Abstract
Hypoxia causes a large increase in [Ca2+]i and attendant contraction in pulmonary artery smooth muscle cells (PASMCs), but not in systemic artery SMCs. The different responses meet the respective functional needs in these two distinct vascular myocytes; however, the underlying molecular mechanisms are not well known. We and other investigators have provided extensive evidence to reveal that voltage-dependent K+ (KV) channels, canonical transient receptor potential (TRPC) channels, ryanodine receptor Ca2+ release channels (RyRs), cyclic adenosine diphosphate-ribose, FK506 binding protein 12.6, protein kinase C, NADPH oxidase and reactive oxygen species (ROS) are the essential effectors and signaling intermediates in the hypoxic increase in [Ca2+]i in PASMCs and HPV, but they may not primarily underlie the diverse cellular responses in pulmonary and systemic vascular myocytes. Hypoxia significantly increases mitochondrial ROS generation in PASMCs, which can induce intracellular Ca2+ release by opening RyRs, and may also cause extracellular Ca2+ influx by inhibiting KV channels and activating TRPC channels, leading to a large increase in [Ca2+]i in PASMCs and HPV. In contrast, hypoxia has no or a minor effect on mitochondrial ROS generation in systemic SMCs, thereby causing no change or a negligible increase in [Ca2+]i and contraction. Further preliminary work indicates that Rieske iron–sulfur protein in the mitochondrial complex III may perhaps serve as a key initial molecular determinant for the hypoxic increase in [Ca2+]i in PASMCs and HPV, suggesting its potential important role in different cellular changes to respond to hypoxic stimulation in pulmonary and systemic artery myocytes. All these findings have greatly improved our understanding of the molecular processes for the differential hypoxic Ca2+ and contractile responses in vascular SMCs from distinct pulmonary and systemic circulation systems.
1. Introduction
Hypoxia results in vasoconstriction in pulmonary arteries (PAs), but not in systemic arteries, to meet the respective physiological and pathological needs in these different circulation systems. Consistent with a general viewpoint that an increase in [Ca2+]i is a key factor in the initiation and maintenance of contraction in vascular smooth muscle cells (SMCs), hypoxia results in a large increase in [Ca2+]i in pulmonary, but not in systemic artery SMCs [see our recent review (Wang & Zheng, 2010)]. An increase in [Ca2+]i in vascular SMCs results from intracellular Ca2+ release and extracellular Ca2+ influx, which are mediated by multiple ion channels, including ryanodine receptor Ca2+ release channels (RyRs), voltage-dependent K+ (KV) channels and store-operated Ca2+ (SOC) channels. Ion channels are highly sensitive to important intracellular signaling molecules reactive oxygen species (ROS). Intracellular ROS generation primarily occurs at mitochondria and NADPH oxidase (NOX) (Wang & Zheng, 2010). Thus, hypoxia may differentially affect intracellular ROS production and then regulate ion channels, leading to diverse Ca2+ and contractile responses in pulmonary and systemic artery myocytes.
We and other researchers have started to conduct comparative studies to determine the potential role of these important ion channels, signaling intermediates and primary molecules in the diversity of hypoxic Ca2+ and contractile responses in pulmonary and systemic vascular myocytes, with the ultimate objective to elucidate underlying molecular mechanisms and advance relevant clinical practices. This review summarizes the key progresses in current research from our laboratory and others.
2. Different hypoxic Ca2+ and contractile responses in pulmonary and systemic arteries
A detectable hypoxic response in PASMCs and PAs normally starts to occur at an O2 tension (PO2) of 60 mmHg or lower (Aaronson et al., 2006;Wang & Zheng, 2010). Physiological hypoxic levels may vary in lungs in vivo; thus, hypoxia at different levels (10–60 mm Hg PO2) has been often used in experiments in vitro and in vivo. Hypoxia-induced vasoconstriction in PAs (HPV) serves as an important physiological process to preserve the sufficient matching of regional alveolar ventilation and pulmonary perfusion in the lungs, but may also result in pulmonary hypertension and attendant heart failure. In contrast, hypoxia normally does not contract and may even dilate systemic (e.g., cerebral, coronary, mesenteric) arteries, leading to a fall in the arterial blood pressure to maintain a fairly constant blood flow in important organs and tissues.
An increase in [Ca2+]i is a key factor in the initiation and maintenance of contraction in vascular SMCs. The increased Ca2+ signaling in PASMCs has been widely accepted to be a critical event for HPV (Mauban et al., 2005;Moudgil et al., 2005;Aaronson et al., 2006;Wang & Zheng, 2010;Weir et al., 2010). The distinct changes in [Ca2+]i by hypoxia are closely correlated with the diversity of hypoxic contractile responses in pulmonary and systemic artery SMCs. In support of this view, we have shown that hypoxia can greatly increase [Ca2+]i in pulmonary artery SMCs (PASMCs), but not in mesenteric (systemic) SMCs (MASMCs) (Wang et al., 2003;Rathore et al., 2006;Zheng et al., 2008;Rathore et al., 2008). Likewise, the hypoxic increase in [Ca2+]i has been observed in PASMCs, but not in cerebral and renal artery myocytes (Vadula et al., 1993;Waypa et al., 2010).
3. Differential effect of hypoxia on ion channels in pulmonary and systemic artery SMCs
The hypoxic increase in [Ca2+]i in PASMCs results from extracellular Ca2+ influx, which may occur due to the inhibition of KV channels and activation of SOC channels (Mauban et al., 2005;Moudgil et al., 2005;Aaronson et al., 2006;Wang & Zheng, 2010;Weir et al., 2010). Extensive studies have consistently revealed that intracellular Ca2+ release from the sarcoplasmic reticulum (SR) through RyRs plays an important role in the hypoxic increase in [Ca2+]i in PASMCs and attendant HPV. We and other investigators have started to address a fundamental question whether these ion channels may underlie the diversity of hypoxic Ca2+ and contractile responses in pulmonary and systemic artery SMCs. The major findings are summarized below.
(3.1) Voltage-dependent K+ channels
Earlier collaborative research by Drs. Hume, Archer and Weir and their associates reported for the first time that hypoxia inhibits KV channels in PASMCs (Post et al., 1992). These findings were subsequently confirmed by Dr. Yuan’s work (Yuan et al., 1993). Inhibition of KV channels has been thought to cause membrane depolarization and activation of voltage-dependent Ca2+ (CaV) channels, leading to extracellular Ca2+ influx, an increase in [Ca2+]i in PASMCs, and HPV. However, scientists have argued the significance of KV and CaV channels in the hypoxic responses (Turner & Kozlowski, 1997;Ward & Aaronson, 1999;Sylvester, 2001;Sham, 2002;Aaronson et al., 2006;Ward & McMurtry, 2009), considering the fact that the hypoxic increase in [Ca2+]i and associated HPV are preserved in the presence of KV and CaV channel blockers, high extracellular K+, and in the absence of extracellular Ca2+ (under conditions where Ca2+ influx through CaV channels is eliminated) (Hasunuma et al., 1991;Demiryurek et al., 1993;Sham et al., 2000;Robertson et al., 2000;Shimoda et al., 2000;Dipp et al., 2001;Kang et al., 2002). Indeed, it is surprising to note that there has been no reports using the patch clamp technique to determine whether the hypoxic inhibition of KV channels may, in fact, activate CaV channels in cultured and freshly isolated PASMCs from rats, mice and humans that have been often used in relevant studies.
