Abstract
Background
Bromelain, a mixture of proteolytic enzymes typically derived from pineapple stem, decreases production of pro-inflammatory cytokines and leukocyte homing to sites of inflammation. We previously showed that short-term oral treatment with bromelain purified from pineapple stem decreased the severity of colonic inflammation in C57BL/6 Il10−/− mice with chronic colitis. Since fresh pineapple fruit contains similar bromelain enzymes but at different proportions, this study aimed to determine whether long-term dietary supplementation with pineapple (supplied as juice) could decrease colon inflammation and neoplasia in Il10−/− mice with chronic colitis as compared with bromelain derived from stem.
Results
Experimental mice readily consumed fresh pineapple juice at a level that generated mean stool proteolytic activities equivalent to 16 mg bromelain purified from stem, while control mice received boiled juice with inactive enzymes. Survival was increased in the group supplemented with fresh rather than boiled juice (p = 0.01). Mice that received fresh juice also had decreased histologic colon inflammation scores and a lower incidence of inflammation-associated colonic neoplasia (35% vs. 66%; p< 0.02), with fewer neoplastic lesions/colon (p = 0.05). Flow cytometric analysis of murine splenocytes exposed to fresh pineapple juice in vitro demonstrated proteolytic removal of cell surface molecules that can affect leukocyte trafficking and activation.
Conclusions
These results demonstrate that long-term dietary supplementation with fresh or unpasteurized frozen pineapple juice with proteolytically active bromelain enzymes is safe and decreases inflammation severity and the incidence and multiplicity of inflammation-associated colonic neoplasia in this commonly used murine model of inflammatory bowel disease.
Keywords: bromelain, IBD, therapy, chemoprevention
Introduction
Bromelain is a mixture of cysteine proteinases that is typically derived from the stem of the pineapple plant (Ananus comosus) (1). Bromelain has historically been used as a meat tenderizer and to pre-treat cells for adhesion assays (2). We and others have demonstrated that bromelain proteolytically removes cell surface molecules that are required for leukocyte migration and activation, resulting in anti-inflammatory activity (3, 4). Accordingly, bromelain has shown promise as therapy for a variety of immune-mediated diseases, including inflammatory bowel disease (IBD) (reviewed in 5). For example, oral treatment with proteolytically active bromelain decreased spontaneous and piroxicam-triggered colonic inflammation in IL-10-deficient mice in vivo (5). Kane et al. (6) described 2 patients with ulcerative colitis who did not respond to conventional treatment but rapidly improved after self-treatment with oral bromelain. Furthermore, in vitro bromelain treatment of colon biopsies from human IBD patients resulted in decreased secretion of pro-inflammatory cytokines and chemokines (7). Previous work in our laboratory showed that exposure to bromelain purified from pineapple stem alters leukocyte expression of cell surface molecules including CD44, CD62L (L-selectin), CD45RA, and CD8 and can effectively decrease neutrophil migration to sites of acute inflammation in part via proteolytic removal of CD128, the receptor for the chemokine IL-8 (4, 8).
As is common for plant-derived products, the exact composition of bromelain preparations can vary according to source and method of purification. The major proteolytic component of bromelain obtained from pineapple stem is stem bromelain (EC 3.4.22.32; ~90%), with minor amounts of fruit bromelain (EC 3.4.22.23), ananain (EC 3.4.22.31), and comosain (9). In contrast, the major proteolytic component of pineapple fruit is fruit bromelain (~90%), with minor amounts of stem bromelain and ananain (9). Stem bromelain and fruit bromelain are highly homologous cysteine proteinases, but differ in exact amino acid sequence, molecular weight, isoelectric point, and carbohydrate content and have distinct proteolytic activities (9, 10). The differences between their potential anti-inflammatory activities are also currently not understood.
Most experimental studies using bromelain have used commercially available bromelain purified from pineapple stem. Fresh pineapple fruit contains similar enzymes but in different proportions. Thus, the consumption of pineapple fruit may represent a more palatable way to supply active bromelain enzymes long term. This study was designed to determine whether long-term dietary supplementation with pineapple fruit (supplied as fresh, non-pasteurized juice) would affect the severity of colon inflammation and the incidence of inflammation-associated neoplasia in mice with chronic colitis. The anti-inflammatory efficacy of supplementation with pineapple juice was compared with that of stem bromelain at the 5 mg/day dose previously shown to decrease colon inflammation in short-term studies (5). The sensitivity of a panel of murine cell surface molecules to pineapple juice-derived bromelains was also determined.
Materials and Methods
Animal models of colitis
Il10−/− male and female mice on the C57BL/6 background (strain name = B6.129P2-Il10tm1Cgn/J; stock # 002251, Jackson Laboratories, Bar Harbor, ME) were used for this study. Mice were housed in polycarbonate micro-isolator cages or on individually ventilated racks under barrier or BSL-2 conditions, with access to food and water ad libitum. Chronic colitis was triggered in 6-week-old Il10−/− mice by exposure to 200 ppm piroxicam in powdered rodent food for 7 days, as described previously (5, 11). Based on the weights of food consumed during this period, mice ingested an average of 40 mg piroxicam/kg/day. Piroxicam was discontinued on day 7 and mice received either fresh or boiled pineapple juice continuously in their drinking water or 5 mg of active bromelain purified from stem or vehicle administered orally once daily for 16 days (short-term study) or for up to 26 weeks (long-term study). Alternatively, chronic colitis was triggered by infecting 6 – 8 wk old Il10−/− mice with Helicobacter typhlonius and H. rodentium as previously described (12) and mice were treated with 5 mg of active bromelain purified from stem or vehicle administered orally once daily for up to 26 weeks. All mice were observed daily for clinical signs of distress and weight was monitored three times per week. Mice were euthanized by CO2 asphyxiation in accordance with the American Veterinary Medical Association Recommendations on Euthanasia (13) if they developed 15% body weight loss, rectal prolapse (a well-recognized complication of chronic inflammation in the colon), or at defined time points (16 days for short-term and 24 – 26 weeks for long-term studies).
A strictly enforced order of cage handling and scrupulous attention to environmental sanitization were used to prevent unintentional infection with environmental helicobacter bacteria. Sentinel mice exposed repetitively to dirty bedding from the mice used in this study were negative for parasites by microscopic exam, negative for Citrobacter rodentium by fecal culture, and negative by serology for a panel of 22 murine protozoal, bacterial, and viral pathogens, including murine parvovirus, murine hepatitis virus, and murine norovirus. All mice were consistently negative for Helicobacter spp. by fecal PCR except when intentionally infected. All animal studies were approved by the Duke University Institutional Animal Care and Use Committee.
