Abstract
Background
Severe burn induces a sustained hypermetabolic response, which causes long-term loss of muscle mass and decrease in muscle strength. In this study, we sought to determine whether muscle disuse has additional impact on muscle atrophy after severe burn using a rat model combining severe cutaneous burn and hindlimb unloading.
Methods
Forty Sprague-Dawley rats (≈300g) were randomly assigned to sham ambulatory (S/A), sham hindlimb unloading (S/HLU), burn ambulatory (B/A) or burn hindlimb unloading (B/HLU) groups. Rats received a 40% total body surface (TBSA) full thickness scald burn, and rats with hindlimb unloading were placed in a tail traction system. At day 14, lean body mass (LBM) was determined using DEXA scan, followed by measurement of the isometric mechanical properties in the predominantly fast-twitch plantaris muscle (PL) and the predominantly slow-twitch soleus muscle (SL). Muscle weight (wt), protein wt, and wet/dry wt were determined.
Results
At day 14, body weight had decreased significantly in all treatment groups; B/HLU resulted in significantly greater loss compared to the B/A, S/HLU and S/A. The losses could be attributed to loss of LBM. PL muscle wt and Po were lowest in the B/HLU group (<0.05 vs. S/A, S/HLU or B/A). SL muscle wt and Po were significantly less in both S/HLU and B/HLU compared that of S/A and B/A; no significant difference was found between S/HLU and B/HLU.
Conclusions
Cutaneous burn and hindlimb unloading have an additive effect on muscle atrophy, characterized by loss of muscle mass and decrease in muscle strength in both fast (PL) and slow (SL) twitch muscles. Of the two, disuse appeared to be the dominant factor for continuous muscle wasting after acute burn in this model.
Keywords: Hindlimb Unloading, Thermal Injury, Skeletal Muscle, Muscle Function
Introduction
Severe trauma and burn induce a sustained hypermetabolic response characterized by increased protein catabolism causing profound loss of lean body mass [1,2], which is associated with immunologic compromise [3], slowed wound healing, and in children, growth delay [4]. Catabolism with sustained loss of muscle mass, as well as loss of muscle strength, delays the return to customary pre-injury activities after severe burn. Although administration of nutrient support during hospitalization has been shown to reduce weight loss in severely burned [5] and other critically ill patients [6], these reductions are only partial, and do not fully compensate for the massive wasting of peripheral musculature [7].
It is well established that the hypermetabolic state is not resolved rapidly after burn and complete wound healing, but lasts for at least 9–12 months after burns over 40% TBSA [2,9]. This results in continuous erosion of lean body mass, from a reduction in muscle protein synthesis, and increase in protein catabolism [2] thus delayed recovery in muscle strength [8] during convalescence. Mechanisms leading to the long-term effect of burn on muscle catabolism have not been fully elucidated. However, one of the known factors associated with long-term muscle catabolism is muscle disuse caused by inactivity, which is common during and after hospitalization in severely burned patients. Wolfe et al showed that muscle inactivity caused by bed rest resulted in a significant decrease in skeletal muscle and whole body protein synthesis in human subjects [10]. They also found that bed rest amplified the normal catabolic responses of skeletal muscle to cortisol, one of the catabolic hormones released after surgery or injury. Prolonged bed rest and hypercortisolaemia exacerbated strength and lean muscle loss via a chronic reduction in muscle protein synthesis [11,12]. These results suggest that either burn and muscle disuse or more likely as a combination contribute to chronic and long-term hypermetabolism after severe injury. However, this notion has not been clearly identified in either animal models or in patients.
A rat model of 40% total body surface area (TBSA) full-thickness burn induces the hypermetabolic response and muscle catabolism independent of effects of inactivity, as activity in these animals was not restricted [13,14]. However, inactivity as an additional component to the catabolic factors associated with severe burn has not been tested in this familiar model, and thus the clinical scenario of severe burn combined with inactivity during the healing period seen in patients has not been fully elucidated in the laboratory. We hypothesize that muscle disuse plays an additive role in burn induced muscle catabolism. Therefore, in this study, we sought to test whether combining the standard Walker-Mason rat burn model with hindlimb unloading (HLU) to simulate the clinical scenario accompanying treatment of the severely burned elucidates additive effects compared to burn or inactivity alone. In this study, we tested the effects of burn and HLU independently and combined to identify the role of burn, HLU or the combination on muscle mass and strength after injury.
Methods
All procedures were reviewed and approved by Institutional Animal Care and Use Committees (IACUC) at the US Army Institute of Surgical Research. Forty male Sprague-Dawley rats weighing approximately 300gm were randomly and evenly assigned to four groups; one animal died due to anesthesia at the time of injury, thus 39 animals were studied: Sham/Ambulatory (S/A; n=10); Burn/Ambulatory (B/A; n=9); Sham/Hindlimb Unloading (S/HLU; n=10), and Burn/Hindlimb Unloading B/HLU; n=10). The study was conducted in the animal facility at the US Army Institute of Surgical Research. Rats were housed individually in specialized HLU metabolic cages [15] in a temperature-controlled environment with a 12-hour light/dark cycle. The animals were acclimatized in HLU metabolic cages and fed powdered food (Harlan Teklad #2018) for five days before the study.
Burn
Animals were anesthetized with continuous 1.5–3% isoflurane (Forane®, Baxter Healthcare Corp. IL 60015) in 100% oxygen using a nose cone. Rats in both sham and burn groups were shaved on the dorsal and ventral surface of the trunk. Animals in the burn groups received a 40% total burn surface area (TBSA) by immersing the dorsum in 100°C heated water for 10 seconds and ventral surface for 2 seconds according to the modified Walker-Mason burn model [13,16]. Burned rats were resuscitated with 20 ml intraperitoneal Ringer’s lactate solution immediately following the burn.
