visible light. Ideal PSs are non-toxic in the absence of activating light. The targeting of the cancer in PDT has a dual nature: the selectivity of the photosensitizing drugs employed and the confinement of the activating light to the tumor site alone. Due to the dual selectivity in PDT the non-tumor tissue largely remains unaffected [17].
Despite the regulatory approvals and the clinical success of PDT in oncology, a limitation of all existing PSs is the lack of high selectivity for target tissue at complex anatomical sites. PSs fluoresce upon light activation, thus enabling online imaging of drug for both image-guided drug delivery and for image guided, active, light dosimetry. Simultaneously combining therapy with imaging would help guide treatments and thus enhance treatment response. PS conjugates and supramolecular delivery platforms can improve PDT selectivity by exploiting cellular and physiological markers of targeted tissue [17]. Overexpression of receptors in cancer and angiogenic endothelial cells allows their targeting by affinity-based moieties for the selective uptake of PS conjugates and encapsulating delivery carriers, while the abnormal tumor neovascularisation induces a specific accumulation of PS nanocarriers by the EPR effect [14]. In addition, polymeric prodrug delivery platforms triggered by the acidic nature of the tumor environment or the expression of proteases can be designed [14]. Promising results obtained with recent systemic theranostic carrier platforms are discussed in the next section. These agents will, in due course, be translated into the clinic for highly efficient and selective PDT protocols.
2.1 Photosensitizers for imaging and PDT
2.1.1 Optical imaging
Optical imaging is a non-ionizing, non-invasive technique whose contrast mechanism is based on the optical properties of the tissue constituents such as absorption, scattering and reflectance. Different microscopic to whole body optical imaging techniques based on absorption, scattering, fluorescence, transmission and reflection properties of tissue constituents are available for various biomedical applications. However the primary limitation of various optical imaging techniques is penetration depth due to strong optical scattering properties of tissue. The use of contrast agents in the optical transparent window of 600–900 nm has alleviated this limitation. A recent review on various biomedical optical imaging techniques illustrates the schematics and principles of the techniques [18].
A summary of the PSs currently being used for clinical or pre-clinical research is shown in Table 1. Most approved PSs are porphyrins, consisting of four pyrrole subunits linked together by four methine bridges. Most commonly used photosensitizing agents among them are a photosensitizer precursor ALA (5-aminolevulinic acid) and derivatives and the sensitizers Verteporfin (benzoporphyrin derivative) and Photofrin (hematoporphyrin derivatives), all of which are effective, FDA-approved PS and are often used in clinical applications for imaging and therapy [19]. ALA or its derivatives are administered either locally or systemically and endogenously converted to protoporphyrin IX, the actual PS. This conversion takes place as part of the mitochondrial heme biosynthetic pathway and in case of cancer cells, the higher activity of enzymes involved in this synthesis pathway may contribute to the observed tumor specificity with this PS [19]. ALA has been extensively explored for image guided PDT and 5-ALA hexylester (Hexvix®) is approved for the diagnosis of bladder cancer [20]. Bogaards et al. showed the use of ALA for image guided brain tumor resection with adjuvant PDT [21]. Another PS which is approved for a broad range of applications is Photofrin, which consists of a mixture of four hematoporphyrin derivatives. Photofrin is approved for the therapy of advanced and early lung cancer, superficial gastric cancer, esophageal adenocarcinoma, cervical cancer and dysplasia, superficial bladder cancer and Barrett’s esophagus [22]. Malignant and premalignant lesions in the lung have been detected using Photofrin fluorescence [23]. Kohno et al. also showed the use of hematoporphyrins for early cancer diagnosis in peripheral blood lymphocytes [24]. ALA and Photofrin, often referred to as first generation PSs, are not ideally suited for the imaging or treatment of deeper tissues because of their low absorption capability at longer wavelengths. For an effective PS it is crucial that the absorption peak matches the so called optical window of the tissue for deeper penetration of the light beam. This window describes a wavelength range from 600–900 nm where the light absorption and scattering of the tissue is lower than at other wavelengths. The absorption of hemoglobin and melanin restrict the lower end of this optical window for PDT. The upper end of the optical window is around 900 nm, due to the energy requirement of the light beam for singlet oxygen generation [19].
Table 1.
Current Approvals and Clinical Trials with a Selection of Photosensitizers. The data on clinical trials was obtained from www.clinicaltrials.gov. For clinical approvals see references [17] [22].
| Photosensitizer | Clinical trials | Approvals |
|---|---|---|
| Foscan, meta-tetra(hydroxyphenyl) chlorin) | Nasopharyngeal carcinoma, bile duct carcinoma, head and neck cancer | Palliative head and neck cancer |
| Hexvix, 5-aminolevulinic acid hexyl ester (converted to protoporphyrin IX) | Colorectal cancer, bladder cancer, cervical intraepithelial neoplasma | Diagnosis of bladder cancer |
| Hypericin and Hypericin derivatives | Actinic keratosis, basal cell carcinoma, bladder cancer | |
| Levulan, 5-aminolevulinic acid (converted to protoporphyrin IX) | Bladder cancer, skin cancer, penile cancer, glioma | Actinic keratosis, basal cell carcinoma. |
| Lu-Tex, lutetium texaphyrin | Prostate cancer, non-small cell lung cancer | |
| Metvix, 5-aminolevulinic acid methyl ester (converted to protoporphyrin IX) | Basal cell carcinoma, non-melanoma skin cancer | Actinic keratosis, basal-cell carcinoma. |
| NPe6, mono-L-aspartyl chlorine-e6 | Hepatocellular carcinoma, colorectal cancer patients with recurrent liver metastases, glioma | Early lung cancer. |
| Pc4, silicon phthalocyanine | Cutaneous T-cell lymphoma, skin cancers, pancreatic cancer | |
| Photochlor, Hexyl ether pyropheophorbide-a derivative | Lung carcinoma, basal cell carcinomas, Barrett’s esophagus. | |
| Photofrin, hematoporphyrin derivatives | Intraperitoneal cancer, cholangiocarcinoma, refractory brain tumors, non-small cell lung cancer. | Advanced and early lung cancer, superficial gastric cancer, esophageal adenocarcinoma, cervical cancer and dysplasia, superficial bladder cancer, Barrett’s esophagus. |
| Photolon, chlorin-e6- polyvinylpyrrolidone | Malignant skin and mucosa tumors, myopic maculopathy | |
| Purlytin, tin ethyl etiopurpurin | Skin adenocarcinoma, prostate cancer, breast cancer | |
| Tookad, palladium- bacteriopheophorbide-a | Prostate cancer | |
| Visudyne, benzoporphyrin derivative monoacid ring A | Pancreatic cancer, brain cancer, basal cell carcinoma, brain and central nervous system tumors, melanoma | Age-related macular degeneration |
New PSs have been designed that have higher absorption coefficients in the longer wavelength range. Some of these PS like Tookad®, the palladium complex of bacteriochlorophyll, have expanded the range of usable wavelength to over 800 nm and possess excellent tissue penetration [25]. Tookad has a very high singlet oxygen quantum yield of 0.99 but a very low fluorescence quantum yield, thus limiting the theranostic use of Tookad. An ideal theranostic PS is an agent that has high singlet oxygen quantum yield for therapy and also a reasonably high fluorescence quantum yield for fluorescence detection. These requirements are moderately fulfilled by two PSs Verteporfin (BPD; benzoporphyrin derivative monoacid ring A) and Photochlor (HPPH; pyropheophorbide-alpha-hexyl-ether). With a singlet oxygen quantum yield of 0.76 and a fluorescent quantum yield of 0.05 for the monomer, BPD can be an effective theranostic agent. Another PS which has been receiving increasing attention in the last few years is Hypericin. It is one of the most potent naturally occurring PS and was originally extracted from Hypericum (Saint John’s wort). Various synthetic hypericin derivatives have been synthesized with improved physicochemical properties which can be used for imaging and PDT [26]. Clinical studies have demonstrated the potential of hypericin for diagnosis of bladder cancer [27] as well as oral cancers [28]. Hypericin was successfully tested in clinical trials for actinic keratosis, nonmelanoma skin cancer [29]. It has also been evaluated in combination therapy with bevacizumab in a mouse model for bladder cancer and the treatment response was imaged using confocal fluorescence endomicroscopy [30]. Recently, Trivedi et al. reported the preparation of chiral porphyrazine (pz), H2[pz(trans-A2B2)] (247), and its potential for imaging and therapy [31–32]. Pz-247 exhibits NIR-emission and shows preferential uptake into tumor cells. The authors demonstrated the association of Pz-247 with low density lipoproteins (LDL) and it’s receptor-mediated cellular uptake with localization in lysosomes. NIR optical imaging of mice with subcutaneous breast cancer tumors showed a strong contrast between tumor and surrounding normal tissue 48 hours after intravenous (i.v.) injection of Pz-247.
Most of the clinically used PSs show some inherent selectivity for the diseased tissue probably due to the enhanced permeability and retention (EPR) effect. While some additional selectivity of PDT for localized tumors can be achieved by site-specific administration of light using optical fibers, the non-specific uptake of PS by normal tissue is a major problem for PDT of highly disseminated tumors (e.g. ovarian cancers), as this can cause severe collateral damage. PS tumor selectivity can be improved by conjugation of PS with molecular moieties that are known to target cellular receptors, intracellular organelles, or vasculature of diseased tissue. Antibodies have been used as one of the earliest PDT targeting strategies by covalent conjugation of PS to form the photoimmunoconjugates (PIC). Recently, Savellano et al. conjugated BPD to PEGylated cetuximab, a clinically approved monoclonal antibody which binds to epidermal growth factor receptor (EGFR), that is often over-expressed on the surface of epithelial cancers [33]. At an optimal labeling ratio (BPD:cetuximab = 7 or 10 :1), PIC was found to accumulate at significantly higher level on EGFR-overexpressing cancer cells (A431 and OVCAR-5) as compared to the low EGFR expressing fibroblast cells (NR6). Although the phototoxicity was less compared to BPD at the equivalent dose, the authors showed that they can still effectively destroy cancer cells by increasing the light dose.
In addition to antibody-based PS conjugates, small molecules and synthetic biomolecules such as RNA-aptamers [34] and peptides have been developed to enhance the delivery to cancer cells. For example, conjugation of pyropheophobide to 2-deoxyglucose resulted in delivery and trapping of PS in cancer cells via the glucose transporter (GLUT)/hexokinase pathway, and therefore is useful both as a near-infrared fluorescence imaging probe and as a PDT agent for the destruction of cancers which has higher levels of GLUT and hexohinase activity than normal tissues [35]. Although, most of the biomolecules were chemically modified to overcome potential degradation by proteases and RNases in vivo, the protease susceptibility of peptides has been explored to design target activatable prodrugs. This approach was initially developed as an imaging technique to differentiate between target and background [36]. A typical construct or “molecular beacon” as it has been referred to, consists of a fluorophore attached to an appropriate fluorescence quencher by a short linker. Cleavage of this linker by some stimulus specific to the target can activate the fluorophore for imaging. This approach has been demonstrated to be well suited for monitoring the target activity [37]. In addition, it has the advantage that one target (e.g., an enzyme) can activate several individual beacon molecules leading to amplification of the fluorescence intensity. This strategy has been shown to elicit a 10 to 1,000 fold amplification of the fluorescence signal compared to simple tagging. This activatable imaging strategy was first introduced for PDT by Zheng et al. in 2004 by replacing the fluorophore with a PS and has been explored for its theranostic potential [38]. Since then, several groups have published promising results and the number of activatable PSs has increased dramatically [39–41]. An in depth review of these activatable PSs was recently published by Lovell et al. [22].
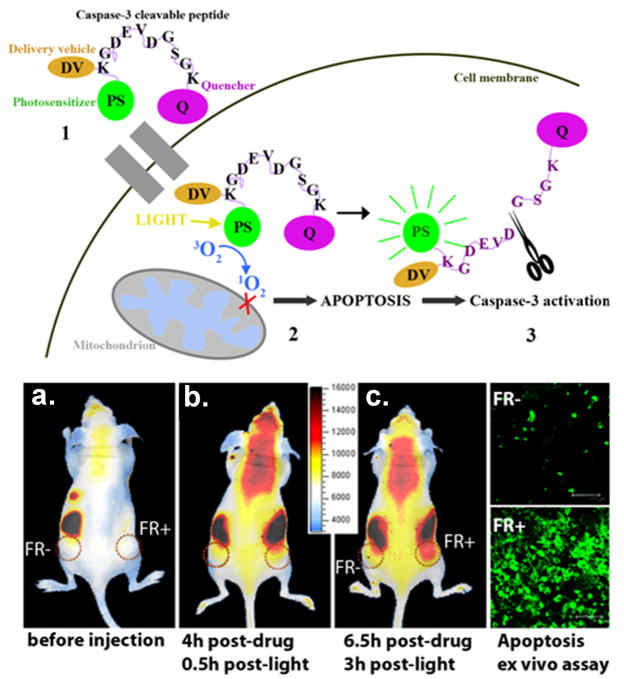
A further development in this field of activatable molecular beacons is the use of a targeting moiety. This targeted molecular beacon strategy was demonstrated by Stefflova et al. [42]. The authors designed a multifunctional, membrane-permeable, and cancer-specific construct that triggers and images apoptosis in cancer cells. This construct contains a fluorescent PS (pyropheophobide) and a cancer-associated folate receptor targeting molecule connected to a caspase-3 cleavable peptide linker that has a fluorescence quencher (BHQ-3) on the opposite side. The double-tumor mouse bearing a folate receptor negative tumor (derived from HT 1080 cells) on one side and a folate receptor positive tumor (derived from KB cells) on the contralateral side was injected intravenously with the construct, followed by PDT. A distinctly higher post-PDT increase in fluorescence was observed in the folate receptor positive tumor compared to the folate receptor negative tumor, confirming the targeting and apoptosis-reporting functions of the construct (Fig. 2). The use of a single molecular agent for targeted PDT and monitoring response to the treatment demonstrated the theranostic potential of such an approach.
Figure 2.
Theranostic molecular beacons for targeted PDT and monitoring treatment response. The top panel is the schematic diagram of structure and function of a targeted PDT agent with a built-in apoptosis sensor: (1) This construct consists of PS, caspase 3 cleavable sequence, fluorescence quencher, and delivery vehicle; (2) The construct accumulates preferentially in cells overexpressing folate receptor, and once activated by light, the PS produces singlet oxygen that destroys the mitochondrial membrane and triggers apoptosis; (3) This leads to activation of caspase 3, which cleaves the peptide linker between the PS and the quencher, thus restoring the PS’s fluorescence and identifying those cells dying by apoptosis by NIR fluorescence imaging. The bottom left panel demonstrated in vivo induction and detection of apoptosis in a mouse bearing folate receptor positive (FR+, KB) cells) and folate receptor negative (FR-, HT 1080 cells) tumors after light treatment (Photodynamic Therapy = PDT, 90 J/cm2) using intravenously administered photoactivatable drug Pyro-K(folate)GDEVDGSGK (BHQ-3) (PFPB, 25 nmol) cleavable by Caspase-3. (a–c): Xenogen images of a mouse bearing FR- (left) and FR+ (right) tumors: a. before i.v. injection of PFPB or PDT; b. 0.5 hour after PDT (4 hours after drug injection); c. 3 hours after PDT (6.5 hours after drug injection). These images are showing a gradual increase in fluorescence in the FR+ compared to FR- tumor. Bottom right: Confocal images of the histology tissue slides of the corresponding FR+ and FR- tumors stained with Apoptag confirmed increased light-induced apoptosis in the FR+ tumor. Adapted from Steffalova et al. [42].
2.1.2 Multimodal imaging (MRI and PET)
Optical fiber-based fluorescence imaging techniques combined with targeting agents have been extensively studied for diagnosis, PDT and treatment response monitoring [17]. However, poor light penetration limits the applicability of light-based imaging and therapies to superficial tumors with depths of 1–2 mm into the tissue. Thus, an emerging trend in the development of theranostic PDT agents is the coupling of optical imaging with other imaging modalities such as positron emission tomography (PET), magnetic resonance imaging (MRI) and ultrasound.
PET imaging agents are most commonly labeled with radioisotopes such as 11C (t1/2=20.4 min) and 18F (t1/2=110 min). However, it is very challenging as synthesis, purification and analysis of these short-lived isotopes has to be done within the order of a few minutes. Large isotopes such as 86Y (t1/2=14.7 hours), 64Cu (t1/2=12.7 hours), and 124I (t1/2=4.2 days) are more suitable candidates. Radiolabeling with 124I for PET studies involving PDT is most appropriate because PS needs relatively long time to accumulate in tumors. A simple method to prepare the 124I-labeled PS is the direct electrophilic aromatic iodination of the trimethylstannyl substituted analogues with Na124I in the presence of commercial iodogen beads. Using this strategy, Pandey et al. prepared 124I-labeled pyropheophorbide and purpurinimide analogues with >95% radioactive specificity [43–44]. It was proposed that this radioactive construct could be used for PET and fluorescence imaging as well as PDT.
MRI is a widely used tool in pharmaceutical research due to its excellent soft-tissue contrast property that provides three-dimensional anatomic images with high spatial resolution. Unlike nuclear scanning, conventional radiography or computed tomography, MRI often relies on contrast enhancers to improve inherent contrast between normal and diseased tissue by altering longitudinal (1/T1) and transverse relaxation rates (1/T2) of tissue protons. Agents containing paramagnetic transition metal ions such as gadolinium (Gd+3) and manganese (Mn+2) have been shown to effectively alter 1/T1 and/or 1/T2. Gd+3 in particular, has seven unpaired electrons within the inner orbital shells and provides a high degree of paramagnetism which causes an increase in the T1 relaxation rates of nearby water molecules. Gd+3 is too large to be accommodated in the macrocyclic center of ordinary porphyrins. Incorporation of Gd+3 with a PS can be achieved by two methods: First method is to insert the ion into an expanded porphyrin which contains five (instead of four) nitrogen atoms in the ring, forms a central chelating cavity 20% larger than that of ordinary porphyrins to accommodate Gd+3 [45]. This metal complex, namely Motexafin gadolinium has been proposed for the treatment of brain cancer but was rejected by FDA in 2007. While the lutetium complex of Motexafin was undergoing clinical trials as a PS agent [46], interestingly, Gd+3 analog had only been investigated as a potential radiation sensitizer by causing redox stress to cancer cells. The second method of incorporating Gd+3 with a PS is to stabilize Gd+3 by attaching PS with a side-chain moiety such as diethylenetriaminepentaacetic acid (DTPA). This strategy is more favorable because it can be applied to virtually any PS. In an early study, two Gd-DTPA moieties were covalently linked to mesoporphyrin (Gadophrin-2) and later to the copper complex (Gadophrin-3) for improved stability and safety. Although such metalloporphyrins may be useful for tumor imaging, they were found to preferentially localize in the periphery of necrotic areas rather than the viable cancer tissue [47].
Pandey and co-workers [48–49] investigated the possibility of delivering the contrast agents to living tumor cells by conjugating gadolinium complexes to HPPH, a tumor-avid chlorophyll derivative at Phase II clinical trials. In this study, up to six Gd+3aminobenzyl DTPA complexes were coupled to HPPH. Most of them had enhanced tumor-imaging potential (T1/T2 relaxivity), which increased with larger number of Gd+3 units. To achieve a comparable signal intensity of the clinical MRI agent Gd-DTPA, only a 10 to 20-fold lower dose of the conjugate was required. The three Gd+3aminobenzyl DTPA conjugate, which showed the best PDT activity in vitro was evaluated in vivo. At 24 h post injection, the accumulation of the conjugate in the Ward tumor was higher than in blood, muscle and most organs. At imaging concentration, the required light dose for the conjugate was lower than the one required for HPPH alone, to achieve comparable tumor response in both radiation induced fibroblast (RIF) and Colon 26 tumor models.
Another promising theranostic system has been developed by Liu et al. to enhance the monitoring of PDT efficacy [50]. In this study, the author investigated a novel PS, prepared from fullerene (C60), which combines the property to produce singlet oxygen and can be conjugated to an MRI agent. Gd3+ was selected as the MRI contrast agent and introduced to the PEG terminal of C60-PEG through metal chelation. Following intravenous injection in the tumor-bearing mice, C60-PEG-Gd maintained an enhanced MRI signal at the tumor tissue for a longer time period in comparison with the commercial contrast agent (Magnevist®). The PEG-conjugated fullerene system showed significant tumor PDT effect although the effect depended on the timing of light irradiation.
To study contrast-enhanced MRI guided PDT with PEG bifunctional polymer conjugate containing an MRI contrast agent and a PS, Vaidya et al. have synthesized PEGylated poly-(L-glutamic acid) conjugates containing mesochlorin e6, a PS, and Gd(III)-DO3A [51–52]. MRI images showed that pegylated conjugate had longer blood circulation, lower liver uptake and higher tumor accumulation than the non-pegylated conjugate. Laser irradiation of tumors resulted in higher therapeutic efficacy for the pegylated conjugate. The PDT treated animals showed a reduced vascular permeability with dynamic contrast-enhanced-MRI and reduced microvessel density on histopathological analysis. They concluded that PEGylation of the bifunctional polymer conjugates reduced non-specific liver uptake and increased tumor uptake, resulting in significant tumor contrast enhancement and higher therapeutic efficacy [52].
Ultrasound as a label-free technique has been used in vascular and interventional imaging. Its therapeutic effects in treatment of solid tumors and its efficacy and safety were confirmed in clinical investigations [53]. There have been several reports on the ability of certain porphyrins (sonosensitizers) to enhance the low-intensity ultrasound-induced cytotoxicity, both in cell culture and in tumor model. This treatment modality is named Sonodynamic therapy (SDT). Although the mechanism of this enhancement effect has not yet established, the experimental evidence suggests that sonodynamic effect may due to the chemical activation of sonosensitizers inside or in the close vicinity of hot collapsing cavitation bubbles to form sensitizer-derived free radicals, and/or due to mechanical stress of physical disruption of cellular membrane by sensitizers [54]. It was also reported that the combination of SDT with PDT can induce tumor necrosis more extensively than in mice receiving only SDT or PDT [55].
The use of PSs as imaging agents for diagnosis or fluorescence guided tumor resection is emerging in the last years. PSs were combined with imaging techniques including fluorescence, MRI, PET, and ultrasound. Some of these approaches have already entered clinical trials or are approved like 5-ALA hexyl ester for the diagnosis of bladder cancer in Sweden. Hexvix was recently approved in the US for bladder cancer detection and fluorescence-guided resection. New technologies for molecular targeting will increase the tumor specificity thereby enabling sensitive and specific detection and site specific treatments.
2.2 Nanoparticles for imaging and PDT
Over the last few years, nanoparticle (NP) based PDT has emerged as an alternative to conventional PDT to efficiently target cancer cells. The dual selectivity provided by the target localizing ability of NP and the spatial control of illumination could significantly reduce the systemic toxicity associated with classical PDT therapy. Besides the systemic toxicity, most PSs used in PDT, have other limitations. Mainly, they are hydrophobic or have a limited water solubility and therefore could aggregate in biological media which leads to the modification of their optical properties and the decrease of singlet oxygen production [56]. Although recently a lot of work has focused on developing several new strategies to improve the performance of PDT agents, including conjugation with oligonucleotides, monoclonal antibodies, carrier proteins, lipids, carbohydrates, or hydrophilic polymers for selective delivery of the agents into tumor tissues [57], the lack of specific targets and the dark cytotoxicity is still a principal challenge for PDT. Many efforts are ongoing to develop new conjugated PS with a covalently linked vector to target receptors over-expressed in cancer cells, however very few have been evaluated in clinic mainly because of their lower in vivo selectivity [58].
Nanotechnology provides a platform for integration of multiple functionalities in a single construct [59]. Here we provide an update of simultaneous tumor targeting and imaging with a number of different nanosystems that have the potential for theranostic PDT. Various nanoprobes have been developed for in vivo magnetic resonance and optical imaging, which include quantum dots, upconverting nanophosphors, gold and Silica NPs, PS containing nanoparticulate carriers such as liposomes, ceramic, polymeric. The in vitro and in vivo fate of these systems after administration is discussed. Although several challenges remain before this modality can be adopted in clinic, multifunctional NPs offer a good tool to treat deep tumors efficiently with PDT.
2.2.1 Optical imaging
Recent breakthroughs in the synthesis of mesoporous silica NP (MSNP) with high surface areas and tunable pore diameter (2–10 nm) have led to the design of new delivery systems, where different molecules, such as pharmaceutical drugs or fluorescent imaging agents, could be absorbed into the mesopores and released later into various solutions [60]. Furthermore, some reports on the design of PS based MSNP have been published, among them, Roy et al. have studied a ceramic system based NP with an average diameter of 30 nm. The particularity of this NP is their capacity to optically protect 2-devinyl-2-(1-hexyloxyethyl) pyropheophorbide (HPPH) PS resulting in a drug-doped, highly monodispersed, and stable NP in an aqueous system. In vitro imaging study of this system demonstrated active uptake of HPPH-doped NPs into the cytosol of tumor cells and efficient cytotoxicity upon irradiation [61].
To circumvent the problem of the mesoporosity of MSNP and the release of the PS during systemic circulation Prasad’s group has succeeded to covalently incorporate iodobenzylpyropheophorbide PS into a novel nano-formulation named Organicaly Modified Silica NPs (ORMOSIL) [62]. These NPs are were taken up by tumor cells in vitro and demonstrated phototoxic action. Kim et al. reported a promising modality using ORMOSIL system where the photosensitizing unit (energy acceptor) is indirectly excited through fluorescence resonance energy transfer (FRET) from the two-photon absorbing dye unit (energy donor [63]. In this study, the authors showed use of nanophotonic tools to produce singlet oxygen and to induce tumor cell death following two-photon PDT. They also demonstrated the potential for co-encapsulation of two drugs to provide contrast in fluorescence images of live tumor cells under two-photon excitation and under near infrared (NIR) light.