Bromelain Administration and Activity
A commercial juicer was used to prepare juice from fresh pineapples purchased from a club grocery store. The juice was centrifuged at 10,000 × g for 15 minutes and then filtered through coffee filters to remove the majority of the pulp. Juice with inactive bromelain enzymes was prepared by boiling fresh filtered juice for 10 minutes. Aliquots of fresh and boiled juice were stored at −20°C until used. Juice was continuously administered to mice in drinking water at dilutions adjusted to maintain similar daily consumption of fresh and boiled juice. To minimize any potential microbial contamination, autoclaved water was used to dilute the juice, which was placed into previously autoclaved bottles and replaced every 2 days. Random sampling of the residual juice showed that proteolytic activity was ~90% of the initial value after 2 days on the cage. Colony counts averaged <105/ml for both boiled and fresh juice (n = 6 each). The bromelain proteolytic activity of each batch of juice was determined by a model substrate assay using the benzoyl (Bz)-Phe-Val-Arg substrate that measures activity of fruit bromelain (14).
Stool from mice that received fresh or boiled juice was extracted with PBS + 1% bovine serum albumin containing 0.1% Kathon (a microbiocide; Supelco, Bellefonte, PA) at 1 ml/100 mg stool and soybean trypsin inhibitor (2 mg/ml) was added to inhibit endogenous trypsin activity prior to measurement of bromelain activity. To assess the effects of anti-bromelain antibodies on the proteolytic activity of bromelain or juice, a defined quantitiy of juice or bromelain was pre-incubated with serum (1:100 final dilution) for 30 minutes prior to addition of substrate.
Bromelains purified from pineapple stem (called “bromelain”) were obtained from Sigma-Aldrich (catalog #B4882; St. Louis, MO). Doses of 5 mg were formulated in 40 µl of 100 mg/ml NaHCO3 to enhance its proteolytic activity within the gastrointestinal tract (15) and administered directly into the mouth in two 20 µl aliquots once daily using pipette tip. Control mice received vehicle (NaHCO3) only.
Tissue and Serum Analysis
After euthanasia, the colon was divided into segments representing the cecum, proximal, middle, distal, and terminal colon/rectum. Segments were fixed in Carnoy’s solution (60% v/v ethanol, 30% CHCl3, 10% glacial acetic acid) for 2 – 4 hours, and then processed into paraffin blocks. Hematoxylin and eosin-stained sections were evaluated blinded to treatment group to determine severity of colon inflammation. Histologic scores were calculated as described (5), using a scale that takes into account hyperplasia, ulceration, degree of inflammation, and % of each of the 5 bowel segments affected by these changes. Using this scale, the maximum score is 75 and a score >12 indicate the presence of colitis. Sections were also scored for non-invasive or invasive neoplasia (16). Gastrointestinal intraepithelial neoplasia (synonymous with atypical hyperplasia, microadenoma, carcinoma in situ) and adenoma were considered to be non-invasive lesions. A diagnosis of invasive carcinoma required the presence of a desmoplastic response to differentiate invasion from mucosal herniation or pseudoinvasion. Regions of neoplasia that were separated by regions of normal mucosa were scored as separate lesions.
Serum anti-bromelain IgG titers were determined using an enzyme immunoassay as described previously (17).
Effects of fresh pineapple juice on cell surface molecules and cytokines
Single cell suspensions of splenocytes from juice-naïve mice were prepared by pressing tissue through a 70µm tissue strainer followed by lysis of red RBC. The cell suspensions were then centrifuged and cell pellet was resuspended in serum-free RPMI1640 media. The surface phenotypes of splenocytes were analyzed using fluorochrome-conjugated mAbs after in vitro treatment with bromelain, fresh or boiled pineapple juice, or sham treatment. Pineapple juice was diluted 1:64 with RPMI plus 10 mM HEPES to maintain neutral pH. Antibodies used were: CD3ε(145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD44 (IM-7), CD45R (B220), CD62L (MEL-14), and F4/80 (BM8), purchased as fluorescent conjugates from BD Pharma (San Jose, CA) or Invitrogen (Carlsbad, CA). Cells were reacted with mAbs for 30 min on ice, then washed with PBS. Cell fluorescence was measured by flow cytometry analyzed with Guava Express Pro software (Guava Technologies, Hayward, CA). Forward vs. side scatter dot plots were used to gate on lymphocytes, monocytes, and granulocytes. The mean fluorescence intensity (MFI) was determined by including all events analyzed in each gated population. Only markers with a MFI for saline-treated cells that were at least fivefold higher than that observed using isotype-matched control IgG mAb were used in the analysis. The percentage of reactivity that remained for each marker after juice treatment was calculated as the MFI after treatment divided by the MFI after saline treatment × 100. Markers were classified as resistant (having > 75% of the reactivity of saline-treated cells after treatment), partially sensitive (having 25–75% of saline reactivity after treatment), or sensitive (having <25% of saline reactivity after treatment).
To assess the effects of exposure to pineapple juice and stem bromelains on cytokine protein stability, purified cytokines were pre-incubated with fresh or boiled pineapple juice (diluted 1:64 with RPMI plus 10 mM HEPES as above) or an equivalent (mg protein) amount of bromelain purified from stem for 30 min. Then the amount of immunoreactive cytokine remaining was quantitated using a Luminex bead-based multiplex fluorescent immunoassay (Invitrogen #LMC0006). The cytokines analyzed were basic FGF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α, TNF, and VEGF.
Statistical analysis
Statistical comparison of histologic scores was performed using Student’s t-test. The chi square test was used to compare the incidence of neoplasia. Survival rates were calculated using Kaplan-Meier test with p-values calculated using the log rank test. A value of p ≤ 0.05 was considered to be significant.
