Abstract
Previous research in our laboratory has demonstrated robust circadian variations of cytokines and cytolytic factors in enriched NK cells from rat spleen, strongly suggesting these functions may be subject to circadian regulation. The SCN mediates timing information to peripheral tissues by both humoral and neural inputs. In particular, noradrenergic (NE) sympathetic nervous system (SNS) terminals innervate the spleen tissue communicating information between central and peripheral systems. However, whether these immune factors are subject to timing information conveyed through neural NE innervation to the spleen remained unknown. Indeed, we were able to characterize a circadian rhythm of NE content in the spleen, supporting the role of the SNS as a conveyor of timing information to splenocytes. By chemically producing a local splenic sympathectomy through guanethidine treatment, the splenic NE rhythm was abolished or shifted as indicated by a blunting of the expected peak at ZT7. Consequently, the daily variations of cytokine, TNF-α, and cytolytic factors, granzyme-B and perforin, in NK cells and splenocytes were altered. Only time-dependent mRNA expression of IFN-γ was altered in splenocytes, but not protein levels in NK cells, suggesting non-neural entrainment cues may be necessary to regulate specific immune factors. In addition, the rhythms of clock genes and proteins, Bmal1 and Per2, in these tissues also displayed significantly altered daily variations. Collectively, these results demonstrate rhythmic NE input to the spleen acts as an entrainment cue to modulate the molecular clock in NK cells and other spleen cells possibly playing a role in regulating the cytokine and cytolytic function of these cells.
Keywords: sympathectomy, guanethidine, spleen, interferon-γ, tumor necrosis factor-α, granzyme-B, perforin
INTRODUCTION
There is accumulating evidence linking the immune system with circadian regulation. For instance, several light input pathways, including the suprachiasmatic nucleus (SCN), may modulate the immune system (Chacon et al., 2002; Roberts, 2000). Further, several studies have described circadian variations of different immune parameters such as lymphocyte proliferation, antigen presentation, and cytokine gene expression (Demas et al., 2003; Levi et al., 1991; Maestroni and Conti, 1996). Importantly, the activity of natural-killer (NK) cells also varies depending on time of day (Arjona et al., 2004; Esquifino et al., 1996; Fernandes et al., 1979), and is associated with the circadian variation of tumor clearance (Shakhar et al., 2001). Previously, we have characterized the rhythmic expression of putative clock genes and proteins in splenocytes (Arjona et al., 2004), and NK cells (Arjona and Sarkar, 2005), isolated from the rat spleen. Cytolytic factors granzyme-B and perforin, in addition to cytokines TNF-α and IFN-γ, are expressed rhythmically in these cells (Arjona et al., 2004; Arjona and Sarkar, 2005). Importantly, the clock gene Per2 may drive the rhythm of IFN-γ in the spleen (Arjona and Sarkar, 2006). However, the influence of the SCN in the control of canonical clock gene expression and cellular function of the spleen, particularly in NK cells, is not known.
Circadian rhythms generated by the central biological clock of the SCN govern many aspects of physiology and behavior, including locomotor activity, sleep-wake cycles, autonomic and endocrine function. The SCN is entrained by an array of internal and external cues, including light input through the retinohypothalamic tract (RHT), interacting across several feedback pathways that allows for coordination and synchronization among many physiological systems. At the cellular level, individual SCN neurons generate spontaneous firing patterns oscillating near 24-hr rhythms (Herzog et al., 1998; Honma et al., 1998; Welsh et al., 1995), which may become synchronized by synaptic and/or non-synaptic mechanisms (e.g., Drouyer et al., 2010; Dudek et al., 1993; Kent and Meredith, 2008; Prosser et al., 1994; Welsh et al., 1995). This coordinated potentially rhythmic SCN output is transmitted to peripheral tissues by direct and indirect neural and endocrine pathways (Albrecht and Oster, 2001; Silver et al., 1996) to regulate physiological responses of peripheral systems, including metabolic and immune systems. Furthermore, the canonical clock genes, including Per1, Per2, Per3, Cry1, Cry2, Clock, and Bmal1, acting in series of positive and negative interlocking transcriptional-translational feedback loops in SCN cells, are also rhythmically expressed in cells from peripheral tissues, including the spleen (Arjona et al., 2004; Arjona and Sarkar, 2005). Thus, the SCN may synchronize and coordinate appropriate tissue-specific physiological and metabolic responses (Hastings et al., 2003; Yoo et al., 2004) by humoral and neural inputs.
The primary neural pathways carrying timing information from the SCN to the periphery are the sympathetic (SNS) and parasympathetic nervous systems. From both neuroanatomical and neurochemical studies, the sole neural input to the spleen is of sympathetic origin (Bellinger et al., 1993; Cano et al., 2001; Nance and Burns, 1989), originating from preautonomic neurons in the hypothalamus (Katafuchi et al., 1993), and upon stimulation of noradrenergic fibers, releases norepinephrine (NE) that targets immune cells, including NK cells, lymphocytes, and macrophages (Dokur et al., 2004; Elenkov et al., 2000). In general, NE release from sympathetic nerve terminals acts on β-adrenergic receptors increasing cAMP accumulation, in turn activating PKA I pathway (Peng et al., 2004; Torgersen et al., 1997), and ultimately suppressing NK cell cytolytic activity and mRNA expression of several cytolytic factors, such as granzyme-B, perforin, and interferon-gamma (IFN-γ) (Dokur et al., 2004). Together, this evidence points toward an integrative axis from the SCN through the SNS to the spleen that is regulated by the catecholamine NE.