More recently Prasad et al. investigated the biological issue raised over the use and safety of ORMOSIL system for diagnosis and therapeutic purposes using different bioimaging modalities including PET, MRI and optical imaging [64]. A NIR PS DY776 was encapsulated in ORMOSIL NP, resulting in a ~20 nm diameter size NIR optical probe. Further the PET imaging probe iodine-124 was conjugated with the NPs, which allow imaging of deep tissue. To study the in vivo clearance of the NPs, animals injected with DY776 conjugated ORMOSIL were imaged daily over a period of 15 days. The results showed accumulation of NPs in liver and spleen over a period of 24 hours, whereas the skin showed a maximum concentration at 72 hours post injection of NPs. Furthermore the clearance studies confirmed that all of the injected ORMOSIL was excreted out of the animal via the hepatobiliary excretion without any sign of organ toxicity [64]. This in vivo bio-imaging, bio-distribution, clearance, and toxicity studies showed that combining this multimodal nano-formulation with PDT, make ORMOSIL NPs an exciting modality for treatment and monitoring.
In a recent report He et al. explored the use of methylene blue-encapsulated silica NPs (MB-PSiNPs) for simultaneous in vivo imaging and PDT [65]. MB-PSiNPs were synthesized with an average diameter of 105 nm. Using a chemical trap, the authors confirmed singlet oxygen production after irradiation. To investigate the biological environment effects on the encapsulated MB, the decreased absorption of leukomethylene blue (LMB) which is the reduced form of MB after contact with enzymes was probed at 660 nm. The results showed that encapsulated MB stayed intact in biological system and PSiNPs prevented the MB from being reduced by enzymes [65]. In vivo monitoring of PDT post irradiation was also performed on subcutaneous-Hela-tumor-xenografted mice. Twelve hours after the injection of MB-PSiNPs, the induced fluorescence was used to guide treatment with 635 nm laser light (500 mW/cm2, 5 min). Following treatment, in vivo imaging was performed using a hyperspectral imaging system to assess treatment response. The tumors treated, with NPs and light irradiation were found to shrink gradually while the control tumors did not show any significant effect [65].
MSNPs have also been designed by Zhang et al. [66]. This multifunctional core-shell NP contains a nonporous dye-doped silica core with an average diameter of ~ 37 nm and a ~ 57 nm of mesoporous silica shell containing PS molecules, hematoporphyrin (HP). These nanocomposites are stable and can be stored for over 1 month at room temperature. The elegance of the bi-functionality of this system is its capacity to act not only as a carrier for the photoactivable drug which is covalently linked to the mesoporous silica shell but also as a nanoreactor to facilitate the photo-oxidation reaction. The author demonstrated that doping of fluorescence dyes into the nonporous core allows for simultaneous PDT and fluorescence imaging in vitro.
Low-density lipoprotein (LDL) provides a highly polyvalent natural nanoplatform for delivery of various imaging and therapeutic agents to neoplastic cells that over express LDL receptors (LDLR) [67]. Covalent attachment of other ligands to the lysine side chain amino groups could be used to target other receptors [68]. The incorporation of NIR fluorescent probes into LDL showed promising results using optical imaging [69–70]. In addition, Gadolinium based agents could also be attached to LDL to improve tumors detection using magnetic resonance imaging (MRI) [71]. More recently Song et al. designed a novel naphthalocyanine (Nc)-based PS as a PDT agent to be delivered by a LDL NP [72]. This Nc-LDL NP was prepared by reconstituting the tetra-t-butyl silicon naphthalocyanine bisoleate (SiNcBOA) into LDL lipid core. In vitro results indicated that Nc-LDL NPs are internalized into Hep G2 cells specifically via the LDLR mediated pathway. This preferential uptake of Nc-LDL NPs by tumor tissue was confirmed in vivo by noninvasive optical imaging technique. Human serum albumin (HSA), the most abundant protein in human blood plasma has been used recently as a platform to deliver a photoactivable drug Pheophorbide (Pheo) into tumor for PDT [73]. In vitro studies on Jurkat cells using fluorescence lifetime imaging showed that Pheo-HSA NPs efficiently decomposed in the cellular lysosomes resulting in higher phototoxicity.
Solid lipid NPs (SLN) represent a novel carrier system that have various advantages compared to liposomes and polymeric NPs [74]. Stevens et al. have used this platform to synthesize a folate receptor (FR)-targeted SLN in which hematoporphyrin PS has been encapsulated to target FR-overexpressing tumor cells. In vitro cytotoxicity study of these NPs showed an IC50 of 1.57 μM in human oral epidermal carcinoma cells and non-targeted SLN gave an IC50 of 5.17 μM. The selectivity of FR-targeted NP was confirmed by fluorescence microscopy [75].
Up-converting Phosphor Technology, based on lanthanide-containing, submicrometer-sized, ceramic particles that can absorb infrared light and emit visible light, has been used by Chatterjee et al. to design NPs as transducers for PDT [76–77]. The up-converting NPs investigated in this study are composed of sodium yttrium fluoride (NaYF4) nanocrystals co-doped with the rare earth ions ytterbium (Yb3+) and erbium (Er3+) with a polymeric coat of poly(ethylene imine) (PEI). The PS zinc phthalocyanine (ZnPC) was superficially non-covalently adsorbed to the NPs. The researchers have succeeded in this study to image cellular uptake by fluorescence imaging microscopy of PEI/NaYF4:YB3+, Er3+ NPs, and they found significant cell death following irradiation with NIR laser light. A potential clinical use of these NPs is to image and photodynamically treat cancers situated in deep tissue.
Liposomes represent a valuable carrier and delivery system due to their high loading capacity and their flexibility to accommodate different PS with variable physicochemical properties [78]. The PDT agents could be targeted by modifying the design and the surface chemistry of liposomes. However conventional liposome could give promising results when administered topically. Bendsoe et al. have reported a clinical study using liposomal Meso-tetra(hydroxyphenyl)chlorin (mTHPC) gel formulation for topical application in connection with PDT of non-pigmented skin malignancies [79]. In this study the authors reported that the treated area did not show any swelling or reddening, as is often seen in PDT using topical ALA. Further, no pain during or after treatment were reported. One week after treatment, healing progress was observed in several patients and no complications were registered. Beside the clinical efficiency of this formulation, liposomal NPs could be used as probe to monitor the sensitizer distribution within tumor and surrounding normal skin using fluorescence imaging before, during, and after PDT.
In our group, Zhong et al. have used liposomally encapsulated benzoporphyrin derivative monoacid ring A (L-BPD) to image, treat and monitor PDT response in vivo in a mouse model of disseminated ovarian cancer [80]. L-BPD (Visudyne) was originally developed for its application in ophthalmology and is currently approved for the treatment of age related macular degeneration (AMD). Additional details about its use in AMD are discussed in section 5.2 of this review. Visudyne is also currently in clinical trials for treatment of ovarian [81–82] and pancreatic cancer [83]. In the study of Zhong and Celli et al. high-resolution fiber-optic fluorescence imaging was used for the detection of microscopic ovarian cancer and for monitoring PDT treatment response. After administration, L-BPD serves as both an imaging agent and a light-activated therapeutic agent. By comparison with histopathology based method, Zhong et al. showed a sensitivity of 86% for in vivo tumor detection using the microendoscope. The results showed that PDT treated mice exhibit an average decrease of 59% in tumor volumes. The author concluded the potential of the approach used to treat and monitor the treatment outcome [80]. Additional studies are necessary to compare feedback from imaging with long-term outcomes to evaluate the potential of this approach for early reporting following treatment and, by extension, as a tool to aid in rational treatment planning.
Using the same platform Derycke et al. have studied the tumor selective behavior of phthalocyanine tetrasulfonate (AlPcS4) when its applied intravesically in transferrin-conjugated liposomes (Tf-Lip–AlPcS4) [84]. The results reported show an efficacy of the PDT treatment using Tf-Lip-ALPCS4 on AY-27 rat bladder carcinoma cells. The authors concluded that transferrin-mediated liposomal targeting of ALPcS4 drugs is a promising tool for PDT of superficial bladder tumors. Thus the selective accumulation of Tf-Lip–AlPcS4 in bladder transitional-cell carcinoma cells would allow photodiagnosis and fluorescence-guided transurethral resection of lesions with a high sensitivity and specificity. Another liposome based formulation has been developed by Meerovich et al. using hydroxyaluminium tetra-3-phenylthiophthalocyanine (3-(PhS)4-PcAlOH) as a NIR PS. Experiments on mice with solid Ehrlich tumor and subcutaneously transplanted P-388 leukemia revealed high selectivity of accumulation of 3-(PhS)4-PcAlOH in tumors in comparison with normal tissues and high PDT activity. The authors concluded that the high selective accumulation of 3-(PhS)4-PcAlOH in tumor could be used for fluorescent diagnosis [85].
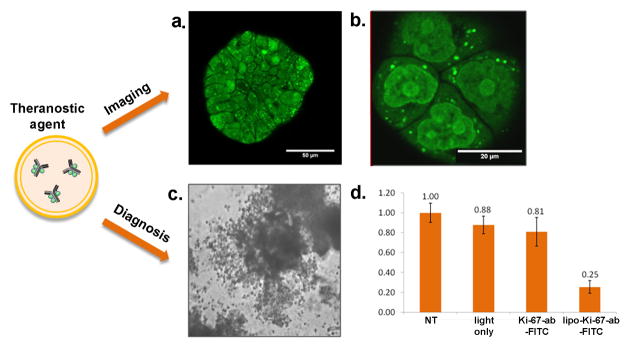
Recently, our group has demonstrated a new approach to target and photoinactivate the nuclear proliferation marker pKi-67 [86]. pKi-67 is a marker that is strongly expressed in all cells that have the ability to divide and proliferate [87]. In many cancers pKi-67 expression is correlated with poor prognosis for disease free progression and overall survival. Therefore, antibodies against pKi-67 are widely used in diagnostics to access the growth fraction of tumors from patient biopsies [88]. Despite the interest of using a diagnostic valuable marker as target for therapy, the inactivation of a nuclear protein has been challenging so far. To target the Ki-67 protein we used dye-labeled antibodies which are encapsulated into PEGylated liposomes (photoimmunoconjugate encapsulated liposomes, PICELS) [86]. The liposomes deliver the photoimmunoconjugate intracellularly where a fraction is released into the cytoplasm. From the cytoplasm the conjugates localize into the nucleus to the actual pKi-67 site. The efficacy of pKi-67 inactivation was demonstrated in a 3D in vitro model for ovarian cancer. In this model ovarian cancer cells form multicellular acini (Fig. 4a), which mimic the small tumor nodules that are found in vivo all over the peritoneal cavity [86]. Treatment of these cells with PICELS and subsequent light irradiation led to destruction of the acinar structure and to more than 70% dead cells 72 h following treatment (Fig. 4b). As a control, pKi-67 negative confluent lung fibroblasts showed no significant effect on cell viability after pKi-67 PDT [86]. In this approach the target protein is not only utilized for the selective delivery of the PS but the antibody itself is inhibiting the protein. Only after light irradiation and the generation of ROS, the photoimmunoconjugate becomes an inhibitor for the target protein. We refer to this as molecularly targeted PDT. The study demonstrated a potential role of pKi-67 as a molecular target for cancer therapy, besides its important role in diagnostics and demonstrated the nuclear delivery of an antibody with non-cationic liposomes [86]. The fact that pKi-67 is a well established marker in tumor diagnostics makes this approach not only valuable for eliminating aggressive cancer cells, it could in the future also be applied to access the growth fraction of the tumor in real time in vivo (Fig. 4a). One or two days after drug administration the fraction of pKi-67 positive cells could be imaged endoscopically in the patient. Different regions in the tissue could be accessed in short time after each other and the Ki-67 labeling index could be estimated based on image fluorescence data. In this first study anti-pKi-67 antibodies were conjugated to FITC (fluorescein 5(6)-isothiocyanate). FITC can easily be conjugated to antibodies and it has been widely used for specific protein inactivation in living cells [89]. FITC has an excitation maximum of 490 nm where light penetration into tissue is fairly limited. For in vivo application a PS with absorption maximum in the longer wavelength range and with higher singlet oxygen quantum yield seems to be more applicable.
Figure 4.
Photoimmunoconjugate encapsulating liposomes (PICELS) for targeting and inactivation of the nuclear proliferation marker pKi-67. a. PICELS deliver pKi-67-FITC antibodies intracellular and can be imaged with confocal microscopy in ovarian cancer 3D-culture ascini. b. Imaging of monolayer cultures shows the nucleolar localization on the PICELS. c.72 hours after laser irradiation with 5 J/cm2 at 488 nm the 3D-acini have lost their spherical morphology and d. Following treatment with PICELS and light irradiation the 3D-acini show a significant decrease in the number of viable cells as measured by a live-dead assay. Based on work by Rahmanzadeh et al. [86].
Numerous reviews have described and provided results on the use of gold NP (GNP) in many biomedical applications including imaging and therapy of cancer [90]. Beside the availability of rich chemistry regarding GNP, currently it is possible to modify the surface of this NP either covalently or noncovalently with PSs. Recently, Wieder et al. reported the development of a new delivery system based on GNPs, whereby the PS is attached to the surface of the NP [91]. Their results showed that GNP conjugates are an excellent carrier for the delivery of hydrophobic PS for high PDT efficacy toward tumor cells. The uptake of these NPs and their phototoxicity toward Hela cells was confirmed using confocal imaging microscope. Though the results are encouraging using these NPs, the PDT efficiency of this system remains to be evaluated in vivo.
Zaruba et al. recently studied the efficacy of PDT using GNP on which two porphyrin–brucine conjugates were immobilized [92]. The intracellular distribution and tumor cell uptake of these NPs were studied using fluorescence microscopy and the results showed that these NPs localize in lysosomes. The in vivo results showed that the brucine-porphyrin derivatives bound to modified GNPs mediate a complete regression of PE/CA-PJ34 carcinoma after PDT. More recently Russell and Jori groups have investigated the in vivo efficacy of Zn(II)-phthalocyanine disulphide (C11Pc) bound to GNPs for the PDT of amelanotic melanoma [93]. In this paper the authors showed an enhanced accumulation of this NP on subcutaneously implanted amelanotic melanoma. Further, electron microscopy observations of tumor specimens obtained at different times after PDT, showed an extensive damage of the blood capillaries and endothelial cells.
Among the various delivery system of GNP, Cheng et al. investigated the efficiency of PEGylated GNP attached to phthalocyanine 4 (Pc4) for in vivo PDT of cancer [94]. A 35% singlet oxygen quantum yield was obtained for Pc4 on PEGylated GNPs while free Pc4 had 50% singlet oxygen quantum yield. Fluorescence images of tumor-bearing mouse were also taken at 1, 30 and 120 min after i.v. injection of drug conjugated NPs. The results showed that the drugs accumulated at the tumor site through a passive targeting process. After illumination the effect of treatment appeared within one week without any noticeable toxicity or side effects to the animals [94].
2.2.2 Magnetic resonance imaging (MRI)
Considerable research efforts have been directed towards developing efficient chitosan-based NP drug delivery systems. In comparison to other biological polymers, positive charges target the chitosan carriers to the negatively charged cell membrane and have mucoadhesive properties to prolong the retention time of chitosan in the targeted locations [95]. Magnetic chitosan NPs can provide excellent biocompatibility, biodegradability, non-toxicity and water solubility without compromising their magnetic targeting ability [96]. Based on these findings Sun et al. have studied a magnetic targeting chitosan NPs (MTCNPs) which have been prepared and tailored as MRI imaging agents and in which PS - 2,7,12,18-tetramethyl-3,8-di-(1-propoxyethyl)-13,17-bis-(3-hydroxypropyl) porphyrin (PHPP), was encapsulated as photo-activatable agent [97]. The results showed that PHPP-MTCNPs could be used in MRI monitored PDT. Non-toxicity and high PDT efficacy on SW480 carcinoma cells both in vitro and in vivo were achieved with this nano formulation. It is noteworthy that the localization of PHPP-MTCNPs in skin and hepatic tissue was significantly less than in tumor tissue; therefore PDT side-effects could be attenuated using this polymeric NP.
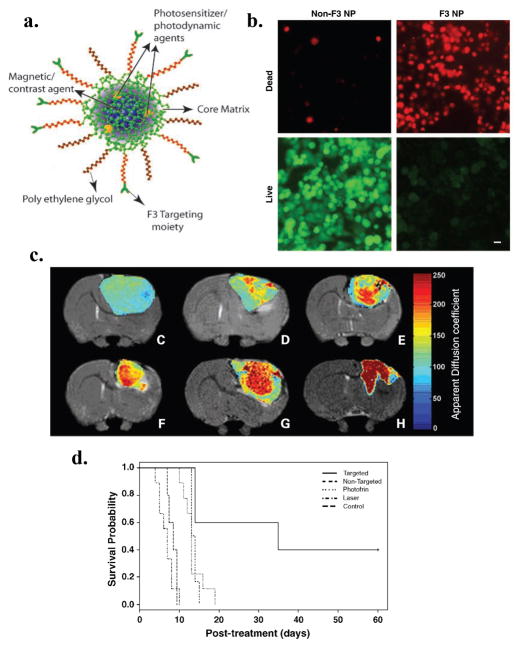
Multifunctional NP (MNP) platforms have been developed by Kopelman and co-workers for in vivo MRI enhancement and PDT of brain cancer [98–100]. The MNPs developed by this group are targeted or enhanced in vivo imaging, diagnostics and therapy. In a recent study by this group, the F3 peptide, which binds to nucleolin expressed on tumor endothelium and cancer cells, was utilized to deliver an imaging agent to brain tumors [100]. The photoactivable agent (Photofrin) and contrast agent (Iron oxide) were encapsulated into amine-functionalized NPs within the core of polyacrylamide matrix. PEG was attached to the surface of the NP along with the targeting peptide (Fig. 5a). After that F3 peptides were conjugated to NP and labeled with Alexa Fluor 594 for optical imaging purpose. To investigate in vitro efficiency of F3 targeted MNP, MDA-MB-435 human breast cancer cells were incubated 4 h with these NPs and irradiated with 630 nm laser light. The resulting combination of light and NPs embedded with Photofrin MNP induced 90% cytotoxicity. Additionally, this study revealed that F3-targeted NPs were bound to, internalized, transported, and concentrated within tumor cell nuclei (Fig. 5b). In vivo studies revealed that iron oxide/Photofrin-encapsulated F3-targeted NPs could be detected in intracranial (i.c.) 9L gliomas using MRI (Fig. 5c). In vivo efficiency of the PDT treatment was monitored after irradiation using T2-weighted and diffusion MRI to follow changes in tumor diffusion for up to 8 days. Based on the correlation of the magnitude of diffusion changes with animal survival [101], F3-targeted NPs were found to have the largest increase in diffusion values and were also found to have the longest survival time over the other treatment groups [100]. The percent of apparent diffusion coefficient showed that there was no statistical difference between the survival of animals treated with Photofrin and those treated with non-targeted Photofrin-encapsulated NP. The T2-weighted MRI image showed an increase of apparent diffusion coefficient 40 days after treatment with the F3-targeted NP, which implied tumor shrinkage. Kaplan-Meier survival plots for the i.c. 9L gliomas tumors showed that PDT based on F3-targeted Photofrin-containing NPs produced a significant improvement in treatment outcome (Fig. 5d).
Figure 5.
Vascular targeted PDT with theranostic agents improves brain cancer therapy as confirmed by MRI a. Schematic representation of the multifunctional nanoparticles. The core of the nanoparticle was synthesized from polyacrylamide, which was embedded with PDT dyes (Photofrin) and/or imaging agents (magnetite/fluorochrome). Polyethylene glycol linker and a molecular address tag (F3 peptide) were attached to target these nanoparticles to cancer cells. b. Cytotoxicity induced by F3-tagged Photofrin-embedded nanoparticles and laser irradiation. MDA-435 cells were incubated 4 hours with nanoparticles with or without F3 tag and irradiated with 1,500 mW of laser for 5 minutes. The Photofrin-mediated cytotoxicity was then monitored by labeling cells with calcein-AM (green, live cells) and propidium iodide (dead, red cells). Bar, 20 μm. c. T2-weighted magnetic resonance images at day 8 after treatment from (C) a representative control i.c. 9L tumor and tumors treated with (D) laser light only, (E) i.v. administration of Photofrin plus laser light, and (F) nontargeted nanoparticles containing Photofrin plus laser light and (G) targeted nanoparticles containing Photofrin plus laser light.The image shown in (H) is from the same tumor shown in (G), which was treated with the F3-targeted nanoparticle preparation but at day 40 after treatment.The color diffusion maps overlaid on top ofT2-weighted images represent the apparent diffusion coefficient (ADC) distribution in each tumor slice shown. d. Kaplan-Meier survival plot for the i.c. 9L tumor groups. Survival curves for brain tumor animals: untreated, laser only, i.v. Photofrin + laser treated, nontargeted nanoparticles containing Photofrin + laser, and F3-targeted Photofrin-containing nanoparticles + laser treated. Adapted from Reddy et al. [100].
In a recent study by our group, L-BPD was used as a PDT agent and Magnevist was used as a MRI agent to monitor tumor development and therapeutic response to PDT in pancreatic cancer xenograft models [102]. To determine pretreatment tumor volume and tumor vascular perfusion volume, MRI images were obtained at 24 to 48 hours pre-PDT and at 48 hours post-PDT. Two tumors cell lines (AsPC-1 and Panc-1) were investigated in this study because of their different levels of aggression. The in vivo and ex vivo data showed that the more aggressive AsPC-1 tumors showed a better response to PDT than the less aggressive Panc-1 tumor. Ex vivo fluorescence image and histological images (H&E stain) were used to assess collateral damage caused by PDT and the results correlate with the in vivo MRI images [102].
Recently Lai et al. have designed and synthesized a tri-functional NP using heavy-transition-metal complexes instead of organic sensitizer [103]. In this study the authors demonstrated that Fe3O4/SiO2 core/shell nanocomposites conjugated by a functionalized iridium complex allow in the same nano-construct the possibility of MRI, phosphorescent labeling and simultaneous singlet oxygen production. The resulting Fe3O4/SiO2(Ir) NP with 55 nm diameter size showed a 62% fluorescence and ~ 10 % phosphorescence quantum yield. In vitro cellular uptake of these nanocomposites were confirmed by MRI. A new class of MSNP was also fabricated by covalent attachment of a PS and by covering their external surface with mannose residues. It was demonstrated in this study that these MSNPs showed a greater in vitro PDT efficiency in MDA-MB-231 cancer cells [104]. The same group also successfully developed a new approach to synthesize multifunctional NPs by using covalent attachment of cyano-bridged coordination polymer Ni2+/[Fe(CN)6]3− to the surface of two-photon dye-doped MSNPs. These hybrid NPs combine effective two-photon excited fluorescence, porosity, high MRI efficiency and superparamagnetic properties [105].
Quantum dots (QD) have gained enormous attention for bio-sensing and bio-monitoring applications. The new generation of QDs have demonstrated promising potential in various applications such as the study of intracellular processes at the single-molecule level, high-resolution cellular imaging, long-term in vivo observation of cell trafficking, tumor targeting, and diagnostics [106]. Bakalova et al. highlighted the potential capacity of QD as candidates for application in PDT and the possibility to conjugate them with appropriate classic PS to increase photosensitizing efficiency [107]. Further, it was also reported that cadmium selenide (CdSe) QD can be used to sensitize a PDT agent via energy transfer mechanism [108]. Recently Bakalova et al. presented a study in which they describe a multimodal QD probe with combined fluorescent and paramagnetic properties, based on silica-shelled single QD micelles with incorporated paramagnetic substances [tris(2,2,6,6-tetramethyl-3,5-heptanedionate)/gadolinium] into the micelle and/or silica coat [109]. The results showed that the developed QD probe is appropriate for in vitro and in vivo tracking of cells and blood vessels via simultaneous use of fluorescent microscopy and MRI.
Tremendous progress of NP-based theranostic PDT has been made in the last few years. Although the majority of researchers are concentrating on developing new formulations and conjugated nano-platforms to enhance selectivity and efficacy, the treatment of deep-seated solid tumors is still a challenge that needs more attention. Recently Cheng et al. have designed a novel PDT agent in which the light is generated by X-ray scintillation of NPs attached with PS [110]. The hypothesis is that the photoactivable agent could be excited without the use of external light source. When the NP-PS conjugates are targeted to tumors and stimulated by X-rays y, the particles generate visible light that can activate the PS for PDT. In this self-lighting PDT regime tumor destruction can be more efficient due to simultaneous PDT and radiotherapy. More importantly, it can be used for deep tumor treatment as X-rays can penetrate deeper through tissue. Further, conjugating this NP with targeting agents could enhance PDT selectivity. Recently this group has reported the synthesis of LaF3:Tb3+–meso-tetra(4-carboxyphenyl) porphine (MTCP) NP targeted conjugates and investigated the energy transfer as well as singlet oxygen generation following X-ray irradiation [111]. The results showed that LaF3:Tb3+-MTCP NP conjugates are efficient photodynamic agents that can be initiated by X-rays at a reasonably low dose. The addition of folic acid to facilitate targeting to folate receptors on tumor cells has no effect on the quantum yield of singlet oxygen production in the NP-MTCP conjugates. The elegance of this novel modality is that it needs lower doses of radiation to produce singlet oxygen and could be used in medical imaging to diagnose diseases.