Hindlimb Unloading
HLU was performed according to the model described by Morey-Holton and Globus [17]. Briefly, the rat’s tail was cleaned with alcohol; tincture of benzoin was applied and allowed to become tacky to the touch. A half inch strip of Skin Tac (Zimmer, San Jose CA) was secured on the tail, wrapped in a stockinette, and one inch strips of filament fiber tape applied at the base, middle and top. The tip of the tail remained exposed in order to monitor circulation. Animals were allowed to fully recover from anesthesia, and then attached to a fish-line swivel hanging from the unloading device, which rides along two parallel sides of the cage. The angle and height of the rats were adjusted to a 30° angle from the cage floor, and thus the hind feet of the rats were not able to touch the grid floor of the cage. The rats were allowed to move on an x-y axis and rotate 360°, and the range of movement was adjusted so that they could freely access food and water but their hindlimbs were kept from contacting the walls of the cage. The ambulatory animals were kept in identical cages without tails attached to the harness. Analgesia was administered for the first 24 hours following injury (0.05 mg/kg Buprenorphine, q12h). Body weight, food and water consumption, and urine and fecal output were monitored and recorded daily.
Muscle Protein Content
At day 14 after injury, the SL and PL from the right hindlimb were isolated under isoflurane anesthesia, dissected and weighed. A small piece of muscle sample from each muscle was dissected, weighed and placed in a drying oven at 50°C for 5 days and weighed again for its dry weight. The ratio of wet weight/dry weight was determined. The remaining muscle samples were snap-frozen and stored at −70°C for later determination of muscle protein content.
PL and SL muscle samples (about 30mg) were processed for total protein extraction. Briefly, the muscle samples were snap-frozen in liquid nitrogen and pulverized in BioPulverizer™ (Biospec Product, USA). The samples were then immediately homogenized in the lysis buffer (Cell Signaling Technology®, Danvers, MA) containing 20 mM Tris-HCL, 150 mM Nacl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1ug/ml leupeptin. The protein suspension was extracted after centrifugation, and protein concentration was measured by BCA Protein Assay (Thermo Scientific, Rockford, IL). The total amount of protein weight per muscle (PL or SL) and percentage of protein content (protein weight per muscle weight of PL or SL; based on whole muscle weight) were then determined.
Isometric Mechanical Properties
Following muscle collection from the right hindlimb, muscle isometric force of PL and SL was measured simultaneously in the left hindlimb under anesthesia. First, the posterior thigh was opened to isolate and expose the sciatic nerve. The distal portion of the sciatic nerve was implanted into an electrode cuff with wires connected to a pulse stimulator (A-M Systems, Inc, Mod. 2100). After securing the electrode cuff, the proximal portion of the sciatic nerve was cut from its connection to the spinal cord. The distal tendons from both PL and SL were dissected and cut carefully without interrupting blood and nerve supply. They were then connected to two lever arms and secured with 4-0 silk suture separately. The lower leg was secured horizontally on the working platform by a combination of a pin drilled through the knee, and tape and bar to stabilize the ankle. The skin and superficial fascia around the opened wound were retracted to make a reservoir to hold warm mineral oil to maintain the temperature between 36.5°C to 37.5°C monitored using a digital thermometer inserted in adjacent muscles. Core temperature was also monitored using a rectal thermometer and maintained at 36.5°C to 37.5°C by manually adjusting the temperature of cir culating water in the rat surgical bed.
The isometric force of the PL and SL muscles was then measured using a modification of an in-situ preparation previously described [18, 19]. The modification consisted of simultaneous determination of mechanical properties for the PL and SL using two dual-mode servo muscle lever system (Aurora Scientific, Inc., Mod. 305b-LR and 305b, respectively). This modification reduced movement artifact and the time required to perform measurements. A personal computer loaded with Labview® software (National Instruments, Inc., Austin, Texas) and a National Instruments A/D board controlled the muscle levers and stimulator, recorded all signals (2000 Hz), and performed real-time analysis using the force and length signals. The nerve was stimulated at two times the voltage required to elicit maximal twitch tension (Pt) at a pulse width of 50 μs using an isolated pulse stimulator (A-M Systems, Inc, Mod. 2100). All measurements were made with the muscles set at optimal length (Lo), which was determined from Pt using an automated routine as follows: starting in a slack position, the muscle was stimulated at 1 Hz for a set of 8 twitches; the last two twitches were averaged and the Pt was stored. The lever was then moved 0.1 mm, and the routine was repeated 2 seconds later. Each twitch set including lever movement took 10 seconds. This procedure continued until the average Pt did not change by more than 2% between 3 consecutive twitch sets. Optimal length was then defined as the second of the three twitch sets.
After PL and SL were adjusted to optimal length respectively, and the twitch and tetanic properties were determined for the PL and SL simultaneously. The twitch tension (Pt), the total contraction time to peak (TPT), and half-relaxation time (1/2RT) from twitch response were measured three consecutive times with an interspersed one minute recovery period to generate averages of twitch tension. The peak isometric tension (tetanic tension, Po) was measured at frequency of 150 Hz. Po, and TPT were also measured three times with interspersed two minute recovery periods to generate an average of tetanic tension. Prior to muscle dissection, muscle length was determined while the muscle was set at Lo using digital micrometer. In order to also express force in terms of specific force (N/cm2), the physiologic cross sectional area (CSA) of PL and SL was calculated using the following formula: CSA= (muscle mass) × cos θ (muscle fiber pennation angle) (1.06 (coefficient of muscle density) × muscle fiber length)−1 [20]. The muscle pennation angle was assumed to be 16.4° and 3.9° for the PL and SL, respectively based on Eng et al., 2008 [21], and the muscle fiber length was calculated from muscle length at Lo and the ratio of muscle fiber length to muscle length of 0.34 and 0.69, for PL and SL, respectively [21].