The role of NE in regulating clock genes and coordinating daily rhythm has been demonstrated in pineal gland and in liver. In mouse liver, acute administration of adrenaline or NE increased mPer1 but not mPer2 expression in vivo and in hepatic slices in vitro (Terazono et al., 2003). Furthermore, under a light-dark cycle, destruction of the SCN flattened the daily rhythms of not only mPer1, mPer2, and mBmal1 genes but also NE content in the liver, whereas, daily injection of catecholamine for 6 days, recovered oscillations of mPer2 and mBmal1 gene expression in the liver of mice with SCN lesion (Terazono et al., 2003). In rat pineal, the expression of Per1 and Cry2 was regulated by the clock-driven changes in NE, in a similar manner to the melatonin rhythm-generating enzyme arylalkylamine N-acetyltransferase. However, the expression of Per3 and Cry1, other clock regulating genes, displayed a daily rhythm not regulated by NE (Simonneaux et al., 2004). These results suggest the possibility that the sympathetic nerves through NE release might be involved in controlling specific clock regulating genes and thereby governing circadian rhythms at the cellular and tissue levels.
In the present study, our focus was to determine if rhythmic expression of molecular clock and immune factors in the spleen were regulated by noradrenergic SNS innervation. We measured gene and protein expression of cytolytics, granzyme-B and perforin, and cytokines, TNF-α, IFN-γ, in intact and chemically sympathectomized by guanethidine rat spleens. These cytolytic factors and cytokines control NK cell functions including the cytolytic activity (Trinchieri, 1989). Also, a circadian rhythm of NE splenic content was characterized. We hypothesized sympathetic denervation would disrupt the time-dependent expression of clock genes as well as cytolytics and cytokines in the rat spleen.
MATERIALS AND METHODS
Animals and Procedures
Male Sprague-Dawley rats (6–8 weeks) were maintained under a 12:12 light-dark (LD) cycle with lights on at 7:00 hr and lights off at 19:00 hr and housed under standard animal facility conditions for the duration of the experiment. All animals were given free access to food and water. Control and experimental rats (total n=46; n=5 per time point for splenic NE characterization (n=30); n=4 per time point per group (n=16) for chemical sympathectomy), were subjected to similar surgical procedures. The experimental rats underwent local sympathectomy by intra-splenic injection of guanethidine, while plain 0.9% saline was injected into the spleen of control rats. Following surgery, animals were returned to respective cages and monitored for several days. At 10 days after surgery, rats were euthanized by decapitation at six successive time points throughout the day corresponding to Zeitgeber times (ZT) 3, 7, 11, 15, 19, and 23. Immediately upon sacrifice, spleens were removed for enrichment of NK cells and whole-tissue assays. Briefly, approximately one-third of the spleen was separated and placed in RPMI (Invitrogen, Carlsbad, CA) for splenocyte and subsequent NK cell separation. The remaining spleen tissue was immediately frozen for additional assays. Animal treatment and surgical procedures were approved by Rutgers Animal Care and Facilities Committee and complied with NIH policy.
Sympathetic denervation of the spleen with guanethidine
Rats were anesthetized with sodium pentobarbital (50–70 mg/kg, i.p.; Henry Schein, Indianapolis, IN). A relatively small (~2 cm) incision was made on the left lateral side of the mid-abdomen caudal to the last rib. Muscular layers were blunt dissected exposing the parietal section of the spleen. Following the longitudinal axis of the spleen, a series of 10 injections were performed. Each injection was approximately 20 μl of guanethidine (1 μg/μl; w/v) or 0.9% saline. The chemical splenic denervation procedure in adult rodents is relatively permanent (Evans et al., 1979), and avoids non-specific effects that may accompany a systemic sympathectomy (Demas and Bartness, 2001).
Determination of NE levels in the spleen
Small spleen tissue aliquouts (~100 mg) were homogenized in 800 μl of 0.1 M perchloric acid. Each homogenate (200 μl) was subjected to alumina extraction of catecholamines. 3,4-Dihydroxybenzlamine (DHBA) was utilized as an internal control during the extraction procedure. Each spleen extract were assayed for NE and DHBA content by high-pressure liquid chromatography/electrochemical (HPLC-EC) detection.