3. Photothermal therapy and imaging for cancer
Photothermal therapy (PTT) is a treatment regime involving irradiation of diseased tissue with electromagnetic radiation (VIS-NIR light) to cause thermal damage. Unlike PDT where ROS are generated by excitation of a PS, in PTT the laser energy is absorbed by the photo-absorbers and is converted to heat. PTT can cause biological changes ranging from protein structural changes to carbonization of the tissue. During PTT the temperature rises to anywhere between 45 °C to 300 °C and the therapeutic effects can be obtained at sufficient depths using NIR radiation. PTT like PDT brings additional specificity to the therapeutic technique as only the diseased tissue is irradiated with light while the surrounding benign tissue is minimally damaged. This spatial specificity and the minimal-invasiveness make PTT an attractive therapeutic modality as compared to open surgery or other invasive therapeutic procedures. In PTT either continuous wave or pulsed lasers are used for tissue irradiation. In case of continuous wave lasers, sufficient laser energy needs to be deposited in the target area before heat loss occurs in the tissue due to blood perfusion. With pulsed lasers, intense heat is built up during PTT as the pulse width used is shorter than the thermal relaxation time of the tissue (thermal confinement condition) [112]. In either case, the laser parameters need to be chosen appropriately to obtain effective thermal therapeutic response. In addition, the laser illumination needs to be chosen at a wavelength where the diseased tissue has higher absorbance than the surrounding tissue i.e., presence of more endogenous chromophores such as hemoglobin and melanin or specific accumulation of photo-absorbers such as NIR dyes in the diseased tissue. Several non-photosensitizing dyes have been introduced to increase the spatial specificity of PTT [113]. Most of these dyes have absorption greater than 600 nm enabling the treatment of deeply situated pathologies. Moreover, as in the case of PDT, the dyes have higher optical absorbance in the “photothermal-therapeutic” window between 600–1000 nm, a range in which the absorption of endogenous chromophores is low. For example, indocyanine-green (ICG) based PTT was used to treat acne vulgaris and the treatment showed significant improvement in 80% of the patients [114]. Similar to PDT photosensitizers, photobleaching of dye molecules is a major limitation in PTT. The advent of non-photobleaching plasmonic metal NPs has enhanced the photodiagnostic and phototherapeutic strategies used for detection and treatment of tumors and infections due to their unique photophysical properties. Especially the development of gold nanoshells by Halas group has further enhanced the efficacy of PTT due to NIR absorption properties of nanoshells [115]. El-sayed et al. have shown the use of gold nanorods for effective treatment of cancer cells [116]. Plasmonic GNPs are excellent PTT agents as they have three to five orders of magnitude higher absorbance than endogenous chromophores and NIR dyes. Moreover, the optical properties of GNPs can be modified by varying their shape, size, coating etc. The rapid heating of non-photobleaching plasmonic NPs has also lead to reduction in treatment time of PTT.
Image guided PTT procedure will enhance therapeutic outcome especially in cancer diagnosis and therapy because 1. Imaging will aid in identifying the precise location of the tumor, 2. Guide and monitor spatial and temporal changes in temperature and tissue morphology during therapeutic procedures and 3. Evaluate the response of the tumor to therapy immediately after therapy procedure and 4. Evaluate the patients for resurgence of the tumors after the therapeutic procedures. A theranostic agent has the potential to be used in one or more of the steps listed above in image guided PTT. Specifically in this section we will review PTT agents that enhance contrast in various imaging modalities such as optical, ultrasound, magnetic resonance imaging and ionizing imaging modalities such as X-ray.
3.1 Optical imaging
PTT agents such as NPs and dyes exhibit intense and narrow optical extinction bands making them ideal contrast agents for optical imaging. Indeed the combination of PTT with various optical imaging modalities is seamless. For example ICG is used for fluorescence imaging as well as PTT. However ICG has short lifetime and is rapidly cleared from the body. To increase the uptake of ICG in tumor location Yu et al. encapsulated ICG in 120-nm polyallylamine capsules [117]. In vitro studies with confocal fluorescence imaging were performed to evaluate the phototherapeutic response of the ICG nanocapsules. A recent review by Jiang et al. featured various fluorescent NPs that were used simultaneously for optical imaging and cancer therapy [118].
In vitro demonstration of the PTT efficacy using various gold based theranostic agents such as nanospheres [119–121], nanoshells [122], nanorods [116], nanocages [123] and nanocubes [124] was performed using traditional optical microscopy techniques such as darkfield imaging, confocal fluorescence imaging etc. Hybrid nanosystems such as silver-gold dendrites [125] and supramolecularly assembled gold nanospheres [126] also show NIR theranostic properties. Novel imaging techniques such as two photon imaging [127] and photothermal imaging [119] have also been used to image cells in conjunction with PTT. One of the first successful in vivo demonstrations for combined optical imaging and PTT using plasmonic NPs was shown by Gobin et al. [128]. Specifically NIR absorbing gold nanoshells were used as dual function theranostic agent for both imaging and cancer therapy. Optical coherence tomorgraphy (OCT), a methodology based on optical backscattering of tissue constituents, was used to monitor uptake of nanoshells in the tumor. Gold nanoshells enhanced the scattering signal for OCT imaging while retaining their photothermal properties i.e., the GNPs can be molded to have high absorption and scattering properties. The results of their therapeutic study showed approximately 40% increase in survival rate in mice that underwent PTT therapy using gold nanoshells as compared to the control study groups. In another in vivo mouse study by Dickerson et al. showcased the potential curative and adjunctive applications of NIR plasmonic gold nanorods [129]. Subcutaneous squamous cell carcinoma xenografts were grown in nude (nu/nu) mice and particles were selectively delivered to tumors by both direct and intravenous injection. In vivo imaging of PEGylated gold nanorod accumulation was monitored by attenuation of NIR transmission at 808 nm using a custom-built CCD device array. PTT was performed with continuous wave laser (0.9–1.1 W/cm2, 6 mm dia., 10 min). The results of the study showed approximately 5 fold decrease in tumor volume as compared to control mice injected with saline solution.
In addition to plasmonic metal NPs and NIR dyes, nanosystems such as carbon nanotubes (CNT) have also been used as theranostic agents. Recently Zhou et al. reported an in vivo photothermal study using single-walled carbon nanotubes (SWNTs) tagged with fluorescently labeled folate antibody as theranostic agents [130]. SWNTs have a high optical absorbance in the NIR region (optical absorption peak at 980 nm) and the results of the study showed the potential of SWNTs combined with suitable tumor markers for selective photothermal cancer treatment. Torti et al. showed the feasibility of using multi-walled carbon nanotubes on cancer cells [131]. Overall, as existing optical probes and new optical in vivo imaging techniques make their way to the clinic in the next few years after through characterization, it will not be uncommon to see routine clinical procedures that incorporate such theranostic probes for cancer diagnosis and therapy.
3.2 Ultrasound-based imaging
Ultrasound imaging is an appealing modality for temperature monitoring during PTT as it is a relatively inexpensive, noninvasive and portable imaging technique. Ultrasound has also been used by Emelianov and coworkers to guide and monitor PTT with GNPs as it has the ability to track spatial and temporal changes in temperature increase throughout the region of interest [132]. During these processes, the elasticity of the tissue is also affected which can be evaluated with ultrasound based elasticity imaging [133]. Recently another study from the same group showed the progression of photothermal treatment by quantifying the mechanical properties of tissue using a novel ultrasound based technique namely magneto-motive ultrasound [134]. Therefore, a comprehensive guidance and assessment of the PTT may be feasible through various ultrasound based imaging techniques [135].
Photoacoustic imaging (optoacoustic or thermoacoustic imaging) is an ultrasound based imaging modality with inherent advantage of high contrast optical imaging techniques. More specifically, it provides information on the optical absorption properties of tissue at spatial resolution on par with ultrasound imaging [136–138]. Photoacoustic transients are generated when nanosecond duration laser pulses interact with the tissue causing thermoelastic expansion. The generated pressure wave is detected by an ultrasound transducer and is digitally processed to obtain a photoacoustic image. Thus, by analyzing photoacoustic images captured at multiple wavelengths, the distribution of optical absorption properties of the tissues can be visualized. Optical backscattering from the tissue limits the penetration depth in optical imaging techniques unlike photoacoustic imaging that is only limited by the penetration of light into tissue. Therefore, photoacoustic technique can image deeper since sound is detected instead of light. In addition, greater penetration depth in tissue can be achieved using NIR wavelengths because endogenous chromophores such as blood absorb less light in the NIR range. The availability of various NPs systems (carbon nanotubes, ICG pebbles, different shapes of gold or silver NPs) that have three to five orders of higher optical absorption has increased the potential of molecular photoacoustic imaging for cancer diagnostics. Many groups have published studies on NP enhanced molecular photoacoustic imaging, however only few of them reported the combination of photoacoustic imaging with PTT. The optical properties of photoacoustic contrast agents such as GNPs also entail them to be good therapeutic agents, given the photothermal stability of NPs. For example, Yun-sheng et al. have shown coating silica gold nanorods increased their thermal stability as compared to PEGylated or CTAB coated nanorods [139].
Combined ultrasound and photoacoustic imaging can be used to plan, guide and monitor the outcome of the PTT. In this approach, combined imaging is first used prior to surgery to identify size, location and functional activity (uptake of the optical contrast agent) of the tumor. Then the ultrasound images obtained during therapy are used to generate temperature maps of the tissue using speckle tracking algorithms. In addition to ultrasound measurements of the temperature, photoacoustic imaging can be used to monitor the temperature changes. The efficacy of using gold nanorods simultaneously as photoacoustic contrast agents and phototherapeutic agents is demonstrated by Shah et al. Gold nanorods (GNR) (Fig. 6a) were directly injected into the subcutaneous tumor in nude mice prior to performing PTT. In vivo ultrasound and photoacoustic imaging performed after the injection showed the presence of NPs in the tumor which was later confirmed by silver staining of tissue slices (Fig. 6b). Photoacoustic thermal imaging performed showed significant temperature elevations within the tumor in response to laser irradiation suggesting thermal damage (Fig. 6c). In addition, tumor necrosis was confirmed by histological assessment [135].
Figure 6.
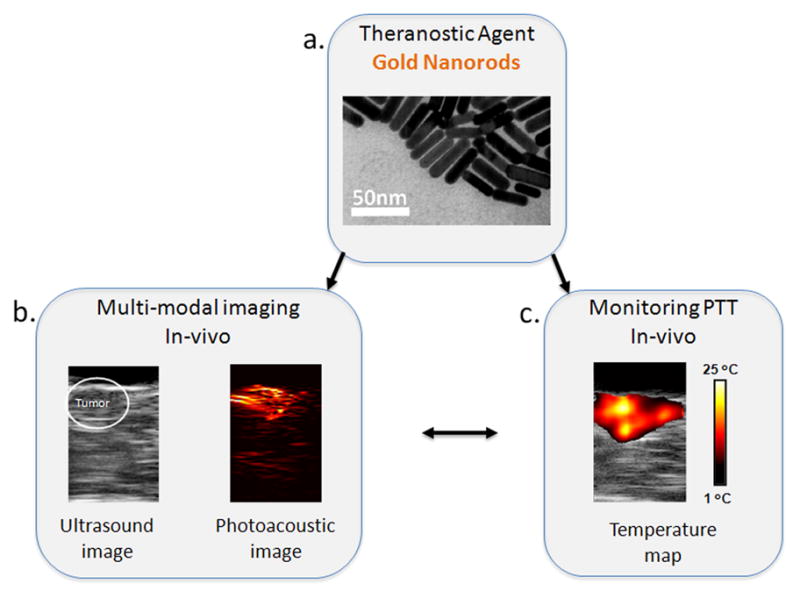
Gold nanorods, a theranostic agent, used for combined ultrasound and photoacoustic imaging and photothermal therapy. a. the TEM images of gold nanorods. b. Ultrasound and photoacoustic images of mouse tumor injected with gold nanorods. The tumor region is shown in white inset in the ultrasound image. The photoacoustic image shows higher contrast in the tumor area due to accumulation of gold nanorods. c. Thermal image of a subcutaneous tumor in nude mouse. During the PTT procedure approximately 25°C temperature rise was observed in the tumor. Adapted from Mallidi et al. [135].
In addition to being photothermal agents, carbon nanotubes have also shown promise as photoacoustic contrast agents for imaging of tumors and infections because they offer high resolution and allow deep tissue imaging. However, carbon nanotubes have relatively low absorption coefficients than the GNPs at near-infrared wavelengths in addition to being more toxic. To overcome this limitation, Kim et al. used gold coated carbon nanotubes (GNT) as photoacoustic and photothermal contrast agents with enhanced near-infrared contrast for targeting lymphatic vessels in mice using extremely low laser fluence levels [140]. The gold coated carbon nanotubes had two orders of magnitude higher absorbance than non-coated nanotubes. In another study by the group, the GNTs were used for ultrasensitive molecular detection and treatment of circulating cancer stem cells [141]. The authors have used a combination of multi-spectral photoacoustic detection and PTT which enabled in vivo detection and treatment of cancer stem cells using the same laser source. Recently, the same group also reported quantum dots as photoacoustic, fluorescent and photothermal contrast agents, thus extending the traditional application of quantum dots beyond being only molecular fluorescent agents. Further in vivo studies are required to validate the usability of these multi-functional quantum dots. ([142]. Ultrasound based PA imaging can provide information on the optical properties of the tissue, the distribution of contrast agents in the tissue and hence guide the PTT therapeutic procedures and finally monitor temperature changes during PTT [143]. When combined with ultrasound imaging all of the above information obtained from PA imaging can be interpreted in context of the anatomical map of the tissue [136]. Overall, the nano-optical contrast agents together with molecular photoacoustic imaging and PTT have opened up new potential for in vivo deep penetrating cancer diagnosis and therapy.
3.3 Magnetic resonance imaging (MRI)
MRI is a useful molecular imaging tool because of its ability to provide anatomical and physiological information simultaneously at high spatial resolution [144]. Being a non-invasive, real-time imaging methodology, MRI was chosen for guiding and monitoring various types of thermal therapies [144–145]. In particular, Hirsch et al. employed MRI to measure temperature during PTT of tumors injected with NIR absorbing nanoshells [115]. The real-time MR temperature imaging (MRTI) provided depth dependence of thermal profiles in irradiated regions. Tumors injected with gold nanoshells and irradiated with NIR light (820 nm, 4 W/cm2, 5-mm spot diameter, <6 min irradiation time) showed a relative temperature increase of approximately 35°C as compared to 15°C in tumors injected with saline. Furthermore, histological examination revealed that MRTI estimation of tissue damage was in good agreement with experimental findings, demonstrating its potential utility in determining tissue damage during therapy. In another study with gold nanoshells by Diagaradjane et al. MRTI was used to monitor mild hyperthermia created in tumors loaded with gold nanoshells using low laser power [146]. The photosensitzation by the mild temperature hyperthermia caused increased tumor perfusion. Using Dynamic contrast enhanced MRI, vascular perfusion in the tumors was also estimated. The DCE-MRI images clearly showed increase in perfusion at the tumor post hyperthermia. MR temperature monitoring during PTT was also used in the study by Bruke et al. In this study multi-walled carbon nanotubes were used as therapy agents [147]. Though carbon nanotubes are good light-heat energy convertors, they donot possess magnetic properties. Only carbon nanotubes for MRI and PT-MR thermometry enables monitoring of heat induction in near real-time and can thus be used to minimize incomplete treatment of tumor margins, a major limitation of current thermal therapies.
Gadolinium forms the basis for many MRI contrast agents despite having low retention time in the body. More recently, super paramagnetic iron oxide particles (SPIOs) have been developed that have high magnetic moment compared to Gd based contrast agents. By controlling the size of the magnetic cores in these NPs, their magnetic properties can be manipulated. SPIO are FDA approved in 1996 and they have been used as MRI contrast enhancers in detecting tumors. Indeed, the efficacy of SPIO NPs as photothermal agents has also explored [148]. However, SPIO NPs have very low optical absorption at NIR wavelength compared to gold or silver NPs. To enhance the photo-theranostic abilities of magnetic NPs, hybrid nanosystems with gold have been proposed. The complexity of these hybrid nanosystems ranged from coating magnetic iron oxide NP with gold [149] to wonton shaped nanosystems containing gold and cobalt [150]. Gold covered cobalt NPs had less toxicity compared to highly toxic cobalt NPs. Apart from gold, magnetic NPs coated with graphite carbon were also used for PTT enhancement. The magnetic and optical properties of various hybrid theranostic agents is listed in Table 2. In vitro cell studies were performed using these hybrid multifunctional nanosystems showcasing the photothermal and magnetic properties. The stability of the hybrid nanosystems in terms of storage, biostability, thermal stability still need to be examined and further in vivo studies are required for the NPs to create significant impact in cancer diagnosis and therapy.
Table 2. Hybrid theranostic NPs with potential for combined MRI and PTT.
The empty spaces in the table indicate unavailability of the data.
| Nanoparticles | Magnetic Properties | Optical absorption (nm) | References | ||
|---|---|---|---|---|---|
| r1 mM−1s−1 | r2 mM−1s−1 | emu/g | |||
| Iron oxide core – gold shell | 23.5 | 540 | [149] | ||
| Magnetic gold nanoshell | 251 | 700–800 | [151] | ||
| Magnetic core-silica and gold coating | 0.65 | 152 | 825–910 | [152] | |
| Magnetic- gold composite | 465 | 2 | 820 | [153] | |
| Nano dumbbell | 80 | 520–538 | [154–155] | ||
| Nano roses | 219 | 34 | 730 | [156] | |
| Nano Wantons | 400–800 | [150] | |||
| Au nanoshell – ICG- FeNPs | 520–600 | [157] | |||
| PLGA/Mn/Au composite | 800–810 | [158] | |||
| FeCo-graphite carbon coating | 70 | 644 | 215 | 700–1100 | [159] |
3.4 Ionizing imaging modalities
Apart from non-ionizing imaging modalities such as MRI and ultrasound, ionizing imaging techniques such as X-ray computed tomography are also widely used for screening and diagnosis of various pathologies including cancer. The ability to visualize deep structures in the body is the main advantage of ionizing imaging modalities. Moreover, a recent review by Gupta et al. highlighted the significant use of PET/CT in planning and guiding radiotherapy in lung cancer, head and neck cancer and cervical cancer [160]. Recently the study performed by Hainfeld et al. showcased gold nanospheres as excellent X-ray contrast agent, in addition to enhancement of radiotherapy in vivo [161–162]. Gold has higher atomic number and a higher absorption coefficient than standard iodinized contrast agents and hence provides a 2–2.7 folds greater contrast per unit weight. Therefore lower concentration of contrast agent can be used leading to higher sensitivity of the imaging technique. On the other hand, Melancon et al. developed radiolabeled GNPs to observe the accumulation of targeted particles in the tumor [163]. Specifically immuno-hollow gold nanospheres targeted to EGFR have been shown to selectively bind to EGFR-positive cells and destroy these cells via a photothermal effect in vitro. Melancon et al. predict the usage of these EGFR targeted NPs to increase efficacy of PTT as their initial mouse experiments showed approximately 7% of the injected dose reached the tumor site. The hollow gold nanospheres used in the study did not display their “theranostic” capabilities (i.e., no imaging on tissues or whole body was performed for diagnosis or treatment monitoring, however by utilizing a γ counter, in vivo tissue distribution of the hollow nanospheres was determined.
The theranostic nature of GNPs was used in a study done by Maltzahn et al. where the gold nano-antennas (gold nanorods of dimension 13 nm × 47 nm) were employed as X-ray contrast agent and photothermal therapeutic agent [164]. Briefly, the study involved development of PEGylated gold nanorods for longer half-life in blood (approximately 17 hours). The nanorods acted as antennas for accepting the external applied photo energy. The PEGylated nanorods exhibited greater photothermal heat generation than gold nanoshells due to enhanced optical absorption. An integrated approach was developed for laser irradiation protocol using the biodistribution of NPs obtained from X-ray CT images. These quantitative protocols enabled estimation of temperature rise during PTT. Minimal damage to the surrounding benign tissue was seen due to the specificity of the therapy procedure that is obtained on two levels – 1. Higher accumulation of nanorods in tumor due to enhanced permeation and retention effect and 2. Laser illumination at the tumor site instead of whole body illumination. Indeed, the study showcased highly selective ablation of the subcutaneous MDA-MB-435 tumors (breast adenocarcinoma) in mice that were injected with 20 mg/kg PEGylated gold nanorods and laser irradiation (2 W/cm2 at 810 nm wavelength). The results of the study are summarized in Figure 7. Maltzahn et al. anticipate using gold nano-antennas together with PTT and whole subject X-ray CT as a route towards personalized diagnosis, radiation planning, therapy optimization and monitoring response [164]. In another study by Lu et al. melanoma-targeted hollow gold nanospheres (HAuNS) were used for selective photothermal ablation (PTA) [165]. The surface of HAuNs had a chelation agent, S-2-(4-[5-Dithiolane-3-pentanamide]benzyl)diethylenetriamine pentaacetic acid (DTPA-TA) which facilitated PET imaging. PTA effect of the NPs was evaluated functionally by [18F] fluorodeoxyglucose positron emission tomography ([18F]FDG-PET). The HAuNS were specifically taken up by melanoma cells and were successfully treated using low dose NIR laser irradiation. The success of the PTT was confirmed by histological and [18F]FDG-PET evaluation.
Figure 7.
Gold nanorods as theranostic agents for in-vivo X-ray CT imaging and PTT therapy. a and b show the feasibility of using nanorods as X-ray CT contrast agent. Clearly the images in b identify the location of the tumor marked by arrows. c and d showcase the feasibility of using nanorods as photothermal agents. d. shows the temperature rise in the tumor injected with gold nanorods during PTT. Finally, the in-vivo mouse survival studies indicate mice injected with PEGylated nanorods and undwernet NIR laser irradiation had greater survival rate than the control groups. Adapted from von Maltzahn et al. [164].
In vivo animal studies performed by various groups suggest that the future of PTT with hybrid plasmonic NPs is promising. Recently Linder et al. have published phase I clinical trials on treating low risk and low volume prostate cancer with image guided focal photothermal ablation. MRI and ultrasound imaging were used to guide the therapy and monitor the response [166]. The histological analysis also showed cancerous regions could be ablated while surrounding tissue underwent minimal adverse effects. These studies did not involve any theranostic agents but showcases the need for image-guided therapy. Overall, further studies are required to demonstrate the effectiveness of this treatment concept with the multi-functional theranostic agents.
Currently, the main hurdle for new theranostic agents is to deliver the diagnostic/therapeutic probe with high molecular specificity. Indeed, researchers are aiming at targeting multiple biomarkers and use multiplex imaging techniques to facilitate early detection of cancer thereby leading to better therapeutic outcome. Multi-functional probes are being developed with an ambitious aim to diagnose and treat cancer at an early stage. In addition there is also thrust for development of multi-modal imaging techniques that can provide complementary information regarding the tumors. For example, Davis et al. have recently reported an MRI-coupled fluorescence tomography technique to quantify EGFR activity in brain tumors [167]. MR provided the anatomical information of the tumor while the florescence tomography provided information on the functional activity of the NIR dye. For these theranostic agents to obtain a foothold in the clinic, they will need to be characterized thoroughly on various issues such as biocompatibility, toxicity and stability. For example, stability of the plasmonic NPs in vivo, loss of polymer coating and leaching out of metal ions need to be investigated further. In addition, the theranostic probes should provide highly sensitive contrast enhancement in diagnostic images. A highly biocompatible, stable, molecular specific theranostic agent has the potential to elevate minimally invasive phototherapeutics as a viable method for cancer treatment. A promising future for cancer theranostics is feasible with the availability of combined imaging techniques and multi-modality, multi-functional, photo-triggered theranostic probes.
4. Photo-triggered drug release and imaging for cancer
The irregular drug release due to variation in physiological conditions and notched distribution of drug in body leading to adverse reactions are the major drawbacks of conventional drug delivery systems (DDS). Externally activated DDS that can trigger release of a drug at the “right” site and at a rate that adjusts in response to the progression of the disease are attractive [14]. Biocompatible materials sensitive to certain physiological variables or external physicochemical stimuli, often referred to as “intelligent” or “smart” materials, can be used for achieving this aim. Light-responsiveness is receiving increasing attention owing to the possibility of developing materials sensitive to innocuous electromagnetic radiation (mainly in the UV, visible and near-infrared range), which can be applied on demand at well delimited sites of the body. Some light-responsive DDS are of a single use (i.e., the light triggers an irreversible structural change that provokes the delivery of the entire dose) while others able to undergo reversible structural changes when cycles of light/dark are applied, behave as multi-switchable carriers (releasing the drug in a pulsatile manner) [14]. The high level of control which can be exerted on light delivered to a molecule in terms of wavelength, duration, intensity, and location can be exploited through a light-controlled drug liberation reaction to give control of the quantity of drug released (the dose), the timing of the release event, and its location. Importantly, this control operates potentially at the level of the single molecule, allowing conjugate-incorporated media to act as drug dosing devices, with potential for dosing controlled at the molecular scale. Two of the most common routes used for light-triggered drug release involve either photochemical or a photothermal mechanism of release and are reviewed below. For completeness, we will also discuss theranostic agents involving optical imaging in combination with therapy that does not involve use of light as a trigger. A scheme illustrating these mechanisms of light triggered drug release is shown in Figure 8.
Figure 8.
Photo-activatable theranostic agents for triggered release of drug. a. Use of light for gene delivery using the principle of photochemical internalization. Adapted from Nishiyama et al. [170] b. NIR light triggered release of drugs trapped in PLGA microspheres that also encapsulate hollow gold nanoshells. NIR light is absorbed by the HAuNSs and is converted to heat which triggers release of the encapsulated drug. Adapted from You et al. [171] c. Use of fluorescence optical imaging for highly selectively tumor imaging with an activatable fluorescence probe–antibody conjugate. The probe is nonfluorescent when outside the tumor cells. After internalization by endocytosis, the probe is accumulated in late endosomes or lysosomes, where the acidic pH activates the probe, making it highly fluorescent which is captured by optical imaging. Adapted from Urano et al. [172].