Results
Short-term treatment with fresh pineapple juice decreases colonic inflammation
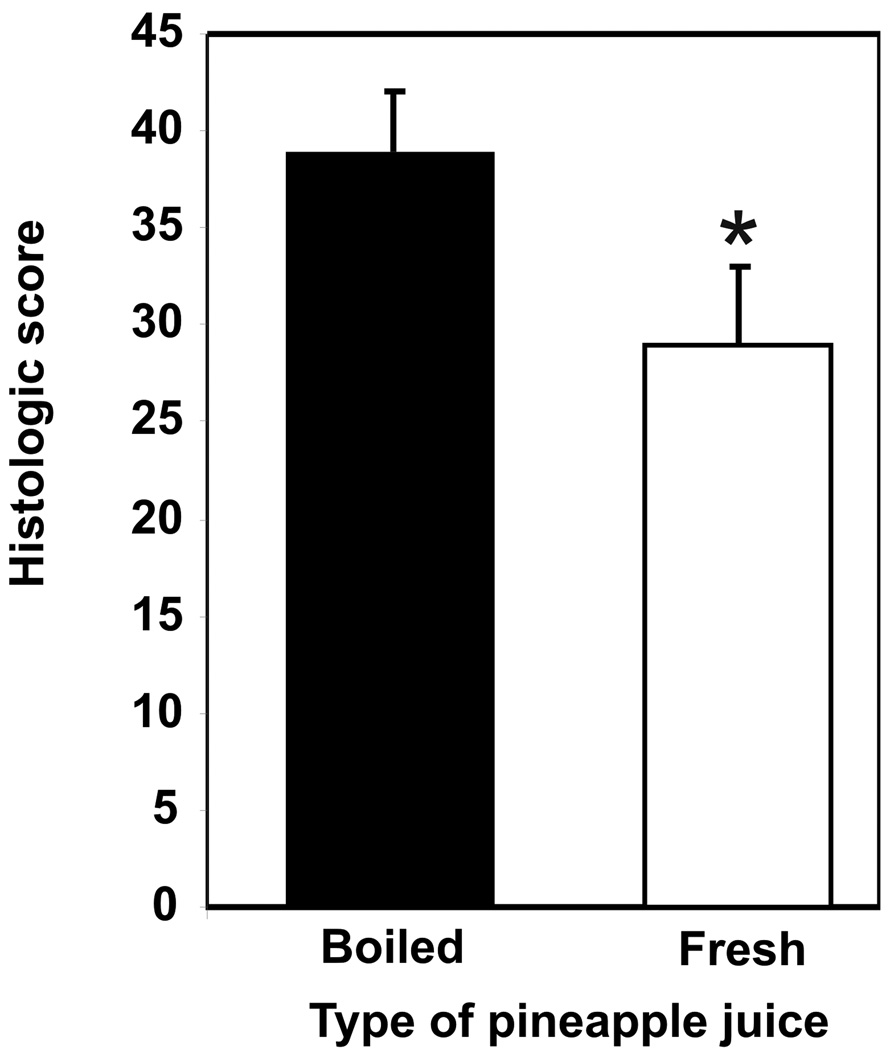
The severity of colon inflammation in Il10−/− mice with colitis triggered by piroxicam was assessed histologically after mice had consumed fresh or boiled pineapple juice in their drinking water for 16 days. Mice consumed 2.1 ml fresh juice per day at a 1:2 dilution with water. This quantity of juice provided proteolytic activity equivalent to 36 mg bromelain purified from stem per day and generated a stool activity equivalent to 14 ± 3 µg bromelain purified from stem per 100 mg stool. Control mice that received boiled pineapple juice with inactive proteolytic enzymes (14) consumed 2.0 ml of boiled juice per day at a dilution of 1:3 (p = 0.46 for differences in juice intake) and no bromelain-specific activity was detected in their stool. Colonic inflammation was significantly decreased in mice that received fresh juice (mean histologic score ± SEM = 29 ± 4; n = 9) compared with mice that received boiled juice (mean histologic score ± SEM = 39 ± 3; n = 9; p = 0.05 ) (Figure 1). However, although significant histologic improvement was seen in mice supplemented with fresh pineapple juice, this treatment was not able to completely diminish colonic inflammation (to a histologic score < 12) over the 16 day treatment period. Mice treated with boiled juice had moderate to severe colon inflammation that was similar to that typically observed in untreated historical controls.
Figure 1. Short-term (16 day) supplementation with fresh pineapple juice decreases severity of colitis in mice.
The mean histologic score ± standard error of the mean (SEM) is shown for groups of 9 mice (* indicates p = 0.05).
Effects of long-term treatment with pineapple juice on chronic colonic inflammation
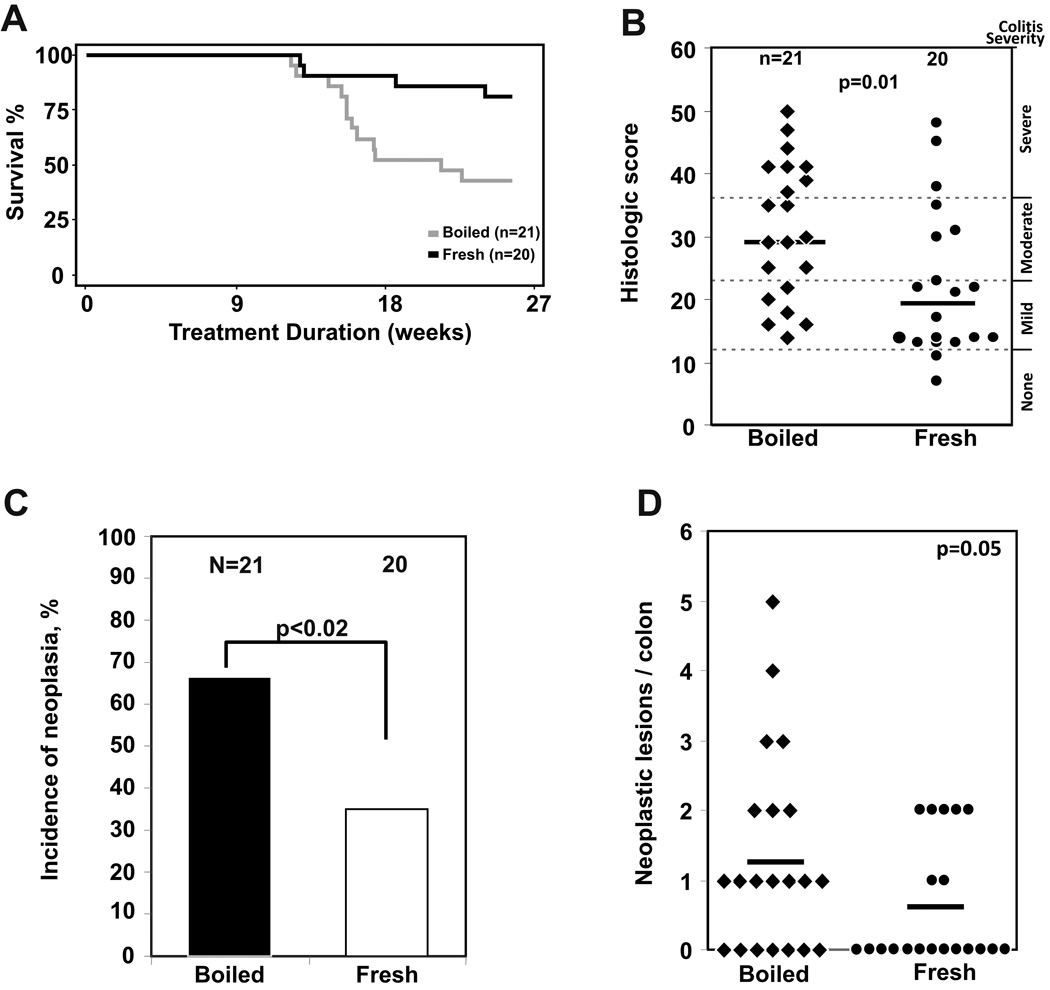
To determine whether bromelains from pineapple juice could sustain their anti-inflammatory effects over a longer treatment period, Il10−/− mice with chronic colitis triggered by exposure to piroxicam were treated with fresh or boiled pineapple juice for up to 26 weeks. Consumption of fresh juice generated a mean stool activity equivalent to 14 ± 2 µg bromelain purified from stem per 100 mg stool. Mortality prior to the endpoint of this study was exclusively due to the development of rectal prolapse that required euthanasia for humane reasons. Mice that received fresh pineapple juice had a significantly greater survival to the 6 month planned study end point (83%, n = 20) compared with mice that received boiled juice (43%, n = 21; p = 0.01) (Figure 2A). Mice that received fresh juice also had significantly decreased histologic colon inflammation scores, with a median histologic score of 19 compared with a median score of 30 for mice that received boiled juice (p= 0.01) (Figure 2B). Inflammation-associated colonic neoplasia was observed in 66% of 21 mice that received boiled juice compared with 35% of 20 mice that received fresh juice (p< 0.02) (Figure 2C), with reduced numbers of neoplastic lesions/colon also observed in mice given fresh juice (p = 0.05; Figure 2D).