Interestingly, we have recently demonstrated that membrane depolarization can cause a direct activation of Gq protein-coupled receptors (GqPCRs) to activate inositol 1,4,5-triphosphate receptor Ca2+ release channels (IP3Rs) to induce Ca2+ release from the SR, which subsequently open neighboring RyRs, leading to further Ca2+ release and contraction without the involvement of CaV channels and associated extracellular Ca2+ influx in airway SMCs (Liu et al., 2009b). This novel Ca2+ signaling mechanism may perhaps exist in PASMCs as well; as such, the hypoxic inhibition of KV channels would cause membrane depolarization, activation of GqPCRs and then Ca2+ release, contributing to the hypoxic increase in [Ca2+]i in PASMCs and HPV. If this hypothesis is confirmed, it may explain why the hypoxic responses are preserved when extracellular Ca2+ influx through CaV channels is eliminated.
Comparable data indicate that hypoxia inhibits KV channels in PASMCs, but not in MASMCs (Yuan et al., 1993). Further studies have reported that hypoxia for 24–72 h significantly decreases KV1.1, 1.2, 1.5 and 2.1 channel α-subunit mRNA and protein expression in cultured rat PASMCs, while their expressions are unaltered in MASMCs (Wang et al., 1997a;Platoshyn et al., 2001). Similarly, a recent study has shown that mRNA and protein expression levels of KV1.1, 1.5, 1.6, 2.1, and 4.3 channel α-subunits are decreased in PAs, but not in aorta from rats with chronic hypoxia for 3 weeks (Wang et al., 2005b). On the other hand, patch clamp recordings have found that membrane depolarization produces equivalent KV channel currents in PASMCs and MASMCs (Yuan et al., 1993). Taken together, KV channels are the hypoxic effectors in PASMCs, but not in systemic artery myocytes; as such, these ion channels may not be the specific, necessary contributors to the difference in hypoxic Ca2+ and contractile responses in distinct pulmonary and systemic vascular myocytes.
(3.2) Canonical transient receptor potential Ca2+ channels
A previous report by Robertson et al has shown that pretreatment with La3+ to inhibit SOC channels or with the SR Ca2+ pump inhibitor cyclopiazonic acid to deplete the SR Ca2+ significantly inhibits HPV in isolated rat PAs (Robertson et al., 2000), indicating the involvement of SOC channels. Similar results have been observed in isolated rat lung preparations (Weigand et al., 2003). Hypoxia also significantly increases Ca2+ entry via SOC channels in pig, rabbit and rat PASMCs (Kang et al., 2003;Lin et al., 2004;Ng et al., 2005;Wang et al., 2005a;Wang et al., 2006;Ng et al., 2007). SOC channels may be encoded by canonical transient receptor potential (TRPC) genes, which are known to consist of seven members designated TRPC1-7 (Nilius et al., 2007;Abramowitz & Birnbaumer, 2009). Several studies have demonstrated that hypoxia for hours upregulates mRNA and protein expression levels of TRPC1, TRPC4 and/or TRPC6, and increases SOC channel currents, leading to Ca2+ entry in PASMCs (Fantozzi et al., 2003;Lin et al., 2004;Wang et al., 2006;Zhang et al., 2007). Therefore, Ca2+ entry via TRPC-encoded SOC channels may contribute to the hypoxic increase in [Ca2+]i and contraction in PASMCs. Supportively, a recent report has shown that the hypoxic Ca2+ influx in PASMCs and HPV in lungs are reduced in TRPC6−/− mice (Weissmann et al., 2006a). These TRPC-encoded channels are highly expressed and functional as well in systemic vascular SMCs (Inoue et al., 2006); however, they cannot be activated in response to hypoxia and hence fail to cause an increase in [Ca2+]i and contraction in this type of cells.
(3.3) Ryanodine receptor Ca2+ release channels
Considering that Ca2+ release from the SR via RyRs is a major component of Ca2+ signaling in vascular SMCs, we have sought to determine the important function of RyRs in hypoxic responses in PASMCs. Our studies using pharmacological agents and specific RyR gene deletion mice have found that RyRs are essential for the hypoxic increase in [Ca2+]i in PASMCs and HPV (Wang et al., 2003;Zheng et al., 2004;Zheng et al., 2005;Li et al., 2009;Liao et al., 2010). Other investigators have also provided pharmacological data to support the central role of RyRs in hypoxic cellular responses (Vadula et al., 1993;Jabr et al., 1997;Dipp et al., 2001;Kang et al., 2002;Morio & McMurtry, 2002;Ng et al., 2005).
The importance of RyR-mediated Ca2+ release in hypoxic responses in PASMCs is reinforced by the findings that the hypoxic inhibition of KV channels is likely to be secondary to RyR-mediated Ca2+ release from the SR. A previous study has shown that the hypoxic inhibition of KV channels is mimicked by application of the RyR activator caffeine to induce RyR-dependent Ca2+ release, and prevented by pretreatment with caffeine to deplete RyR-expressing Ca2+ stores as well as the Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid to buffer [Ca2+]i (Post et al., 1995). Moreover, inhibition of Ca2+ release with ryanodine, caffeine and the SR Ca2+ pump inhibitor thapsigargin all decrease outward K+ currents (Vandier et al., 1998). A series of well-designed studies have further provided evidence that acute hypoxia causes Ca2+ release from the SR by opening RyRs, which activates SOC channels, leading to Ca2+ influx in PASMCs (Ng et al., 2005;Ng et al., 2007).
Three subtypes of RyRs (RyR1, RyR2 and RyR3) are expressed in mammalian cells, and each is encoded by a distinct gene. A series of our experiments have demonstrated that all three RyR subtypes are present and functional in PASMCs (Zheng et al., 2005;Zheng et al., 2008;Li et al., 2009;Liao et al., 2010). Importantly, RyR1 gene deletion blocks the hypoxic increase [Ca2+]i in PASMCs and HPV (Li et al., 2009). Similarly, RyR2 or RyR3 gene deletion produces similar effects (Zheng et al., 2005;Liao et al., 2010). These data reveal that all three subtypes of RyRs are important for hypoxic Ca2+ and contractile responses in PASMCs.
With a consideration of the importance of RyRs in the hypoxic increase in [Ca2+]i in PASMCs and HPV, we have started to assess their potential role in the diversity of hypoxic Ca2+ and contractile responses in pulmonary and systemic vascular SMCs. The results indicate the RyRs show overall high expression levels and/or functional activities in both vascular myocytes (Zheng et al., 2008). In addition, we have found that RyRs are highly expressed and functional as well in other (e.g., airway) SMCs (Liu et al., 2007;Liu et al., 2009a;Liu et al., 2009b). Collectively, RyRs are imperative for the hypoxic increase in [Ca2+]i in PASMCs and HPV, but fail to be activated by hypoxia and thus are unable to lead to Ca2+ and contractile responses in SMCs from systemic arteries.
(3.4) Other ion channels
Inositol triphosphate receptor Ca2+ release channels
Bright et al have shown that cyclopiazonic acid inhibits chemical hypoxia-induced rise in [Ca2+]i in cultured rat PASMCs; and the inhibitory effect of cyclopiazonic acid has been attributed to the depletion of IP3-sensitive Ca2+ stores (Bright et al., 1995). In contrast, it has been reported that cyclopiazonic acid or thapsigargin significantly enhances HPV in freshly isolated canine pulmonary arteries (Jabr et al., 1997).
In spite of these inconsistent reports, we and other investigators have provided evidence to indicate that IP3Rs and RyRs are functionally coupled in PASMCs, by which Ca2+ release from the SR through IP3Rs can activate neighboring RyRs, causing further Ca2+ release, a local Ca2+-induced Ca2+ release (CICR) process, amplifying neurotransmitter-induced increase in [Ca2+]i and contraction (Jabr et al., 1997;Janiak et al., 2001;Zheng et al., 2005;Li et al., 2009). We have also found that this local CICR process due to the interaction of IP3Rs with neighboring RyRs exists as well in airway SMCs (Liu et al., 2007;Liu et al., 2009b). Nevertheless, further studies are necessary to directly evaluate the role of IP3Rs in the hypoxic Ca2+ release in PASMCs. It should be pointed out that to date there is no studies to determine the role of IP3Rs in the diversity of hypoxic cellular responses in pulmonary and systemic artery myocytes.