NK cell enrichment
To obtain homogenous splenocyte suspensions in RPMI (Invitrogen), spleens were individually processed as described previously (Boyadjieva et al., 2001). RBCs and granulocytes were separated by centrifugation (30 min. at 400 g) with Histopaque-1083 (Sigma, St. Louis, MO). After washing RPMI, splenocytes (~10 × 107, each spleen) were resuspended in running buffer (PBS, 0.5% BSA). NK cells were then enriched by magnetic separation (negative selection) with an AutoMACS Magnetic Separator (Miltenyi Biotec, Auburn, CA) after incubating the splenocytes with anti-pan T-cell (OX52), anti-CD45RA (mature B-cells), anti-MHC II (OX6; dendritic cells and macrophages) microbeads following manufacturer’s indications (Miltenyi Biotec). The yield in the negative (enriched) fraction was consistently ~5 × 106 cells per spleen. The purity of the enriched fraction was assessed by flow-cytometry using the FITC-conjugated anti-rat CD161a (NKR-P1A) mAb (BD Biosciences, San Jan, CA) together with appropriate isotypic IgG controls. This enrichment method, which is completed within 60–80 min., consistently yields purity of ~70% of untouched NKRP1A positive cells. The percentage of CD3 and CD8 positive cells in the NK-enriched fraction was also assessed by flow-cytometry with PE-conjugated anti-rat CD3 and anti-rat CD8 mAbs (BD Biosciences, San Jose, CA), and it was consistently below 3%. Because Histopaque-1083 (Sigma-Aldrich, USA) separates out all polymorphonuclear leukocytes and magnetic separation removes a majority of macrophages, T cells, and dendritic cells, it is believed that the remaining cells may be a mixture of premature hematopoietic cells and endothelial cells. Immediately after the separation procedure, enriched NK cells were lysed in appropriate buffers for later protein analysis.
RNA extraction, RT-PCR, and real-time
Total RNA was isolated from approximately 30 mg of spleen tissue using Trizol reagent (Invitrogen, USA) according to manufacturer’s protocol. RNA was treated with DNase I (QIAGEN, Valencia, CA) following provided protocol guidelines to remove DNA contamination. RNA was quantified spectrophotometrically by absorbance measurements at 260 and 280 nm using the NanoDrop system (NanoDrop Technologies). A total of 2 μg RNA per sample was reverse transcribed following the manufacturer’s protocol (Applied Biosystems, Foster City, CA). Relative quantification of mRNA levels was performed by real-time PCR (SYBR Green and TaqMan Gene Expression Assays; Applied Biosystems) using an ABI prism 7700 Sequence Detector. The following primer sequences were used: Per2 forward 5′-GCAGCCTTTCGATTATTCTTC-3′, reverse 5′-GCTCCACGGGTTGATGAAG-3′, Bmal1 forward 5′-TCCGATGACGAACTGAAACAC-3′, reverse 5′-CTCGGTCACATCCTACGACAA-3′; perforin 5′-GCATCGGTGCCCAAGCCAGTG-3′, reverse 5′-GCCAGCGAGCCCCTGCTCATCA-3′ (Arjona and Sarkar, 2005), and predesigned probes for granzyme-B and IFN-γ (Applied Biosystems), and TNF-α. For each sample, the mRNA level was normalized with respective GAPDH (primers; Applied Biosystems) mRNA levels to determine ratios for treatment group and time points.
Western Blot
For protein analysis, 2 × 106 enriched NK cells (each spleen) were subjected to standard SDS-PAGE electrophoresis. Proteins were transferred to PVDF membranes following standard procedures. Protein blots were probed with Granzyme-B mAb (Becton Dickinson, Franklin Lakes, NJ), Perforin polyclonal Ab (Torrey Pines Biolabs, San Marcos, CA), IFN-γ polyclonal antibody (Chemicon, Temecula, CA), TNF-α polyclonal Ab (Chemicon) and actin mAb (Oncogene, San Diego, CA) to normalize values. After chemiluminiscence detection (ECL plus, Amersham, Arlington Heights, IL), densitometric analyses were performed using Scion Image software.
Statistical analyses
A one-way ANOVA with Dunnett’s post-hoc tests for multiple comparisons was used to compare splenic NE content across time points. All time points were compared to the lowest value. A two-way ANOVA was used to determine the daily variations of mRNA and protein expression levels between groups and across time points followed by Bonferroni post-hoc tests. Statistical significance was set at α = 0.05.
RESULTS
Daily rhythm of NE content in the spleen
Neural signals originating from the SCN and communicated through the SNS may maintain rhythmic gene and protein patterns in peripheral tissues (Buijs et al., 2003; Guo et al., 2005). If NE secreted from SNS terminals coordinates clock, cytokine and cytolytic factors rhythmic gene expression in the spleen, it would be expected splenic NE content follows a circadian pattern. Therefore, splenic content of NE was determined at 4-hr intervals through the day and pictured in Fig. 1. Indeed, we demonstrate a significant peak in NE content during the light period (ZT3) and trough during the dark period (ZT19) (p < 0.05), similar to NE daily variations in the rat SCN (Cagampang et al., 1994). These results provide a correlative evidence to support an inhibitory role of NE in controlling NK cell cytolytic activity (Dokur et al., 2004) and suggest the possibility that NE input might govern circadian rhythm of NK cell cytolytic function.
Fig. 1.