Figure 2. Long-term dietary supplementation with fresh pineapple juice decreases mortality, colon inflammation, and inflammation-associated neoplasia in mice with colitis.
A. Mortality in this study was due to rectal prolapse, an indicator of severe colitis in mice. 83% of mice given fresh juice survived to the 6 month study endpoint compared with 43% in mice given boiled juice with inactive enzymes (p = 0.01). B. Dietary supplementation with fresh pineapple juice for up to 26 wks also decreases colonic inflammation, with a median histologic score of 30 for mice receiving boiled juice, compared with 19 for mice receiving fresh juice (p = 0.01). C, D. Dietary supplementation with fresh pineapple juice decreased the incidence (C; p < 0.02) and multiplicity (D; p = 0.05) of inflammation-associated neoplastic lesions in the colon of Il10−/− mice with chronic colitis.
Effects of long-term treatment with bromelain purified from pineapple stem on chronic colonic inflammation
In contrast to their enthusiastic consumption of pineapple juice, mice refused to voluntarily consume food or water containing comparable amounts of bromelain derived from stem. Efforts to disguise its taste by compounding with sugar, chocolate, or peanut butter were unsuccessful. Micro-encapsulation with several different coating types (Gelucire 43/01, shellac, Eudragit S100, or Ethocel Std 10) was able to mask the taste of stem-derived bromelain, but the amount of non-nutritive polymer required resulted in weight loss independent of bromelain content. Therefore, these long-term studies were performed using the 5 mg/day bromelain dose previously demonstrated to have anti-inflammatory efficacy in short-term studies (5). Il10−/− mice with chronic colitis triggered by exposure to piroxicam were treated orally with bromelain derived from stem or vehicle once daily for up to 6 months. There was no difference in survival between bromelain- and vehicle-treated mice (p = 0.20). Mice treated with bromelain had moderate colitis with histologic scores of 31 ± 4 (n = 15), similar to those treated with vehicle alone (mean histologic score ± SEM = 30 ± 3; n = 17) (p = 0.7). Fifty three percent of bromelain-treated mice developed colonic neoplasia with a range of 0 – 5 neoplastic lesions/mouse. This was similar to what was observed in vehicle-treated mice, where 65% of mice developed colonic neoplasia (range of lesions 0 – 3). Thus, despite the efficacy of bromelain derived from pineapple stem in reducing inflammation when given short-term (5), this benefit was not sustained over 6 months of treatment given as a single dose of 5 mg bromelain/day.
The effect of daily oral treatment with 5 mg bromelain derived from stem was also determined in Il10−/− mice with chronic colitis triggered by helicobacter infection. Eighty eight percent (15 out of 17) of bromelain-treated and 94% (17 out of 18) of vehicle-treated mice developed rectal prolapse that required euthanasia prior to the scheduled 6 month end point. The mean survival for the mice treated with bromelain was 16.8 ± 1.7 wks, which was not significantly different from the survival of vehicle-treated controls, 20.1 ± 1.5 wks (p = 0.4). Histologic scoring showed that bromelain-treated mice had severe inflammation (mean ± SEM histologic score = 44 ± 1; n = 18) which was slightly greater than that seen with mice treated with vehicle alone (histologic score, mean ± SEM = 40 ± 2; n = 17) (p = 0.03). The increased severity of colitis in Il10−/− mice triggered by Helicobacter infection versus piroxicam is consistent with our previous studies using these models of inflammatory bowel disease (12, 18).
The total incidence of invasive + non-invasive colonic neoplasia in mice infected with H. typhlonius and H. rodentium and treated with bromelain was 94% (mean lesions ± SEM = 2 ± 1; range 0 – 5). Sixty seven percent of these mice had at least one invasive adenocarcinoma. This did not differ from mice that received vehicle, who had an 82% incidence of neoplasia, with a mean ± SEM = 2 ± 1 lesions (range = 0 – 5). The number of invasive neoplastic lesions (adenocarcinomas) was also similar in mice that received bromelain or vehicle alone (67% vs. 65%, respectively). Thus long term treatment with 5 mg bromelain derived from stem did not decrease inflammation nor did it affect the development of inflammation-associated colonic neoplasia in this model of colitis triggered by helicobacter infection.