Lean Mass Determination
After the muscle function test, the rat carcass was taken to a dual-image x-ray absorptiometry (DEXA) scanner (Lunar Prodigy, GE). Rats were placed independently into the scanner for measurement of whole body lean body mass.
Statistics
Statistical analysis was performed using one-way or two-way ANOVA (Tukey’s test). Significant difference was accepted at p<0.05. Data are expressed as mean ± standard error of mean (SEM).
Results
Body Weight
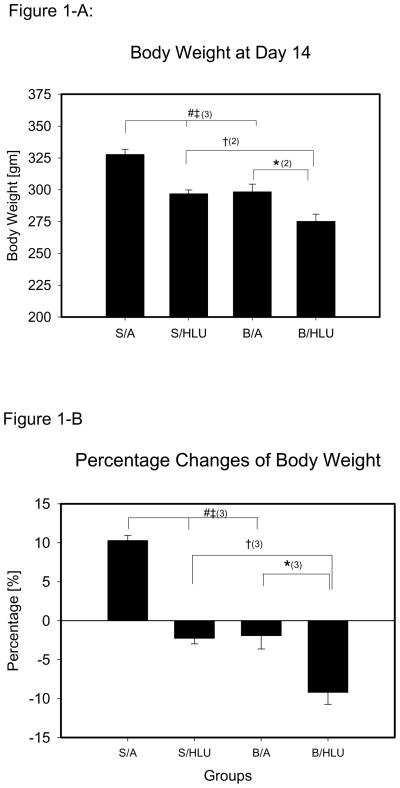
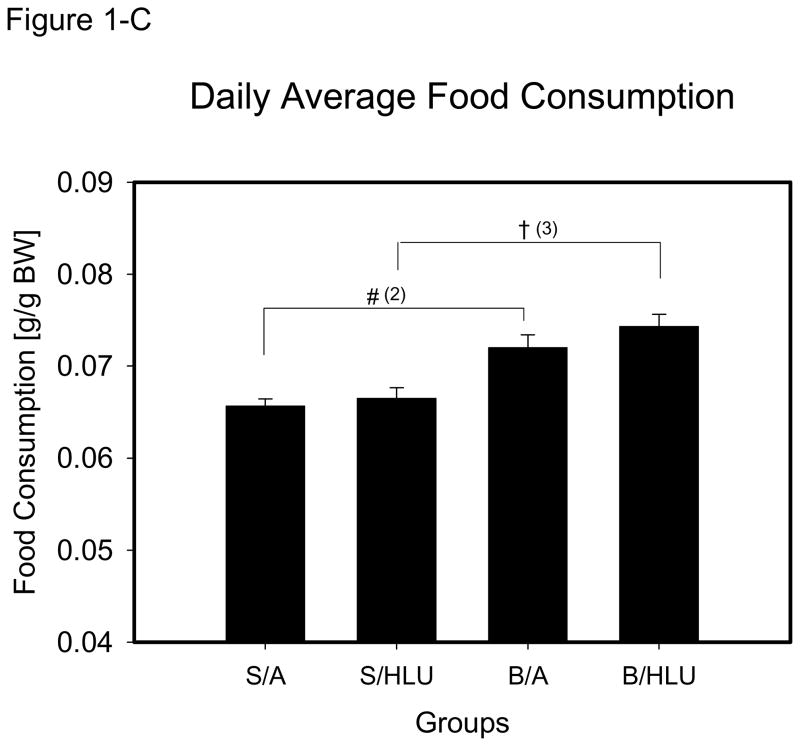
The body weight of the rats was not different between groups at the beginning of the study. The overall initial body mass was 302.0±1.3g. Body weight was measured daily, and S/A animals had constant weight gain. B/HLU rats had lower body weight at all measurement times compared to S/A, S/HLU and B/A. At day 14, body weight of B/HLU was significantly lower than B/A, S/HLU and S/A (Fig. 1-A), and body weight in S/HLU and B/A groups was significantly lower than S/A. However, no significant difference of body weight was found between B/A and Sham/HLU at day 14. Rats in B/HLU lost more than 10% body weight at day 14 (Fig. 1-B), which was significantly greater than the other three groups.
Figure 1.
S/A: Sham Ambulation; S/HLU: Sham Hindlimb Unloading; B/A: Burn Ambulation; B/HLU: Burn Hindlimb Unloading. Significant difference: #: S/A vs. B/A; ‡: S/A vs. S/HLU; †: S/HLU vs. B/HLU; *: B/A vs. B/HLU; (1): p<0.05; (2): p<0.01; (3): p<0.001. 1-A: body weight at day 14. B/HLU was significantly lower than S/HLU and B/A. S/HLU, and B/A was significantly lower than S/A. 1-B: Percentage change of body weight from the original body weight. B/HLU was significantly less than S/HLU and B/A. S/HLU, and B/A was significantly less than S/A. 1-C: Daily Average Food Consumption per Gram of Body Mass. B/HLU was significantly greater than S/HLU. B/A was significantly greater than S/A.
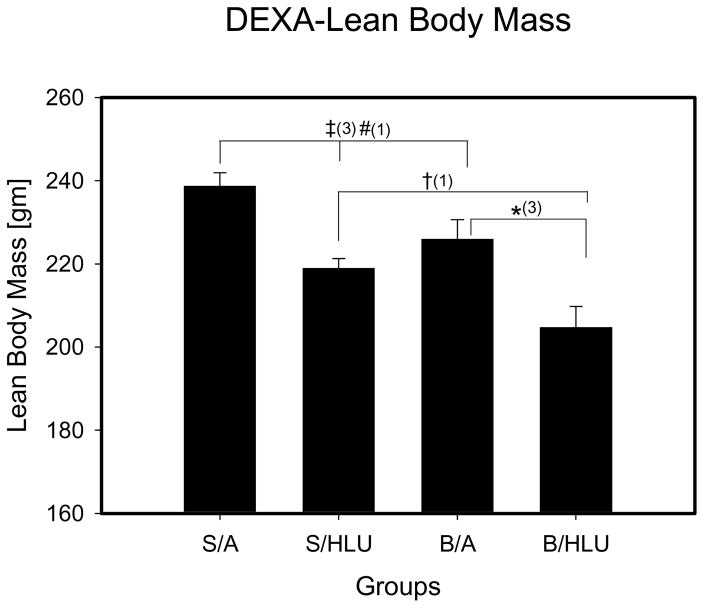
B/HLU lean body mass (LBM) was significantly lower than all other groups (Fig. 2). LBM was not significantly different between S/HLU and B/A, but both were significantly decreased compared to S/A.