Ca2+-activated Cl− channels
We have found that hypoxia causes the opening of Ca2+-activated Cl− (ClCa) channels in PASMCs (Wang et al., 1997b;Wang et al., 2003). The equilibrium potential for ClCa channels is between −20 – −30 mV; thus, hypoxia activates ClCa channels to produce inward (depolarizing) currents at the resting potential of about −55 mV. It is interesting to note that ClCa currents produced by hypoxia are sustained. The sustained currents are likely to result from the removal of phosphorylation-dependent inactivation of the channels, which is mediated by Ca2+/calmodulin-dependent protein kinase II (Pacaud et al., 1992;Wang & Kotlikoff, 1997;Greenwood et al., 2001). Moreover, mild chemical hypoxia increases the frequency and amplitude of RyR-mediated spontaneous local Ca2+ release (Ca2+ sparks) and spontaneous transient inward currents (STICs) (Wang et al., 2003). Thus, hypoxia may perhaps activate RyRs and then ClCa channels, contributing to the hypoxic extracellular Ca2+ influx in PASMCs and HPV.
Voltage-dependent Ca2+ channels
There is a report showing that hypoxia shifts the voltage-dependence of CaV channel opening towards the negative membrane potential in rabbit PASMCs, thereby potentiating Ca2+ influx (Franco-Obregon & Lopez-Barneo, 1996). Studies of oxygen sensing in carotid body cells by the same group, however, have failed to find an effect on CaV channel gating (Lopez-Barneo et al., 1993). These results, together with previous reports that relaxation of rabbit pulmonary arteries is usually seen during hypoxic stimulation (Marriott & Marshall, 1990;MacLean et al., 1993;Vadula et al., 1993), suggest that such a mechanism may not play an important role in hypoxic increase in [Ca2+]i. As described above, a number of reports have shown that hypoxic contraction in isolated lungs, PAs and PASMCs are relatively insensitive to multiple CaV channel blockers (Demiryurek et al., 1993;Jabr et al., 1997;Robertson et al., 2000;Shimoda et al., 2000;Sham et al., 2000;Dipp & Evans, 2001;Kang et al., 2002).
Ca2+-activated K+ channels
Whether hypoxia in fact inhibits Ca2+-activated K+ (KCa) channels remains unclear, since during hypoxia this channel has been shown to be inhibited (Park et al., 1995;Peng et al., 1997;Vandier et al., 1998), activated (Archer et al., 1996;Cornfield et al., 1996;Nossaman et al., 1997), and unaffected (Post et al., 1995).
Taken together, the role of IP3R, ClCa, CaV and KCa channels in hypoxic cellular responses in PASMCs need further experiments for confirmation. In addition, these ion channels show similar expression levels and functional activities in pulmonary and systemic vascular SMCs, nevertheless, none of these ion channels would be able to be responded to hypoxia and accordingly can cause cellular responses in vascular SMCs from the systemic circulation system.
4. Effect of hypoxia on cyclic ADP-ribose, protein kinase C, and reactive oxygen species in pulmonary and systemic artery SMCs
Multiple intracellular molecules and proteins such as cyclic adenosine diphosphate-ribose (cADPR), protein kinase C (PKC) and ROS are known to be important intermediates to participate in Ca2+ signaling in vascular SMCs. Numerous studies have provided interesting information with respect to the potential important role of these intermediates in the hypoxic increase in [Ca2+]i in PASMCs and HPV as well as the difference in hypoxic cellular responses in pulmonary and systemic artery myocytes, as described below.
(4.1) Cyclic adenosine diphosphate-ribose
Hypoxia has been shown to increase the activity of CD38/ADP-ribosyl cyclase and then generation of cADPR in PAs (Wilson et al., 2001), and the cADPR antagonist 8-Br cADPR blocks HPV (Dipp & Evans, 2001). Thus, cADPR may perhaps serve as a mediator in the hypoxic Ca2+ release and contraction in PASMCs. However, cADPR has been shown to induce Ca2+ release from the SR by opening RyRs in variety of cell types including systemic (coronary) artery SMCs (Tang et al., 2002).
We and other investigators have provided evidence to suggest that cADPR induces Ca2+ release from the SR by dissociating FK506 binding protein 12.6 (FKBP12.6) from RyRs in airway and coronary artery SMCs, pancreatic cells, and adrenal chromaffin cells (Noguchi et al., 1997;Tang et al., 2002;Wang et al., 2004;Morita et al., 2006). Our studies have further discovered that FKBP12.6 is involved in the hypoxic Ca2+ release and contraction in PASMCs (Zheng et al., 2004;Liao et al., 2010).
These results indicate that cADPR and FKBP12.6 function as important intermediate signaling molecules to mediate the hypoxic increase in [Ca2+]i in PASMCs and HPV, but they may not be the major primary factors in determining the divergent hypoxic responses in pulmonary and systemic vascular SMCs.
(4.2) Protein kinase C
Pharmacological studies have shown that PKC may play an important role in mediating hypoxic Ca2+ and contractile responses in PASMCs. The PKC inhibitors prevent, whereas the PKC activators mimic and subsequently block, HPV in isolated canine and rabbit lungs, as well as isolated rat PAs (Orton et al., 1990;Jin et al., 1992;Barman, 1999;Weissmann et al., 1999;Tsai et al., 2004). It has also been reported that HPV is inhibited in isolated lungs from PKCε−/− mice (Littler et al., 2003).
Using multiple biochemical, pharmacological and genetic approaches, we have demonstrated that PKCε is a major isoform of the PKC family to mediate the hypoxic increase in [Ca2+]i and contraction in PASMCs (Rathore et al., 2006). Interestingly, the hypoxic activation of PKCε is mimicked by exogenous H2O2, but abolished by pharmacological and genetic inhibition of mitochondrial ROS generation. Our studies have also found that hypoxia fails to activate PKC in MASMCs. Moreover, PKCε expression levels and responses to the PKC activator phorbol 12-myristate 13-acetate (PMA) are comparable in pulmonary and systemic artery myocytes. Apparently, hypoxia activates PKCε by increasing mitochondrial ROS generation to play a critical role in hypoxic responses in PASMCs, but this signaling molecule is not a specific, important participant in the divergent effects of hypoxia on [Ca2+]i and contractility in pulmonary and systemic artery myocytes,.
(4.3) Intracellular ROS generation
Many studies have revealed that hypoxia increases intracellular ROS generation/concentration ([ROS]i) in isolated rabbit and lamb lungs, rat and dog pulmonary arteries, and cultured calf, dog and rat PASMCs (Marshall et al., 1996;Killilea et al., 2000;Waypa et al., 2001;Paddenberg et al., 2003;Brennan et al., 2003;Liu et al., 2003;Jernigan et al., 2004;Waypa et al., 2006), although hypoxia has also been reported to decrease [ROS]i in isolated rat lungs and PASMCs (Archer et al., 1993;Michelakis et al., 2002), as well as in microsome-enriched fractions of calf pulmonary arteries (Mohazzab & Wolin, 1994a;Mohazzab & Wolin, 1994b). Interestingly, hypoxia for minutes decreases, whereas for 48 hours increases, oxidation of dihydroethidium ([ROS]i) in cultured PASMCs (Wu et al., 2007).
We have also looked into the effect of hypoxia on [ROS]i in PASMCs using multiple ROS detection approaches, and found that hypoxia results in a large increase in [ROS]i (Rathore et al., 2006;Wang et al., 2007;Rathore et al., 2008;Liao et al., 2010). Recent reports using a novel, ratiometric, redox-sensitive fluorescence resonance energy transfer (FRET) probe reveal that hypoxia augments ROS signals as well in PASMCs (Waypa et al., 2006;Waypa et al., 2010). Using a newly developed, genetically-encoded, specific ROS biosensor HyPer (Belousov et al., 2006), we have further demonstrated the hypoxic increase in [ROS]i in PASMCs (Korde & Wang, 2008).