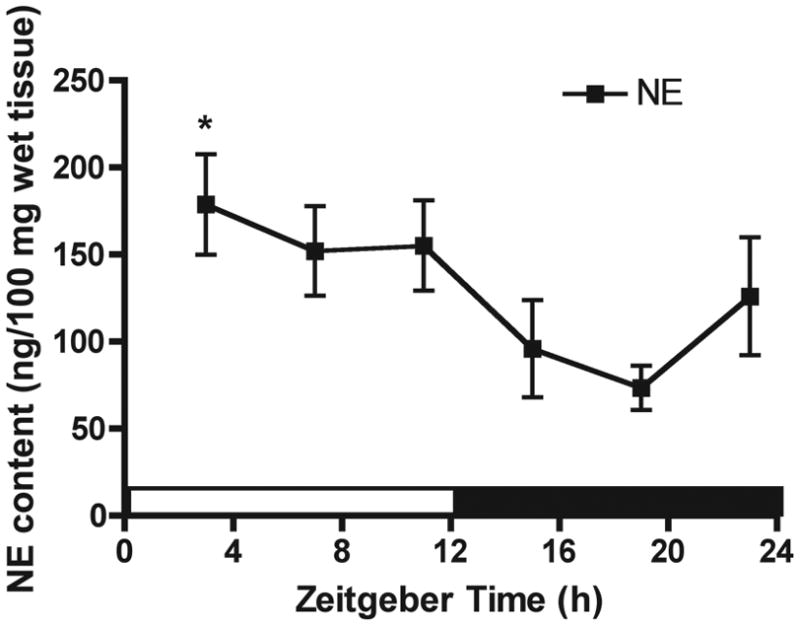
The daily rhythm of NE content in the rat spleen. Male rats were sacrificed at 4-hr intervals across a full day and spleens were collected for determination of NE content by HPLC-EC. Data are mean ± SEM, n=5 per time-point. *, p < 0.05, significantly different from the lowest value (ZT19) per one-way ANOVA with Dunnett’s post-hoc test.
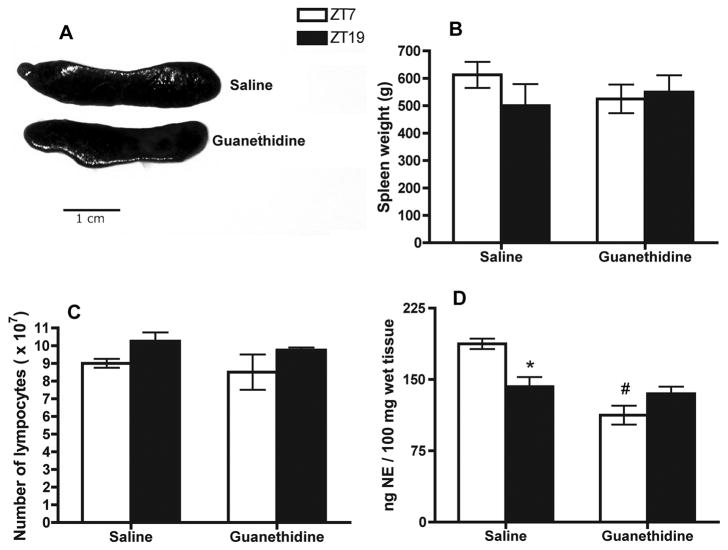
Guanethidine does not induce non-specific effects on general spleen physiology
In order to determine general physiological influence of local guanethidine injections to the spleen, spleens were weighed prior to gene and protein assays. Macroscopic appearance, size, spleen weight, and lymphocyte numbers did not differ between treated groups or between time points (Fig. 2A, 2B, 2C, respectively), suggesting local guanethidine injections did not have any toxicity on spleen cells. The total number of enriched NK cells obtained from saline and guanethidine-treated spleens were nearly identical (data not shown), confirming a previous study using a similar sympathectomy procedure (Reder et al., 1989).
Fig. 2.
Effect of local splenic sympathectomy on spleen size and macroscropic appearance (A), spleen weight (B), and total number of lymphocytes (C) in NK cells, and splenic NE content (D) enriched at ZT7 and ZT19. Data are mean ± SEM, n=4. *, significantly different (p < 0.05) from the other timepoint of the same group. #, significant difference (p < 0.01) between saline and guanethidine.
Guanethidine induced local sympathectomy disrupts NE content in the spleen at ZT7 and ZT19
Guanethidine effects are specific for sympathetic neurons and include blockade of NE release, depletion of neuronal NE stores, and blockade of NE reuptake into the neurons, which collectively creates a functional sympathectomy (Robertson and Smith, 1981). We hypothesized if guanethidine is effective in blocking SCN neural input to the spleen, the circadian peak of NE would be significantly altered. NE content was determined at two representative time points, ZT7 and ZT19. These two time points correspond with the peak and nadir (or vice versa) of most the rhythms described in this study (i.e., splenic NE content, granzyme-B, perforin,, IFN-γ, TNF-α, Per2, and Bmal1; Arjona et al., 2004; Arjona and Sarkar., 2005). Thus, the variations observed between these two time points should be representative of the daily changes in the mentioned variables. As expected, saline-treated spleens exhibited reduced NE content at ZT19 relative to ZT7 (p < 0.05), indicating that the surgical procedure per se does not hinder the physiological splenic NE rhythm. However, guanethidine-treated spleens exhibited no differences between ZT19 and ZT7 (Fig. 2D). Thus, guanethidine treatment significantly affects the splenic NE rhythm by blunting or shifting the peak during the light period.