Figure 2. Lean Body Mass.
S/A: Sham Ambulation; S/HLU: Sham Hindlimb Unloading; B/A: Burn Ambulation; B/HLU: Burn Hindlimb Unloading. Significant difference: #: S/A vs. B/A; ‡: S/A vs. S/HLU; †: S/HLU vs. B/HLU; *: B/A vs. B/HLU; (1): p<0.05; (2): p<0.01; (3): p<0.001. B/HLU was significantly less than S/HLU and B/A. S/HLU and B/A were significantly less than S/A.
Daily food consumption was recorded and expressed by the amount of food consumed per 100 grams of body weight (F/BW, gm/100 gm), and was analyzed by daily average from day 3 to day 14, when the rats regained constant intake following the injury. The daily F/BW was constant in S/A during the study period. There was a significant increase in F/BW in B/HLU and B/A compared to S/HLU and S/A (p<0.001, Fig. 1–C), and no significant difference was found between B/HLU and B/A, or between S/HLU and S/A.
Muscle Weight and Morphology
PL and SL muscle wt was significantly reduced in all treatment groups (B/A, S/HLU and B/HLU) compared to S/A (Table 1). In PL, the muscle wt of the HLU groups was significantly lower than ambulatory groups (S/A vs. S/HLU; B/A vs. B/HLU), and the muscle wt of B/HLU was significantly lower than S/HLU. In SL, the muscle wt of HLU groups was significantly lower than ambulatory groups (S/A vs. S/HLU; B/A vs. B/HLU), however, no significant difference was found between S/HLU and B/HLU. Muscle wt was reduced more in SL (−101% in S/HLU, −102% in B/HLU) than in PL (−27% in S/HLU, −44% in B/HLU) compared to control. In addition, no significant difference for muscle dry/wet weight ratio was shown in either PL or SL among the groups.
Table 1.
Muscle Mass, Lo, and CSA
| Group | PL |
SL |
||||
|---|---|---|---|---|---|---|
| Mass (mg) | Lo (mm) | CSA (mm2) | Mass (mg) | Lo (mm) | CSA (mm2) | |
| S/A | 413.3±8.7 | 39.94±0.36 | 26.29±0.80 | 154.9±4.7 | 28.78±0.40 | 5.86±0.21 |
| B/A | 359.0±13.6#(2) | 38.70±0.46 | 24.34±0.85#(1) | 133.2±2.7#(3) | 27.78±0.33 | 5.26±0.21#(1) |
| S/HLU | 324.3±11.4‡(3) | 37.52±0.53‡(3) | 21.93±0.43‡(3) | 77.2±4.3‡(3) | 26.49±0.32‡(3) | 3.55±0.08‡(3) |
| B/HLU | 278.5±8.8*(3)†(2) | 38.02±0.48 | 19.51±0.49*(3)†(1) | 76.8±2.5*(3) | 26.31±0.33*(2) | 3.59±0.15*(3) |
PL: Plantaris; SL: Soleus; Lo: Optimal length; CSA: Cross-sectional area; S/A: Sham Ambulation; S/HLU: Sham Hindlimb Unloading; B/A: Burn Ambulation; B/HLU: Burn Hindlimb Unloading. Data are expressed by mean ± SEM. Significant difference:
S/A vs. B/A;
S/A vs. S/HLU;
S/HLU vs. B/HLU;
B/A vs. B/HLU;
p<0.05;
p<0.01;
p<0.001.
The muscle optimal length (Lo) of PL and SL were measured immediately following the tests of muscle function. In PL, Lo of S/HLU was significantly less than that of S/A. However, no significant difference of Lo was found between B/HLU and S/HLU or B/A. CSA of both PL and SL was significantly decreased in HLU groups compared to ambulatory groups (S/A vs. S/HLU; B/A vs. B/HLU) (Table 1). CSA of PL in B/HLU was also significantly lower than that of S/HLU. In SL, Lo of S/HLU and B/HLU was significantly less than S/A and B/A respectively (Table 1). No significant difference in SL Lo was found between B/HLU and S/HLU. The greatest reduction of CSA occurred in SL of S/HLU and B/HLU (−49% compared to S/A), however, no significant difference of CSA of SL was observed between B/HLU and S/HLU.
Muscle Protein Content
The total protein content of PL and SL was significantly reduced in all treatment groups (B/A, S/HLU and B/HLU) compared to S/A (Table 2). In PL, the total protein wt of HLU groups was significantly less than ambulatory groups (S/A vs. S/HLU; B/A vs. B/HLU). The total protein wt was the least in PL of B/HLU, which was significantly less than S/HLU. In SL, the total protein wt of HLU groups was significantly less than ambulatory groups (S/A vs. S/HLU; B/A vs. B/HLU), however, no significant difference was found between S/HLU and B/HLU. In addition, the percentage of protein content in both PL and SL was not significantly different among the groups (Table 2).
Table 2.