Exposure to pineapple juice removes a subset of cell surface molecules on splenocytes
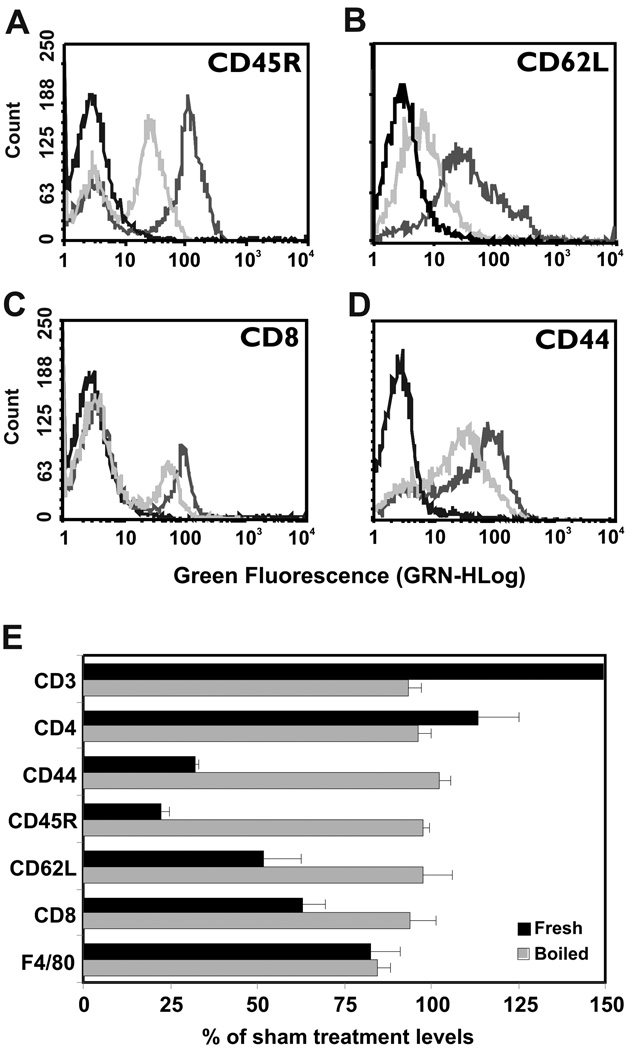
Our laboratory previously reported that in vitro treatment of human peripheral blood mononuclear cells with bromelain purified from stem removed at least 8 cell surface molecules involved in lymphocyte adhesion and activation (4). In the present study, we treated murine splenocytes with fresh or boiled pineapple juice and then analyzed these cells for reactivity with mAbs specific for 7 cell surface markers. All antibodies demonstrated reactivity with splenocytes exposed to boiled pineapple juice that was indistinguishable from that of sham-treated cells. As was previously observed for bromelain purified from stem (3, 14), exposure to fresh pineapple juice had differential effects on the cell surface antigens recognized by these antibodies. The CD45R marker was juice-sensitive, with a mean fluorescence intensity (MFI) after juice treatment of 23% that of sham-treated cells (Figure 5A, E). CD62L (Figure 5B), CD8α (Figure 5C), and CD44 (Figure 5D) were partially juice-sensitive, with MFI decreases to 32, 52, and 63% of sham-treated controls, respectively (Figure 5E). In contrast and similar to what was previously observed with bromelain purified from stem, reactivity with CD3, CD4, and F4/80 antibodies was juice-resistant, with MFI values similar to or greater than saline-treated controls (Figure 5E). The increased reactivity of CD3 and CD4 antibodies with cells treated with fresh vs. to boiled juice suggests potential proteolytic unmasking of antigen by the fresh juice that contains active enzymes.
Figure 5. Flow cytometric analysis of splenocytes treated in vitro with pineapple juice.
A – D. Fresh pineapple juice that contains active bromelain enzymes (medium gray lines), but not boiled juice with inactive enzymes (light gray lines) specifically removes some cell surface molecules associated with leukocyte adhesion and activation (A=CD45R; B=CD62L; C= CD8; D= CD44). The reactivity of sham-treated cells was virtually super-imposable with that for boiled juice and is omitted for clarity. The black line indicates reactivity with isotype-matched control antibody. Juice was diluted 1:64 for this study. E. A summary of effects of fresh juice on cell surface molecules presents the MFI ± SEM for 3 independent experiments. Reactivity with CD45R antibody was highly sensitive to removal by fresh juice, while reactivity with CD44, CD62L, and CD8 antibodies was partially sensitive. Reactivity with CD3, CD4, and F4/80 antibodies was resistant to removal by juice.
Exposure to pineapple juice decreases stability of a subset of pro-inflammatory chemokines
We previously showed that in vitro treatment of human colon tissues with bromelain purified from stem decreased secretion of cytokine proteins (7). Increased proteolytic degradation of secreted cytokines is another mechanism by which consumption of pineapple juice containing active bromelain enzymes could potentially decrease inflammation in the colon. To model this situation, we assessed the effects of fresh or boiled pineapple juice on the stability of a panel of 20 purified cytokines and chemokines in vitro. Results showed that only 3 of the chemokines and chemokines tested were sensitive to proteolysis by fresh pineapple juice: IP-10 (71±1% of control; p=0.0002), KC (66±2% of control; p = 0.0007), and MCP-1 (83±1% of control; p =0.03). IP-10 and KC were also sensitive to proteolysis by bromelain purified from stem (IP-10 = 35±1% of control; KC= 41±1% of control). Neither fresh juice nor bromelain purified from stem affected the stability of basic FGF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2,I L-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, MIG, MIP-1α, TNF, or VEGF under the same conditions used to determine effects of juice on cell surface molecules.
Anti-bromelain responses in juice-supplemented vs. bromelain-treated mice
We previously showed that mice given multiple oral boluses of bromelain purified from stem developed strong anti-bromelain IgG antibody responses that could quantitatively immunoprecipitate bromelain but did not affect bromelain activity (15). Development of these anti-bromelain responses following oral exposure required proteolytically active bromelain (17). Serum samples from mice in the studies reported here were analyzed to determine whether similar anti-bromelain antibody responses occurred when mice were continuously exposed orally to pineapple juice-derived bromelains. Il10−/− mice with piroxicam-triggered colitis failed to develop anti-bromelain IgG responses when supplemented with boiled juice (all titers were <1:256, the lowest dilution tested; n = 21). In contrast, all mice supplemented with fresh juice for up to 6 months developed strong anti-bromelain responses (geometric mean titer = 1:72,214; range from 1:32,768 to 1:262,144; n = 22). These anti-bromelain responses developed rapidly, since mice in the short-term juice study developed geometric mean anti-bromelain titers of 1:8841 (range = 1:1024 to 1:32,768; n = 9) after just 16 days of supplementation with fresh juice. The anti-bromelain titers elicited by long-term exposure of Il10−/− mice with piroxicam-triggered colitis to fresh pineapple juice were slightly less than those elicited by long-term daily oral bolus administration of bromelain purified from stem, where the geometric mean titer was 1: 136,875 (range = 1:32,768 to 1:524,288; n = 15; p = 0.04). However, it is important to note that although the anti-bromelain IgG responses mounted by both groups of mice were strong, they did not prevent anti-inflammatory effects in mice supplemented with fresh juice. Furthermore, pre-incubation of bromelain with serum from bromelain-treated mice (1:100 final dilution) did not alter its proteolytic activity in our model substrate assay compared with bromelain pre-incubated with similar concentrations of serum from vehicle-treated mice (proteolytic activities of 72 ± 3 vs 69 ± 5 µg/ml, respectively; p = 0.14) Likewise, serum from mice supplemented with fresh juice did not alter the proteolytic activity of juice compared to serum from mice supplemented with boiled juice (proteolytic activities of 40 ± 2 vs 39 ± 1 µg/ml, respectively; p = 0.25)
Discussion
This study found that short- and long-term dietary supplementation with fresh frozen pineapple juice was both safe and effective for the treatment of chronic colitis in Il10−/− mice. Mice that received fresh, enzymatically active juice had decreased colitis-associated mortality, decreased colon inflammation, and decreased inflammation-associated neoplasia compared with mice that received boiled, proteolytically inactive juice. Taste preferences of the mice prevented an analogous dietary supplementation with bromelain purified from stem. Oral administration of bromelain purified from stem once daily was found to have no long-term effect on inflammation and inflammation-associated neoplasia in this model. Variables that may explain the observed differential effects of fresh pineapple juice vs. bromelain purified from stem include the manner of administration, the total dose achieved, and/or differences in enzyme composition or activity against critical cell surface molecules.