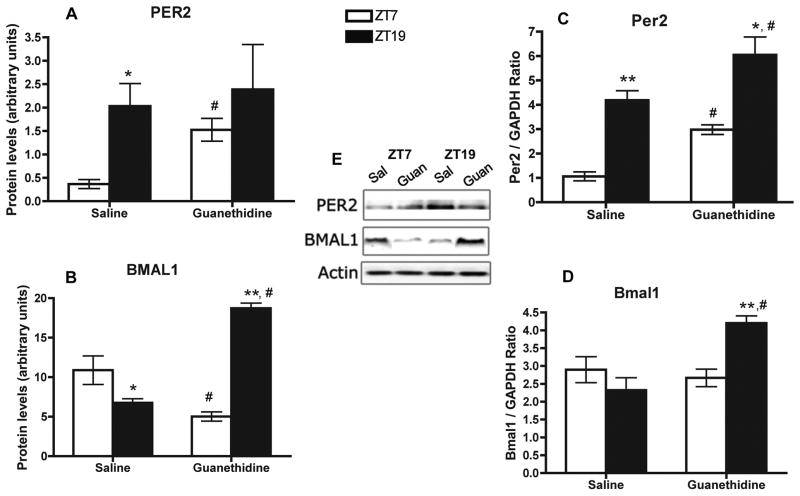
The daily rhythms of canonical clock proteins in enriched NK cells and clock genes in whole-spleen tissue are altered following splenic sympathectomy
It is known that adrenergic agents can modulate clock gene expression in a variety of cell types (Simonneaux et al., 2004; Takekida et al., 2000; Terazono et al., 2003). For example, a blunted NE rhythm in the liver was associated with abnormal rhythms of Per1, Per2, and Bmal1 (Terazono et al., 2003). Hence, we determined guanethidine treatment effects on these clock-regulating genes. As expected from our previous study in NK cells (Arjona et al., 2005), in control saline-treated spleens, BMAL1 and PER2 proteins levels in NK cells exhibited higher levels at ZT7 and ZT19, respectively (p < 0.05; Fig. 3A, 3B). In NK cells enriched from guanethidine-treated, PER2 protein levels were increased at ZT7 (P<0.001) and resulted in abolishment of rhythms as indicated by no difference between ZT7 and ZT19 time points (Fig. 3A). In addition, BMAL1 protein in NK cells from guanethidine-treated spleens displayed an inverse of rhythm compared to control spleens (time × treatment, p < 0.0001), indicated by an increase of BMAL1 protein expression at ZT19 in guanethidine-treated (p < 0.001), but at ZT7 in saline-treated spleens (p < 0.05; Fig. 3B). In saline-treated control spleens, mRNA levels of Per2 but not Bmal1 displayed increased levels at ZT19 (p < 0.001; Fig. 3C, 3D). Following guanethidine treatment, levels of Bmal1 and Per2 were increased at ZT19 (Fig. 3C, 3D). Thus, these results indicate NE neural input to spleen appears important for maintaining the functional rhythms of the molecular clock in NK cells and splenocytes.
Fig. 3.
Effect of local splenic sympathectomy in daily clock gene expression. Protein levels of PER2 (A), and BMAL1 (B) in NK cells enriched at ZT7 and ZT19 from saline and guanethidine-treated spleens. Representative immunoblots, including actin (control), are shown. Levels of mRNA Per2 (C) and Bmal1 (D) in whole-spleen at ZT7 and ZT19. Data are mean ± SEM, n=4. * (p < 0.05), ** (p < 0.001), significant differences between time points within groups. #, significant difference (p < 0.01) between saline and guanethidine.
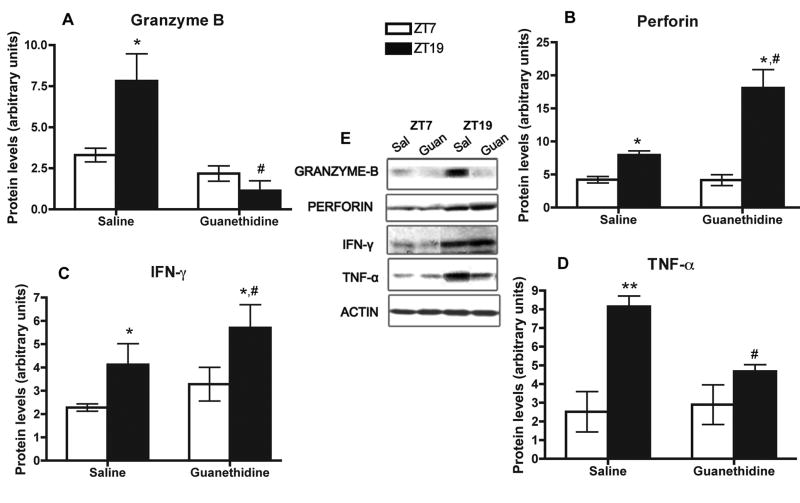
The daily rhythms of cytokines and cytolytic factors in NK cells are selectively disrupted following splenic sympathectomy
NK cells obtained from control (saline-treated) spleens presented the normal daily changes in the levels of granzyme-B, perforin, IFN-γ, and TNF-α, since the protein abundance of the mentioned cytolytic factors and cytokines was higher at ZT19 (Arjona and Sarkar, 2005). We show here NK cells obtained from saline-treated spleens exhibits higher protein expression at ZT19 than ZT7 (Fig. 4A–D). Strikingly, NK cells obtained from guanethidine-treated spleens failed to show significant daily changes in granzyme-B and TNF-α protein expression, which demonstrates the role of the SNS in the entrainment of granzyme-B and TNF-α rhythms in NK cells. Additionally, the protein levels of these two factors were significantly reduced at ZT19 (p < 0.05; Fig. 4A and 4D). In contrast, sympathectomy did not hamper perforin daily changes, albeit perforin levels were significantly higher at ZT19 in NK cells obtained from guanethidine-treated spleens (p < 0.05, Fig. 4B). Guanethidine treatment was not able to suppress the nocturnal increase in IFN-γ protein levels in NK cells (Fig. 4C). Therefore, rhythmic NE input to the spleen may not be necessary for circadian expression of perforin and IFN-γ, but critical to maintaining the rhythms of TNF-α and granzyme-B, in splenic NK cells.