4.1 Photochemically triggered drug release and imaging
The utilization of macromolecules in therapy of cancer and other diseases is becoming increasingly relevant. Recent advances in molecular biology and biotechnology have made it possible to improve targeting and design of cytotoxic agents, DNA complexes, and other macromolecules for clinical applications [168]. To achieve the expected biological effect of these macromolecules, in many cases, internalization to the cell cytosol is crucial. At an intracellular level, the most fundamental obstruction for cytosolic release of the therapeutic molecule is the membrane-barrier of the endocytic vesicles. There is a need for DDS that can enhance cytosolic delivery of anti-cancer drugs trapped in the endo-lysosomal compartments. Kristian Berg and co-workers [168] have used photochemical mechanisms to release drugs that are often trapped and destroyed by the endo-lysosomal compartments. Photochemical internalization (PCI) is a novel technology for release of endocytosed macromolecules into the cytosol. Exposure of cells to specific PSs followed by light irradiation results in the transfer of agents from the endocytic compartment into the cytosol. This technology is under development for clinical use in treatment of soft tissue sarcomas and other solid tumors. PCI has been shown to potentiate the biological activity of a large variety of macromolecules and other molecules that do not readily penetrate the plasma membrane, including type I ribosome-inactivating proteins (RIPs), gene-encoding plasmids, adenovirus, oligonucleotides, and the chemotherapeutic bleomycin [169]. PCI of an epidermal growth factor receptor (EGFR)-targeted protein toxin (Cetuximab-saporin) linked via streptavidin-biotin for screening of targeted toxins as well as PCI of nonviral polyplex-based gene therapy have also been described[169]. PCI of other gene therapy vectors (e.g., viral vectors), peptide nucleic acids (PNA), small interfering RNA (siRNA), polymers, NPs, and some chemotherapeutic agents have been reviewed in detail elsewhere [168–170]. A few representative examples demonstrating the potential of PCI are reviewed below.
Norum et al. have shown that PCI may release endocytosed bleomycin (BLM) into the cytosol by photochemical rupture of the endocytic vesicles [173]. In this study, the human fibrosarcoma xenograft HT1080 was transplanted into the leg muscle of athymic mice. The PS disulfonated aluminum phthalocyanine (AlPcS2a) and BLM were systemically administrated 48 h and 30 min, respectively, prior to light exposure at 670 nm (30 J/cm2). They compared the treatment response to AlPcS2a-PDT and AlPcS2a-PDT in combination with BLM (i.e. PCI of BLM) in an orthotopic, invasive and clinically relevant tumor model and to explore the underlying response mechanisms caused by PDT and PCI of BLM. The treatment response was evaluated by measuring tumor growth, contrast-enhanced magnetic resonance imaging (CE-MRI), histology and fluorescence microscopy. The results show that PCI of BLM is superior to PDT in inducing tumor growth retardation and acts synergistically as compared to the individual treatment modalities. The CE-MRI analyses 2 h after AlPcS(2a)-PDT and PCI of BLM identified a treatment-induced nonperfused central zone of the tumor and a well-perfused peripheral zone. While there were no differences in the vascular response between PDT and PCI, the histological analyses showed that PDT caused necrosis in the tumor center and viable tumor cells were found in the tumor periphery. PCI caused larger necrotic areas and the regrowth in the peripheral zone was almost completely inhibited after PCI. The results indicate that PDT is less efficient in the tumor periphery than in the tumor center and that the treatment effect of PCI is superior to PDT in the tumor periphery [173].
PCI has also been shown to enhance the treatment effect of targeted therapeutic macromolecules. Selbo et al. have combined PCI with a recombinant, targeted therapeutic and demonstrated that this non-invasive, multimodal approach enhances the in vivo efficacy without sacrificing selectivity or enhancing systemic toxicity [174]. They used a recombinant single-chain fusion construct scFvMEL/rGel, composed of an antibody targeting the progenitor marker HMW-MAA/NG2/MGP/gp240 and the highly effective toxin gelonin (rGel). They have demonstrated enhanced tumor cell selectivity, cytosolic delivery and anti-tumor activity by applying PCI of scFvMEL/rGel. PCI performed by light activation of cells co-incubated with scFvMEL/rGel and the endo-lysosomal targeting PSs AlPcS2a or TPPS2a resulted in enhanced cytotoxic effects against antigen-positive cell lines, while no differences in cytotoxicity between the scFvMEL/rGel and rGel were observed in antigen-negative cells. Mice bearing well-developed melanoma (A-375) xenografts (50–100 mm3) were treated with PCI of scFvMEL/rGel. By 30 days after injection, approximately 100% of mice in the control groups had tumors >800 mm3. In contrast, by day 40, 50% of mice in the PCI of scFvMEL/rGel combination group had tumors <800 mm3 with no increase in tumor size up to 110 days. PCI of scFvMEL/rGel resulted in a synergistic effect (p<0.05) and complete regression (CR) in 33% of tumor-bearing mice (n = 12). Such an approach warrants further evaluation of its clinical potential [174].
Multiple drug resistance (MDR) is a problem that seriously reduces the efficacy of many chemotherapy agents. One mechanism for MDR is increased acidification of endocytic vesicles and increased cytosol pH, so weak base chemotherapeutic agents, including doxorubicin, are trapped in endocytic vesicles and exhibit a drug resistant phenotype [175]. Treatments that selectively reverse this accumulation may therefore reverse the MDR phenotype. Lou PJ et al. evaluated the potential of PCI for release of doxorubicin from endocytic vesicles in MDR cells [175]. Two breast cancer cell lines, MCF-7 and MCF-7/ADR (the latter resistant to doxorubicin), were selected. They were found equally sensitive to photochemical treatment with the photosensitiser TPPS2a (disulfonated meso-tetraphenylporphine) and light. On exposure to doxorubicin alone, the IC50 (drug concentration for 50% reduction in colony formation) was 0.1 μM for MCF-7 and 1 μM for MCF-7/ADR. After PCI (photochemical treatment followed by doxorubicin), the IC50 concentration was 0.1 μM for both cell lines. Comparable changes were seen with assay of cell viability using 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). On fluorescence microscopy in MCF-7/ADR cells, doxorubicin localized in granules identified as lysosomes. After PCI, doxorubicin was released into the cytosol and entered cell nuclei, as was seen in MCF-7 cells without PCI. PCI reversed the MDR phenotype of doxorubicin resistant breast cancer cells by endo-lysosomal release of the drug[175]. The technique is a promising new approach to tackling the problem of MDR.
Febvay et al. have presented a method for precise spatial and temporal control over cytosolic delivery of compounds that would otherwise be cell impermeable by using PCI mechanism within a cell targetable mesoporous silica nano-carrier [176]. Size-tunable (30–200 nm), highly monodispersed mesoporous silica NPs that can be biofunctionalized and targeted to specific cell surface proteins were first developed As an example, they delivered a cell-impermeable fluorescent compound exclusively to the cytosol of multidrug resistant cancer cells in a mixed population that was verified by confocal microscopy. These NPs can be loaded with a wide variety of compounds and can mediate cytosolic release of cell-impermeable molecules in P-gp-expressing cells via light-mediated endosomal breakage. Such an approach is promising in expanding the pharmacological arsenal for cell-impermeable compounds to overcome multidrug resistance. In addition, these novel NPs may be useful vectors for highly specific protein and nucleic acid delivery [176].
Recently, light has also been used to release therapeutic agents from delivery systems or to activate cytotoxic drugs. Pashkovskaya et al. have studied photosensitized damage to liposome membranes by using different dye-leakage assays based on fluorescence dequenching of a series of dyes upon their release from liposomes [177]. Their work demonstrated the participation of oxidative damage to membrane lipids in the photosensitized membrane destruction which could play a role in many of the toxic as well as therapeutic effects of photodynamic action [177]. In particular, photosensitized damage to lipids in liposome membranes was shown to manifest itself in the formation of pores with rather high selectivity. These pores proved to be permeable not only for fluorescent markers but also for PSs, e.g., AlPcS3. Thus, the basis of targeted delivery of PSs via encapsulation and subsequent light-induced release from liposomes as an approach for PDT was experimentally confirmed in a very simple model system. Moreover, the present data provide the rationale for selective liposome-based drug delivery [177].
Further, liposomes made with light-sensitive lipids have been developed to release their contents when exposed to near-UV light due to changes in membrane permeability [178]. Photo-sensitive liposomes have been studied for a few decades and various photo-sensitive triggers have been developed so far. A recent review by Wang et al. offers an overview of the different photo-triggering mechanisms for controlled pulsatile content release from liposomes, which have the potential of finding clinical applications as intelligent DDS [178]. Lu et al. have reported the use of nanoimpeller-controlled mesostructured silica NPs to deliver and release anticancer drugs into living cells upon external command [179]. Using light-activated mesostructured silica (LAMS) NPs, luminescent dyes and anticancer drugs are only released inside of cancer cells that are illuminated at the specific wavelengths that activate the impellers. The quantity of molecules released is governed by the light intensity and the irradiation time. Human cancer cells (a pancreatic cancer cell line, PANC-1 and a colon cancer cell line, SW480) were exposed to suspensions of the particles and the particles were taken up by the cells. Confocal microscopy imaging of cells containing the particles loaded with the membrane impermeable dye, propidium iodide (PI), shows that the PI is released from the particles only when the impellers are photoexcited, resulting in staining of the nuclei. The anticancer drug camptothecin (CPT) was also loaded into and released from the particles inside the cells under light excitation, and apoptosis was induced. Intracellular release of molecules is sensitively controlled by the light intensity, irradiation time, and wavelength, and the anticancer drug delivery inside of cells is regulated under external control. The delivery and release capability of light-activated mesostructured silica particles containing molecular impellers is the first step towards a novel platform for the next generation of nanotherapeutics with both spatial and temporal external control [179].
Dvir et al. have recently described the use of light to target NP binding (as compared to single release events) in specific illuminated areas [180]. The basic design is a drug-loaded NP whose surface is covalently modified with a targeting moiety consisting of an avid but nonspecific ligand that is rendered biologically nonfunctional (“caged”) and prevented from binding by chemical modification with a photoremovable protecting group. The caging group is removed at the desired site by illumination. For proof of concept, the authors used commercially available carboxylated polystyrene NPs with diameters of 328 ± 2 nm as model NPs. The nonspecific ligand was a small peptide YIGSR, an amino acid sequence in laminin that is crucial for adhesion to integrin β1 on the cell membrane of a broad range of potential target cell types including stromal and endothelial cells, which are present in all tissues. The biological activity of YIGSR can be greatly attenuated by mutation or deletion of tyrosine. Therefore caging the tyrosine with 4,5-dimethoxy-2-nitrobenzyl (DMNB) would inactivate the peptide until the cage was removed by illumination. This work by Kohane and co-workers is the first example of a targeting system capable of binding NPs to cells selectively upon illumination [180]. In contrast to previous work where NPs have been triggered to produce a single drug release event by light, this approach results in the deposition of a sustained release system at the desired site. Another important point is that this system allows tissue targeting without specific markers (since the receptor for the ligand used in these experiments is ubiquitous throughout the body), provided the tissue can be illuminated. Furthermore, this approach could be used with specific ligands to perhaps further enhance specificity. The potential applicability of caging to a spectrum of potential ligands is seen in the fact that it has been used to inactivate a wide range of biomolecules including peptides, enzymes, nucleotides, mRNA and DNA [180]. In vivo work will require the use of NPs with direct applicability in drug delivery, such as liposomes and biodegradable polymeric nanospheres. Other modifications may also be necessary, such as surface optimization to minimize uptake by the reticuloendothelial system, for example, by PEGylation. The wavelengths at which DMNB can be made to uncage (350–400 nm) limits this particular application of this technology to areas of the body that can be illuminated directly, like for skin cancers. However, the use of lasers and minimally invasive fiber-optic tools, and the development of new caging groups that respond to wavelengths with better tissue penetration such as near-infrared, may make direct targeting of deep tissues possible.
Drug release triggered by two-photon excitation in NIR using FRET has not yet been developed despite progress in photo-triggered drug release. The approach of two-photon induced intraparticle FRET for drug release, based on the use of two-photon fluorescent nano-assembly as a donor and a photosensitive linker as an acceptor, offers a novel design for developing formulations of smart drug-carrier nanoassemblies for more superior control over the location and the onset of drug release [181]. Banerjee et al. have developed a multifunctional NP that can efficiently up-convert the energy of NIR light for triggering drug release by cleavage of a photosensitive linker [181]. The NP and the underlying nanophotonics approach described in this work represents a significant breakthrough in developing a two-photon-triggered drug delivery vehicle that combines imaging, sensing and therapy and drug targeting at the same time [181].
Light-triggered theranostic nanocarriers could revolutionize cancer chemotherapy. Most chemotherapeutic compounds are nonspecific and are taken up by all cell types – and this nonselective nature of the agents usually causes severe toxicity [182]. The drug nanocarriers reviewed here have the potential to dramatically improve the treatment of cancer by selectively providing the optimum dosage of the drug at the tumor site, ultimately even to the individual tumor cell. However, most of these stimuli-responsive DDS are indirectly triggered, as they induce a macroscopic change in the matrix into which the drug is incorporated [13–14]. To date, no method to externally and directly trigger precise drug doses to a targeted area has been demonstrated. McCoy et al. show a molecular method for light-triggered drug delivery of various drug classes using low energy, long wavelength radiation, with the drug dose being precisely controlled by the duration of applied light[183]. Together with an appropriate polymer matrix, the molecular unit acts as a molecule-scale drug dosing device, with control potentially at the level of a single drug molecule [13].
4.2 Photothermally triggered drug release and imaging
Combination therapies are commonly employed in a wide range of cancer treatments as they have better therapeutic response compared to monotherapies. In particular, hyperthermia can increase the concentration of an administered therapeutic NP in a tumoral region by increasing blood flow and vessel permeability. Cavitation bubbles produced following irradiation of GNPs can also be used for intracellular drug delivery of cell-impermeable biomolecules like antibodies. For e.g., Yao et al. recently demonstrated intracellular delivery of an antibody following light irradiation using GNPs [184]. In this work lymphoma cells were targeted with GNPs that were conjugated to anti-CD-30 antibody. Pulsed laser irradiation led to transient permeabilization of cell membranes and this lead to subsequent internalization of a secondary dye-labeled antibody. Delivery of the secondary dye-labeled antibody was confirmed by flow cytometry. The membrane permeabilization only occurred in cells expressing the CD30 receptor. No effect was seen with CD30 negative cells.
Hyperthermia can also enhance drug toxicity in cancer cells that are otherwise resistant to chemotherapeutics [185]. Furthermore, local hyperthermia can improve the accumulation of a drug, which is encapsulated in a thermosensitive carrier [185]. The combination of hyperthermia and chemotherapeutics can, therefore, be employed synergistically to treat high-risk tumors with a goal of total tumor eradication. From a clinical perspective, precise and site-specific heat transfer to a diseased site would improve the safety and efficacy of thermal cancer therapies [185]. Park et al. have demonstrated that a pair of synthetic NPs can work together to detect a diseased site and more effectively deliver chemotherapeutics to the site than individual NP treatments [186]. This system relies on GNR transduction of an external optical signal into a tumor-specific thermal signal that enhances recruitment of circulating drug carriers into the tumor and triggers drug release from the carriers. GNRs localized in tumors can be identified in vivo by their intense SERS signals, making it possible to use these nanoagents in both diagnostic and therapeutic applications and illustrates the potential advantage of dual therapeutic nanomaterials in the precision treatment of more drug-resistant cancers [186].
Photosensitive caged compounds have enhanced our ability to address the complexity of biological systems by generating effectors with remarkable spatial/temporal resolutions. The caging effect is typically removed by photolysis with ultraviolet light to liberate the bioactive species. Although this technique has been successfully applied to many biological problems, it suffers from a number of intrinsic drawbacks [187]. For example, it requires dedicated efforts to design and synthesize a precursor compound for each effector. The ultraviolet light may cause damage to biological samples and is suitable only for in vitro studies because of its rapid attenuation in tissue. Yavuz et al. have addressed these issues by developing a platform based on the photothermal effect of gold nanocages [187]. Gold (Au) nanocages represent a class of nanostructures with hollow interiors and porous walls. They can have strong absorption (for the photothermal effect) in the near-infrared while maintaining a compact size. When the surface of Au nanocage is covered with a smart polymer, the pre-loaded effector can be released in a controllable fashion using NIR irradation. This system works well with various effectors without involving sophisticated syntheses, and is well suited for in vivo studies owing to the high transparency of soft tissue in the near-infrared region. They have demonstrated a platform based on Au nanocages covered with smart polymers for controlled release with NIR light [187]. When combined with optical manipulation, this platform offers many extra advantages such as high spatial/temporal resolution. In addition, Au nanocages are bio-inert and the surface can be readily functionalized with targeting ligands such as antibodies using the gold-thiolate chemistry.
Silica–gold (SiO2–Au) nanoshells are a new class of NPs that consist of a silica dielectric core that is surrounded by a gold shell. These nanoshells are unique because their peak extinctions are very easily tunable over a wide range of wavelengths particularly in the NIR region of the spectrum. Light in this region is transmitted through tissue with relatively little attenuation due to absorption. As discussed in the PTT section, irradiation of SiO2–Au nanoshells at their peak extinction coefficient results in the conversion of light to heat energy that produces a local rise in temperature. Thus, to develop a photothermal modulated drug delivery system, Bikram et al. have fabricated nanoshell-composite hydrogels in which SiO2–Au nanoshells of varying concentrations have been embedded within temperature-sensitive hydrogels, for the purpose of initiating a temperature change with light. N-isopropylacrylamide-co-acrylamide (NIPAAm-co-AAm) hydrogels are temperature-sensitive hydrogels that were fabricated to exhibit a lower critical solution temperature (LCST) slightly above body temperature [188]. The resulting composite hydrogels had the extinction spectrum of the SiO2–Au nanoshells in which the hydrogels collapsed reversibly in response to temperature (50 °C) and laser irradiation. The degree of collapse of the hydrogels was controlled by the laser fluence as well as the concentration of SiO2–Au nanoshells. Modulated drug delivery profiles for methylene blue, insulin, and lysozyme were achieved by irradiation of the drug-loaded nanoshell-composite hydrogels, which showed that drug release was dependent upon the molecular weight of the therapeutic molecule [188].
As discussed, the combination of chemotherapeutics and hyperthermia has been an emerging approach for cancer therapy. However, this combinatorial therapy is highly requisite to deliver drugs and localized heating to the cancerous area. Recently, Cheng et al. have developed stabilizer-free taxol-loaded poly(lactic-co-glycolic acid) (PLGA) NPs [189]. These can be directly surface conjugated to other NPs, like gold nanorods (GNR), iron oxide NP (Fe3O4), quantum dots (QD) and the inner core can be used to encapsulate drugs to potentially serve as multifunctional probes. In this work, they have demonstrated efficacy of a nanoplatform that combined chemotherapy and PTT in vitro, in mammalian cells and in vivo, in an animal model of lung cancer. A significant enhancement of the anticancer effect was observed when chemotherapy and photothermal destruction were combined, with GNR/QD/Fe3O4/Taxol-loaded PLGA NPs injection being followed by laser irradiation [189]. Since the iron oxide NPs are decorated on PLGA NPs, they can potentially serve as a contrast agent for MRI. The GNR/QD/Fe3O4/Taxol loaded PLGA NPs were further administered to A549 (lung cancer cells)-induced SCID mice and the tumor was imaged using MRI. In mice that received binary treatment, the tumor growth was suppressed and the tumors tended to shrink as the test period went on. The mice treated with chemotherapy and photothermal destruction remained alive after two months, and the tumors of treated mice either decreased 100% or showed no sign of regrowth after therapy [189].
Liposomes show great promise as intravenous drug delivery vehicles, but it is often difficult to combine stability in the circulation with rapid, targeted release at the site of interest. Targeting to specific tissues requires developing highly specific ligands with strong affinities to receptors overexpressed on diseased cells; a new cellular target requires developing new ligands and identifying new receptors. A novel proof of principle demonstration for contents release from liposomes that can be selectively activated by light irradiation is presented by Paasonen et al. [190]. The content release temperature was adjusted to slightly above body temperature, and hydrophobic or hydrophilic GNPs were incorporated into the lipid bilayer or the core of the liposomes, respectively. The release of a fluorescent marker was monitored upon exposure of the liposomes to UV light. GNP-containing liposomes remained intact at 37 °C but contents release was triggered by UV light induced heating of the GNPs [190].
Drug release has also been triggered by irradiating liposomes conjugated to hollow gold nanoshells with a near-infrared (NIR) pulsed laser [191]. Novel photoactivated, hollow, gold nanoshell (HGN)/liposome composites provide a new approach to both controlled release and specific targeting. HGN are extremely efficient NIR light absorbers, and are not susceptible to photobleaching like conventional dyes. Near-complete liposome contents release can be initiated within seconds by irradiating HGNs with an NIR pulsed laser. Targeting the drug is limited only by the dimensions of the laser beam; no specific ligands or antibodies are required, so different tissues and cells can be targeted with the same HGN/liposomes. HGNs can be encapsulated within liposomes or tethered to the outer surface of liposomes for the most efficient drug release. HGNs in liposome solutions can also trigger release, but with lower efficiency. Drug release is induced by adsorbing femto- to nanosecond NIR light pulses that cause the HGNs to rapidly increase in temperature. The resulting large temperature gradients lead to the formation of vapor microbubbles in aqueous solutions, similar to the cavitation bubbles induced by sonication. The collapse of the unstable vapor bubbles causes liposome-membrane rupture and contents release, with minimal damage to the surroundings, and little overall heating of the solution. Wu et al. have reviewed the use of such hybrids for photothermally-triggered drug release and imaging [191]. NIR light can penetrate up to 10 cm into tissue, which should allow these liposome/HGN complexes to be addressed noninvasively within a reasonable fraction of the human body. Any liposome carrier could be modified by tethering or encapsulating HGN to produce a system for rapid release on demand via NIR irradiation. This should eventually allow for better control of drug delivery to selected disease sites while minimizing systemic toxicity [191].
The hypothesis that the photothermal effect mediated by a NIR laser and hollow gold nanospheres (HAuNSs) could modulate the release of anticancer agents was tested by You Jian et al. using biodegradable and biocompatible microspheres (1–15μm) containing the antitumor drug paclitaxel (PTX) and HAuNSs (~35 nm in diameter), which display surface plasmon absorbance in the NIR region [171]. HAuNS-containing microspheres exhibit a NIR-induced thermal effect similar to that of plain HAuNSs. Rapid, repetitive PTX release from the PTX/HAuNS-containing microspheres is observed upon irradiation with NIR light (808nm), whereas PTX release is insignificant when the NIR light is switched off. The release of PTX from the microspheres is readily controlled by the output power of the NIR laser, duration of irradiation, treatment frequency, and concentration of HAuNSs embedded inside the microspheres. In vitro, cancer cells incubated with PTX/HAuNS-loaded microspheres and irradiated with NIR light display significantly greater cytotoxic effects than cells incubated with the microspheres alone or cells irradiated with NIR light alone, owing to NIR-light-triggered drug release. Treatment of human U87 gliomas and MDA-MB-231 mammary tumor xenografts in nude mice with intratumoral injections of PTX/HAuNS-loaded microspheres followed by NIR irradiation results in significant tumor-growth delay compared to tumors treated with HAuNS-loaded microspheres (no PTX) and NIR irradiation or with PTX/HAuNS-loaded microspheres alone. The data support the feasibility of a therapeutic approach in which NIR light is used for simultaneous modulation of drug release and induction of photothermal cell killing [171]. Dual-functional hollow gold nanospheres (HAuNS, ~40-nm diameter) capable of mediating both photothermal ablation of cancer cells and drug release upon near-infrared (NIR) light irradiation has been reported by You Jian et al. [192]. As high as 63% DOX by weight could be loaded to polyethylene glycol (PEG)-coated HAuNS since DOX was coated to both the outer and the inner surfaces of HAuNS. Irradiation with NIR laser induced photothermal conversion, which triggered rapid DOX release from DOX-loaded HAuNS. The release of DOX was also pH-dependent, with more DOX released in aqueous solution at lower pH. Significantly greater cell killing was observed when MDA-MB-231 cells incubated with DOX-loaded HAuNS were irradiated with NIR light, attributable to both HAuNS-mediated photothermal ablation and cytotoxicity of released free DOX [192]. This approach is advantageous in several aspects. HAuNS displayed exceptionally high drug loading capacity and stability as exemplified by DOX owing to the unique physicochemical characteristics of HAuNS. In particular, the hollow interior of the NPs allowed significant increase in effective surface area for DOX attachment, resulting in 3.5-fold increase in DOX payload compared with solid GNP of the same size, surface charge, and weight. HAuNS mediated strong photothermal effect owing to their strong surface plasmon absorption in the NIR region. This property was exploited for controlled release of DOX from DOX loaded HAuNS using NIR light as the external stimulus to trigger drug release. Dual modality of cell killing were integrated into a single nanodevice, that is, photothermal ablation mediated by HAuNS and antitumor activity of DOX released from HAuNS upon NIR laser irradiation. This “two-punch” approach is expected to significantly increase the likelihood of cell killing and potentially overcome resistance to chemotherapeutic agents, making it a promising approach to cancer therapy [192].