Our studies showed that fresh pineapple juice has anti-inflammatory activity while boiled juice does not. This demonstrates definitively that the anti-inflammatory components of juice are heat-sensitive, since no components are added or subtracted from these samples. By using a variety of methods of chemical inactivation, we and others previously showed that the anti-inflammatory activity of bromelain required proteolytically active bromelain enzymes (3, 5, 7, 19–21). In separate studies, we showed the proteolytic activities of both pineapple juice and purified bromelain were also abrogated by heating (14). For these reasons, we feel that our results strongly implicate proteolytic effects of the bromelain enzymes present in the fresh juice as the mechanism for the anti-inflammatory activity that we observed. It is important to note, however, that our study design cannot rule out the possibility that the beneficial anti-inflammatory effects are due to a heat-sensitive non-proteolytic component of pineapple juice.
We have previously shown that the amount of any given cell surface molecule that is removed by bromelain enzymes is a function of both the enzyme concentration and the length of exposure (4). The time required for re-expression of cell surface molecules following proteolysis varies according to the cell surface molecule and the cell on which it is expressed, ranging from <30 minutes in the case of CD62L on murine peripheral blood leukocytes (8) to >48 hours for CD44 or CD45RA on human lymphocytes (3). The proteolytic spectrum of fruit bromelain, the major enzyme present in pineapple fruit, toward cell surface molecules is different than that of stem bromelain, the major enzyme present in bromelain purified from stem (14). However, we do not currently know which molecule(s) must be removed to achieve a long-term decrease in inflammation. Indeed, the full spectrum of cell surface molecules that are sensitive to proteolysis by either of these bromelain enzymes remains to be determined. This is further complicated by our lack of knowledge of whether the critical proteolytic effects are on leukocytes, epithelial cells, colonic bacteria, or other cell types that are present within the colonic microenvironment. Therefore, the question of whether fruit bromelain or stem bromelain proteolytic activity or some other heat-sensitive component of juice is most critical for anti-inflammatory efficacy remains unresolved. The enthusiastic consumption of pineapple juice by the mice allowed achievement of a higher daily dose of fruit bromelain (equivalent to the fruit bromelain content in 36 mg of bromelain derived from stem) than could be achieved by a single daily administration of bromelain derived from stem. It is possible that these differences in dose account for the lack of long-term effects associated with treatment with bromelain derived from stem as compared with fresh juice. The more frequent exposure to proteolytic enzymes allowed by dietary ad libitum consumption in drinking water may have further enhanced the efficacy of juice compared with bromelain administered once daily. Mice that received fresh juice developed anti-bromelain IgG responses that were substantial and were only slightly lower than those developed by mice treated with bromelain purified from stem. Our studies showed that these antibodies did not affect the proteolytic activity of bromelain. Thus, differential immune responses against bromelain in mice treated with fresh juice vs. bromelain purified from stem probably contribute minimally to the differences in efficacy observed between these 2 groups.
The lack of long-term efficacy of bromelain purified from stem was somewhat surprising, given our prior findings that long term oral bromelain treatment decreased the incidence of spontaneous colitis in Il10−/− mice and that short term (16 day) treatment with oral bromelain decreased the severity of piroxicam-triggered colitis in this model (5). Resistance to the proteolytic effects of bromelain via mutation is not expected. The data do suggest that mechanisms by which long-term bromelain treatment may prevent onset of spontaneous colitis differ from its effects on established colitis. The differences between the short-term (16 day) efficacy of oral bromelain on severity of piroxicam-triggered colitis and its lack of efficacy in the model when given long-term (6 months) is more difficult to reconcile. It is possible, however, that continuous or episodic absence of bromelain-sensitive molecules on leukocytes may affect gene expression patterns in a manner that favors ongoing inflammation. It is also possible that the pathways that maintain chronic inflammation long-term differ from those that predominate early in the course of colitis. Daily bromelain treatment also lacked long-term efficacy in the helicobacter-triggered colitis model, where helicobacter organisms remain present and provide a continuous stimulus for inflammation. Further studies of interim time points will be needed to begin to address these mechanisms. However, our results do highlight the importance of carrying out long-term studies of efficacy prior to adopting long-term use of anti-inflammatory treatments previously proven to be efficacious only in short-term studies.
It is important to note that although consumption of fresh juice increased survival of these mice with colitis (Figure 2A), decreased their median severity of colon inflammation (Figure 2B), and also decreased the incidence of inflammation-associated neoplasia Figure 2C, D), a few mice treated with fresh juice continued to exhibit severe inflammation. The neoplasias observed in the fresh juice group were limited to those mice with histologic scores higher than the median. This suggests that fresh juice primarily inhibits the development of inflammation-associated colon cancers by decreasing inflammation; however our studies cannot rule out an additional effect of fresh juice on tumor growth.
We previously showed that in vitro bromelain treatment of human colonic tissue resulted in decreased secretion of pro-inflammatory cytokines (7). The results presented here additionally show that exposure to juice or bromelain purified from stem can increase the degradation of some chemokines that may be secreted into the lumen. This provides an additional mechanism by which consumption of pineapple juice may decrease inflammatory activity in vivo.
In addition to determining the efficacy of fresh pineapple juice treatment on chronic colitis, we also demonstrated that, similar to bromelain purified from stem, bromelains present in fresh pineapple juice can also remove cell surface molecules known to affect leukocyte migration and function. CD44, CD45R, CD62L, and CD8 were found to be at least partially sensitive to removal by exposure to fresh juice (Figure 5). Note that the bromelain concentration in the diluted juice used for these assays (340 µg/ml) is less than the 1 mg/ml used in prior reports using bromelain purified from stem (4). Differences in juice bromelain concentration, amino acid-based differences in the sensitivity of murine cell surface molecules to bromelain proteolysis, and/or differences in the epitopes of the specific antibody clones used for this study can explain quantitative differences in bromelain sensitivity of between the current and previously published studies (4).