Muscle Protein Content
| Group | PL |
SL |
||
|---|---|---|---|---|
| Total Protein (mg) | Protein Content (%) | Total Protein (mg) | Protein Content (%) | |
| S/A | 25.66±1.60 | 6.21±0.37 | 8.34±0.37 | 5.37±0.11 |
| B/A | 21.35±1.03#(1) | 5.76±0.20 | 7.26±0.30#(1) | 5.65±0.18 |
| S/HLU | 19.93±1.62‡(2) | 6.10±0.49 | 4.51±0.35‡(3) | 5.83±0.26 |
| B/HLU | 15.25±1.24*(1)†(1) | 5.44±0.44 | 4.43±0.28*(3) | 5.76±0.29 |
PL: Plantaris; SL: Soleus; S/A: Sham Ambulation; S/HLU: Sham Hindlimb Unloading; B/A: Burn Ambulation; B/HLU: Burn Hindlimb Unloading. Data are expressed by mean ± SEM. Significant difference:
S/A vs. B/A;
S/A vs. S/HLU;
S/HLU vs. B/HLU;
B/A vs. B/HLU;
p<0.05;
p<0.01;
p<0.001.
Muscle Isometric Force
The average Pt as well as TPT and ½RT of PL were not significantly different among groups (Table 3). The Pt of SL in HLU groups was significantly less than ambulatory groups (S/A vs. S/HLU and B/A vs. B/HLU) (Table 3). The TPT of Pt in SL was significantly decreased in HLU groups compared to ambulatory groups (S/A vs. S/HLU; B/A vs. B/HLU), and accordingly, the ½ RT was also decreased in HLU groups compared to ambulatory groups, however, the statistical significant difference was occurred between S/HLU and S/A.
Table 3.
Muscle Contractile Properties
| PL | SL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S/A (n=10) | B/A (n=9) | S/HLU (n=10) | B/HLU (n=10) | S/A (n=10) | B/A (n=9) | S/HLU (n=6) | B/HLU (n=9) | ||
| Twitch Force | Pt (N) | 1.62±0.09 | 1.82±0.13 | 1.80±0.11 | 1.88±0.07 | 0.28±0.02 | 0.29±0.02 | 0.16±0.01‡(3) | 0.22±0.02*(1) |
| TPT (ms) | 18.64±0.70 | 19.94±1.04 | 20.10±0.73 | 19.95±0.82 | 43.48±1.22 | 43.81±2.22 | 32.67±0.68‡(2) | 37.58±2.28*(1) | |
| ½ RT (ms) | 16.92±1.30 | 18.28±2.08 | 17.85±1.15 | 17.83±1.72 | 50.00±2.48 | 46.02±2.97 | 39.50±3.03‡(1) | 41.55±4.12 | |
| Tetanic Force | Po (N) | 8.03±0.22 | 7.48±0.28 | 6.84±0.20‡(3) | 6.10±0.20*(3)†(1) | 1.29±0.07 | 1.15±0.06 | 0.62±0.03‡(3) | 0.75±0.07*(3) |
| Po/CSA (N/cm2) | 30.67±0.93 | 31.15±1.52 | 31.28±1.05 | 31.18±1.01 | 22.04±1.26 | 21.35±1.19 | 17.34±0.78‡(2) | 20.28±1.45 | |
| Pt/Po | 0.20±0.01 | 0.26±0.01#(2) | 0.26±0.01‡(2) | 0.31±0.02*(2)†(1) | 0.22±0.01 | 0.26±0.01#(1) | 0.26±0.02‡(2) | 0.29±0.02*(1)†(1) | |
| Fatigue Index (100%) | 11.9±1.1 | 16.1±0.9#(1) | 16.6±1.3‡(2) | 14.6±1.2 | 73.3±5.5 | 80.8±3.6 | 79.0±2.1 | 79.9±3.4 | |
PL: Plantaris; SL: Soleus; S/A: Sham Ambulation; S/HLU: Sham Hindlimb Unloading; B/A: Burn Ambulation; B/HLU: Burn Hindlimb Unloading. Data are expressed by mean ± SEM. Significant difference:
S/A vs. B/A;
S/A vs. S/HLU;
S/HLU vs. B/HLU;
B/A vs. B/HLU;
p<0.05;
p<0.01;
p<0.001.
The absolute Po in PL significantly decreased in the HLU groups (compared to ambulatory groups (S/A vs. S/HLU; B/A vs. B/HLU) respectively, and Po of B/HLU was significantly less than S/HLU (Table 3). However, there was no significant difference of specific Po (Po/CSA) of PL among the groups. The absolute Po in SL was significantly declined in HLU groups compared to ambulatory groups (S/A vs. S/HLU and B/A vs. B/HLU), and no significant difference was found between S/HLU and B/HLU. The specific Po in SL was significantly declined in S/HLU compared to S/A, but no significant difference of specific Po was found either between S/A and B/A or between B/A and B/HLU (Table 3). There was no significant difference of TPT of Po among the groups in both PL and SL. The specific tension of PL was significantly greater than SL in each group respectively. In addition, Pt/Po ratio of both PL and SL was significantly increased in every treatment group (B/A, S/HLU, and B/HLU) compared to S/A respectively, and Pt/Po ratio of B/HLU was significantly greater than S/HLU.
Discussion
There are numerous studies showing that hypermetabolic response and muscle catabolism are induced in the standard Walker Mason rat burn model [13,14], however, this response is relatively modest and does not continue long into the recovery phase. In our previous study, we found that both muscle weight and muscle strength had a trajectory recovery after 14 days of burn using this model [33]. This is in contrast to findings from severely burned subjects who display sustained muscle catabolism up to 9 months after burn [2,8]. One of the major differences is that these patients are treated in an ICU beds for weeks to months after injury, but the animals are ambulatory. Although both bed rest and severe burn clearly show tremendous independent impact on muscle catabolism, it has not been fully investigated whether bed rest, decreased muscle activity or weight loading plays a role in the induction and development of burn induced muscle atrophy. HLU in rats has been commonly used to study inactivity-induced muscle atrophy, such as muscle disuse and micro-gravity of space flight [22,23,17]. More importantly, it has been reported that HLU mimics the physiologic change of long-term bed rest [24,17]. Using this model of combined burn and HLU, we have shown that the combination of burn and HLU result in greater muscle atrophy compared to either condition alone. We also show that burn and HLU independently caused reductions in body weight, lean body mass, muscle mass, and muscle strength, but the two together caused even further decrements in an additive fashion.