Hollow layer-by-layer (LbL) capsules can be refilled with various molecules for drug delivery. Drug release can be activated on demand by several remote physical stimuli. For example, Skirtach et al. demonstrated the selective addressing of intra cellular LbL microcapsules with laser light. The release of encapsulated material from polyelectrolyte-multilayer capsules has been demonstrated inside living cells. Metal NPs were incorporated inside the walls of the capsules, and served as energy absorbing centers for illumination by laser light. AF-488 dextran was successfully incorporated into the capsules using a novel heat-shrinking method. The capsules obtained by such a method exhibit improved mechanical stability—properties important for the delivery of encapsulated material. Upon illumination by laser light, the encapsulated dextran leaves the interior of a capsule inside a living cancer cell. This study serves as a significant step toward the use of polyelectrolyte-multilayer capsules for the delivery of medicine into biological cells. The presented method is different from previous, albeit also important, studies in that it is conducted on an individual capsule level and offers an improved degree of control and monitoring. Release from polyelectrolyte microcapsules functionalized with metal NPs, by burst opening and deformation, has been demonstrated by Volodkin et al. [193]. They have also demonstrated temperature-triggered release of a liposome cargo from surface supported vesicles embedded inside biocompatible polyelectrolyte multilayers. In an effort to enlarge the scope of application of remote release and to extend it further to other surface-supported drug delivery vesicles, Volodkin et al. [194] have applied remote release to liposome–GNPs, referred to as assemblies or complexes (Lip-NP). The goals of this work were to show that Lip-NP assemblies could be prepared in a controlled manner in terms of size and NP state and then to use near-IR light to selectively release encapsulated dye from the assemblies. Functionalized liposome–NP assemblies can be used for transdermal applications in which an active compound is delivered through the skin, which is easily accessible by light. Due to quite deep IR-light tissue penetration, the light-responsive liposome assemblies could serve as active constituents of implanted devices. In a recent study, Volodkin et al. have reported on the functionalization of layer-by-layer films with GNPs, microcapsules, and DNA molecules by spontaneous incorporation into the film [195]. Exponentially growing films from biopolymers, namely, hyaluronic acid (HA) and poly-L-lysine (PLL), and linearly growing films fromthe synthetic polymers, namely, poly(styrene sulfonate) (PSS) and poly(allylamine hydrochloride) (PAH), were examined for the embedding. The HA/PLL film studied here possesses high loading capacity as a result of polymer doping onto the film surface that results in the accumulation of a large amount of adsorbing material, which is many times less for the PSS/PAH film that has low polymer mobility. Microcapsules, GNPs, and DNA can be embedded in the HA/PLL film and located on the film surface. The diffusion of embedded DNA into the film can be triggered by heating. The HA/PLL film with adsorbed GNPs and DNA possesses remote release features by stimulation with “biofriendly” IR light. DNA release from the film modified with GNPs is supposed to be caused by local destruction of the polymer network in the film followed by the blocking of PLL-DNA bonding and, as a result, the release of DNA molecules from the film. Laser activation of film-supported capsules shows the remote release of encapsulated dextran. This study can serve future biomedical applications in tissue engineering and biocoatings where high loading capacity together with remote release functionalities is demanded. Light-triggered DNA transfection to a single cell can also be achieved by this approach. The mechanism of the release is dependent on the disturbance in bonding between “doping” PLL and DNA, which is induced by local thermal decomposition of the HA/PLL network in the film when the film is exposed to IR light. Remote IR-light activation of dextran-filled microcapsules modified by GNPs and integrated into the HA/PLL film is also demonstrated, revealing an alternative release pathway using immobilized light-sensitive carriers (microcapsules) [195].
Carbon nanotubes are unique materials that absorb infrared (IR) radiation, especially between 700 and 1100 nm, where body tissues are most transparent. Absorbed IR promotes molecular oscillation leading to efficient heating of the surrounding environment. A method to enhance drug localization for peritoneal malignancies is perfusion of warm (40–42 °C) chemotherapeutic agents in the abdomen. However, all tissues in the peritoneal cavity are subjected to enhanced drug delivery due to increased cell membrane permeability at hyperthermic temperatures. Levi et al. have shown that rapid heating (within ten seconds) of colorectal cancer cells to 42 °C, using infrared stimulation of nanotubes as a heat source, in the presence of the drugs oxaliplatin or mitomycin C, is as effective as two hours of radiative heating at 42 °C for the treatment of peritoneal dissemination of colorectal cancer [196]. This approach has the potential to be used as a rapid bench to bedside clinical therapeutic agent with significant impact for localizing chemotherapy agents during the surgical management of peritoneal dissemination of colorectal cancer. The method is quite simple since no attachment of the NP to the drug is required. Yet, the effect is significant increase in the amount of agent that is retained in the cells. The evaluations of nanotubes and other NPs reported to date have focused on therapeutic delivery or thermal ablation. However, most cancer treatments involve a combination of surgery, chemotherapy, and radiation. Intraperitoneal hyperthermic chemoperfusion using carbon nanotubes could be utilized as a rapid bench-to-bedside technique since it can be applied during surgical procedures, all the nanomaterial can be removed from the body following delivery, and hyperthermia can be localized. MWNT induced chemotherapy for metastatic peritoneal cancer can be clinically applied during open abdominal procedures by filling the abdomen with a solution of nanotubes and chemotherapeutic agent and applying infrared light only to the tumor nodules. MWNT would be directly introduced into the treatment region and not intravenously introduced due to the lack of systemic delivery of therapeutic agents from the bloodstream to the peritoneum. It is beneficial to minimize the surgical time to reduce the time that a patient is anesthetized, to maximize recovery and minimize complications due to prolonged anesthesia. For example, to surgically remove a tumor nodule may take five minutes but laser application would take only a few seconds. This would significantly reduce the overall time of the surgery. Furthermore, the nanotubes could be easily removed from the abdomen following hyperthermic chemotherapy delivery by flushing the abdomen with saline after the procedure. Future evolutions of nanotubes used for dissemination of metastatic peritoneal cancers include (1) targeting nanotubes to specific tumor types using antibodies, bacteriophages, or other moieties such as folic acid; or (2) using nanotubes to induce hyperthermic chemotherapy in a closed abdominal procedure using laproscopic techniques and a fiber optic infrared source. IPHC using carbon nanotubes has the clinical potential to reduce treatment times for hyperthermic chemotherapy by localizing heat, thus aiding in the penetration of chemotherapeutics into malignant tumor cells, and hence reducing the overall treatment time and increasing the effectiveness of treatment and patient survival [196].
The development of systems for releasing guest molecules from mesoporous silica supports using molecular and supramolecular concepts is currently taking chemistry to the forefront of nanoscience. Aznar et al. have shown that it is possible to obtain simple and very effective guest release control using polyalcohol entities anchored onto mesoporous materials and boronic acid functionalized GNPs as effective caps [197]. Both pH-controlled and NIR light-controlled delivery effects have been observed to occur in pure water. In relation to the pH-controlled delivery, the release of the cargo is inhibited at pH 5, whereas there is rapid release of the guest molecule from the mesoporous silica scaffolding at pH 3. The pH-controlled release is reversible, and the entrapped guests can be delivered in installments by simple changes in the pH. The pH-controlled “open-close” mechanism is associated with the reversible formation of boroesters between alcohol groups and boronic acid functionalized NPs (closed-gate) and their quick and easy hydrolysis (open-gate). At the same time the use of GNPs opens the way to employing light as a suitable stimulus for release procedures. This is related to the ability of GNPs to raise their temperature locally by absorbing laser light. Plasmonic heating results in cleavage of the boronic ester linkage that anchors the NPs to the surface of the mesoporous silica-based material, allowing the release of the cargo. A finetune of the amount of cargo delivered by simply controlling the laser irradiation is also possible. Both pH and light are easy-to-use external stimuli and appealing methods of releasing entrapped guests that could be used to develop new controlled delivery systems for a wide range of different applications. The possibility of using these stimuli for delivering cargo in small portions also opens the possi bility of designing stimuli-induced pulsatile release supports. Plasmonic heating results in cleavage of the boronic ester linkage that anchors the NPs to the surface of the mesoporous silica-based material, allowing the release of the cargo. A fine-tune of the amount of cargo delivered by simply controlling the laser irradiation is also possible. Both pH and light are easy-to- use external stimuli and appealing methods of releasing entrapped guests that could be used to develop new controlled delivery systems for a wide range of different applications. The possibility of using these stimuli for delivering cargo in small portions also opens the possibility of designing multi-stimuli-induced pulsatile release supports [197].
4.3 Combined optical imaging and therapy
Several agents that can be used for therapy in combination with optical imaging are also being developed for cancer applications and a few of these agents are reviewed here. NIR fluorophores have several advantages over visible fluorophores, including improved tissue penetration and lower autofluorescence [172, 198–200]; however, only indocyanine green (ICG) is clinically approved. Its use in molecular imaging probes is limited because it loses its fluorescence after protein binding. This property can be harnessed to create an activatable NIR probe. After cell binding and internalization, ICG dissociates from the targeting antibody, thus activating fluorescence. In work done by Ogawa et al. ICG was conjugated to the therapeutic antibodies daclizumab (Dac), trastuzumab (Tra), or panitumumab (Pan) [201].The conjugates had almost no fluorescence in PBS but became fluorescent after SDS and 2-mercaptoethanol, with a quenching capacity of 10-fold for 1:1 conjugates and 40- to 50-fold for 1:5 conjugates. In vitro microscopy showed activation within the endolysosomes in target cells. In vivo fluorescent imaging in mice showed that CD25-expressing tumors were specifically visualized with Dac-ICG. Furthermore, tumors over-expressing HER1 and HER2 were successfully characterized in vivo by using Pan-ICG(1:5) and Tra-ICG(1:5), respectively. Thus, they have developed an activatable NIR optical probe that “switches on” only in target cells. Because both the antibody and the fluorophore are Food and Drug Administration approved, the likelihood of clinical translation is improved [201].
A long-term goal of cancer diagnosis is to develop tumor imaging techniques that have sufficient specificity and sensitivity. To achieve this goal, minimizing the background signal originating from non-target tissues is crucial. Urano et al. have achieved highly specific in vivo cancer visualization by using a newly designed targeted ‘activatable’ fluorescent imaging probe [172]. This agent is activated after cellular internalization by sensing the pH–change in the lysosome. Novel acidic pH activatable probes based on the boron-dipyrromethene fluorophore were synthesized and then conjugated to a cancer-targeting monoclonal antibody. As proof of concept, ex vivo and in vivo imaging of human EGFR type 2–positive lung cancer cells in mice was performed. The probe was highly specific for tumors with minimal background signal. Furthermore, because the acidic pH in lysosomes is maintained by the energy-consuming proton pump, only viable cancer cells were successfully visualized. The design concept can be widely adapted to cancer-specific, cell surface–targeting molecules that result in cellular internalization. They have developed small-molecule, pH-activatable fluorescence probes and have targeted them to viable cancer cells using macromolecule conjugates. These probe conjugates can potentially be used as in vitro tools for evaluating intracellular receptor kinetics, cell viability and real-time monitoring of cell death, although their main potential application will be as a clinical tool for cancer detection and real-time monitoring of therapy [172].
Luminescent semiconductor nanocrystals, also known as quantum dots (QDs), have advanced the fields of molecular diagnostics and nano-therapeutics [202]. Much of the initial progress for QDs in biology and medicine has focused on developing new biosensing formats to push the limit of detection sensitivity. Nevertheless, QDs can be more than passive bio-probes or labels for biological imaging and cellular studies. The high surface-to-volume ratio of QDs enables the construction of a smart multifunctional nanoplatform, where the QDs serve not only as an imaging agent but also a nano-scaffold catering for therapeutic and diagnostic (theranostic) modalities. Ping Ho et al. have recently highlighted the emerging applications of functionalized QDs as fluorescence contrast agents for imaging or as nanoscale vehicles for delivery of therapeutics, with special attention paid to the promise and challenges towards QD-based theranostics [202].
Ma et al. [203] have developed a two-step method for synthesis of multifunctional core-shell NPs with an improved structure as compared with those prepared by traditional methods used independently. The NPs comprise a superparamagnetic core, an inner insulating dye-free silica shell, an outer luminescent silica shell encapsulating thousands of dye molecules and a functionalizeable surface. The innovative insertion of the isolating silica shell benefits the NPs’ architecture in two ways. Firstly, by keeping the dye molecules away from the magnetic core, the silica shell prevents dye luminescence quenching. Secondly, the non-magnetic shell decreases magnetic interparticle coupling, which, by reducing aggregation and preventing agglomeration, facilitates the formation of the high-quality luminescent shell in the second step of the synthesis procedure. The final NPs being both superparamagnetic and luminescent have a great potential for theranostic applications such as ultra-sensitive detection, and in vitro and in vivo imaging [203].
The delivery of therapeutic nucleic acids such as siRNA, antisense agents, transcription factor decoys, and plasmid DNA (pDNA) offers an unprecedented opportunity for developing highly specific treatments for many devastating diseases including cancer. The parallel development of novel nucleic acid drugs and theranostic vehicles that offer disease diagnosis, treatment, and the ability to understand the delivery mechanisms/kinetics on a range of biological scales will advance this field toward the discovery of personalized treatment strategies. Bryson et al. have developed polymer beacons that allow the delivery of nucleic acids to be visualized at different biological scales [204]. The poly-cations have been designed to contain repeated oligoethyleneamines, for binding and compacting nucleic acids into NPs, and lanthanide (Ln) chelates [either luminescent europium (Eu3+) or paramagnetic gadolinium (Gd3+)]. The chelated Lns allow the visualization of the delivery vehicle both on the nanometer scale via microscopy and on the sub-mm scale via MRI. Bryson et al. demonstrate that these delivery beacons effectively bind and compact plasmid pDNA into NPs and protect nucleic acids from nuclease damage. These delivery beacons efficiently deliver pDNA into cultured cells and do not exhibit toxicity. Micrographs of cultured human cervix adenocarcinoma (HeLa) cells exposed to the NP complexes formed with fluorescein- labeled pDNA and the europium-chelated polymers reveal effective intracellular imaging of the delivery process. MRI of bulk cells exposed to the complexes formulated with pDNA and the gadolinium-chelated structures show bright image contrast, allowing visualization of effective intracellular delivery on the tissue scale. Because of their versatility, these delivery beacons posses remarkable potential for tracking and understanding nucleic acid transfer in vitro, and have promise as in vivo theranostic agents [204].
Thus to summarize, light triggered DDS has been introduced as a novel scientific approach leading to macroscopic changes in the system for controlled release of drug in terms of quantity, location and time thereby overcoming the shortcomings of conventional DDS. Nanotechnology offers novel insights and concepts for drug delivery and diagnostics. The release of encapsulated materials remotely is desired in drug delivery for minimizing drug toxicity, controlling the properties of surfaces and interfaces, and studying intracellular processes. Light-stimulated remote release is of special interest because of the possibility for external control of the light intensity and modulation and because of its noninvasive character, which is desirable for bioapplications. Low energy radiation and accurate control on applied light by sophisticated equipment are the unique features of the system. Photochemical internalization (PCI) is a novel technology developed for site-specific enhancement of the therapeutic efficacy and has considerable translational potential. Other potential applications of such a light-triggered approach are expected in PTT, in which the dynamic photothermal effect can be augmented by the delivery cargo. Optical imaging-based diagnosis can be used to guide therapy and monitor post-treatment response. The synthesis of novel photoresponsive conjugates and incorporation of more drugs in these light-triggered theranostics may enhance patient compliance by reduction in side effect and promote the scientific research towards sensitive and specific diagnosis and precisely controlled drug delivery for personalized theranostics.
5. Photo-triggered theranostic agents for non-cancer pathologies
5.1 Infectious Diseases
Theranostic agents have a well known impact in oncology but it also set a treatment ground for drug resistant infection diseases. Infectious diseases are caused by pathogenic microorganisms; which can be viruses, bacteria, fungi or protozoa. Today the major challenges for the treatment of infectious diseases are the increase in therapy-resistant pathogen strains together with the need for fast diagnosis methods to specify the infecting organism and enable better initial treatment of patients and more efficient use of antimicrobials [205]. Bacterial identification and antibacterial susceptibility testing methods, currently used in clinical microbiology laboratories, require at least two days because they rely on the growth and isolation of microorganisms. However, the delay of initiation of adequate treatment is a major determinant of success in therapy of infectious diseases, underlying the urgent need for rapid and accurate diagnostic tests. In recent years, a number of different molecular methods for the rapid detection of Methicillin-resistant Staphylococcus aureus (MRSA) which is a major cause of nosocomial infection have been described. The Infection Diagnostic Inc- MRSA test (Cepheid, Sunnyvale, CA) is highly specific for detecting MRSA in nasal swabs [206]. Polymerase chain reaction screening for MRSA with this test at admission to critical care units has been demonstrated to be feasible in routine clinical practice, and to provide quicker results than culture- based screening [207]. Disadvantages include the need for specific DNA primers and advanced equipment. In contrast, NP-based theranostic methods have the potential to be rapid, easy to use and inexpensive, while maintaining a high level of accuracy [208].
5.1.1 Photodynamic therapy
PDT has been successfully applied for elimination of pathogens. The dual selectivity of PDT (i.e., the selective PS and the localized selective illumination) is an advantage in the treatment of infectious diseases [209]. The ROS produced during PDT have multiple cellular targets [17]. Zeina et al. demonstrated the elimination of several cutaneous microbial species with methylene blue and visible light [210]. As control, the human keratinocyte cell line (H103) resisted killing under the same treatment conditions and showed no immediate or delayed genotoxic damage. Recently, Dai et al. [211] showed the efficacy of PDT for the treatment of MRSA infection in skin abrasion wounds in a mouse model. PDT with polyethylenimine (PEI)–ce6 as PS and red light accelerated the wound healing on average by 8.6 days in comparison to the untreated infected wounds. Our group has recently demonstrated a new way to target ampicillin resistant pathogens in taking advantage of their resistance mechanism [212]. A target-activated drug (β–LEAP) was developed, for which two phenothiazinium PS (EtNBS) were attached to a cephalosporin linker. These PSs are quenched in the uncleaved construct, but activated by cleavage of the lactam ring by beta-lactamase, which is synthesized only by resistant bacteria (Fig. 9a). The selectivity of β–LEAP was shown in co-culture experiments with human foreskin fibroblasts (HFF-1) and S. aureus strain 8179. Fluorescence intensity in S. aureus was much higher than in fibroblasts (Fig. 9b) and elimination after light illumination was more successful than with Penicillin G (Figs. 9c and 9d). This novel targeting strategy of the resistance mechanism itself has, besides the specificity for resistant bacteria, the potential advantage to distinguish between human and microbial cells. We anticipate that this strategy will be able to be used in combination with standard antibiotic treatment to eliminate resistant and nonresistant bacteria. Because of the increase in fluorescence intensity upon cleavage of the β–LEAP construct, it can also be used in diagnostics, to identify resistant pathogens. Engelhardt et al. showed the efficacy of the PSs hypericin and fospeg against S. aureus [213]. 100nM of water-soluble formulations of hypericin (PVP-hypericin) and m-tetrahydroxyphenylchlorin (Fospeg) were incubated for 5 min with S. aureus and illuminated for 30 min at a power density of 75 mW/cm2. Both PSs led to an impressive 4–5 log reduction in bacterial burden [213].
Figure 9.
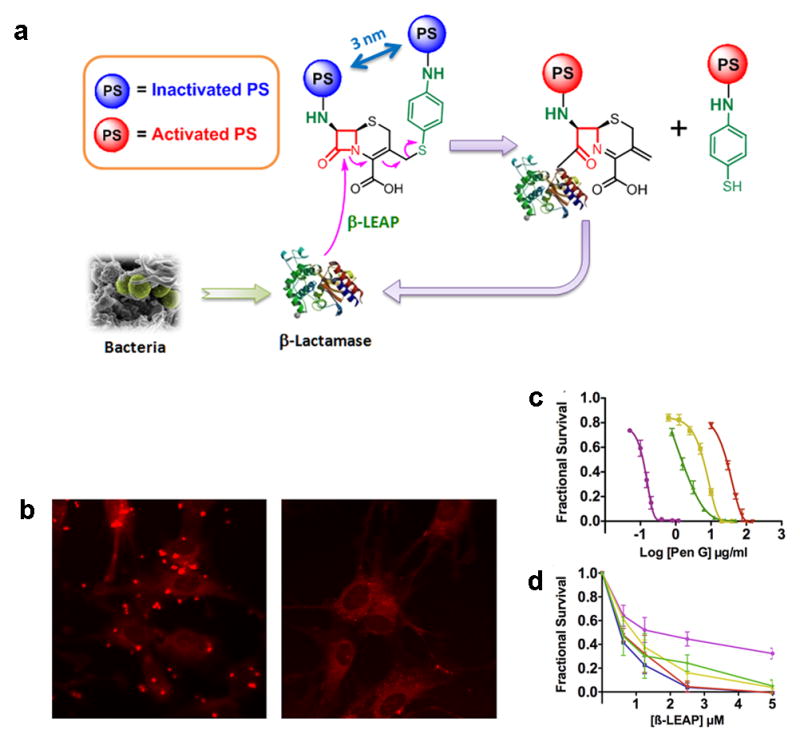
Enzyme activated theranostic prodrug for overcoming bacterial resistance a. Mechanism for the activation of β-LEAP. In resistant bacteria β-LEAP is cleaved by β-lactamase, releasing the quenched PS (blue balls) into an unquenched state (red balls). b. The released PS is activated by light irradiation, as shown in coculture of HFF-1 cells and strain 8179 (left panel) and HFF-1 cells alone (right panel). c. Inhibition profiles for selected strains of S. aureus with penicillin G for comparison are shown in. d. β-LEAP hydrolysis by selected strains of S. aureus leads to loss of viability in bacterial cells. Key for c and d: purple 29213, green 9307, gold 8150, red 8179, blue 8140 (d only). Based on work by Zheng et al. [212].
PDT has been successfully applied for the treatment of Leishmaniasis, which is caused by parasites of the species Leishmania and can lead to ulcerate lesions on exposed skin, scar formation, blood vessel and nerve damage, and secondary infection. Akilov et al. demonstrated effective PDT in vitro and in a mouse model of Leishmaniasis with the phenothiazinium PS EtNBS and PPA904 in vitro [214]. Multiple treatments where required for optimal outcome of parasite elimination. A clinical trial with PDT against Cutaneous Leshmaniasis was reported by Asilian et al. [215]. Topical PDT, repeated weekly over 4 weeks resulted in significant better treatment outcome than standard paromomycin treatment. Patients who received PDT showed a 100 % parasitological cure compared to 63 % in the paromomycin treatment group. Successful PDT was also demonstrated for the treatment of fungal or virus infections. Smijs et al. showed photodynamic killing of Trichophyton rubrum, an anthropophilic dermatophyte with the porphine. Sylsens B [216]. Zane and coworkers showed a modest efficacy of 20% ALA in Eucerin cream and red light illumination for treatment of interdigital mycosis of the feet [217]. A clinical trial carried out by Wulf and co-workers showed the efficacy of ALA-PDT against warts and proposed future randomized clinical trials for efficient cure of warts, mosaic warts and condylomata [218]. And recently Marotti et al. demonstrated PDT efficacy in patients with methylene blue (MB) against the herpes virus [219].
It has also been demonstrated that coupling of GNPs to PSs enhanced their PDT capability for cell elimination. Perni et al. embedded MB and GNPs in polysiloxane polymers. 5 min light irradiation at low power at 660 nm led to significant elimination of Staphylococcus aureus and Escherichia coli [220]. While the bacterial death was related to singlet oxygen production from, MB, GNP significantly enhanced the PDT-efficacy of MB. The reason for this synergistic effect is not clear. Due to the wavelength and laser power, a thermal effect of GNP has been excluded by the authors as a mechanism of bactericidal effect. Due to their optical properties, GNPs have a strong potential for diagnosis of pathogenic microorganisms. An optical absorption change in aggregating GNPs, has been utilized to detect bacterial DNA after hybridization. For this, GNP where functionalized with thiol modified oligonucleotides, which bind complementary DNA. This binding is followed by clustering of the GNP [221]. Also as demonstrated by Huang et al. in their work, the aggregation of bacteria bound to magnetic particles in an external magnetic field can be used for diagnostics following the same principle of detecting clustering of GNPs [222].
5.1.2 Photothermal therapy
Photothermal therapy (PTT) offers innovative new technologies for the treatment of infectious diseases, but as compared to PDT, it is in the experimental- and in vitro- stage. During the last decade the use of GNPs (GNPs) for the application of localized heat in biological tissue and bacteria has been greatly emerging. GNPs have attracted interest because of their optical properties resulting from their plasmon resonance absorption. Gold nano-spheres, nanorods or nanotubes have been developed for shifting the absorption band into NIR-wavelength range and for further functionalization. Another advantage is the localization of the damaging effects in the nanometer range around GNP. One of the first reports of bacterial inactivation with GNPs was given by Zharov et al. [223], who demonstrated the elimination of Staphylococcus aureus after pulsed irradiation of 40 nm spherical GNPs. The GNP where functionalized with antibodies against protein A on the bacterial cell surface. Cavitation bubbles, which emerged upon irradiation around clustered GNP led to elimination of the bacteria. Recently Huang et al. [222] used multifunctional Fe3O4/Au nanoparticles (NPs) with magnetic properties. Vancomycin was immobilized on the surface of these NPs to provide selective binding to bacteria. 3 min irradiation with NIR light led to a temperature increase to 55 °C and to the elimination of different bacterial strains. A second characteristic of these NPs, their magnetism, was used to cluster bacteria in an external magnetic field before light irradiation. This clustering led to a more pronounced effect in killing after NIR light exposure. Wang et al. [224] showed a similar approach with Fe3O4/Au hybrid NPs, and demonstrated their tunable magnetic and plasmonic functionality.
Similar approaches have been successfully explored with carbon nanotubes (CNT) to achieve antimicrobial diagnostics and therapy as NIR-light photothermal contrast agents for pathogens. CNT have high binding affinity to bacteria and also bacterial internalization has been observed and utilized to transport peptides, DNA and RNA into cells [225–226]. Kim et al. [227] have shown the ability of SWNTs and MWNTs as PT contrast agents for diagnosis, and their potential in causing irreversible damage to pathogens upon NIR laser irradiation. Upon incubation of Escherichia coli with CNTs and binding of CNTs to the bacteria surface CNTs self-assemble to clusters, which led to an increase in PT response. After a nanosecond-pulsed laser irradiation at 532 nm and 1064 nm a decrease in bacteria viability was observed, demonstrating the therapeutic potential of this approach. The authors point out, that this technology may also be used for purification of drinking water, food processing, and disinfection of medical instrumentation and transplants.