The bromelain-sensitive and partially-sensitive molecules identified in this study are important in leukocyte adhesion, migration, and activation. CD8α is a co-activation molecule for MHC class I-restricted cellular immune responses and also regulates the activation threshold for intestinal intraepithelial lymphocytes (22, 23). CD45R is a tyrosine phosphatase that regulates T cell activation threshold (23). CD44 and CD62L were shown to be homing molecules for leukocytes (25, 26). CD44 has also been shown to affect lymphocyte adhesion (3, 27) and activation (3, 28). Thus full or partial removal of these molecules by bromelains in juice could affect leukocyte trafficking and activation.
In summary, this study shows that long-term dietary supplementation with fresh frozen pineapple juice containing proteolytically active bromelain enzymes does not negatively affect the health or body weight of mice with colitis. Consumption of fresh juice decreases inflammation severity and the incidence and multiplicity of inflammation-associated colonic neoplasia in the commonly used Il10−/− murine model of inflammatory bowel disease. In contrast, long-term once-daily treatment with bromelain purified from pineapple stem was not effective in decreasing inflammation or neoplasia in this model. Additional studies will be required to determine how the differential effects of fresh pineapple juice vs. bromelain purified from stem are related to the manner of administration, the total dose achieved, or to the differences in enzyme activity between their formulations, since the factors that limited bromelain dosing in these murine studies can be mitigated in humans through use of enteric coated pills taken multiple times daily. Studies to better understand the mechanisms by which bromelain affects colon inflammation and inflammation-associated neoplasia, including identification of the full range of bromelain-sensitive molecules and the cell signaling pathways affected, will aid translation of these findings to effective IBD therapies. In the interim however, the safety and efficacy of fresh pineapple juice in this commonly used murine model of inflammatory bowel disease provides a strong rationale for trials of dietary supplementation with fresh pineapple in humans with inflammatory bowel disease.
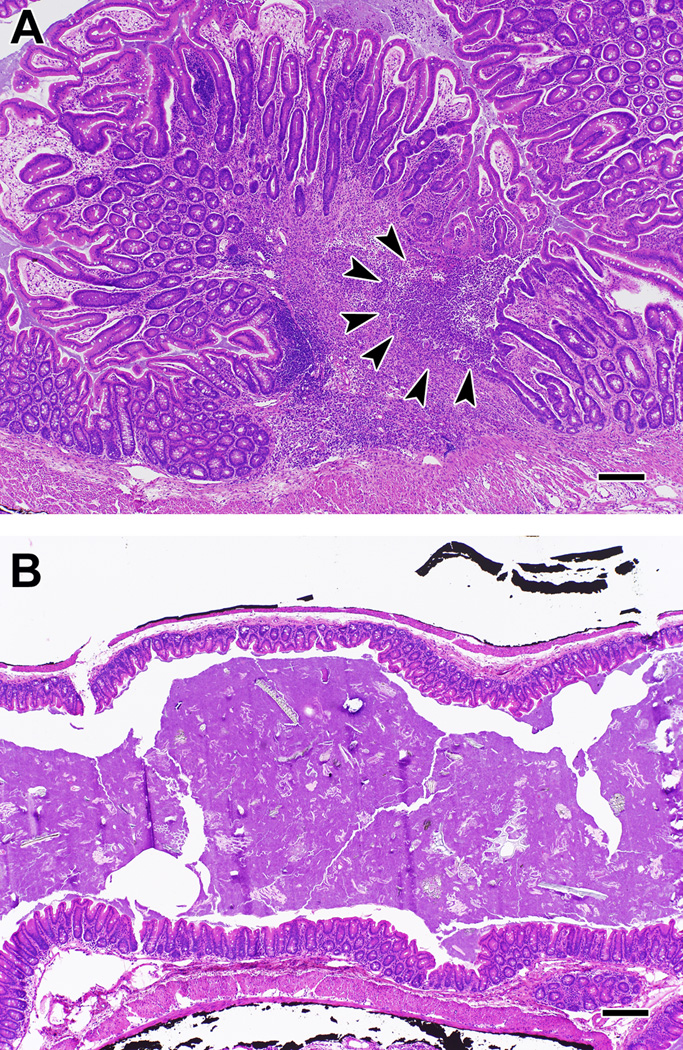
Figure 3. Inflammation in Il10−/− mice with colitis that were supplemented with either fresh or boiled pineapple juice for up to 6 months.
Severe mucosal hyperplasia with architectural distortion and ulceration were commonly observed in the cecum (shown) and colon of mice fed boiled juice (A). Arrows point out the loss of mucosal epithelium and underlying inflammation and granulation tissue characteristic of an ulcer. Severe mucosal hyperplasia is demonstrated by the marked increase in mucosal thickness in (A) compared with mice fed fresh juice (B) who typically had only mild mucosal hyperplasia and inflammation. Panels were taken at the same magnification to emphasize the difference in mucosal hyperplasia between the 2 groups. Bar = 50 µm.
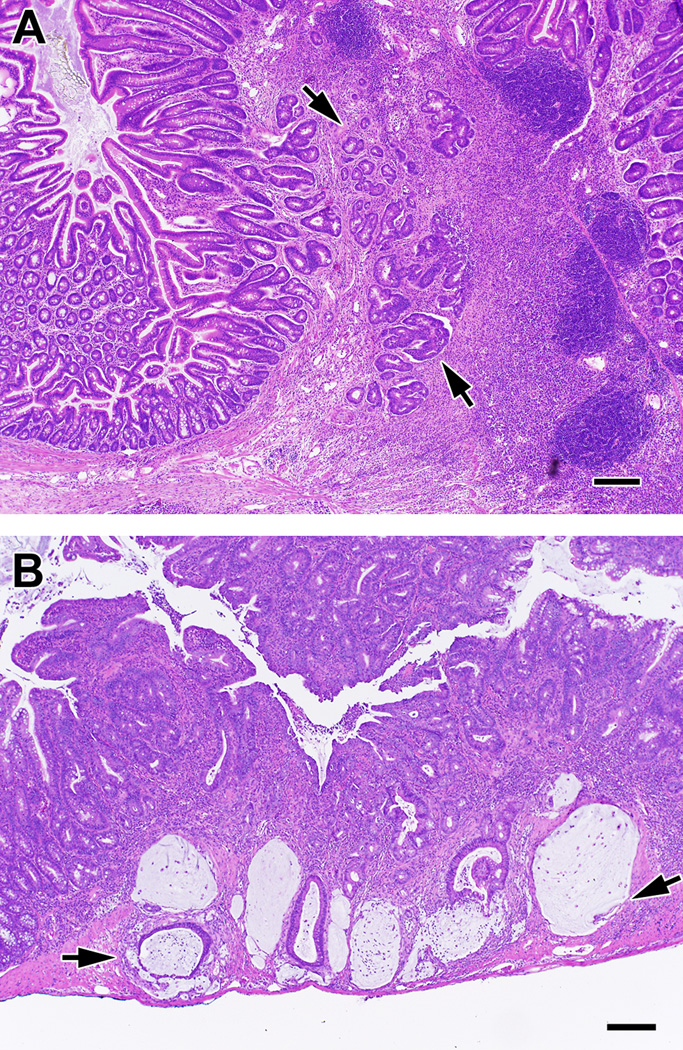
Figure 4. Neoplasia in Il10−/− mice with colitis that were supplemented with boiled pineapple juice.