Body mass
The results of this study are consistent with previous investigations that report decreases in body mass after burn [13,14] and after HLU [25]. In this study, we found that rats in B/HLU lost more body wt than rats in burn or HLU alone. This was not due to reduced food intake on the part of B/HLU, as the food intake of this group was significantly higher than that of the other three groups (Figure 1C). At the same time the body wt gain of B/HLU was actually reduced compared to B/A and S/HLU (Figure 1B), indicating a marked reduction in food efficiency (weight gain per gram of food eaten). The loss in body mass following all treatments is attributable to a loss of LBM, as determined by DEXA scan (Figure 2). The pattern among groups for LBM is consistent with the changes in body wt. (Figure 1B).
Muscle mass
The loss in LBM following burn or HLU is primarily due to the loss of muscle mass. The effects of disuse, due to hindlimb unloading, on muscle wasting in rats are well documented in both whole muscle and isolated single muscle fibers [29,30]. HLU results in preferential atrophy of planter flexor muscles compared to dorsi flexor muscles, and among planter flexors, slow-twitch muscle fibers are much more affected than fast-twitch muscle [25–29]. Because planter flexors are more affected by HLU, we chose to use the PL and SL in the current study. The PL and the SL are composed of predominantly fast-twitch and slow-twitch muscle fibers, respectively. It has been reported by ourselves as well as others [31–33], that the greatest decrease of muscle mass after burn occurs in predominately fast-twitch muscles, with little change in the slow-twitch SL. It was therefore unexpected to find that the loss of mass following burn (B/A) in the current study was independent of fiber type, i.e., the PL and SL underwent identical declines in mass as a result of burn alone (Table 1). One distinction between those studies [31–32] and this one is that they used the extensor digitorum longus (EDL) as the representative fast-twitch muscle. The EDL is a dorsi flexor, while the PL is a planter flexor. It is possible, as with HLU, plantar flexors and dorsi flexors respond differently, even when composed of the same fiber type.
It has been shown that muscle protein degradation increases, and protein synthesis decreases in the beginning of both HLU and burn [31,32,38] so that the negative protein balance causes reduction of lean body mass (LBM). In human, muscle inactivity caused by bed rest had a significant decrease in skeletal muscle and whole body protein synthesis in human subjects [10], and prolonged bed rest combined with hypercortisolaemia exacerbated strength and lean muscle loss via a chronic reduction in muscle protein synthesis [11,12]. In this study, the B/HLU simulates the burn and bed rest, and B/HLU has the least LBM compared to S/A, B/A and S/HLU, indicating the whole body’s net balance of protein synthesis and protein degradation is significantly declined in the combination of burn and muscle disuse caused by HLU compared to burn or muscle disuse alone. We found that the catecholamine and urine cortisol level were significantly increased in B/HLU at day 2–3 compared to B/A and S/HLU, which may contribute to increased protein degradation in B/HLU.
Muscle function
Studies examining the impact of HLU on muscle isometric properties have consistently demonstrated a preferential impact on slow-twitch muscle [27,28]. Previous studies have shown that Pt increases, Po decreases, and Pt/Po is increased after two or more weeks of HLU in SL [34,35]. This is consistent with the current results in both of the HU groups in the present study. In contrast to SL, the mechanical properties of the PL is less affected than SL by HLU, where the changes are very temporary, modest or not significant [36]. Compared to HLU, there are few reports on the impact of burn on mechanical properties. In a previous investigation [33], we found that the tibia antarialis (TA) underwent a significant reduction of muscle mass and muscle strength in response to burn, and the loss was attributable solely to the reduction in muscle mass.
In general, the reduction of muscle maximum force (Po) in response to B/A, S/HLU and B/HLU is paralleled by the reduction of muscle mass and CSA without significant difference of Po/CSA ratio among the groups. The lack of change in specific force (Po (N/cm2) supports the contention that the reduction in Po is a result of muscle quantity, not quality. This is supported by the lack of differences in % protein content among muscles (Table 2). The exception to this is the SL in S/HLU (Table 3), which displayed a significant reduction in specific force. Since the % protein content was not changed, it is unlikely that this is due to a reduction in myofibular protein. A loss of myofibrillar proteins such as myosin and actin has been reported to occur in the SL in response to HLU alone [28, 37], with a resultant reduction in specific force [36]. Based on this it would have been predicted that SL in B/HLU should have undergone a reduction in specific force. At this time, we are unable to explain these contractions with the literature.
In this study, we found a significant increase of Pt/Po in all treatment groups of B/A, S/HUL and B/HLU, this was due to a relatively greater decrease for Po compared to Pt. The Pt/Po ratio is higher in fast twitch muscle than slow twitch muscle [40]. Microgravity in spaceflight and HLU leads to a loss of slow twitch fibers and a shift of myosin isoform expression from slow to fast [39, 41–44]. It is therefore likely that in the SL the increase in Pt/Po reflects an increased contribution of fast twitch fibers, which would also explain the increase in contractile speed (TPT and 1/2RT) for the SL in the HLU groups. Additionally, other factors such Ca2+ affinity and handling of Ca2+ by the sarcoplasmic reticulum may be altered after HLU [45], which may contribute to the faster speed in SL in response to HLU.