Light-based theranostics have the potential to overcome resistance against antibiotics– one of the major problems for the treatment of infectious diseases. A great potential of these technologies in diagnosis and treatment with the same agent has been demonstrated. Rapid and sensitive diagnosis for infectious diseases is especially important for the success of the treatment. PDT has already proven to be successful for treatment of several infectious diseases. The development of so called “smart” drugs that are activated by resistant bacteria and differentiate between human and microbial cells will increase target specificity and efficacy. PTT has proven to be well suited for selective anti-microbial treatment.
5.2 Other diseases
Cardiovascular diseases (CVD) are the leading cause of death worldwide, a fact that eclipses the mortalities due to any type of cancer or infectious disease in the United States, Europe and much of Asia [228–229]. This high mortality highlights the importance of developing useful applications of light based theranostic agents for CVD and for other non-cancerous and non-infectious diseases. Though pathogenesis occurs latently over decades, theranostic agents will be able to address this problem in symptomatic as well as the asymptomatic high risk but subclinical populations. Theranostic regimens are finding potential application for other diseases such as atherosclerosis, arthritic diseases, AMD and psoriasis [228–233].
AMD is the leading cause of vision loss in elderly populations of the developed world [233]. The disease can be either characterized as non-neovascular or neovascular with the later being routinely treated with either bevacizucimab or visudyne-based PDT or a combination of both [234]. In neovascular AMD the proliferation of endothelial cells to form choroidal neovascularization behind the retina is implicated in the cause for vision loss [235]. PDT as a means to occlude CNV avoids collateral damage to the retinal tissue because the PS, Visudyne, is preferentially retained in CNV [236]. After photoactivation, the generation of ROS and other reactive species damage endothelial cells which leads to the occlusion of CNV. Visudyne is a thus useful therapeutic for management of AMD. Following treatments fluorescein angiography is used to observe choroidal closure.
Atherosclerosis is an inflammatory disease which results in the emergence of lesions mostly in medium to large sized elastic and muscular arteries. These lesions can be present for decades or even throughout a person’s life, presenting as early as infanthood where the early stage lesions consist exclusively of macrophages and T lymphocytes [229]. Instable, dangerous plaques are difficult to diagnose by angiography and plaque rupture accounts for approximately 75% of acute coronary events and 60% of symptomatic carotid artery disease [229, 232]. In atherosclerogenesis, macrophages drive the inflammatory response by secreting cytokines to recruit other cells to lesions as well as produce metalloproteinase which promotes plaque instability [229]. Given that macrophages also tend to accumulate in the atherosclerotic plaques, they make promising targets for imaging, diagnosis and targeted cytotoxic therapy in atherosclerogenesis. It has been previously reported that macrophages have dextran receptors on the cell surface and this has been exploited to selectively deliver dextranated nanoconstructs to macrophages because the dextran coat promotes phagocytosis of the NP by the cell. Using dextranated nanoconstructs McCarthy et al. and Lim et al. have been able to target macrophages in vitro and also promote death using two different light activated cytotoxic therapies, PDT and PTT respectively [228, 237]. McCarthy et al. synthesized multimodal magnetofluorescent NP (MFNP) to aid in the diagnosis and therapy of atherosclerotic plaques. The dextranated particle, produced by cross-linking amine-dextran to iron oxide was conjugated to Alexa Fluor 750 (AF750) and also a PS, 5-(4-carboxyphenyl)-10,15,20-triphenyl-2,3-dihydroxychlorin (TPC). When the nanoconstructs were excited at the therapeutic wavelength (650 nm) there was minimal intramolecular energy transfer between the therapeutic agent, TPC, and the fluorescent diagnostic agent (AF750). At the imaging wavelength (750 nm) no therapeutic effect was observed. Uptake of the nanoconstructs was observed in vitro in human macrophage monolayer cultures and cytotoxicity was observed upon irradiation with 650 nm light. Given that this moiety consists of an MRI contrast agent in addition to the fluorescent probe, it provides what will be a useful imaging mode in vivo. In addition to PDT agent, PTT agents have been dextranated to selectively target macrophages [237]. Dextranated hollow-type GNPs were synthesized to be responsive to near-infrared light and shown to be internalized by RAW264.7 cells in vitro. Light scattering properties offered a way to image the internalization of the nanoconstructs while their light absorbing properties provided the energy for the thermal based therapy.
Rheumatoid arthritis (RA), like atherosclerosis, is an inflammatory disease and most of the cellular agents implicated in lesion formation in atherosclerosis are also present in RA. The inflammation of the joint is driven by activated macrophages secreting cytokines and recruiting immune cells [230]. As a result there is an increase in local angiogenesis and hyperplasia along the synovium. PDT based reduction of hyperplastic synovium is a conservative approach to treating RA. Selectivity of any treatment is of importance because of potential to damage other tissues. Gabriel et al. synthesized a novel polymeric PS (Pheophorbide a) prodrug that is cleavable by the protease thrombin, which is upregulated in a synovial tissue of RA patients (Fig. 10a). PS fluorescence was directly observable within arthritic joints which had levels 4-fold more than in non diseased joints (Fig. 10b) and also the increase in the fluorescence caused by the peptide cleavage correlates to the clinical grade of arthritis which will be useful in diagnosis. The group also demonstrated the cytotoxicity of the activated PS to in vitro primary human synoviocytes [230]. This could be a valuable tool to diagnose the thrombin status or aggressiveness of the inflammation and also treat arthritis, perhaps at an earlier stage.
Figure 10.
Theranostic agents for in vivo imaging and PDT of rheumatoid arthritis. a. Scheme illustrating the concepts involved in the design of the theranostic prodrug. Self-quenching of PS occurs when tethered to lysine backbone. After proteolytic cleavage and light activation, PS forms reactive oxygen species. b. Fluorescence of arthritic joints and non-arthritic joints pre (left image) and 4 h post i.v.administration (right image) of prodrug. Adapted from Gabriel et al. [230].
Psoriasis is a dermatological autoimmune disease. Fluorescence diagnosis with ALA-induced porphyrins (FDAP) has been used in the clinic to visualize psoriatic plaque and lesions. After topical administration, ALA is taken up by the diseased tissue and then converted to PPIX due to metabolic activity. The fluorescence of PPIX is detectable when it is excited with blue light. Fluorescence signal is negatively correlated to corneum thickness so heterogeneity in lesion thickness can be observed [238]. PPIX also serves as a PS to target the psoriatic lesions and plaques and thus provide the patient with targeted therapy. By exploiting the endogenous upregulation of the metabolism of ALA to PPIX, lesions convert the prodrug into the theranostic PS [239].
Light activated theranostic agents promise to enable minimally invasive therapeutic and diagnostic strategy with potential to diagnose conditions early as well as monitor them during treatment. These agents whether on the nanoscale or microscale offer the ability to refine existing photosynthetic agents for targeting or use entirely new methods to diagnose conditions. Given the push for theranostics we expect that in the future there will be more revision on existing therapies to combine therapeutics, imaging and vice versa. This in addition to cancers and infectious diseases will bring us towards a standard of treating the most devastating health conditions that ail humankind.
6. Future directions and discussion
Light-responsiveness is a fairly attractive phenomenon for developing advanced theranostics capable of not only sensitive and specific diagnosis but also a precise external modulation of the site and the rate of delivery. A wide range of approaches are currently being studied to optimize the light-responsive materials in order to achieve therapeutically efficient and reproducible release profiles. It is a challenge to develop complex theranostic systems that are responsive to biochemical signals or biomarkers typically present in a less than nanomolar concentration range. Such systems-within-systems need a complex, hierarchical organization of the responsive particles (as discussed in this article) to accommodate various possible amplification mechanisms. A hierarchical organization (for example, hierarchical compartmentalization) will also be important for the development of systems where the functions of ‘receiving’ the signal and ‘responding’ by changing the material’s properties are separate because, in some cases, the changes affected by the stimuli may interfere with the desired changes in the material’s properties. In living systems, nature broadly exploits the principle of partitioning; local dynamic changes take place in compartments that are separated by perm selective membranes. This type of organization in stimuli-responsive materials will provide great opportunities with regard to a programmable, complex response of the materials. Another challenge in the design of theranostic agents is to develop systems that can respond to several external stimuli in an intelligent way. Much research remains to be done before practical applications become viable. The clinical use of the light-sensitive theranostics is still in its infancy and requires considerable efforts on several aspects listed below.
Design and synthesis of new biocompatible materials in order to increase the range of light-sensitive compounds that fulfill the requirements of generally recognized safe products.
Specialized equipment capable of providing the adequate irradiation intensity in the target place without altering surrounding tissues. The relative impermeability of the human body to the light makes a direct irradiation at a significant depth of the body difficult and confines the applicability of the UV visible light-sensitive DDS to treatments of the surface layers of the skin or a few millimeters beyond. NIR lasers and NIR-sensitive light materials appear to be feasible alternatives to their UV visible counterparts.
In vivo evaluation of the performance of the new delivery systems. Currently, most of the reviewed systems have been tested in vitro with limited or no toxicity studies performed in vivo. These studies must be supplemented by extensive studies in vivo as the DDS make progress toward clinical use.
Imaging systems for extremely sensitive and specific detection of microscopic disease.
7. Conclusions
Medicine, as we move into the third millennium, still targets therapy to the broadest patient population that might possibly benefit from it, and it relies on statistical analysis of this population’s response for predicting therapeutic outcome in individual patients. This “one drug fits all” approach could, with the use of theranostics, evolve into an individualized approach to therapy where optimally effective drugs are matched to a patient’s unique molecular profile and their activity is triggered using light to localize the therapeutic effects only in and around the diseased tissue with limited collateral damage. Monitoring the effects of light-triggered therapy and initiating a second treatment if required would also be possible. Light-triggered theranostics in combination with multi-modal imaging techniques can thus help provide personalized medicine; that is, unique, individualized for each patient. Pharmaceutical, diagnostics and biotechnological companies are all interested in this rapidly emerging field. Already the U.S. Department of Health and Human Services has publicly recognized personalized medicine as one of its top priorities. Although significant awareness has been created about personalized medicine, its full potential is yet to be tapped. Nanotechnology could provide a platform for the successful integration of diagnostics and therapy, which is vital for the future of customized treatments. Factors such as cost and regulatory timelines are amongst the major hurdles that need to be addressed at the moment. The concepts presented in this review will allow the introduction of new possibilities in the field of biomedical theranostics.
Figure 3.
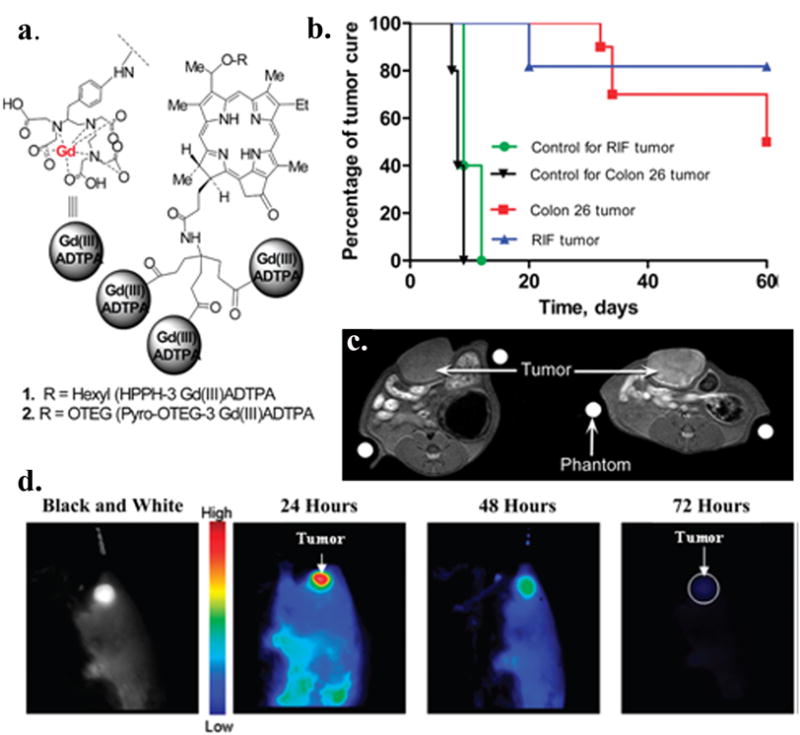
Tumor-Avid PS-Gd(III)DTPA for imaging and therapy. Conjugate shows potential for in vivo imaging (MR, Fluorescence) and PDT. a. Structure of Gd+3aminobenzyl DTPA conjugates of HPPH; b. In vivo PDT efficacy of HPPH-3Gd in C3H mice bearing RIF tumors and BALB/c mice bearing Colon 26 tumors. At an imaging/therapeutic dose of 10 umol/kg; c. Increase in signal intensity was seen in rat Ward Colon tumors (arrow) from preinjection (left) to postinjection (right, 24 hour postinjection) of HPPH-3Gd; d. Fluorescent images of HPPH-3Gd in BALB/c mice at 24, 48, and 72 hour. Adapted from Spernyak et al. [49].
Acknowledgments
We would like to thank Prof. Gang Zheng and his postdoctoral fellow Dr. Klara Stefflova for their help with making Figure 2 of this manuscript. We would also like to thank Dr. Bryan Spring for his helpful suggestions. This work was supported by National Institutes of Health Grant Numbers P01 CA084203 and R44CA128346 National Cancer Institute/National Institutes of Health Grant Numbers R01 CA119388 and RC1 CA146337, the Department of Defense Air Force Office of Scientific Research FA9550-04-1-0079, and the Wellman Center for Photomedicine core funds. Y.M. acknowledges funding from Fonds québécois de la recherche sur la nature et les technologies (FQRNT), Québec, Canada. A.K. would like to acknowledge funding from the Higher Education Commission (HEC) of Pakistan.
Abbreviations
- 3-(PhS)4-PcAlOH
hydroxyaluminium tetra-3-phenylthiophthalocyanine
- Ag
silver
- ALA
5-aminolevulinic acid
- AlPcS4
phthalocyanine tetrasulfonate
- Au
gold
- BDP-MA
benzoporphyrin derivative monoacid ring A
- BLM
bleomycin
- C11Pc
Zn(II)-phthalocyanine disulphide
- C60
fullerene
- CdSe
cadmium selenide
- CE
contrast enhanced
- CNT
carbon nanotubes
- CPT
camptothecin
- CR
complete regression
- CT
computed tomography
- CVD
cardiovascular disease
- Dac
daclizumab
- DDS
drug delivery system
- DMNB
dimethoxy-2-nitrobenzyl
- DO3A
1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid
- DOX
doxorubicin
- DPBF
1,3-diphenylisobenzofuran
- DTPA
diethylenetriaminepentaacetic acid
- E. coli
Escherichia coli
- EGFR
epidermal growth factor receptor
- EPR
enhanced permeability and retention
- Er3+
erbium
- Eu
europium
- FDA
food and drug administration
- Fe3O4
iron oxide
- FITC
(fluorescein 5(6)-isothiocyanate)
- FR
folate receptor
- FRET
fluorescence resonance energy transfer
- EtNBS
carboxybutylamino diethylaminobenzo phenothiazinium
- GLUT
glucose transporter
- Gd
gadolinium
- GNP
gold nanoparticle
- GNT
gold-coated carbon nanotube
- GNR
gold nanorod
- HA
hyaluronic acid
- HAuNS
hollow gold nanosphere
- HGN
hollow gold nanoshell
- HFF-1
human foreskin fibroblasts
- HP
hematoporphyrin
- HPPH
pyropheophorbide-alpha-hexyl-ether
- HSA
human serum albumin
- i.c
intracranial
- ICG
indocyanine green
- i.p
intra peritoneal
- IR
infra red
- i.v
intra venous
- LAMS
light-activated mesostructured silica
- LbL
layer-by-layer
- L-BPD
liposomal benzoporphyrin derivative monoacid ring A
- LCST
lower critical solution temperature
- β–LEAP
β-lactamase enzyme-activated photosensitizer
- LDL
low-density lipoprotein
- LDLR
LDL receptors
- Lip-NP
liposome-nanoparticle assembly
- LMB
leuko methylene blue
- Ln
lanthanides
- MB
methylene blue
- Mce6
mesochlorin e6
- MDR
multi drug resistance
- MFNP
magnetofluorescent nano particle
- MRI
magnetic resonance imaging
- MRSA
methicillin-resistant Staphylococcus aureus
- MSNP
mesoporous silica nanoparticle
- MTCNPs
magnetic targeting chitosan NPs
- MTCP
meso-tetra(4-carboxyphenyl) porphine
- mTHPC
meso-tetra(hydroxyphenyl) chlorine
- MTT
3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MWNT
multi wall carbon nanotubes
- NaYF4
sodium yttrium fluoride
- Nc
naphthalocyanine
- NIPAAm-co-AAm
N-isopropylacrylamide-co-acrylamide
- NIR
near infrared
- NP
nanoparticle
- OCT
optical coherence tomography
- ORMOSIL
organically modified silica
- PA
photoacoustic
- PAH
poly(allylamine hydrochloride)
- Pan
panitumumab
- Pc4
phthalocyanine 4
- PCI
photochemical internalization
- PDD
photodynamic diagnosis
- pDNA
plasmid DNA
- PDT
photodynamic therapy
- PEG
polyethylene glycol
- PEI
poly(ethylene imine)
- PET
positron emission tomography
- Pheo
pheophorbide
- PHPP
2,7,12,18-tetramethyl-3,8-di-(1-propoxyethyl)-13,17-bis-(3-hydroxypropyl) porphyrin
- PI
propidium iodide
- PIC
photoimmunoconjugate
- PICEL
photoimmunoconjugate encapsulating liposome
- PLGA
poly-L-co-glycolic-acid
- PS
photosensitizer
- PSiNPs
phosphonate-terminated silica nanoparticles
- PSS
poly(styrene sulfonate)
- PT
photothermal
- PTT
photothermal therapy
- PTX
paclitaxel
- pz
porphyrazine
- QD
quantum dots
- rGel
gelonin toxin
- RA
rheumatoid arthritis
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- SDT
sonodynamic therapy
- SiNcBOA
silicon naphthalocyanine bisoleate
- SiO2
silica
- siRNA
small interfering RNA
- SLN
solid lipid nanoparticles
- S. aureus
Staphylococcus aureus
- SWNTS
single wall carbon nanotubes
- TEOS
tetraEthOxy silane
- Tf-Lip
transferring-conjugated liposomes
- THPMP
tri-hydroxyl silyl propyl methyl phosphonate
- TPC
5-(4-carboxyphenyl)-10,15,20-triphenyl-2,3-dihydroxychlorin
- TPPS2A
disulfonated meso-tetraphenylporphine
- Tra
trastuzumab
- UV
ultraviolet
- VIS
visible light
- Yb3+
ytterbium
- ZnPC
zinc phthalocyanine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rising Health Costs a Global Problem, in, 2010.