In support of the view that ROS are critical for hypoxic Ca2+ and contractile responses in PASMCs, many research groups have found that exogenous H2O2, similar to hypoxia, induces an increase in [Ca2+]i in PASMCs (Waypa et al., 2002;Lin et al., 2007) and vasoconstriction in PAs (Burghuber et al., 1986;Seeger et al., 1986;Rhoades et al., 1990;Kjaeve et al., 1991;Sheehan et al., 1993;Yamaguchi et al., 1994;Wilhelm & Herget, 1995;Jin & Rhoades, 1997;Jones et al., 1997). It should be pointed out that in most of these previous studies, the concentrations of H2O2 used are above several hundred μM. As such, the pulmonary vasoconstriction produced is often irreversible. Despite the fact that physiological concentrations of H2O2 in PASMCs are unknown, our investigations have discovered that acute hypoxia (10 – 20 Torr) yields a ROS signal equivalent to that generated by exogenous H2O2 at 51 μM (Wang et al., 2007). Using 30 μM, Pourmahram et al have shown that H2O2 evokes a reversible contraction in isolated PAs (Pourmahram et al., 2008). Furthermore, pharmacological and genetic inhibition of intracellular ROS generation block the hypoxic increase in [Ca2+]i in PASMCs and HPV, as summarized in our recent review (Wang & Zheng, 2010).
An earlier comparative study has shown that hypoxia decreases [ROS]i in freshly isolated rat PASMCs, but increases [ROS]i in renal (systemic) artery myocytes (Michelakis et al., 2002). We have also wondered whether hypoxia differentially affects ROS signaling to be essential for the different hypoxic Ca2+ and contractile responses in pulmonary and systemic artery SMCs. Our data reveal that hypoxia causes a large increase in [ROS]i in freshly isolated mouse PASMCs, but not in MASMCs (Rathore et al., 2006;Rathore et al., 2008). In addition, hypoxia has been found to decrease [ROS]i in both cultured, passaged human pulmonary and coronary (systemic) artery SMCs (Mehta et al., 2008). More perplexedly, a recent study indicates that hypoxia produces a similar increase in ROS signals in cultured, passaged pulmonary and renal artery myocytes (Waypa et al., 2010). The reason for these inconsistent data is unclear; however, different hypoxic responses may occur in freshly isolated and cultured, passaged vascular SMCs under different experimental conditions.
Nevertheless, our further investigations demonstrate that exogenous H2O2 mimics the hypoxic response, leading to a large increase in the activity of PKCε to contribute to the hypoxic increase in [Ca2+]i and contraction in PASMCs; and exogenous H2O2 produces similar cellular responses in MASMCs (Rathore et al., 2006;Rathore et al., 2008). Evidently, a distinct increase in [ROS]i in pulmonary and mesenteric artery SMCs may principally account for the diverse hypoxic Ca2+ and contractile responses in these two different types of vascular myocytes.
5. Effect of hypoxia on NADPH oxidase in pulmonary and systemic artery SMCs
NOX has been thought to be a major source for intracellular ROS generation to mediate hypoxic responses in PASMCs (Weissmann et al., 2006b;Wolin et al., 2007). In phagocytic cells, NOX is well characterized to include the membrane-bound subunits p22phox and gp91phox (NOX2) subunits, and the cytosolic subunits p47phox and p67phox. The association of these membrane-bound and cytosolic subunits is required to assemble the active NOX. Numerous studies have demonstrated that NOX inhibition by iodonium compounds attenuates the hypoxic increase in [ROS]i (Mohazzab et al., 1995;Marshall et al., 1996), hypoxic inhibition in KV currents (Weir et al., 1994), hypoxic increase in [Ca2+]i and contraction in PASMCs (Thomas, III et al., 1991;Marshall et al., 1996;Zhang et al., 1997), and HPV in isolated lungs and PAs (Mohazzab & Wolin, 1994a;Grimminger et al., 1995;Mohazzab et al., 1995;Weissmann et al., 2000;Weissmann et al., 2006c). The specificity of iodonium compounds as NOX inhibitors has been disputed, since these agents inhibit voltage-dependent Ca2+ currents in PASMCs and mitochondrial functions in heart cells (Ragan & Bloxham, 1977;Weir et al., 1994). While gp91phox−/− mice show normal or reduced hypoxic responses (Archer et al., 1999;Liu et al., 2006), HPV is inhibited in p47phox−/− mice (Weissmann et al., 2006c).
We have recently unveiled that hypoxia largely increases the activity of NOX in PASMCs; and pharmacological and genetic inhibition of NOX decrease the hypoxic increase in [ROS]i, [Ca2+]i and contraction (Rathore et al., 2008). The hypoxic activation of NOX is mimicked by the PKC activator PMA, whereas blocked by PKCε inhibitors and gene deletion, indicating that hypoxia activates NOX by stimulating PKCε in PASMCs (Rathore et al., 2008). These data, together with the previously described findings that the hypoxic activation of PKCε is secondary to mitochondrial ROS (Rathore et al., 2006), suggest that hypoxia may enhances mitochondrial ROS generation, activates PKCε, and then augments NOX activity to cause further generation of intracellular ROS. This PKCε-dependent ROS-induced ROS generation plays a crucial role in the hypoxic increase in [Ca2+]i in PASMCs and HPV. Supportively, exogenous H2O2 mimics the hypoxic effect, leading to a large increase in the activity of NOX, while pharmacological and genetic inhibition of mitochondrial ROS generation both prevent the hypoxic activation of NOX.
Our studies have also found that the major NOX subunit gp91phox analogues (Nox1 and Nox4, but not itself), p22phox, p47phox, and p67phox proteins are equally expressed in PASMCs and MASMCs. Hypoxia increases NOX activity in former cells, but not in the latter. In agreement with similar NOX subunit expression levels, PMA and H2O2 both cause an equivalent increase in the activity of NOX in PASMCs and MASMCs (Rathore et al., 2008). Accordingly, NOX, like other hypoxic signaling molecules, plays a significant role in hypoxic responses in PASMCs, but not in dissimilar hypoxic responses in pulmonary and systemic systems.
6. Effect of hypoxia on mitochondrial ROS generation in pulmonary and systemic artery SMCs
As aforementioned, a series of studies from our laboratory and others have provided pharmacological and genetic evidence to indicate that mitochondria are a primary source of the hypoxic ROS generation in PASMCs (Wang & Zheng, 2010). Consistent with this view, mitochondrial inhibitors block the hypoxic increase in [Ca2+]i in PASMCs, and contraction in PASMCs, pulmonary arteries and isolated lungs (Leach et al., 2001;Waypa et al., 2001;Waypa & Schumacker, 2002;Paddenberg et al., 2003;Rathore et al., 2006;Wang et al., 2007;Rathore et al., 2008;Liao et al., 2010). It is also worth stating that pharmacological studies have suggested that the mitochondrial complex III is a main site of the hypoxic effect on ROS generation in PASMCs (Archer et al., 1993;Leach et al., 2001;Waypa et al., 2001;Michelakis et al., 2002;Waypa & Schumacker, 2002;Weissmann et al., 2003;Waypa et al., 2006;Rathore et al., 2006;Weissmann et al., 2006c;Wang et al., 2007;Rathore et al., 2008). Excitingly, our recent preliminary study has disclosed that siRNA-mediated gene silencing of Rieske iron–sulfur protein in the complex III blocks the hypoxic ROS generation, increase in [Ca2+]i and contraction in PASMCs (Korde & Wang, 2008).