Conclusion
This study improves knowledge in that it is the first time an animal model has been produced that closely mimics the metabolic changes seen in patients weeks after injury. The results of this study show that both burn and HLU are independent factors associated with muscle atrophy, and the effects of the combination are additive. We suggest that muscle disuse is the dominant cause of long-term muscle catabolism in burned patients. Clinical therapeutic approaches to ameliorate muscle wasting after injury should therefore be aimed at both diminishing the injury response as well as directed at muscle inactivity and lacking of weight bearing especially at lower extremities. The components of injury/inflammation have been shown to respond well to metabolic interventions such as oxandrolone, growth hormone, insulin, and propranolol treatment [46–48]. Meanwhile, a directed exercise program to increase physical activity also shows advantages in enhancing and improving recovery from muscle catabolism after severe burn [49]. Similarly, both anabolic agents and increased muscle activity is beneficial to reduce muscle wasting and improve muscle recovery after HLU [43,50]. The combination treatment applying both exercise and long-term growth hormone and oxandrolone treatment had additive beneficial effect on reduction of long-term muscle catabolism in pediatric burned patients [51,52]. Since the impact of muscle disuse begins immediately after injury, future clinical studies should investigate potential approaches to improve and regulate metabolic alteration by earlier mobilization and weight bearing exercise.
Acknowledgments
Grant Information: Combat Casualty Care Division United States Army Medical Research and Materiel Command and The Technologies for Metabolic Monitoring (TMM)/Julia Weaver Fund, A Congressionally Directed Program Jointly Managed by the USA MRMC, NIH, NASA and the Juvenile Diabetes Research Foundation and The National Institutes of Health (1 R01 GM063120-04)
Footnotes
The opinions or assertions contained herein are the private views of the author and are not to be construed as official or reflecting the views of the US Department of Defense or the US Government. The author is an employee of the US Government. This work was prepared as part of his official duties and, as such, there is no copyright to be transferred.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newsome TW, Mason AD, Jr, Pruitt BA., Jr Weight loss following thermal injury. Ann Surg. 1973;178(2):215–7. doi: 10.1097/00000658-197308000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo R, Herndon DN, Kobayashi M, Pollard RB, Suzuki F. CD4- CD8- TCR alpha/beta+ suppressor T cells demonstrated in mice 1 day after thermal injury. J Trauma. 1997;42(4):635–40. doi: 10.1097/00005373-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study. Lancet. 1999;354(9192):1789. doi: 10.1016/s0140-6736(99)02741-5. [DOI] [PubMed] [Google Scholar]
- 5.Gore DC, Rutan RL, Hildreth M, Desai MH, Herndon DN. Comparison of resting energy expenditures and caloric intake in children with severe burns. J Burn Care Rehabil. 1990;11(5):400–4. doi: 10.1097/00004630-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bower RH. Nutrition during critical illness and sepsis. New Horiz. 1993;1(2):348–52. [PubMed] [Google Scholar]
- 7.Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987;27(3):262–6. doi: 10.1097/00005373-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 8.St-Pierre DM, Choinière M, Forget R, Garrel DR. Muscle strength in individuals with healed burns. Arch Phys Med Rehabil. 1998 Feb;79(2):155–61. doi: 10.1016/s0003-9993(98)90292-1. [DOI] [PubMed] [Google Scholar]
- 9.Milner EA, Cioffi WG, Mason AD, McManus WF, Pruitt BA., Jr A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma. 1994 Aug;37(2):167–70. doi: 10.1097/00005373-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996 Apr;270(4 Pt 1):E627–33. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 11.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006 Dec;91(12):4836–41. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- 12.Ferrando AA, Stuart CA, Sheffield-Moore M, Wolfe RR. Inactivity amplifies the catabolic response of skeletal muscle to cortisol. J Clin Endocrinol Metab. 1999 Oct;84(10):3515–21. doi: 10.1210/jcem.84.10.6046. [DOI] [PubMed] [Google Scholar]
- 13.Herndon DN, Wilmore DW, Mason AD., Jr Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res. 1978 Nov;25(5):394–403. doi: 10.1016/s0022-4804(78)80003-1. [DOI] [PubMed] [Google Scholar]
- 14.Barrow RE, Meyer NA, Jeschke MG. Effect of varying burn sizes and ambient temperature on the hypermetabolic rate in thermally injured rats. J Surg Res. 2001 Aug;99(2):253–257. doi: 10.1006/jsre.2001.6183. [DOI] [PubMed] [Google Scholar]
- 15.Harper JS, Mulenburg GM, Evans J, Navidi M, Wolinsky I, Arnaud SB. Metabolic cages for a space flight model in the rat. Lab Anim Sci. 1994 Dec;44(6):645–7. [PubMed] [Google Scholar]
- 16.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968 Nov;8(6):1049–51. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 18.Walters TJ, Sweeney HL, Farrar RP. Aging does not affect contractile properties of type IIb FDL muscle in Fischer 344 rats. Am J Physiol. 1990 Jun;258(6 Pt 1):C1031–5. doi: 10.1152/ajpcell.1990.258.6.C1031. [DOI] [PubMed] [Google Scholar]
- 19.Walters TJ, Sweeney HL, Farrar RP. Influence of electrical stimulation on a fast-twitch muscle in aging rats. J Appl Physiol. 1991 Nov;71(5):1921–8. doi: 10.1152/jappl.1991.71.5.1921. [DOI] [PubMed] [Google Scholar]
- 20.Powell PL, Roy RR, Kanim P, Bello M, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- 21.Eng CM, Smallwood LH, Rainiero MP, Lahey M, Ward SR, Lieber RL. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol. 2008;211:2336–2346. doi: 10.1242/jeb.017640. [DOI] [PubMed] [Google Scholar]