- 2.WORLD HEALTH STATISTICS 2010. World Health Organization; France: 2010. pp. 1–177. [Google Scholar]
- 3.Rai AJ, Yee J, Fleisher M. Biomarkers in the era of personalized medicine - a multiplexed SNP assay using capillary electrophoresis for assessing drug metabolism capacity. Scand J Clin Lab Invest Suppl. 2010;242:15–18. doi: 10.3109/00365513.2010.493355. [DOI] [PubMed] [Google Scholar]
- 4.Blanchet KD. Redefining personalized medicine in the postgenomic era: developing bladder cancer therapeutics with proteomics. BJU Int. 2010;105:i–iii. doi: 10.1111/j.1464-410X.2009.09168.x. [DOI] [PubMed] [Google Scholar]
- 5.Francke U. On the bumpy road towards ‘personalized medicine’. EMBO Mol Med. 2010;2:1–2. doi: 10.1002/emmm.200900056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates S. Progress towards personalized medicine. Drug Discov Today. 2010;15:115–120. doi: 10.1016/j.drudis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Espina V, Liotta LA, Petricoin EF., 3rd Reverse-phase protein microarrays for theranostics and patient tailored therapy. Methods Mol Biol. 2009;520:89–105. doi: 10.1007/978-1-60327-811-9_7. [DOI] [PubMed] [Google Scholar]
- 8.Falk J. Epigenetics & Sequencing - Gene Expression Systems’ Second International Meeting. Chromatin methylation to disease biology & theranostics. IDrugs. 2008;11:650–652. [PubMed] [Google Scholar]
- 9.Buxton DB. Current status of nanotechnology approaches for cardiovascular disease: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:149–155. doi: 10.1002/wnan.8. [DOI] [PubMed] [Google Scholar]
- 10.Morris K. Nanotechnology crucial in fighting infectious disease. Lancet Infect Dis. 2009;9:215. doi: 10.1016/s1473-3099(09)70100-8. [DOI] [PubMed] [Google Scholar]
- 11.Nazem A, Mansoori GA. Nanotechnology solutions for Alzheimer’s disease: advances in research tools, diagnostic methods and therapeutic agents. J Alzheimers Dis. 2008;13:199–223. doi: 10.3233/jad-2008-13210. [DOI] [PubMed] [Google Scholar]
- 12.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 13.McCoy CP, Brady C, Cowley JF, McGlinchey SM, McGoldrick N, Kinnear DJ, Andrews GP, Jones DS. Triggered drug delivery from biomaterials. Expert Opin Drug Deliv. 2010;7:605–616. doi: 10.1517/17425241003677731. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 15.McBride JW, Balestrero A, Ghezzi L, Tribulato G, Cross KJ. Optical fiber imaging for high speed plasma motion diagnostics: applied to low voltage circuit breakers. Rev Sci Instrum. 2010;81:055109. doi: 10.1063/1.3428737. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 17.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balas C. Review of biomedical optical imaginga a powerful, non-invasive, non-ionizing technology for improving in vivo diagnosis. Measurement Science and Technology. 2009;20:104020. [Google Scholar]
- 19.Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter BO, Angell-Petersen E, Warloe T, Frandsen N, Hogset A. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc-Oxf. 2005;218:133–147. doi: 10.1111/j.1365-2818.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 20.Ray ER, Chatterton K, Khan MS, Thomas K, Chandra A, O’Brien TS. Hexylaminolaevulinate ‘blue light’ fluorescence cystoscopy in the investigation of clinically unconfirmed positive urine cytology. BJU Int. 2009;103:1363–1367. doi: 10.1111/j.1464-410X.2008.08238.x. [DOI] [PubMed] [Google Scholar]
- 21.Bogaards A, Varma A, Zhang K, Zach D, Bisland SK, Moriyama EH, Lilge L, Muller PJ, Wilson BC. Fluorescence image-guided brain tumour resection with adjuvant metronomic photodynamic therapy: pre-clinical model and technology development. Photochem Photobiol Sci. 2005;4:438–442. doi: 10.1039/b414829k. [DOI] [PubMed] [Google Scholar]
- 22.Lovell JF, Liu TW, Chen J, Zheng G. Activatable photosensitizers for imaging and therapy. Chem Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 23.Ohsaki Y, Takeyama K, Nakao S, Tanno S, Toyoshima E, Nakanishi K, Nishigaki Y, Ogasa T, Osanai S, Kikuchi K, Nakajima S. Detection of Photofrin Fluorescence From Malignant and Premalignant Lesions in the Bronchus using a Full-color Endoscopic Fluorescence Imaging System. Diagn Ther Endosc. 2001;7:187–195. doi: 10.1155/DTE.7.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohno M, Takeda M, Niwano Y, Saito R, Emoto N, Tada M, Kanazawa T, Ohuchi N, Yamada R. Early diagnosis of cancer by detecting the chemiluminescence of hematoporphyrins in peripheral blood lymphocytes. Tohoku J Exp Med. 2008;216:47–52. doi: 10.1620/tjem.216.47. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q, Huang Z, Luck D, Beckers J, Brun PH, Wilson BC, Scherz A, Salomon Y, Hetzel FW. Preclinical studies in normal canine prostate of a novel palladium-bacteriopheophorbide (WST09) photosensitizer for photodynamic therapy of prostate cancer. Photochemistry and Photobiology. 2002;76:438–445. doi: 10.1562/0031-8655(2002)076<0438:PSINCP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Karioti A, Bilia AR. Hypericins as potential leads for new therapeutics. Int J Mol Sci. 2010;11:562–594. doi: 10.3390/ijms11020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kah JC, Lau WK, Tan PH, Sheppard CJ, Olivo M. Endoscopic image analysis of photosensitizer fluorescence as a promising noninvasive approach for pathological grading of bladder cancer in situ. J Biomed Opt. 2008;13:054022. doi: 10.1117/1.2981827. [DOI] [PubMed] [Google Scholar]
- 28.Thong PS, Olivo M, Chin WW, Bhuvaneswari R, Mancer K, Soo KC. Clinical application of fluorescence endoscopic imaging using hypericin for the diagnosis of human oral cavity lesions. Br J Cancer. 2009;101:1580–1584. doi: 10.1038/sj.bjc.6605357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kacerovska D, Pizinger K, Majer F, Smid F. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract--a pilot study. Photochem Photobiol. 2008;84:779–785. doi: 10.1111/j.1751-1097.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 30.Bhuvaneswari R, Thong PS, Gan YY, Soo KC, Olivo M. Evaluation of hypericin-mediated photodynamic therapy in combination with angiogenesis inhibitor bevacizumab using in vivo fluorescence confocal endomicroscopy. J Biomed Opt. 2010;15:011114. doi: 10.1117/1.3281671. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi ER, Harney AS, Olive MB, Podgorski I, Moin K, Sloane BF, Barrett AG, Meade TJ, Hoffman BM. Chiral porphyrazine near-IR optical imaging agent exhibiting preferential tumor accumulation. Proc Natl Acad Sci U S A. 2010;107:1284–1288. doi: 10.1073/pnas.0912811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi ER, Vesper BJ, Weitman H, Ehrenberg B, Barrett AG, Radosevich JA, Hoffman BM. Chiral bis-acetal porphyrazines as near-infrared optical agents for detection and treatment of cancer. Photochem Photobiol. 2010;86:410–417. doi: 10.1111/j.1751-1097.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 33.Savellano MD, Hasan T. Photochemical targeting of epidermal growth factor receptor: A mechanistic study. Clinical Cancer Research. 2005;11:1658–1668. doi: 10.1158/1078-0432.CCR-04-1902. [DOI] [PubMed] [Google Scholar]
- 34.Mallikaratchy P, Tang ZW, Tan WH. Cell specific aptamer-photosensitizer conjugates as a molecular tool in photodynamic therapy. ChemMedChem. 2008;3:425–428. doi: 10.1002/cmdc.200700260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Zhang ZH, Blessington D, Li H, Busch TM, Madrak V, Miles J, Chance B, Glickson JD, Zheng G. Pyropheophorbide 2-deoxyglucosamide: A new photosensitizer targeting glucose transporters. Bioconjugate Chem. 2003;14:709–714. doi: 10.1021/bc034038n. [DOI] [PubMed] [Google Scholar]
- 36.Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 37.Zlokarnik G, Negulescu PA, Knapp TE, Mere L, Burres N, Feng L, Whitney M, Roemer K, Tsien RY. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science (New York, NY. 1998;279:84–88. doi: 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Stefflova K, Niedre MJ, Wilson BC, Chance B, Glickson JD, Zheng G. Protease-triggered photosensitizing beacon based on singlet oxygen quenching and activation. J Am Chem Soc. 2004;126:11450–11451. doi: 10.1021/ja047392k. [DOI] [PubMed] [Google Scholar]
- 39.Choi Y, Weissleder R, Tung CH. Selective antitumor effect of novel protease-mediated photodynamic agent. Cancer Res. 2006;66:7225–7229. doi: 10.1158/0008-5472.CAN-06-0448. [DOI] [PubMed] [Google Scholar]
- 40.Zheng G, Chen J, Stefflova K, Jarvi M, Li H, Wilson BC. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc Natl Acad Sci U S A. 2007;104:8989–8994. doi: 10.1073/pnas.0611142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nesterova IV, Erdem SS, Pakhomov S, Hammer RP, Soper SA. Phthalocyanine Dimerization-Based Molecular Beacons Using Near-IR Fluorescence. J Am Chem Soc. 2009;131:2432. doi: 10.1021/ja8088247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefflova K, Chen J, Li H, Zheng G. Targeted photodynamic therapy agent with a built-in apoptosis sensor for in vivo near-infrared imaging of tumor apoptosis triggered by its photosensitization in situ. Mol Imaging. 2006;5:520–532. [PubMed] [Google Scholar]
- 43.Pandey SK, Gryshuk AL, Sajjad M, Zheng X, Chen Y, Abouzeid MM, Morgan J, Charamisinau I, Nabi HA, Oseroff A, Pandey RK. Multimodality agents for tumor imaging (PET, fluorescence) and photodynamic therapy. A possible “see and treat” approach. J Med Chem. 2005;48:6286–6295. doi: 10.1021/jm050427m. [DOI] [PubMed] [Google Scholar]
- 44.Pandey SK, Sajjad M, Chen YH, Pandey A, Missert JR, Batt C, Yao RT, Nabi HA, Oseroff AR, Pandey RK. Compared to Purpurinimides, the Pyropheophorbide Containing an Iodobenzyl Group Showed Enhanced PDT Efficacy and Tumor Imaging (I-124-PET) Ability. Bioconjugate Chem. 2009;20:274–282. doi: 10.1021/bc8003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young SW, Qing F, Harriman A, Sessler JL, Dow WC, Mody TD, Hemmi GW, Hao YP, Miller RA. Gadolinium(III) texaphyrin: A tumor selective radiation sensitizer that is detectable by MRI. Proc Natl Acad Sci U S A. 1996;93:6610–6615. doi: 10.1073/pnas.93.13.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rockson SG, Kramer P, Razavi M, Szuba A, Filardo S, Fitzgerald P, Cooke JP, Yousuf S, DeVault AR, Renschler MF, Adelman DC. Photoangioplasty for human peripheral atherosclerosis - Results of a phase I trial of photodynamic therapy with motexafin lutetium (Antrin) Circulation. 2000;102:2322–2324. doi: 10.1161/01.cir.102.19.2322. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann B, Bogdanov A, Marecos E, Ebert W, Semmler W, Weissleder R. Mechanism of gadophrin-2 accumulation in tumor necrosis. JMRI-J Magn Reson Imaging. 1999;9:336–341. doi: 10.1002/(sici)1522-2586(199902)9:2<336::aid-jmri28>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Li GL, Slansky A, Dobhal MP, Goswami LN, Graham A, Chen YH, Kanter P, Alberico RA, Spernyak J, Mazurchuk R, Oseroff A, Grossman Z, Pandey RK. Chlorophyll-a analogues conjugated with aminobenzyl-DTPA as potential bifunctional agents for magnetic resonance imaging and photodynamic therapy. Bioconjugate Chem. 2005;16:32–42. doi: 10.1021/bc049807x. [DOI] [PubMed] [Google Scholar]
- 49.Spernyak JA, White WH, Ethirajan M, Patel NJ, Goswami L, Chen YH, Turowski S, Missert JR, Batt C, Mazurchuk R, Pandey RK. Hexylether Derivative of Pyropheophorbide-a (HPPH) on Conjugating with 3Gadolinium(III) Aminobenzyldiethylenetriaminepentaacetic Acid Shows Potential for in Vivo Tumor Imaging (MR, Fluorescence) and Photodynamic Therapy. Bioconjugate Chem. 2010;21:828–835. doi: 10.1021/bc9005317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Ohta S, Sonoda A, Yamada M, Yamamoto M, Nitta N, Murata K, Tabata Y. Preparation of PEG-conjugated fullerene containing Gd3+ ions for photodynamic therapy. J Control Release. 2007;117:104–110. doi: 10.1016/j.jconrel.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Vaidya A, Sun Y, Ke T, Jeong EK, Lu ZR. Contrast enhanced MRI-guided photodynamic therapy for site-specific cancer treatment. Magn Reson Med. 2006;56:761–767. doi: 10.1002/mrm.21009. [DOI] [PubMed] [Google Scholar]
- 52.Vaidya A, Sun Y, Feng Y, Emerson L, Jeong EK, Lu ZR. Contrast-enhanced MRI-guided photodynamic cancer therapy with a pegylated bifunctional polymer conjugate. Pharm Res. 2008;25:2002–2011. doi: 10.1007/s11095-008-9608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu F, Wang ZB, Chen WZ, Zou JZ. Extracorporeal high intensity focused ultrasound for treatment of solid carcinomas: four-year Chinese clinical experience. 2nd International Symposium on Therapeutic Ultrasound; Seattle, WA, USA. 2002. p. 11. [Google Scholar]
- 54.Hiraoka W, Honda H, Feril LB, Kudo N, Kondo T. Comparison between sonodynamic effect and photodynamic effect with photo sensitizers on free radical formation and cell killing. Ultrasonics Sonochemistry. 2006;13:535–542. doi: 10.1016/j.ultsonch.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Jin ZH, Miyoshi N, Ishiguro K, Umemura S, Kawabata K, Yumita N, Sakata I, Takaoka K, Udagawa T, Nakajima S, Tajiri H, Ueda K, Fukuda M, Kumakiri M. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J Dermatol. 2000;27:294–306. doi: 10.1111/j.1346-8138.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 56.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–1637. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Gravier J, Schneider R, Frochot C, Bastogne T, Schmitt F, Didelon J, Guillemin F, Barberi-Heyob M. Improvement of meta-tetra(hydroxyphenyl)chlorin-like photosensitizer selectivity with folate-based targeted delivery. synthesis and in vivo delivery studies. J Med Chem. 2008;51:3867–3877. doi: 10.1021/jm800125a. [DOI] [PubMed] [Google Scholar]
- 58.Taquet JP, Frochot C, Manneville V, Barberi-Heyob M. Phthalocyanines covalently bound to biomolecules for a targeted photodynamic therapy. Curr Med Chem. 2007;14:1673–1687. doi: 10.2174/092986707780830970. [DOI] [PubMed] [Google Scholar]
- 59.Fang C, Zhang M. Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slowing, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J Am Chem Soc. 2007;129:8845–8849. doi: 10.1021/ja0719780. [DOI] [PubMed] [Google Scholar]
- 61.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: A novel drug-carrier system for photodynamic therapy. Journal of the American Chemical Society. 2003;125:7860–7865. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 62.Ohulchanskyy TY, Roy I, Goswami LN, Chen Y, Bergey EJ, Pandey RK, Oseroff AR, Prasad PN. Organically modified silica nanoparticles with covalently incorporated photosensitizer for photodynamic therapy of cancer. Nano Lett. 2007;7:2835–2842. doi: 10.1021/nl0714637. [DOI] [PubMed] [Google Scholar]
- 63.Kim S, Ohulchanskyy TY, Pudavar HE, Pandey RK, Prasad PN. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J Am Chem Soc. 2007;129:2669–2675. doi: 10.1021/ja0680257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, Prasad PN. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano. 2010;4:699–708. doi: 10.1021/nn901146y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He X, Wu X, Wang K, Shi B, Hai L. Methylene blue-encapsulated phosphonate-terminated silica nanoparticles for simultaneous in vivo imaging and photodynamic therapy. Biomaterials. 2009;30:5601–5609. doi: 10.1016/j.biomaterials.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 66.Zhang R, Wu C, Tong L, Tang B, Xu QH. Multifunctional core-shell nanoparticles as highly efficient imaging and photosensitizing agents. Langmuir. 2009;25:10153–10158. doi: 10.1021/la902235d. [DOI] [PubMed] [Google Scholar]
- 67.Glickson JD, Lund-Katz S, Zhou R, Choi H, Chen IW, Li H, Corbin I, Popov AV, Cao W, Song L, Qi C, Marotta D, Nelson DS, Chen J, Chance B, Zheng G. Lipoprotein nanoplatform for targeted delivery of diagnostic and therapeutic agents. Mol Imaging. 2008;7:101–110. [PubMed] [Google Scholar]
- 68.Glickson JD, Lund-Katz S, Zhou R, Choi H, Chen IW, Li H, Corbin I, Popov AV, Cao W, Song L, Qi C, Marotta D, Nelson DS, Chen J, Chance B, Zheng G. Lipoprotein nanoplatform for targeted delivery of diagnostic and therapeutic agents. Adv Exp Med Biol. 2009;645:227–239. doi: 10.1007/978-0-387-85998-9_35. [DOI] [PubMed] [Google Scholar]
- 69.Zheng G, Li H, Yang K, Blessington D, Licha K, Lund-Katz S, Chance B, Glickson JD. Tricarbocyanine cholesteryl laurates labeled LDL: new near infrared fluorescent probes (NIRFs) for monitoring tumors and gene therapy of familial hypercholesterolemia. Bioorg Med Chem Lett. 2002;12:1485–1488. doi: 10.1016/s0960-894x(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 70.Li H, Zhang Z, Blessington D, Nelson DS, Zhou R, Lund-Katz S, Chance B, Glickson JD, Zheng G. Carbocyanine labeled LDL for optical imaging of tumors. Acad Radiol. 2004;11:669–677. doi: 10.1016/j.acra.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 71.Corbin IR, Li H, Chen J, Lund-Katz S, Zhou R, Glickson JD, Zheng G. Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia. 2006;8:488–498. doi: 10.1593/neo.05835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song L, Li H, Sunar U, Chen J, Corbin I, Yodh AG, Zheng G. Naphthalocyanine-reconstituted LDL nanoparticles for in vivo cancer imaging and treatment. Int J Nanomedicine. 2007;2:767–774. [PMC free article] [PubMed] [Google Scholar]
- 73.Chen K, Preuss A, Hackbarth S, Wacker M, Langer K, Roder B. Novel photosensitizer-protein nanoparticles for photodynamic therapy: photophysical characterization and in vitro investigations. J Photochem Photobiol B. 2009;96:66–74. doi: 10.1016/j.jphotobiol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Saupe A, Wissing SA, Lenk A, Schmidt C, Muller RH. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) -- structural investigations on two different carrier systems. Biomed Mater Eng. 2005;15:393–402. [PubMed] [Google Scholar]
- 75.Stevens PJ, Sekido M, Lee RJ. Synthesis and evaluation of a hematoporphyrin derivative in a folate receptor-targeted solid-lipid nanoparticle formulation. Anticancer Res. 2004;24:161–165. [PubMed] [Google Scholar]
- 76.Chatterjee DK, Yong Z. Upconverting nanoparticles as nanotransducers for photodynamic therapy in cancer cells. Nanomedicine (Lond) 2008;3:73–82. doi: 10.2217/17435889.3.1.73. [DOI] [PubMed] [Google Scholar]
- 77.Chatterjee DK, Rufaihah AJ, Zhang Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials. 2008;29:937–943. doi: 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 78.Derycke AS, de Witte PA. Liposomes for photodynamic therapy. Adv Drug Deliv Rev. 2004;56:17–30. doi: 10.1016/j.addr.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 79.Bendsoe N, Persson L, Johansson A, Axelsson J, Svensson J, Grafe S, Trebst T, Andersson-Engels S, Svanberg S, Svanberg K. Fluorescence monitoring of a topically applied liposomal Temoporfin formulation and photodynamic therapy of nonpigmented skin malignancies. J Environ Pathol Toxicol Oncol. 2007;26:117–126. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i2.60. [DOI] [PubMed] [Google Scholar]
- 80.Zhong W, Celli JP, Rizvi I, Mai Z, Spring BQ, Yun SH, Hasan T. In vivo high-resolution fluorescence microendoscopy for ovarian cancer detection and treatment monitoring. Br J Cancer. 2009;101:2015–2022. doi: 10.1038/sj.bjc.6605436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hendren SK, Hahn SM, Spitz FR, Bauer TW, Rubin SC, Zhu T, Glatstein E, Fraker DL. Phase II trial of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Ann Surg Oncol. 2001;8:65–71. doi: 10.1007/s10434-001-0065-x. [DOI] [PubMed] [Google Scholar]
- 82.Hahn SM, Fraker DL, Mick R, Metz J, Busch TM, Smith D, Zhu T, Rodriguez C, Dimofte A, Spitz F, Putt M, Rubin SC, Menon C, Wang HW, Shin D, Yodh A, Glatstein E. A phase II trial of intraperitoneal photodynamic therapy for patients with peritoneal carcinomatosis and sarcomatosis. Clin Cancer Res. 2006;12:2517–2525. doi: 10.1158/1078-0432.CCR-05-1625. [DOI] [PubMed] [Google Scholar]
- 83.Sandanayake NS, Huggett MT, Bown SG, Pogue BW, Hasan T, Pereira SP. In: Photodynamic therapy for locally advanced pancreatic cancer: early clinical results. Kessel DH, editor. SPIE; San Francisco, California, USA: 2010. pp. 75510L–75518. [Google Scholar]
- 84.Derycke AS, Kamuhabwa A, Gijsens A, Roskams T, De Vos D, Kasran A, Huwyler J, Missiaen L, de Witte PA. Transferrin-conjugated liposome targeting of photosensitizer AlPcS4 to rat bladder carcinoma cells. J Natl Cancer Inst. 2004;96:1620–1630. doi: 10.1093/jnci/djh314. [DOI] [PubMed] [Google Scholar]
- 85.Meerovich IG, Smirnova ZS, Oborotova NA, Luk’yanets EA, Meerovich GA, Derkacheva VM, Polozkova AP, Kubasova IY, Baryshnikov AY. Hydroxyaluminium tetra-3-phenylthiophthalocyanine is a new effective photosensitizer for photodynamic therapy and fluorescent diagnosis. Bull Exp Biol Med. 2005;139:427–430. doi: 10.1007/s10517-005-0313-3. [DOI] [PubMed] [Google Scholar]
- 86.Rahmanzadeh R, Rai P, Celli JP, Rizvi I, Baron-Luhr B, Gerdes J, Hasan T. Ki-67 as a molecular target for therapy in an in vitro 3D model for ovarian cancer. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-1190. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int JCancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 88.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. JCell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 89.Surrey T, Elowitz MB, Wolf PE, Yang F, d,lec FN, Shokat K, Leibler S. Chromophore-assisted light inactivation and self-organization of microtubules and motors. Proc Natl Acad Sci USA. 1998;95:4293–4298. doi: 10.1073/pnas.95.8.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 91.Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell DA. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: cancer therapy using a ‘Trojan horse’. Photochemical & Photobiological Sciences. 2006;5:727–734. doi: 10.1039/b602830f. [DOI] [PubMed] [Google Scholar]
- 92.Zaruba K, Kralova J, Rezanka P, Pouckova P, Veverkova L, Kral V. Modified porphyrin-brucine conjugated to gold nanoparticles and their application in photodynamic therapy. Org Biomol Chem. 2010 doi: 10.1039/c002823a. [DOI] [PubMed] [Google Scholar]
- 93.Camerin M, Magaraggia M, Soncin M, Jori G, Moreno M, Chambrier I, Cook MJ, Russell DA. The in vivo efficacy of phthalocyanine-nanoparticle conjugates for the photodynamic therapy of amelanotic melanoma. Eur J Cancer. 2010;46:1910–1918. doi: 10.1016/j.ejca.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 94.Cheng Y, CSA, Meyers JD, Panagopoulos I, Fei B, Burda C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc. 2008;130:10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 96.Yan D, Li L, Chen DY, Zhang YH, Hu CH, Deng ZH. Detection of fungi in liquor workers with tinea corporis and tinea cruris using arbitrarily primed polymerase chain reaction. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007;25:133–135. [PubMed] [Google Scholar]
- 97.Sun Y, Chen ZL, Yang XX, Huang P, Zhou XP, Du XX. Magnetic chitosan nanoparticles as a drug delivery system for targeting photodynamic therapy. Nanotechnology. 2009;20:135102. doi: 10.1088/0957-4484/20/13/135102. [DOI] [PubMed] [Google Scholar]
- 98.Koo YE, Fan W, Hah H, Xu H, Orringer D, Ross B, Rehemtulla A, Philbert MA, Kopelman R. Photonic explorers based on multifunctional nanoplatforms for biosensing and photodynamic therapy. Appl Opt. 2007;46:1924–1930. doi: 10.1364/ao.46.001924. [DOI] [PubMed] [Google Scholar]
- 99.Tang W, Xu H, Kopelman R, Philbert MA. Photodynamic characterization and in vitro application of methylene blue-containing nanoparticle platforms. Photochem Photobiol. 2005;81:242–249. doi: 10.1562/2004-05-24-RA-176.1. [DOI] [PubMed] [Google Scholar]
- 100.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YE, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 101.Moffat BA, Chenevert TL, Meyer CR, McKeever PE, Hall DE, Hoff BA, Johnson TD, Rehemtulla A, Ross BD. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8:259–267. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Samkoe KS, Chen A, Rizvi I, O’Hara JA, Hoopes PJ, Pereira SP, Hasan T, Pogue BW. Imaging tumor variation in response to photodynamic therapy in pancreatic cancer xenograft models. Int J Radiat Oncol Biol Phys. 2010;76:251–259. doi: 10.1016/j.ijrobp.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lai CW, Wang YH, Lai CH, Yang MJ, Chen CY, Chou PT, Chan CS, Chi Y, Chen YC, Hsiao JK. Iridium-complex-functionalized Fe3O4/SiO2 core/shell nanoparticles: a facile three-in-one system in magnetic resonance imaging, luminescence imaging, and photodynamic therapy. Small. 2008;4:218–224. doi: 10.1002/smll.200700283. [DOI] [PubMed] [Google Scholar]
- 104.Brevet D, Gary-Bobo M, Raehm L, Richeter S, Hocine O, Amro K, Loock B, Couleaud P, Frochot C, Morere A, Maillard P, Garcia M, Durand JO. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem Commun (Camb) 2009:1475–1477. doi: 10.1039/b900427k. [DOI] [PubMed] [Google Scholar]
- 105.Chelebaeva E, Raehm L, Durand JO, Guari Y, Larionova J, Guerin C, Trifonov A, Willinger M, Thangavel K, Lascialfari A, Mongin O, Mir Y, Blanchard-Desce M. Mesoporous silica nanoparticles combining two-photon excited fluorescence and magnetic properties. Journal of Materials Chemistry. 2010;20:1877–1884. [Google Scholar]
- 106.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Baba Y. Quantum dots as photosensitizers? Nat Biotechnol. 2004;22:1360–1361. doi: 10.1038/nbt1104-1360. [DOI] [PubMed] [Google Scholar]
- 108.Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125:15736–15737. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- 109.Bakalova R, Zhelev Z, Aoki I, Masamoto K, Mileva M, Obata T, Higuchi M, Gadjeva V, Kanno I. Multimodal silica-shelled quantum dots: direct intracellular delivery, photosensitization, toxic, and microcirculation effects. Bioconjug Chem. 2008;19:1135–1142. doi: 10.1021/bc700431c. [DOI] [PubMed] [Google Scholar]
- 110.Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. Journal of Nanoscience and Nanotechnology. 2006;6:1159–1166. doi: 10.1166/jnn.2006.327. [DOI] [PubMed] [Google Scholar]
- 111.Liu YF, Chen W, Wang SP, Joly AG. Investigation of water-soluble x-ray luminescence nanoparticles for photodynamic activation. Applied Physics Letters. 2008;92 [Google Scholar]
- 112.Jacques S. Laser-tissue interactions. Photochemical, photothermal, and photomechanical. The Surgical clinics of North America. 1992;72:531. doi: 10.1016/s0039-6109(16)45731-2. [DOI] [PubMed] [Google Scholar]
- 113.Jori G, Spikes JD. Photothermal sensitizers: possible use in tumor therapy. Journal of photochemistry and photobiology B, Biology. 1990;6:93–101. doi: 10.1016/1011-1344(90)85078-b. [DOI] [PubMed] [Google Scholar]
- 114.Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Tuchin V, Altshuler GB. Low-intensity indocyanine-green laser phototherapy of acne vulgaris: pilot study. Journal of Biomedical Optics. 2004;9:828–834. doi: 10.1117/1.1756596. [DOI] [PubMed] [Google Scholar]