Shown are examples of neoplasia in the cecum (A) and proximal colon (B) of mice fed boiled juice for up to 6 months. Arrows indicate areas of invasive adenocarcinoma. Bar = 50 µm.
Acknowledgments
Supported by the American Institute for Cancer Research #06A057 and by the National Institutes of Health 1R01-CA115480.
References
- 1.Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem. J. 1990;266:869–875. [PMC free article] [PubMed] [Google Scholar]
- 2.Hale LP, Singer KH, Haynes BF. CD44 antibody against In(Lu)-related p80, lymphocyte homing receptor molecule, inhibits the binding of human erythrocytes to T cells. J. Immunol. 1989;143:3944–3948. [PubMed] [Google Scholar]
- 3.Hale LP, Haynes BF. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J Immunol. 1992;149:3809–3816. [PubMed] [Google Scholar]
- 4.Hale LP, Greer PK, Sempowski GD. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin Immunol. 2002;104:183–190. doi: 10.1006/clim.2002.5254. [DOI] [PubMed] [Google Scholar]
- 5.Hale LP, Greer PK, Trinh CT, Gottfried MR. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin Immunol. 2005;116:135–142. doi: 10.1016/j.clim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Kane S, Goldberg MJ. Use of bromelain for mild ulcerative colitis. Ann Int Med. 2000;132:680. doi: 10.7326/0003-4819-132-8-200004180-00026. [DOI] [PubMed] [Google Scholar]
- 7.Onken JE, Greer PK, Calingaert B, Hale LP. Bromelain treatment decreases secretion of pro-inflammatory cytokines and chemokines by colon biopsies in vitro. Clin Immunol. 2008;126:345–352. doi: 10.1016/j.clim.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzhugh DJ, Shan S, Dewhirst MW, Hale LP. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin Immunol. 2008;128:66–74. doi: 10.1016/j.clim.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. San Diego: Academic Press; 1998. pp. 566–567. [Google Scholar]
- 10.Yamada F, Takahashi N, Murachi T. Purification and characterization of a proteinase from pineapple fruit, fruit bromelain FA2. J Biochem. 1976;79:1223–1234. doi: 10.1093/oxfordjournals.jbchem.a131176. [DOI] [PubMed] [Google Scholar]
- 11.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 12.Chichlowski M, Sharp JM, Vanderford DA, Myles MH, Hale LP. Helicobacter typhlonius and H. rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL-10-deficient mice. Comp Med. 2008;58:534–541. [PMC free article] [PubMed] [Google Scholar]
- 13.American Veterinary Medical Association. [Accessed May 6, 2009];AVMA guidelines on euthanasia: Jun 2007 update. Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf.
- 14.Hale LP, Greer PK, Trinh CT, James CL. Proteinase activity and stability of natural bromelain preparations. Intl Immunopharmacol. 2005;5:783–793. doi: 10.1016/j.intimp.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Hale LP. Proteolytic activity and immunogenicity of oral bromelain within the gastrointestinal tract of mice. Intl. Immunopharmacol. 2004;4:255–264. doi: 10.1016/j.intimp.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterol. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 17.Hale LP, Fitzhugh DJ, Staats HF. Oral immunogenicity of the plant proteinase bromelain. Intl. Immunopharmacol. 2006;6:2038–2046. doi: 10.1016/j.intimp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Hale LP, Perrera D, Gottfried MR, Maggio-Price L, Srinivasan S, Marchuk DA. Neonatal infection with Helicobacter species markedly accelerates the development of inflammation-associated colonic neoplasia in IL-10−/− mice. Helicobacter. 2007;12:598–604. doi: 10.1111/j.1523-5378.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 19.Mynott TL, Ladhams A, Scarmato P, Engwerda CR. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J Immunol. 1999;163:2568–2575. [PubMed] [Google Scholar]
- 20.Mynott TL, Crossett B, Prathalingam SR. Proteolytic inhibition of Salmonella enterica Serovar Typhimurium -induced activation of the MAP kinases ERK and JNK in cultured human intestinal cells. Infect. Immun. 2002;70:86–95. doi: 10.1128/IAI.70.1.86-95.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleef R, Delohery TM, Bovbjerg DH. Selective modulation of cell adhesion molecules lymphocytes by bromelain protease 5. Pathobiol. 1996;64:339–346. doi: 10.1159/000164070. [DOI] [PubMed] [Google Scholar]
- 22.Konig R. Interactions between MHC molecules and co-receptors of the TCR. Curr Opin Immunol. 2002;14:75–83. doi: 10.1016/s0952-7915(01)00300-4. [DOI] [PubMed] [Google Scholar]
- 23.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang H-C, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interactions between CD8αα and the nonclassical MHC molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 24.Alexander DR. The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Semin Immunol. 2000;12:349–359. doi: 10.1006/smim.2000.0218. [DOI] [PubMed] [Google Scholar]
- 25.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
- 26.Steeber D, Tedder T. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2000;22:299–317. doi: 10.1385/IR:22:2-3:299. [DOI] [PubMed] [Google Scholar]
- 27.Munzig E, Eckert K, Harrach T, Graf H, Maurer HR. Bromelain protease F9 reduces the CD44 mediated adhesion of human peripheral blood lymphocytes to human umbilical vein endothelial cells. FEBS Letters. 1994;351:215–218. doi: 10.1016/0014-5793(94)00860-4. [DOI] [PubMed] [Google Scholar]
- 28.Denning SM, Le PT, Singer KH, Haynes BF. Antibodies against the CD44 p80, lymphocyte homing receptor molecule augment human peripheral blood T cell activation. J Immunol. 1990;144:7–15. [PubMed] [Google Scholar]