- 22.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol. 1992 Nov;73(5):2172–8. doi: 10.1152/jappl.1992.73.5.2172. [DOI] [PubMed] [Google Scholar]
- 23.Berg HE, Dudley GA, Häggmark T, Ohlsén H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol. 1991 Apr;70(4):1882–5. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenfeld G. Use of animal models for space flight physiology studies, with special focus on the immune system. Gravit Space Biol Bull. 2005 Jun;18(2):31–5. [PubMed] [Google Scholar]
- 25.Musacchia XJ, Steffen JM, Deavers DR. Rat hindlimb muscle responses to suspension hypokinesia/hypodynamia. Aviat Space Environ Med. 1983;54:1015–1020. [PubMed] [Google Scholar]
- 26.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol. 1987;63:558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 27.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol. 1987 Aug;63(2):558–63. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 28.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1988;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Wronski TJ, Morey-Holton ER. Skeletal response to simulated weightlessness: a comparison of suspension techniques. Aviat Space Environ Med. 1987:5863–68. [PubMed] [Google Scholar]
- 30.Gardetto PR, Schluter JM, Fitts RH. Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol. 1989;66(6):2739–49. doi: 10.1152/jappl.1989.66.6.2739. [DOI] [PubMed] [Google Scholar]
- 31.Fang CH, Li BG, Tiao G, Wang JJ, Fischer JE, Hasselgren PO. The molecular regulation of protein breakdown following burn injury is different in- fast- and slow-twitch skeletal muscle. Int J Mol Med. 1998 Jan;1(1):163–9. doi: 10.3892/ijmm.1.1.163. [DOI] [PubMed] [Google Scholar]
- 32.Hasselgren PO, James JH, Benson DW, et al. Total and myofibrillar protein breakdown in different types of rat skeletal muscle: effects of sepsis and regulation by insulin. Metabolism. 1989;38(7):634–40. doi: 10.1016/0026-0495(89)90100-5. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Wolf SE, Thomas WJ. Muscle Contractile Properties in Severely Burned Rats. Burns 2010 Burns. 2010 Apr 7; doi: 10.1016/j.burns.2010.02.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leterme D, Cordonnier C, Mounier Y, Falempin M. Insluence of chromic stretching upon rat soleus muscle during non-weight-bearing conditions. Pflugers Arch. 1994;429:274–279. doi: 10.1007/BF00374323. [DOI] [PubMed] [Google Scholar]
- 35.Winiarski Am, Roy RR Alford EK, Chiang PC, Edgerton VR. Mechanical properties of rat skeletal muscle after hindlimb suspension. Exp Neurol. 1987;96:650–660. doi: 10.1016/0014-4886(87)90226-3. [DOI] [PubMed] [Google Scholar]
- 36.Diffee GM, Caiozzo VJ, Herrick RE, Baldwin KM. Contractile and biochemical properties of rat soleus and plantaris after hindlimb suspension. Am J Physiol Cell Physiol. 1991;260:C528–C534. doi: 10.1152/ajpcell.1991.260.3.C528. [DOI] [PubMed] [Google Scholar]
- 37.Babij P, Booth FW. Alpha-actin and cytochrome c mRNAs in atrophied adult rat skeletal muscle. Am J Physiol. 1988;254(5 Pt 1):C651–6. doi: 10.1152/ajpcell.1988.254.5.C651. [DOI] [PubMed] [Google Scholar]
- 38.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68(1):1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Steppen JM, Musacchia XJ. Effect of hypokinesia and hypodynamia on protein, RNA and DNA in rat hindlimb muscles. Am J Physiol. 1984;247:R728–R732. doi: 10.1152/ajpregu.1984.247.4.R728. [DOI] [PubMed] [Google Scholar]
- 40.Celichowski J, Grottel K. Twitch/tetanus ratio and its relation to other properties of motor units. NeuroReport. 1993;5(3):201–204. doi: 10.1097/00001756-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Caiozzo VJ, Haddad F, Baker MJ, Herrick RE, Prietto N, Baldwin KM. Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J Appl Physiol. 1996;81:123–132. doi: 10.1152/jappl.1996.81.1.123. [DOI] [PubMed] [Google Scholar]
- 42.Allen DL, Yasui W, Tanaka T, Ohira Y, nagaoka S, Sekiguchi c, Hinds WE, Roy RR, Edgerton VR. Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J Appl Physiol. 1996;81:145–151. doi: 10.1152/jappl.1996.81.1.145. [DOI] [PubMed] [Google Scholar]
- 43.Tsika RW, Herrick RE, Baldwin KM. Effect of anabolic steroids on skeletal muscle mass during hindlimb suspension. J Appl Physiol. 1987;63(5):2122–2127. doi: 10.1152/jappl.1987.63.5.2122. [DOI] [PubMed] [Google Scholar]
- 44.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol. 1987;63(2):558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 45.Gardetto PR, Schulter JM, Fitts RH. Contractile function of single muscle fibers following hindlimb suspension. J Appl Physiol. 1989;66:2739–2749. doi: 10.1152/jappl.1989.66.6.2739. [DOI] [PubMed] [Google Scholar]
- 46.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000 Mar;15(1):12–7. doi: 10.1053/jcrc.2000.0150012. [DOI] [PubMed] [Google Scholar]
- 47.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004 Jun 5;363(9424):1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 48.Hart DW, Herndon DN, Klein G, Lee SB, Celis M, Mohan S, Chinkes DL, Wolf SE. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233(6):827–34. doi: 10.1097/00000658-200106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001 Sep;91(3):1168–75. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 50.Hurst JE, Fitts RH. Hindlimb unloading-induced muscle atrophy and loss of function: protective effect of isometric exercise. J Appl Physiol. 2003;95:1405–1417. doi: 10.1152/japplphysiol.00516.2002. [DOI] [PubMed] [Google Scholar]
- 51.Suman OE, Thomas SJ, Wilkins JP, Mlcak RP, Herndon DN. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol. 2003 June;94(6):2273–81. doi: 10.1152/japplphysiol.00849.2002. [DOI] [PubMed] [Google Scholar]
- 52.Przkora R, Herndon D, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007 Jan;119(1):e109–16. doi: 10.1542/peds.2006-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]