Archer and his colleagues have proposed a model that suggests different mitochondrial functions may account for the diversity of hypoxic Ca2+ and contractile responses in pulmonary and renal (systemic) artery SMCs (Michelakis et al., 2002). In this study, they have reported that hypoxia and mitochondrial inhibitors can inhibit the tonic production of mitochondrial ROS to elicit contraction in PASMCs, but increase mitochondrial ROS generation to cause relaxation in renal artery SMCs. Likewise, we and others have also revealed that hypoxia produces a different change in [ROS]i in pulmonary and systemic artery SMCs; however, hypoxia increases [ROS]i in the former cells, and produces no effect or a minor increase in the latter cells (Rathore et al., 2006;Rathore et al., 2008;Waypa et al., 2010).
Taking in account of all these results along with the generally agreed concept that pharmacological and genetic inhibition of mitochondrial ROS production block the hypoxic increase in [Ca2+]i in PASMCs and attendant HPV, we believe that different generation of mitochondrial ROS is a primary determinant factor in the diversity of hypoxic Ca2+ and contractile responses in pulmonary and systemic artery SMCs.
7. Role of ROS in hypoxic effect on ion channels in pulmonary and systemic artery SMCs
Ion channels are highly regulated by the biologically important intracellular signaling molecules ROS; thus, hypoxia may differently regulate ion channels by affecting intracellular ROS generation, which leads to diverse Ca2+ and contractile responses in pulmonary and systemic artery myocytes. Indeed, an earlier comparative research has shown that mitochondrial inhibitors, similar to hypoxia, can cause a decrease in [ROS]i to produce KV channel inhibition and contraction in PASMCs; on the contrary, mitochondrial inhibitors and hypoxia increase [ROS]i to lead to KV channel augmentation and relaxation in renal artery SMCs (Michelakis et al., 2002). However, it should be noted that H2O2-induced increase in [Ca2+]i in PASMCs is unaffected by either Ca2+ removal in extracellular solution or the CaV channel blocker nifedipine (Lin et al., 2007). Similarly, neither H2O2-evoked vasoconstriction nor the increase in [Ca2+]i in PAs is affected by removal of extracellular Ca2+ (Pourmahram et al., 2008). Extracellular Ca2+ influx contributes to HPV, although the importance of KV channels in the hypoxic extracellular Ca2+ influx has been argued (Turner & Kozlowski, 1997;Ward & Aaronson, 1999;Sylvester, 2001;Sham, 2002;Aaronson et al., 2006;Ward & McMurtry, 2009). Collectively, the ionic mechanisms for H2O2-evoked pulmonary vasoconstriction are at least in part different from those for HPV. Moreover, additional experiments are needed to further evaluate the different generation of mitochondrial ROS in the hypoxic inhibition of KV channels and associated cellular responses in pulmonary and systemic artery SMCs.
Previous reports indicate the involvement of TRPC channels in the hypoxic increase in [Ca2+]i in PASMCs and HPV (Wang & Zheng, 2010); however, no study has determined the role of ROS in the hypoxic activation of these channels in PASMCs. In spite of this, SKF-96365 or La3+, known to block TRPC channels, does not affect H2O2-induced increase in [Ca2+]i in PASMCs (Lin et al., 2007).
In contrast, H2O2-elicited increases in [Ca2+]i in PASMCs and contraction in PAs are consistently shown to be inhibited or abolished by the RyR antagonist dantrolene or ryanodine (Lin et al., 2007;Pourmahram et al., 2008), which is comparable to their inhibitory effect on the hypoxic increase in [Ca2+]i in PASMCs and HPV (Wang & Zheng, 2010). Interestingly, we have very recently provided biochemical and genetic evidence that hypoxia results in the dissociation of FKBP12.6 from RyR2 to augment the Ca2+ release channel activity in PASMCs (Liao et al., 2010). The hypoxic dissociation of FKBP12.6 is mimicked by exogenous H2O2, and inhibited by blocking mitochondrial ROS generation with myxothiazol and enhancing mitochondrial ROS degradation with glutathione peroxidase-1 gene overexpression, demonstrating that the hypoxic response is secondary to an increase in mitochondrial ROS generation. Chemical and genetic removal of FKBP12.6 enhance, whereas RyR2 gene deletion blocks, the hypoxic increase in [Ca2+]i in PASMCs and HPV (Zheng et al., 2004;Liao et al., 2010). These findings, together with the fact that FKBP12.6 and RyRs are highly expressed and functional in pulmonary and systemic vascular as well as other SMCs (Coussin et al., 2000;Lohn et al., 2001;Tang et al., 2002;Wang et al., 2004;Zheng et al., 2008;Liu et al., 2009a;Liu et al., 2009b), imply that hypoxia largely enhances mitochondrial ROS generation to lead to the opening of RyRs, Ca2+ release and contraction in PASMCs, but causes no effect or an insufficient increase in mitochondrial ROS formation to produce cellular responses in systemic artery myocytes.
8. Conclusion
Hypoxia contracts pulmonary arteries, but does not contract or dilate systemic arteries. These different hypoxic contractile responses meet the unique functional needs of these two different circulation systems. Consistent with the well-established, general concept that Ca2+ signaling is obligatory for the initiation and maintenance of contraction in vascular SMCs, hypoxia causes a large increase in [Ca2+]i in pulmonary, but not in systemic artery SMCs. The cellular and molecular mechanisms underlying the difference of the hypoxic Ca2+ and contractile responses in pulmonary and systemic artery SMCs are poorly understood.
As diagrammed in Figure 1, we and other scientists have provided extensive evidence to reveal that RyRs, TRPC channels, KV channels, FKBP12.6, cADPR, PKC, ROS and NOX are the essential effectors or signaling intermediates in the hypoxic increase in [Ca2+]i in PASMCs and HPV. However, these imperative hypoxic effectors and intermediates, on the whole, show high expression levels and/or intrinsic functional activities in both pulmonary and systemic vascular myocytes, and thus may not serve as primary determinants for the diverse cellular responses in vascular myocytes from these two distinct circulation systems. On the other hand, hypoxia significantly increases mitochondrial ROS generation in pulmonary, but not in systemic artery myocytes. The increased mitochondrial ROS are not only able to induce intracellular Ca2+ release from the SR by opening RyRs, but may also cause extracellular Ca2+ influx by inhibiting KV channels and activating TRPC channels, leading to a large increase in [Ca2+]i in PASMCs and HPV; in contrast, hypoxia has no or a minor effect on mitochondrial ROS generation to influence the activity of RyR, KV or TRPC channels, causing no or a negligible increase in [Ca2+]i and contraction in systemic SMCs.
Figure 1.
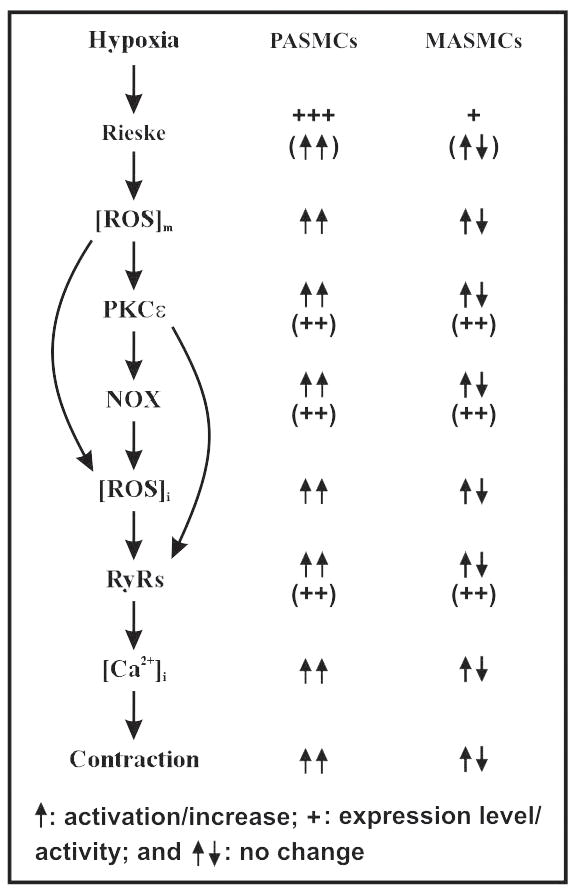
A diagram depicting a potential important primary hypoxic sensing molecule (Rieske iron–sulfur protein in the mitochondrial complex III), intermediate signaling molecules (ROS, PKCε and NOX) and effectors (RyRs) in the hypoxic Ca2+ and contractile responses in PASMCs, and their potential roles in the diversity of hypoxic responses in PASMCs and MASMCs. ROS, reactive oxygen species; PKCε, protein kinase C-ε; NOX, NADPH oxidase; and RyRs, ryanodine receptor Ca2+ release channels.