Fig. 4.
Effect of local splenic sympathectomy on cytokines and cytolytic factors in NK cells. Protein levels of granzyme-B (A), perforin (B), IFN-γ (C), and TNF-α (D), in NK cells enriched at ZT7 and ZT19 from saline and guanethidine-treated spleens. Representative immunoblots, including actin (control), are shown. Data are mean ± SEM, n=4. * (p < 0.05), ** (p < 0.001), significant differences between time points within groups. #, significant difference (p < 0.01) between saline and guanethidine.
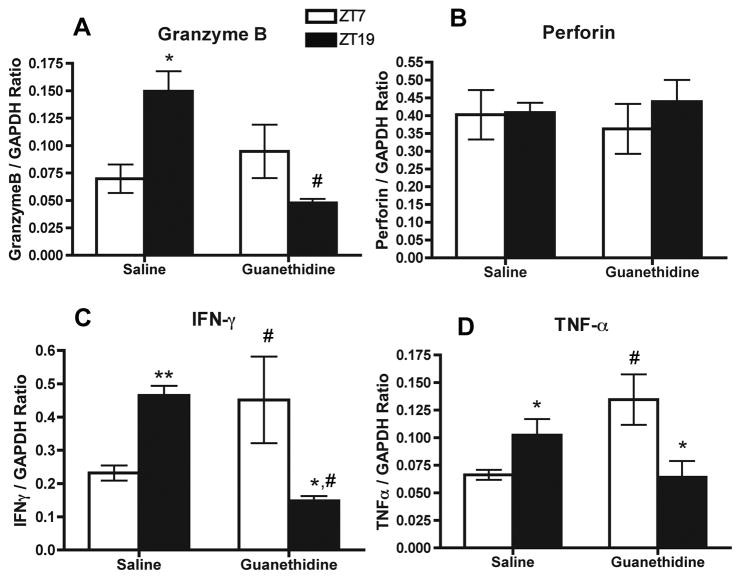
The daily rhythms of cytokines and cytolytic factors mRNA in whole-spleen tissue are selectively disrupted following splenic sympathectomy
Overall, mRNA expression of cytokines and cytolytic factors in control spleen tissue were similar across time points as previously shown (Arjona et al., 2004). As expected, granzyme-B mRNA displayed an increase during the dark period in saline-treated spleens (p < 0.05) that was abolished by guanethidine treatment (Fig. 5A). In contrast, no differences in rhythm were found for perforin mRNA in saline or guanethidine-treated spleens (Fig. 5B). Further, the increases during the dark period of both cytokines IFN-γ and TNF-α mRNA levels in the spleen were reversed by guanethidine treatment. For IFN-γ, the mRNA rhythm was inversed in guanethidine treated spleens (time × treatment, p = 0.002), indicated by a decrease in levels at ZT19 (p < 0.05), and an observable rhythm in saline-treated spleens (p < 0.001; Fig. 5C). A similar inverse expression pattern was found for TNF-α mRNA in guanethidine-treated spleens (time × treatment, p = 0.005), indicated by a decrease in levels at ZT19 (p < 0.05), and an observable rhythm in saline-treated spleens (p < 0.05; Fig. 5D). Therefore, rhythmic NE input to the spleen is necessary for maintaining the daily rhythms of mRNA expression of cytokines and cytolytic factors in heterogeneous cell populations in the whole-spleen.
Fig. 5.
Effect of local splenic sympathectomy on cytokines and cytolytic factors in whole-spleen tissue. Levels of mRNA of granzyme-B (A), perforin (B), IFN-γ (C), and TNF-α (D), in splenocytes at ZT7 and ZT19 from saline and guanethidine-treated spleens. Data are mean ± SEM, n=4. * (p < 0.05), ** (p < 0.001), significant differences between time points within groups. #, significant difference (p < 0.01) between saline and guanethidine.
DISCUSSION
The present study demonstrates rhythmic noradrenergic sympathetic input to the spleen with highest level during the day. It also provides evidence for a role of the noradrenergic input in governing the rhythms of canonical clock genes, cytolytic factors, and cytokines in enriched NK cells and splenocytes. In line with our previous results, granzyme-B, perforin, IFN-γ, and TNF-α proteins in enriched NK cells, were expressed in a rhythmic manner with highest levels during the dark period (Arjona and Sarkar, 2005), while mRNA of granzyme-B, IFN-γ, and TNF-α in the whole-spleen tissue displayed similar expression patterns (Arjona et al., 2004). In general, chemically induced local splenic sympathectomy altered the nocturnal increase in cytokines and cytolytic factors levels in NK cells and splenocytes. Interestingly, the daily variation of clock genes Bmal1 and Per2 were disrupted following splenic sympathectomy. Taken together, these data suggest that the rhythmic sympathetic noradrenergic neural input to the spleen entrains the molecular clock in NK cells and other lymphocytes potentially regulating the daily rhythms of specific cytokines and cytolytic factors in the spleen.