- 115.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Sayed I, Huang X, El-Sayed M. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Letters. 2006;239:129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 117.Yu J, Javier D, Yaseen MA, Nitin N. Self-Assembly Synthesis, Tumor Cell Targeting, and Photothermal Capabilities of Antibody-Coated Indocyanine Green Nanocapsules. Journal of the American Chemical Society. 2010 doi: 10.1021/ja908139y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang S, Gnanasammandhan M, Zhang Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. Journal of The Royal Society Interface. 7:3. doi: 10.1098/rsif.2009.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zharov V, Galitovskaya E, Johnson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: potential for cancer therapy. Lasers in Surgery and Medicine. 2005;37:219–226. doi: 10.1002/lsm.20223. [DOI] [PubMed] [Google Scholar]
- 120.Skala M, Crow M, Wax A, Izatt J. Photothermal Optical Coherence Tomography of Epidermal Growth Factor Receptor in Live Cells Using Immunotargeted Gold Nanospheres. Nano Letters. 2008;8:3461–3467. doi: 10.1021/nl802351p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X, Lloyd M, Fedorenko I, Bapat P, Zhukov T, Huo Q. Enhanced imaging and accelerated photothermalysis of A549 human lung cancer cells by gold nanospheres. Nanomedicine (London, England) 2008;3:617–626. doi: 10.2217/17435889.3.5.617. [DOI] [PubMed] [Google Scholar]
- 122.Lal S, Clare S, Halas N. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Accounts of Chemical Research. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 123.Skrabalak SE, Chen J, Au L, Lu X, Li X, Xia Y. Gold Nanocages for Biomedical Applications. Advanced materials (Deerfield Beach, Fla) 2007;19:3177–3184. doi: 10.1002/adma.200701972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu X, Ming T, Wang X, Wang P, Wang J, Chen J. High-photoluminescence-yield gold nanocubes: for cell imaging and photothermal therapy. ACS Nano. 2010;4:113–120. doi: 10.1021/nn901064m. [DOI] [PubMed] [Google Scholar]
- 125.Hu KW, Huang CC, Hwu JR, Su WC, Shieh DB, Yeh C. A new photothermal therapeutic agent: core-free nanostructured Au x Ag1-x dendrites. Chemistry (Weinheim an der Bergstrasse, Germany) 2008;14:2956–2964. doi: 10.1002/chem.200800114. [DOI] [PubMed] [Google Scholar]
- 126.Wang S, Chen K, Wu TH, Wang H. Angewandte Chemie (International ed. in English) 2010. Photothermal Effects of Supramolecularly Assembled Gold Nanoparticles for the Targeted Treatment of Cancer Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huff T, Hansen M, Tong L, Zhao Y, Wang H, Zweifel D, Cheng J, Wei A. Plasmon-resonant nanorods as multimodal agents for two-photon luminescent imaging and photothermal therapy. Society of Photo-Optical Instrumentation Engineers (SPIE) Conference Series. 2007;6448:5. [Google Scholar]
- 128.Gobin AM, Lee MH, Halas N, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Letters. 2007;7:1929–1934. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 129.Dickerson EB, Dreaden EC, Huang X, El-Sayed I, Chu H, Pushpanketh S, McDonald JF, El-Sayed M. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Letters. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. Journal of Biomedical Optics. 2009;14:021009. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torti S, Byrne F, Whelan O, Levi N, Ucer B, Schmid M, Torti F, Akman S, Liu J, Ajayan P. Thermal ablation therapeutics based on CNx multi-walled nanotubes. International Journal of Nanomedicine. 2007;2:707. [PMC free article] [PubMed] [Google Scholar]
- 132.Shah J, Aglyamov SR, Sokolov K, Milner TE, Emelianov SY. Ultrasound imaging to monitor photothermal therapy - feasibility study. Optics Express. 2008;16:3776–3785. doi: 10.1364/oe.16.003776. [DOI] [PubMed] [Google Scholar]
- 133.Aglyamov S, Emelianov S, Shah J, Sokolov K, Milner T. Ultrasound-Based Thermal and Elasticity Imaging to Assist Photothermal Cancer Therapy - Preliminary Study. IEEE Ultrasonics Symposium. 2006:1029–1032. [Google Scholar]
- 134.Mehrmohammadi M, Ma LL, Chen YS, Qu M. Combined photothermal therapy and magneto-motive ultrasound imaging using multifunctional nanoparticles. Proceedings of SPIE. 2010;7574:1–8. [Google Scholar]
- 135.Mallidi S, Wang B, Mehrmohammadi M, Qu M, Chen Y, Joshi P, Kim S, Homan KA, Karpiouk AB, Smalling RW, Sokolov K, Emelianov S. Ultrasound-based imaging of nanoparticles: From molecular and cellular imaging to therapy guidance. IEEE Ultrasonics Symposium. 2009:27–36. [Google Scholar]
- 136.Emelianov SY, Li PC, O’Donnell M. Photoacoustics for molecular imaging and therapy. Physics today. 2009;62:34–39. doi: 10.1063/1.3141939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L. Multiscale photoacoustic microscopy and computed tomography. Nature Photonics. 2009;3:503–509. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu M, Wang L. Photoacoustic imaging in biomedicine. Review of Scientific Instruments. 2006;77:041101. [Google Scholar]
- 139.Chen Y, Frey W, Kim S, Homan K, Kruizinga P. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Optics Express. doi: 10.1364/OE.18.008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim J, Galanzha E, Shashkov E, Moon H, Zharov V. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nature Nanotechnology. 2009;4:688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Galanzha E, Kim J, Zharov V. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform forin-vivodetection and killing of circulating cancer stem cells. Journal of Biophotonics. 2009;2:725–735. doi: 10.1002/jbio.200910078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008;8:3953–3958. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shah J, Park S, Aglyamov S, Larson T, Ma L, Sokolov K, Johnston K, Milner T, Emelianov SY. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. Journal of Biomedical Optics. 2008;13:034024. doi: 10.1117/1.294036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caruthers S, Winter P, Wickline S, Lanza G. Targeted magnetic resonance imaging contrast agents. Methods in molecular medicine. 2006;124:387. doi: 10.1385/1-59745-010-3:387. [DOI] [PubMed] [Google Scholar]
- 145.Morawski AM, Lanza GA, Wickline SA. Targeted contrast agents for magnetic resonance imaging and ultrasound. Current Opinion in Biotechnology. 2005;16:89–92. doi: 10.1016/j.copbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 146.Diagaradjane P, Shetty A, Wang J, Elliott A, Schwartz J, Shentu S, Park H, Deorukhkar A, Stafford R, Cho S. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Letters. 2008;8:1492. doi: 10.1021/nl080496z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Burke A, Ding X, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, Szot C, Buchanan C, Whitney J, Fisher J, Hatcher HC, D’Agostino R, Kock ND, Ajayan PM, Carroll DL, Akman S, Torti FM, Torti SV. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12897–12902. doi: 10.1073/pnas.0905195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim J, Oh J, Kang H, Feldman M, Milner T. Photothermal response of superparamagnetic iron oxide nanoparticles. Lasers in Surgery and Medicine. 2008;40:415–421. doi: 10.1002/lsm.20650. [DOI] [PubMed] [Google Scholar]
- 149.Larson T, Bankson J, Aaron J, Sokolov K. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology. 2007;18:325101–325101. [Google Scholar]
- 150.Bouchard LS, Anwar MS, Liu GL, Hann B, Xie ZH, Gray JW, Wang X, Pines A, Chen FF. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4085–4089. doi: 10.1073/pnas.0813019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim J, Park S, Lee JE, Jin SM, Lee JH, Lee IS, Yang I, Kim JS, Kim SK, Cho MH, Hyeon T. Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angewandte Chemie (International ed in English) 2006;45:7754–7758. doi: 10.1002/anie.200602471. [DOI] [PubMed] [Google Scholar]
- 152.Ji X, Shao R, Elliott AM, Stafford RJ, Esparza-Coss E, Liang G, Luo ZP, Park K, Markert JT, Li C. Bifunctional Gold Nanoshells with a Superparamagnetic Iron Oxide-Silica Core Suitable for Both MR Imaging and Photothermal Therapy, The journal of physical chemistry C. Nanomaterials and interfaces. 2007;111:6245. doi: 10.1021/jp0702245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee J, Yang J, Ko H, Oh S, Kang J, Son J, Lee K, Lee S, Yoon H, Suh J. Multifunctional magnetic gold nanocomposites: human epithelial cancer detection via magnetic resonance imaging and localized synchronous therapy. Advanced Functional Materials. 2008;18:258. [Google Scholar]
- 154.Kirui DK, Rey DA, Batt CA. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology. 2010;21:105105. doi: 10.1088/0957-4484/21/10/105105. [DOI] [PubMed] [Google Scholar]
- 155.Yu H, Chen M, Rice P, Wang S, White R, Sun S. Dumbbell-like Bifunctional Au–Fe3O4 Nanoparticles. Nano Letters. 2005;5:379–382. doi: 10.1021/nl047955q. [DOI] [PubMed] [Google Scholar]
- 156.Ma L, Feldman M, Tam J, Paranjape A, Cheruku K, Larson T, Ingram D, Paramita V, Villard J. Small multifunctional nanoclusters (nanoroses) for targeted cellular imaging and therapy. ACS Nano. 2009;3:2686–2696. doi: 10.1021/nn900440e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen W, Xu N, Xu L, Wang L, Li Z, Ma W, Zhu Y, Xu C, Kotov N. Multifunctional Magnetoplasmonic Nanoparticle Assemblies for Cancer Therapy and Diagnostics (Theranostics) Macromolecular Rapid Communications. 2010;31:228–236. doi: 10.1002/marc.200900793. [DOI] [PubMed] [Google Scholar]
- 158.Park H, Yang J, Seo S, Kim K, Suh J, Kim D, Haam S, Yoo KH. Multifunctional nanoparticles for photothermally controlled drug delivery and magnetic resonance imaging enhancement. Small. 2008;4:192–196. doi: 10.1002/smll.200700807. [DOI] [PubMed] [Google Scholar]
- 159.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, Dai H. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nature Materials. 2006;5:971–976. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 160.Gupta T, Beriwal S. PET/CT-guided radiation therapy planning: from present to the future. Indian J Cancer. 2010;47:126–133. doi: 10.4103/0019-509X.63000. [DOI] [PubMed] [Google Scholar]
- 161.Hainfeld J, Slatkin D, Focella T, Smilowitz H. Gold nanoparticles: a new X-ray contrast agent. British Journal of Radiology. 2006;79:248. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 162.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Physics in medicine and biology. 2004;49:N309–315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 163.Melancon M, Lu W, Yang Z, Zhang R, Cheng Z, Elliot A, Stafford J, Olson T, Zhang J, Li C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Molecular Cancer Therapeutics. 2008;7:1730–1739. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer research. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clinical cancer research. 2009;15:876–886. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M, Wilson BC, Fenster A, Trachtenberg J. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. The Journal of urology. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 167.Davis SC, Samkoe KS, O’Hara JA, Gibbs-Strauss SL. MRI-coupled Fluorescence Tomography Quantifies EGFR Activity in Brain Tumors. Academic Radiology. 2010;17:271–276. doi: 10.1016/j.acra.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hogset A, Prasmickaite L, Selbo PK, Hellum M, Engesaeter BO, Bonsted A, Berg K. Photochemical internalisation in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:95–115. doi: 10.1016/j.addr.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 169.Berg K, Selbo PK, Prasmickaite L, Hogset A. Photochemical drug and gene delivery. Curr Opin Mol Ther. 2004;6:279–287. [PubMed] [Google Scholar]
- 170.Nishiyama N, Iriyama A, Jang WD, Miyata K, Itaka K, Inoue Y, Takahashi H, Yanagi Y, Tamaki Y, Koyama H, Kataoka K. Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer. Nat Mater. 2005;4:934–941. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]
- 171.You J, Shao R, Wei X, Gupta S, Li C. Near-infrared light triggers release of Paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activity. Small. 2010;6:1022–1031. doi: 10.1002/smll.201000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, Kobayashi H. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–109. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Norum OJ, Gaustad JV, Angell-Petersen E, Rofstad EK, Peng Q, Giercksky KE, Berg K. Photochemical internalization of bleomycin is superior to photodynamic therapy due to the therapeutic effect in the tumor periphery. Photochem Photobiol. 2009;85:740–749. doi: 10.1111/j.1751-1097.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 174.Selbo PK, Rosenblum MG, Cheung LH, Zhang W, Berg K. Multi-modality therapeutics with potent anti-tumor effects: photochemical internalization enhances delivery of the fusion toxin scFvMEL/rGel. PLoS One. 2009;4:e6691. doi: 10.1371/journal.pone.0006691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lou PJ, Lai PS, Shieh MJ, Macrobert AJ, Berg K, Bown SG. Reversal of doxorubicin resistance in breast cancer cells by photochemical internalization. Int J Cancer. 2006;119:2692–2698. doi: 10.1002/ijc.22098. [DOI] [PubMed] [Google Scholar]
- 176.Febvay S, Marini DM, Belcher AM, Clapham DE. Targeted cytosolic delivery of cell-impermeable compounds by nanoparticle-mediated, light-triggered endosome disruption. Nano Lett. 2010;10:2211–2219. doi: 10.1021/nl101157z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pashkovskaya A, Kotova E, Zorlu Y, Dumoulin F, Ahsen V, Agapov I, Antonenko Y. Light-triggered liposomal release: membrane permeabilization by photodynamic action. Langmuir. 2010;26:5726–5733. doi: 10.1021/la903867a. [DOI] [PubMed] [Google Scholar]
- 178.Wang JY, Wu QF, Li JP, Ren QS, Wang YL, Liu XM. Photo-sensitive liposomes: chemistry and application in drug delivery. Mini Rev Med Chem. 2010;10:172–181. doi: 10.2174/138955710791185091. [DOI] [PubMed] [Google Scholar]
- 179.Lu J, Choi E, Tamanoi F, Zink JI. Light-activated nanoimpeller-controlled drug release in cancer cells. Small. 2008;4:421–426. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dvir T, Banghart MR, Timko BP, Langer R, Kohane DS. Photo-targeted nanoparticles. Nano Lett. 2010;10:250–254. doi: 10.1021/nl903411s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Banerjee SS, Chen DH. A multifunctional magnetic nanocarrier bearing fluorescent dye for targeted drug delivery by enhanced two-photon triggered release. Nanotechnology. 2009;20:185103. doi: 10.1088/0957-4484/20/18/185103. [DOI] [PubMed] [Google Scholar]
- 182.McCoy CP, Rooney C, Jones DS, Gorman SP, Nieuwenhuyzen M. Rational design of a dual-mode optical and chemical prodrug. Pharm Res. 2007;24:194–200. doi: 10.1007/s11095-006-9145-8. [DOI] [PubMed] [Google Scholar]
- 183.McCoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. Light-triggered molecule-scale drug dosing devices. J Am Chem Soc. 2007;129:9572–9573. doi: 10.1021/ja073053q. [DOI] [PubMed] [Google Scholar]
- 184.Yao C, Qu X, Zhang Z, Huttmann G, Rahmanzadeh R. Influence of laser parameters on nanoparticle-induced membrane permeabilization. J Biomed Opt. 2009;14:054034. doi: 10.1117/1.3253320. [DOI] [PubMed] [Google Scholar]
- 185.Morton JG, Day ES, Halas NJ, West JL. Nanoshells for photothermal cancer therapy. Methods Mol Biol. 2010;624:101–117. doi: 10.1007/978-1-60761-609-2_7. [DOI] [PubMed] [Google Scholar]
- 186.Park H, Yang J, Lee J, Haam S, Choi IH, Yoo KH. Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano. 2009;3:2919–2926. doi: 10.1021/nn900215k. [DOI] [PubMed] [Google Scholar]
- 187.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bikram M, Gobin AM, Whitmire RE, West JL. Temperature-sensitive hydrogels with SiO2-Au nanoshells for controlled drug delivery. J Control Release. 2007;123:219–227. doi: 10.1016/j.jconrel.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 189.Cheng FY, Su CH, Wu PC, Yeh CS. Multifunctional polymeric nanoparticles for combined chemotherapeutic and near-infrared photothermal cancer therapy in vitro and in vivo. Chem Commun (Camb) 2010;46:3167–3169. doi: 10.1039/b919172k. [DOI] [PubMed] [Google Scholar]
- 190.Paasonen L, Romberg B, Storm G, Yliperttula M, Urtti A, Hennink WE. Temperature-sensitive poly(N-(2-hydroxypropyl)methacrylamide mono/dilactate)-coated liposomes for triggered contents release. Bioconjug Chem. 2007;18:2131–2136. doi: 10.1021/bc700245p. [DOI] [PubMed] [Google Scholar]
- 191.Wu G, Mikhailovsky A, Khant HA, Zasadzinski JA. Chapter 14 - Synthesis, characterization, and optical response of gold nanoshells used to trigger release from liposomes. Methods Enzymol. 2009;464:279–307. doi: 10.1016/S0076-6879(09)64014-3. [DOI] [PubMed] [Google Scholar]
- 192.You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010;4:1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Volodkin DV, Delcea M, Mohwald H, Skirtach AG. Remote near-IR light activation of a hyaluronic acid/poly(l-lysine) multilayered film and film-entrapped microcapsules. ACS Appl Mater Interfaces. 2009;1:1705–1710. doi: 10.1021/am900269c. [DOI] [PubMed] [Google Scholar]
- 194.Volodkin DV, Skirtach AG, Mohwald H. Near-IR remote release from assemblies of liposomes and nanoparticles. Angew Chem Int Ed Engl. 2009;48:1807–1809. doi: 10.1002/anie.200805572. [DOI] [PubMed] [Google Scholar]
- 195.Volodkin DV, Madaboosi N, Blacklock J, Skirtach AG, Mohwald H. Surface-supported multilayers decorated with bio-active material aimed at light-triggered drug delivery. Langmuir. 2009;25:14037–14043. doi: 10.1021/la9015433. [DOI] [PubMed] [Google Scholar]
- 196.Levi-Polyachenko NH, Merkel EJ, Jones BT, Carroll DL, Stewart JHt. Rapid photothermal intracellular drug delivery using multiwalled carbon nanotubes. Mol Pharm. 2009;6:1092–1099. doi: 10.1021/mp800250e. [DOI] [PubMed] [Google Scholar]
- 197.Aznar E, Marcos MD, Martinez-Manez R, Sancenon F, Soto J, Amoros P, Guillem C. pH- and photo-switched release of guest molecules from mesoporous silica supports. J Am Chem Soc. 2009;131:6833–6843. doi: 10.1021/ja810011p. [DOI] [PubMed] [Google Scholar]
- 198.Ogawa M, Kosaka N, Longmire MR, Urano Y, Choyke PL, Kobayashi H. Fluorophore-quencher based activatable targeted optical probes for detecting in vivo cancer metastases. Mol Pharm. 2009;6:386–395. doi: 10.1021/mp800115t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Asanuma D, Kobayashi H, Nagano T, Urano Y. Fluorescence imaging of tumors with “smart” pH-activatable targeted probes. Methods Mol Biol. 2009;574:47–62. doi: 10.1007/978-1-60327-321-3_5. [DOI] [PubMed] [Google Scholar]
- 200.Kobayashi H, Ogawa M, Kosaka N, Choyke PL, Urano Y. Multicolor imaging of lymphatic function with two nanomaterials: quantum dot-labeled cancer cells and dendrimer-based optical agents. Nanomedicine (Lond) 2009;4:411–419. doi: 10.2217/nnm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69:1268–1272. doi: 10.1158/0008-5472.CAN-08-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ho YP, Leong KW. Quantum dot-based theranostics. Nanoscale. 2010;2:60–68. doi: 10.1039/b9nr00178f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ma D, Jakubek ZJ, Simard B. A new approach towards controlled synthesis of multifunctional core-shell nano-architectures: luminescent and superparamagnetic. J Nanosci Nanotechnol. 2006;6:3677–3684. doi: 10.1166/jnn.2006.622. [DOI] [PubMed] [Google Scholar]
- 204.Bryson JM, Fichter KM, Chu WJ, Lee JH, Li J, Madsen LA, McLendon PM, Reineke TM. Polymer beacons for luminescence and magnetic resonance imaging of DNA delivery. Proc Natl Acad Sci U S A. 2009;106:16913–16918. doi: 10.1073/pnas.0904860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Picard FJ, Bergeron MG. Rapid molecular theranostics in infectious diseases. Drug Discov Today. 2002;7:1092–1101. doi: 10.1016/s1359-6446(02)02497-2. [DOI] [PubMed] [Google Scholar]
- 206.Huletsky A, Lebel P, Picard FJ, Bernier M, Gagnon M, Boucher N, Bergeron MG. Identification of methicillin-resistant Staphylococcus aureus carriage in less than 1 hour during a hospital surveillance program. Clin Infect Dis. 2005;40:976–981. doi: 10.1086/428579. [DOI] [PubMed] [Google Scholar]
- 207.Cunningham R, Jenks P, Northwood J, Wallis M, Ferguson S, Hunt S. Effect on MRSA transmission of rapid PCR testing of patients admitted to critical care. J Hosp Infect. 2007;65:24–28. doi: 10.1016/j.jhin.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 208.Pissuwan D, Cortie CH, Valenzuela SM, Cortie MB. Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol. 2010;28:207–213. doi: 10.1016/j.tibtech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 209.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zeina B, Greenman J, Corry D, Purcell WM. Antimicrobial photodynamic therapy: assessment of genotoxic effects on keratinocytes in vitro. Br J Dermatol. 2003;148:229–232. doi: 10.1046/j.1365-2133.2003.05091.x. [DOI] [PubMed] [Google Scholar]
- 211.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zheng X, Sallum UW, Verma S, Athar H, Evans CL, Hasan T. Exploiting a bacterial drug-resistance mechanism: a light-activated construct for the destruction of MRSA. Angew Chem Int Ed Engl. 2009;48:2148–2151. doi: 10.1002/anie.200804804. [DOI] [PubMed] [Google Scholar]
- 213.Engelhardt V, Krammer B, Plaetzer K. Antibacterial photodynamic therapy using water-soluble formulations of hypericin or mTHPC is effective in inactivation of Staphylococcus aureus. Photochem Photobiol Sci. 2010;9:365–369. doi: 10.1039/b9pp00144a. [DOI] [PubMed] [Google Scholar]
- 214.Akilov OE, Kosaka S, O’Riordan K, Hasan T. Photodynamic therapy for cutaneous leishmaniasis: the effectiveness of topical phenothiaziniums in parasite eradication and Th1 immune response stimulation. Photochem Photobiol Sci. 2007;6:1067–1075. doi: 10.1039/b703521g. [DOI] [PubMed] [Google Scholar]
- 215.Asilian A, Davami M. Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: a placebo-controlled, randomized clinical trial. Clin Exp Dermatol. 2006;31:634–637. doi: 10.1111/j.1365-2230.2006.02182.x. [DOI] [PubMed] [Google Scholar]
- 216.Smijs TG, Schuitmaker HJ. Photodynamic inactivation of the dermatophyte Trichophyton rubrum. Photochem Photobiol. 2003;77:556–560. doi: 10.1562/0031-8655(2003)077<0556:PIOTDT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 217.Calzavara-Pinton PG, Venturini M, Capezzera R, Sala R, Zane C. Photodynamic therapy of interdigital mycoses of the feet with topical application of 5-aminolevulinic acid. Photodermatol Photoimmunol Photomed. 2004;20:144–147. doi: 10.1111/j.1600-0781.2004.00095.x. [DOI] [PubMed] [Google Scholar]
- 218.Stender IM, Na R, Fogh H, Gluud C, Wulf HC. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: randomised double-blind trial. Lancet. 2000;355:963–966. doi: 10.1016/S0140-6736(00)90013-8. [DOI] [PubMed] [Google Scholar]
- 219.Marotti J, Aranha AC, Eduardo Cde P, Ribeiro MS. Photodynamic therapy can be effective as a treatment for herpes simplex labialis. Photomed Laser Surg. 2009;27:357–363. doi: 10.1089/pho.2008.2268. [DOI] [PubMed] [Google Scholar]
- 220.Perni S, Piccirillo C, Pratten J, Prokopovich P, Chrzanowski W, Parkin IP, Wilson M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials. 2009;30:89–93. doi: 10.1016/j.biomaterials.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 221.Storhoff JJ, Lucas AD, Garimella V, Bao YP, Muller UR. Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat Biotechnol. 2004;22:883–887. doi: 10.1038/nbt977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang WC, Tsai PJ, Chen YC. Multifunctional Fe3O4@Au nanoeggs as photothermal agents for selective killing of nosocomial and antibiotic-resistant bacteria. Small. 2009;5:51–56. doi: 10.1002/smll.200801042. [DOI] [PubMed] [Google Scholar]
- 223.Zharov VP, Mercer KE, Galitovskaya EN, Smeltzer MS. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys J. 2006;90:619–627. doi: 10.1529/biophysj.105.061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang C, Irudayaraj J. Multifunctional magnetic-optical nanoparticle probes for simultaneous detection, separation, and thermal ablation of multiple pathogens. Small. 2010;6:283–289. doi: 10.1002/smll.200901596. [DOI] [PubMed] [Google Scholar]
- 225.Kam NW, Liu Z, Dai H. Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew Chem Int Ed Engl. 2006;45:577–581. doi: 10.1002/anie.200503389. [DOI] [PubMed] [Google Scholar]
- 226.Bianco A, Kostarelos K, Partidos CD, Prato M. Biomedical applications of functionalised carbon nanotubes. Chem Commun (Camb) 2005:571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 227.Kim JW, Shashkov EV, Galanzha EI, Kotagiri N, Zharov VP. Photothermal antimicrobial nanotherapy and nanodiagnostics with self-assembling carbon nanotube clusters. Lasers Surg Med. 2007;39:622–634. doi: 10.1002/lsm.20534. [DOI] [PubMed] [Google Scholar]
- 228.McCarthy JR, Jaffer FA, Weissleder R. A macrophage-targeted theranostic nanoparticle for biomedical applications. Small. 2006;2:983–987. doi: 10.1002/smll.200600139. [DOI] [PubMed] [Google Scholar]
- 229.Ross R. Mechanisms of disease - Atherosclerosis - An inflammatory disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 230.Gabriel D, Busso N, So A, van den Bergh H, Gurny R, Lange N. Thrombin-sensitive photodynamic agents: a novel strategy for selective synovectomy in rheumatoid arthritis. J Control Release. 2009;138:225–234. doi: 10.1016/j.jconrel.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 231.Park K. Selective synovectomy using thrombin-sensitive photodynamic agents. J Control Release. 2009;138:224. doi: 10.1016/j.jconrel.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 232.Slevin M, Badimon L, Grau-Olivares M, Ramis M, Sendra J, Morrison M, Krupinski J. Combining nanotechnology with current biomedical knowledge for the vascular imaging and treatment of atherosclerosis. Mol Biosyst. 6:444–450. doi: 10.1039/b916175a. [DOI] [PubMed] [Google Scholar]
- 233.Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45:195–214. doi: 10.1016/s0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 234.Kaiser PK, Boyer DS, Garcia R, Hao Y, Hughes MS, Jabbour NM, Mieler W, Slakter JS, Samuel M, Tolentino MJ, Roth D, Sheidow T, Strong HA. Verteporfin photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2009;116:747–755. 755 e741. doi: 10.1016/j.ophtha.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 235.Bressler SB, Bressler NM, Fine SL, Hillis A, Murphy RP, Olk RJ, Patz A. Natural course of choroidal neovascular membranes within the foveal avascular zone in senile macular degeneration. Am J Ophthalmol. 1982;93:157–163. doi: 10.1016/0002-9394(82)90410-x. [DOI] [PubMed] [Google Scholar]
- 236.Gryziewicz L. Regulatory aspects of drug approval for macular degeneration. Adv Drug Deliv Rev. 2005;57:2092–2098. doi: 10.1016/j.addr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 237.Lim YT, et al. Diagnosis and therapy of macrophage cells using dextran-coated near-infrared responsive hollow-type gold nanoparticles. Nanotechnology. 2008;19:375105. doi: 10.1088/0957-4484/19/37/375105. [DOI] [PubMed] [Google Scholar]
- 238.Kleinpenning MM, Smits T, Ewalds E, Erp PEJv, Kerkhof PCMvd, Gerritsen MJP. Heterogeneity of fluorescence in psoriasis after application of 5-aminolaevulinic acid: an immunohistochemical study. British Journal of Dermatology. 2006;155:539–545. doi: 10.1111/j.1365-2133.2006.07341.x. [DOI] [PubMed] [Google Scholar]
- 239.Kleinpenning MM, Kanis JH, Smits T, van Erp PEJ, van de Kerkhof P, Gerritsen RMJP. The effects of keratolytic pretreatment prior to fluorescence diagnosis and photodynamic therapy with aminolevulinic acid-induced porphyrins in psoriasis. Journal of Dermatological Treatment. 21:245–251. doi: 10.3109/09546630903271548. [DOI] [PubMed] [Google Scholar]