The findings from pharmacological studies suggest that the hypoxic production of mitochondrial ROS predominantly occurs at the complex III. Our recent preliminary study indicates that Rieske iron–sulfur protein in the complex III is indispensible to the hypoxic ROS generation to cause an increase in [Ca2+]i in PASMCs and HPV. These data, together with the aforementioned fact that hypoxia produces a different effect on mitochondrial ROS generation to cause different Ca2+ and contractile responses in pulmonary and systemic artery SMCs, inspire us to conjecture that Rieske iron–sulfur protein may show different expression levels and/or functional activities to serve as a key, initial molecular determinant for the dissimilar hypoxic cellular responses in vascular SMCs from distinct pulmonary and systemic circulation systems. Clearly, further studies aimed to indentify Rieske iron–sulfur protein and/or potentially other key molecular determinants would greatly improve our understanding of the molecular mechanisms responsible for the hypoxic ROS generation, increase in [Ca2+]i and contraction in PASMCs and the diversity of these hypoxic responses in pulmonary and systemic artery myocytes. New data may also help to create novel therapeutic targets for pulmonary hypertension and other related lung diseases.
Acknowledgments
Our work presented in this review article was supported by AHA Established Investigator Award 0340160N and NIH R01HL64043, HL064043-S1 and HL075190 (Y.-X. W.), as well as AHA Scientist Development Grant 0630236N (Y.-M. Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JP. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol. 2006;570:53–58. doi: 10.1113/jphysiol.2005.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- Archer SL, Huang JM, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–442. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci U S A. 1999;96:7944–7949. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman SA. Potassium channels modulate canine pulmonary vasoreactivity to protein kinase C activation. Am J Physiol Lung Cell Mol Physiol. 1999;277:L558–L565. doi: 10.1152/ajplung.1999.277.3.L558. [DOI] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- Bright RT, Salvaterra CG, Rubin LJ, Yuan XJ. Inhibition of glycolysis by 2-DG increases [Ca2+]i in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 1995;269:L203–L208. doi: 10.1152/ajplung.1995.269.2.L203. [DOI] [PubMed] [Google Scholar]
- Burghuber OC, Strife R, Zirolli J, Mathias MM, Murphy RC, Reeves JT, Voelkel NF. Hydrogen peroxide induced pulmonary vasoconstriction in isolated rat lungs is attenuated by U60,257, a leucotriene synthesis blocker. Wien Klin Wochenschr. 1986;98:117–119. [PubMed] [Google Scholar]
- Cornfield DN, Reeve HL, Tolarova S, Weir EK, Archer S. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc Natl Acad Sci U S A. 1996;93:8089–8094. doi: 10.1073/pnas.93.15.8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussin F, Macrez N, Morel JL, Mironneau J. Requirement of ryanodine receptor subtypes 1 and 2 for Ca2+-induced Ca2+ release in vascular myocytes. J Biol Chem. 2000;275:9596–9603. doi: 10.1074/jbc.275.13.9596. [DOI] [PubMed] [Google Scholar]
- Demiryurek AT, Wadsworth RM, Kane KA, Peacock AJ. The role of endothelium in hypoxic constriction of human pulmonary artery rings. Am Rev Respir Dis. 1993;147:283–290. doi: 10.1164/ajrccm/147.2.283. [DOI] [PubMed] [Google Scholar]
- Dipp M, Evans AM. Cyclic ADP-ribose is the primary triggered for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res. 2001;89:77–83. doi: 10.1161/hh1301.093616. [DOI] [PubMed] [Google Scholar]
- Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L318–L325. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1233–L1245. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Lopez-Barneo J. Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. J Physiol. 1996;491:511–518. doi: 10.1113/jphysiol.1996.sp021235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. J Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger F, Weissmann N, Spriestersbach R, Becker E, Rosseau S, Seeger W. Effects of NADPH oxidase inhibitors on hypoxic vasoconstriction in buffer-perfused rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 1995;268:L747–L752. doi: 10.1152/ajplung.1995.268.5.L747. [DOI] [PubMed] [Google Scholar]
- Hasunuma K, Rodman DM, McMurtry IF. Effects of K+ channel blockers on vascular tone in the perfused rat lung. Am Rev Respir Dis. 1991;144:884–887. doi: 10.1164/ajrccm/144.4.884. [DOI] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol. 1997;122:21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C22–C33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- Jernigan NL, Resta TC, Walker BR. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L947–L955. doi: 10.1152/ajplung.00215.2003. [DOI] [PubMed] [Google Scholar]
- Jin N, Packer CS, Rhoades RA. Pulmonary arterial hypoxic contraction: signal transduction. Am J Physiol Lung Cell Mol Physiol. 1992;263:L73–L78. doi: 10.1152/ajplung.1992.263.1.L73. [DOI] [PubMed] [Google Scholar]
- Jin N, Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol. 1997;272:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- Jones RD, Thompson JS, Morice AH. The effect of hydrogen peroxide on hypoxia, prostaglandin F2 alpha and potassium chloride induced contractions in isolated rat pulmonary arteries. Pulm Pharmacol Ther. 1997;10:37–42. doi: 10.1006/pupt.1997.0071. [DOI] [PubMed] [Google Scholar]
- Kang TM, Park MK, Uhm DY. Characterization of hypoxia-induced [Ca2+]i rise in rabbit pulmonary arterial smooth muscle cells. Life Sci. 2002;70:2321–2333. doi: 10.1016/s0024-3205(02)01497-2. [DOI] [PubMed] [Google Scholar]
- Kang TM, Park MK, Uhm DY. Effects of hypoxia and mitochondrial inhibition on the capacitative calcium entry in rabbit pulmonary arterial smooth muscle cells. Life Sci. 2003;72:1467–1479. doi: 10.1016/s0024-3205(02)02441-4. [DOI] [PubMed] [Google Scholar]
- Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L408–L412. doi: 10.1152/ajplung.2000.279.2.L408. [DOI] [PubMed] [Google Scholar]
- Kjaeve J, Vaage J, Bjertnaes L. Toxic oxygen metabolites induce vasoconstriction and bronchoconstriction in isolated, plasma-perfused rat lungs. Acta Anaesthesiol Scand. 1991;35:65–70. doi: 10.1111/j.1399-6576.1991.tb03243.x. [DOI] [PubMed] [Google Scholar]
- Korde AS, Wang YX. Mitochondrial rieske protein, are you a real hypoxic sensor in pulmonary artery smooth muscle cells? FASEB J. 2008;22:11174. [Google Scholar]
- Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Zheng YM, Rathore R, Ma J, Takeshima H, Wang YX. Genetic evidence for functional role of ryanodine receptor 1 in pulmonary artery smooth muscle cells. Pflugers Arch. 2009;457:771–783. doi: 10.1007/s00424-008-0556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao B, Zheng YM, Yadav VR, Korde AS, Wang YX. Hypoxia induces intracellular Ca2+ release by causing ROS-mediated dissociation of FKBP12.6 with ryanodine receptor 2 in pulmonary artery myocytes. Antioxid Redox Signal. 2010 Jun 2; doi: 10.1089/ars.2009.3047. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1598–L1608. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- Littler CM, Morris KG, Jr, Fagan KA, McMurtry IF, Messing RO, Dempsey EC. Protein kinase C-epsilon-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol. 2003;284:H1321–H1331. doi: 10.1152/ajpheart.00795.2002. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase., gp91phox. Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- Liu QH, Zheng YM, Korde AS, Li XQ, Ma J, Takeshima H, Wang YX. Protein kinase C-ε regulates local calcium signaling in airway smooth muscle cells. Am J Respir Cell Mol Biol. 2009a;40:663–671. doi: 10.1165/rcmb.2008-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci U S A. 2009b;106:11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QH, Zheng YM, Wang YX. Two distinct signaling pathways for regulation of spontaneous local Ca2+ release by phospholipase C in airway smooth muscle cells. Pflugers Arch. 2007;453:531–541. doi: 10.1007/s00424-006-0130-1. [DOI] [PubMed] [Google Scholar]