If the sympathetic nervous system plays a role in coordinating the daily changes in the levels of cytolytic factors and cytokines in splenic NK cells, it would be expected to find daily variations in NE content in the spleen. The data presented in this manuscript demonstrate the existence of a daily rhythm of splenic NE content, and supports the role of the sympathetic nervous system as a circadian messenger between the SCN and the spleen (Guo et al., 2005). A similar daily variation of NE content has been found in the rat SCN (Cagampang et al., 1994) and in the mouse liver (Terazono et al., 2003), but not in the mouse spleen (Kelley et al, 1996). Although the circadian changes in NE expression in the spleen we observed in rats is not consistent with previous reports in various strains of mice (Kelley, 1996), these data are consistent with the circadian changes in the expression of NE in the SCN (Cagampang et al., 1994) and liver (Terazono et al., 2003). The reason for the species difference in the daily expression pattern of splenic NE content is not apparent.
To determine the role of daily sympathetic input in circadian variations of immune factors in the spleen and specifically in NK cells, we characterized the rhythm of splenic NE content and produced a functional sympathectomy by local injection of guanethidine. The effects of guanethidine are specific for sympathetic neurons, and selectively block NE release and reuptake, and induce the depletion of NE stores (Burnstock et al., 1971; Heath et al., 1972), while avoiding non-specific extraneous effects on other tissues or behavior (Demas and Bartness, 2001). The local sympathectomy method that we employed in this study (originally validated for the rat spleen by Demas and Bartness, 2001) has the enormous advantage of avoiding the non-specific effects that accompany a systemic sympathectomy. NE content at ZT7 was reduced by ~45% in guanethidine-treated spleens. There was, however, no such difference in NE content between saline and guanethidine-treated spleens at ZT19, the nadir time of splenic NE rhythm. Since guanethidine acts specifically on neuronal terminals and the treatment was localized to the spleen, similar baseline NE content at ZT19 in both saline and guanethidine-treated spleens may be due to the detection of residual NE present in the blood perfusing the tissue at the time of collection. Although most of the splenic NE is considered to be from neural origin (Felten and Olschowka, 1987), perhaps the contribution of blood NE (that would not be affected by guanethidine) to the overall splenic NE content is augmented at ZT19 since plasma NE levels in the rat are known to increase during the dark period (De Boer and Van der Gugten, 1987). Alternatively, a longer period between treatment and sampling would have likely yielded a larger depletion of splenic NE (Demas and Bartness, 2001). Nevertheless, guanethidine treatment significantly reduced splenic NE content during peak time.
As expected, NE splenic content at ZT7 was higher than the one determined at ZT19 in saline-treated spleens, indicating that the surgical procedure per se does not hinder the physiological splenic NE rhythm. Although, as judged by NE content, we achieved a partial sympathectomy, it is important to note that it was sufficient to blunt the daily variation in NE content in guanethidine-treated spleens. However, only peak and trough time points were measured for NE content in guanethidine-treated spleens and expression of cytolytic factors and cytokines. It is difficult to discuss changes in expression of cytolytic factors and cytokines from a rhythmic perspective, rather than a binary view of NE-mediated increases/decreases of expression. Therefore, it remains a possibility, peak and trough levels of NE content in the spleen and subsequent expression of the immune factors examined may be modified across 24 hrs in guanethidine-treated spleens resembling a “shift” instead of blunting of circadian rhythmicity. Further studies sampling more time points along the circadian time scale would prove useful in addressing these issues.
In previous reports, we have shown granzyme-B, perforin, IFN-γ, and TNF-α oscillate in a circadian manner in NK cells and whole-spleens. Here, each immune factor examined in NK cells displayed the expected peak levels at ZT19. Also, the mRNA expression of these factors was consistent with previous data (Arjona et al., 2004). As we hypothesized, local splenic sympathectomy disrupted the typical daily rhythm of cytokine and cytolytic proteins in NK cells. The lack of NE input to the spleen altered rhythms of granzyme-B, perforin, and TNF-α proteins in NK cells. However, IFN-γ protein rhythms from enriched NK cells were not altered by splenic sympathectomy. Similarly, in splenocytes, mRNA expression rhythms of TNF-α and granzyme-B were significantly altered. Also, alterations in the rhythm were observed for IFN-γ in whole-spleen. Thus, NE input to the spleen regulates the circadian nature of these immune factors in NK cells specifically, and other cell types in the spleen.
In vitro studies suggests NE and other beta-adrenergic agonists suppresses the mRNA expression of granzyme-B, perforin and IFN-y. While the general effects for protein were similar, these changes were not always the same as gene expression levels (Dokur et al., 2004). As alluded to previously (Dokur et al., 2004), NE may be acting quite differently on the transcription and/or stability of the mRNA, or on processes regulating protein expression, especially since here, mRNA and protein levels suggest these processes may also depend on time of day. For example, with the exception of gene expression of perforin, at ZT7, granzyme-B, IFN-y, and TNF-a increased, which is exactly the time when NE levels were most affected by guanethidine treatment. Thus, the effect we may predict would be an increase in mRNA at ZT7 due to reduced levels of NE. Again, the inconsistencies between the protein levels at ZT7 may be due to differences in the process by which the protein is translated at a specific time of day.