- Lohn M, Jessner W, Furstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res. 2001;89:1051–1057. doi: 10.1161/hh2301.100250. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Benot A, Urena J. Oxygen sensing and the electrophysiology of arterial chemoreceptor cells. News Physiol Sci. 1993;8:191–195. [Google Scholar]
- MacLean MR, McCulloch KM, MacMillan JB, McGrath JC. Influences of the endothelium and hypoxia on neurogenic transmission in the isolated pulmonary artery of the rabbit. Br J Pharmacol. 1993;108:150–154. doi: 10.1111/j.1476-5381.1993.tb13455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott JF, Marshall JM. Effects of hypoxia upon contractions evoked in isolated rabbit pulmonary artery by potassium and noradrenaline. J Physiol. 1990;422:15–28. doi: 10.1113/jphysiol.1990.sp017969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- Mauban JR, Remillard CV, Yuan JX. Hypoxic pulmonary vasoconstriction: role of ion channels. J Appl Physiol. 2005;98:415–420. doi: 10.1152/japplphysiol.00732.2004. [DOI] [PubMed] [Google Scholar]
- Mehta JP, Campian JL, Guardiola J, Cabrera JA, Weir EK, Eaton JW. Generation of oxidants by hypoxic human pulmonary and coronary smooth-muscle cells. Chest. 2008;133:1410–1414. doi: 10.1378/chest.07-2984. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- Mohazzab KM, Fayngersh RP, Kaminski PM, Wolin MS. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial PO2-elicited responses. Am J Physiol Lung Cell Mol Physiol. 1995;269:L637–L644. doi: 10.1152/ajplung.1995.269.5.L637. [DOI] [PubMed] [Google Scholar]
- Mohazzab KM, Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery PO2 sensor. Am J Physiol Lung Cell Mol Physiol. 1994a;267:L823–L831. doi: 10.1152/ajplung.1994.267.6.L823. [DOI] [PubMed] [Google Scholar]
- Mohazzab KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 1994b;267:L815–L822. doi: 10.1152/ajplung.1994.267.6.L815. [DOI] [PubMed] [Google Scholar]
- Morio Y, McMurtry IF. Ca2+ release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92:527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- Morita K, Kitayama T, Kitayama S, Dohi T. Cyclic ADP-ribose requires FK506-binding protein to regulate intracellular Ca2+ dynamics and catecholamine release in acetylcholine-stimulated bovine adrenal chromaffin cells. J Pharmacol Sci. 2006;101:40–51. doi: 10.1254/jphs.fp0050991. [DOI] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563:409–419. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LC, Wilson SM, McAllister CE, Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol. 2007;152:101–111. doi: 10.1038/sj.bjp.0707357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J Biol Chem. 1997;272:3133–3136. doi: 10.1074/jbc.272.6.3133. [DOI] [PubMed] [Google Scholar]
- Nossaman BD, Kaye AD, Feng CJ, Kadowitz PJ. Effects of charybdotoxin on responses to nitrosovasodilators and hypoxia in the rat lung. Am J Physiol Lung Cell Mol Physiol. 1997;272:L787–L791. doi: 10.1152/ajplung.1997.272.4.L787. [DOI] [PubMed] [Google Scholar]
- Orton EC, Raffestin B, McMurtry IF. Protein kinase C influences rat pulmonary vascular reactivity. Am Rev Respir Dis. 1990;141:654–658. doi: 10.1164/ajrccm/141.3.654. [DOI] [PubMed] [Google Scholar]
- Pacaud P, Loirand G, Gregoire G, Mironneau C, Mironneau J. Calcium-dependence of the calcium-activated chloride current in smooth muscle cells of rat portal vein. Pflugers Arch. 1992;421:125–130. doi: 10.1007/BF00374818. [DOI] [PubMed] [Google Scholar]
- Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N, Braun-Dullaeus RC, Kummer W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2003;284:L710–L719. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- Park MK, Lee SH, Lee SJ, Ho WK, Earm YE. Different modulation of Ca-activated K channels by the intracellular redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflugers Arch. 1995;430:308–314. doi: 10.1007/BF00373904. [DOI] [PubMed] [Google Scholar]
- Peng W, Hoidal JR, Karwande SV, Farrukh IS. Effect of chronic hypoxia on K+ channels: regulation in human pulmonary vascular smooth muscle cells. Am J Physiol Cell Physiol. 1997;272:C1271–C1278. doi: 10.1152/ajpcell.1997.272.4.C1271. [DOI] [PubMed] [Google Scholar]
- Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L801–L812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol Cell Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med. 2008;45:1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Ragan CI, Bloxham DP. Specific labelling of a constituent polypeptide of bovine heart mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone reductase by the inhibitor diphenyleneiodonium. Biochem J. 1977;163:605–615. doi: 10.1042/bj1630605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCε signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2006;351:784–790. doi: 10.1016/j.bbrc.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCε signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RA, Packer CS, Roepke DA, Jin N, Meiss RA. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol. 1990;68:1581–1589. doi: 10.1139/y90-241. [DOI] [PubMed] [Google Scholar]
- Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger W, Suttorp N, Schmidt F, Neuhof H. The glutathione redox cycle as a defense system against hydrogen-peroxide-induced prostanoid formation and vasoconstriction in rabbit lungs. Am Rev Respir Dis. 1986;133:1029–1036. doi: 10.1164/arrd.1986.133.6.1029. [DOI] [PubMed] [Google Scholar]
- Sham JS. Hypoxic pulmonary vasoconstriction: ups and downs of reactive oxygen species. Circ Res. 2002;91:649–651. doi: 10.1161/01.res.0000039065.10754.de. [DOI] [PubMed] [Google Scholar]
- Sham JS, Crenshaw BR, Jr, Deng LH, Shimoda LA, Sylvester JT. Effects of hypoxia in porcine pulmonary arterial myocytes: roles of KV channel and endothelin-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L262–L272. doi: 10.1152/ajplung.2000.279.2.L262. [DOI] [PubMed] [Google Scholar]
- Sheehan DW, Giese EC, Gugino SF, Russell JA. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol Heart Circ Physiol. 1993;264:H1542–H1547. doi: 10.1152/ajpheart.1993.264.5.H1542. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol. 2000;279:L884–L894. doi: 10.1152/ajplung.2000.279.5.L884. [DOI] [PubMed] [Google Scholar]
- Sylvester JT. Hypoxic pulmonary vasoconstriction: a radical view. Circ Res. 2001;88:1228–1230. doi: 10.1161/hh1201.093167. [DOI] [PubMed] [Google Scholar]
- Tang WX, Chen YF, Zou AP, Campbell WB, Li PL. Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1304–H1310. doi: 10.1152/ajpheart.00843.2001. [DOI] [PubMed] [Google Scholar]
- Thomas HM, III, Carson RC, Fried ED, Novitch RS. Inhibition of hypoxic pulmonary vasoconstriction by diphenyleneiodonium. Biochem Pharmacol. 1991;42:R9–12. doi: 10.1016/0006-2952(91)90440-g. [DOI] [PubMed] [Google Scholar]
- Tsai BM, Wang M, Pitcher JM, Meldrum KK, Meldrum DR. Hypoxic pulmonary vasoconstriction and pulmonary artery tissue cytokine expression are mediated by protein kinase C. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1215–L1219. doi: 10.1152/ajplung.00179.2004. [DOI] [PubMed] [Google Scholar]
- Turner JL, Kozlowski RZ. Relationship between membrane potential, delayed rectifier K+ currents and hypoxia in rat pulmonary arterial myocytes. Exp Physiol. 1997;82:629–645. doi: 10.1113/expphysiol.1997.sp004052. [DOI] [PubMed] [Google Scholar]
- Vadula MS, Kleinman JG, Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 1993;265:L591–L597. doi: 10.1152/ajplung.1993.265.6.L591. [DOI] [PubMed] [Google Scholar]
- Vandier C, Delpech M, Bonnet P. Spontaneous transient outward currents and delayed rectifier K+ current: effects of hypoxia. Am J Physiol Lung Cell Mol Physiol. 1998;275:L145–L154. doi: 10.1152/ajplung.1998.275.1.L145. [DOI] [PubMed] [Google Scholar]
- Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997a;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2005a;288:L1059–L1069. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2005b;288:L1049–L1058. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang YX, Yu M, Kotlikoff MI. Ca2+-activated Cl− currents are activated by metabolic inhibition in rat pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 1997b;273:C520–C530. doi: 10.1152/ajpcell.1997.273.2.C520. [DOI] [PubMed] [Google Scholar]
- Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Kotlikoff MI. Inactivation of calcium-activated chloride channels in smooth muscle by calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1997;94:14918–14923. doi: 10.1073/pnas.94.26.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zheng YM. ROS-dependent signaling mechanisms for hypoxic Ca2+ responses in pulmonary artery myocytes. Antioxid Redox Signal. 2010;12:611–623. doi: 10.1089/ars.2009.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zheng YM, Abdullaev II, Kotlikoff MI. Metabolic inhibition with cyanide induces intracellular calcium release in pulmonary artery myocytes and Xenopus oocytes. Am J Physiol Cell Physiol. 2003;284:C378–88. doi: 10.1152/ajpcell.00260.2002. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, Xin HB, Kotlikoff MI. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol. 2004;286:C538–C546. doi: 10.1152/ajpcell.00106.2003. [DOI] [PubMed] [Google Scholar]
- Ward JP, Aaronson PI. Mechanisms of hypoxic pulmonary vasoconstriction: can anyone be right? Respir Physiol. 1999;115:261–271. doi: 10.1016/s0034-5687(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9:287–296. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Schumacker PT. O2 sensing in hypoxic pulmonary vasoconstriction: the mitochondrial door re-opens. Respir Physiolo Neurobiol. 2002;132:81–91. doi: 10.1016/s1569-9048(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Weigand LA, Wang J, Shimoda LA, Sham JS, Sylvester JT. Inhibitors of capacitative calcium entry block hypoxic pulmonary artery vasoconstriction. HPV) in isolated rat lungs. Am J Respir Crit Care Med. 2003;167:A698. [Google Scholar]
- Weir EK, Cabrera JA, Mahapatra S, Peterson DA, Hong Z. The role of ion channels in hypoxic pulmonary vasoconstriction. Adv Exp Med Biol. 2010;661:3–14. doi: 10.1007/978-1-60761-500-2_1. [DOI] [PubMed] [Google Scholar]
- Weir EK, Wyatt CN, Reeve HL, Huang J, Archer SL, Peers C. Diphenyleneiodonium inhibits both potassium and calcium currents in isolated pulmonary artery smooth muscle cells. J Appl Physiol. 1994;76:2611–2615. doi: 10.1152/jappl.1994.76.6.2611. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006a;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann N, Ebert N, Ahrens M, Ghofrani HA, Schermuly RT, Hanze J, Fink L, Rose F, Conzen J, Seeger W, Grimminger F. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. Am J Respir Cell Mol Biol. 2003;29:721–732. doi: 10.1165/rcmb.2002-0217OC. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Sommer N, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Oxygen sensors in hypoxic pulmonary vasoconstriction. Cardiovasc Res. 2006b;71:620–629. doi: 10.1016/j.cardiores.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Tadic A, Hanze J, Rose F, Winterhalder S, Nollen M, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase- derived H2O2? Am J Physiol Lung Cell Mol Physiol. 2000;279:L683–L690. doi: 10.1152/ajplung.2000.279.4.L683. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Voswinckel R, Hardebusch T, Rosseau S, Ghofrani HA, Schermuly R, Seeger W, Grimminger F. Evidence for a role of protein kinase C in hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 1999;276:L90–L95. doi: 10.1152/ajplung.1999.276.1.L90. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Zeller S, Schafer RU, Turowski C, Ay M, Quanz K, Ghofrani HA, Schermuly RT, Fink L, Seeger W, Grimminger F. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2006c;34:505–513. doi: 10.1165/rcmb.2005-0337OC. [DOI] [PubMed] [Google Scholar]
- Wilhelm J, Herget J. Role of ion fluxes in hydrogen peroxide pulmonary vasoconstriction. Physiol Res. 1995;44:31–37. [PubMed] [Google Scholar]
- Wilson HL, Dipp M, Thomas JM, Lad C, Galione A, Evans AM. ADP-ribosyl cyclase and cyclic adp-ribose hydrolase act as a redox sensor. a primary role for cyclic adp-ribose in hypoxic pulmonary vasoconstriction. J Biol Chem. 2001;276:11180–11188. doi: 10.1074/jbc.M004849200. [DOI] [PubMed] [Google Scholar]
- Wolin MS, Ahmad M, Gao Q, Gupte SA. Cytosolic NAD(P)H regulation of redox signaling and vascular oxygen sensing. Antioxid Redox Signal. 2007;9:671–678. doi: 10.1089/ars.2007.1559. [DOI] [PubMed] [Google Scholar]
- Wu W, Platoshyn O, Firth AL, Yuan JX. Hypoxia divergently regulates production of reactive oxygen species in human pulmonary and coronary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L952–L959. doi: 10.1152/ajplung.00203.2007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Asano K, Mori M, Takasugi T, Fujita H, Suzuki Y, Kawashiro T. Constriction and dilatation of pulmonary arterial ring by hydrogen peroxide--importance of prostanoids. Adv Exp Med Biol. 1994;361:457–463. doi: 10.1007/978-1-4615-1875-4_80. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 1993;264:L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- Zhang F, Carson RC, Zhang H, Gibson G, Thomas HM., III Pulmonary artery smooth muscle cell [Ca2+]i and contraction: responses to diphenyleneiodonium and hypoxia. Am J Physiol Lung Cell Mol Physiol. 1997;273:L603–L611. doi: 10.1152/ajplung.1997.273.3.L603. [DOI] [PubMed] [Google Scholar]
- Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1202–L1210. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- Zheng YM, Mei QB, Wang QS, Abdullaev I, Lai FA, Xin HB, Kotlikoff MI, Wang YX. Role of FKBP12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium. 2004;35:345–355. doi: 10.1016/j.ceca.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Zheng YM, Wang QS, Liu QH, Rathore R, Yadav V, Wang YX. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J Vasc Res. 2008;45:469–479. doi: 10.1159/000127438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]