Other studies have shown noradrenergic input regulates NK cell and immune function in the spleen. For example, splenic denervation increases TNF-α expression (Kees et al., 2003; Meltzer et al., 2004; Molina et al., 2001; Straub et al., 2000), under different methodological conditions, including responses to activated sympathetic nervous system states (e.g., acute stress, LPS challenge). Since our aim was to analyze the role of daily rhythm of NE to the spleen as an entrainment cue, our experiments were conducted under baseline challenge-free conditions. In line with our findings, reduction in TNF-α protein in NK cells of guanethidine treated animals at ZT19 suggests in specific cell types, like macrophages (Johnson et al., 1995; Spengler et al., 1990; Zhou et al., 2001), NE may induce TNF-α protein expression. According to our mRNA TNF-α results from the spleen, NE may differentially affect regulation of this particular cytokine depending on time of day, strongly suggesting NE acts as an entrainment mechanism on TNF-α functionality. While our results show no significant alterations in IFN-γ protein rhythms in NK cells, mRNA rhythms from the spleen were significantly altered, suggesting NE is not necessary for regulating IFN-γ protein in NK cells, but may be important in regulating the transcription in other cells in the spleen. Possibly, non-neural, such as humoral, or other entrainment or circadian cues modulate expression of IFN-γ in NK cells from the spleen. In fact, glucocorticoids influence cytokine release from peripheral blood lymphocytes (Dimitrov et al., 2004; Hermann et al., 2006; Hohagen et al., 1993; Petrovsky et al., 1998).
Transgenic experiments demonstrated that granzyme-B and perforin are critical for NK cell-mediated apoptosis and lysis of target cells (Barry and Bleackley, 2002; Heusel et al., 1994; Shresta et al., 1995), and TNF-α acting in an autocrine manner, promotes NK cell killing activity (Baxevanis et al., 2000; Jewett et al., 1996). IFN-γ acts as a self-activating molecule that stimulates NK cell cytolytic activity (Cifone et al., 1999). Disruptions in normal daily rhythms of cytolytics and cytokines in NK cells and splenocytes due to desynchronization between central and peripheral systems through lack of sympathetic input to the spleen may compromise NK cell function and immune response, subsequently promoting disease. For example, abrupt shifts in the LD cycle modeling chronic jet-lag conditions, increases tumor progression in mice (Filipski et al., 2004). Circadian disruption or desynchronization between central and peripheral systems could cause altered rhythms in sympathetic NE input to the spleen compromising the innate ability of NK cells. Possibly, reduced NK cell function due to altered rhythms in immune effectors, such as granzyme-B, perforin, TNF-α, and IFN-γ, could play a role metastatic tumor growth, or other diseases.
It is known adrenergic agents can modulate clock gene expression in a variety of cell types (Simonneaux et al., 2004; Takekida et al., 2000; Terazono et al., 2003). Our present results indicate NE input to the spleen is necessary for the daily rhythmic expression of clock genes in NK cells and splenocytes. As seen in other cell types (Jilg et al., 2005), BMAL1 and PER2 protein expression in NK cells peaked at ZT7 and ZT19, respectively, and the lack of NE input disrupted the daily variation of clock genes in not only NK cells, but also a heterogeneous population of splenocytes. Thus, splenic NE originating from the SNS may regulate the molecular clock in cells of the spleen possibly governing the daily rhythms of cytokines and cytolytic immune factors. Supporting this notion, in other tissues, such as the liver, it has been shown rhythmic clock gene expression depends upon neural input rather than humoral signals (Guo et al., 2003; Terazono et al., 2003).
The possible involvement of the NK cell molecular clock itself in the expression of cytolytic factors and cytokines have been previously determined in a limited fashion (Arjona and Sarkar, 2006). Successful Per2 knockdown by Per2-siRNA has been shown to decrease both PER2 proteins levels as well as granzyme-B and perforin levels in NK cells (Arjona and Sarkar, 2006). Considering that reduced levels of granzyme-B and perforin would certainly compromise NK cell cytolytic activity, PER2 appears as a positive modulator for NK cell function. Supporting this concept, there is the observation that Per2 mutant (Brdm1) mice lacked the physiological daily rhythm of IFN-γ mRNA and protein expression in the spleen (Arjona and Sarkar 2006b). These observations were associated with a significant alteration in the expression of canonical clock genes. In line with this observation Per2 mutant (Brdm1) mice show a higher incidence of neoplastic growth (Fu et al., 2002), possibly due to an impaired NK cell function. Similarly, abnormal Per2 rhythms were associated with faster tumor growth in mice subjected to chronic jet lag (Filipski et al., 2004). However, the mechanism by which PER2 regulates cytotoxic factors and cytokines expression in immune cells remains unknown. It is possible that other clock regulating proteins separately or in co-ordinance with PER2 may modulate the expression of cytokines and cytolytic factors. Additional studies are needed to address these issues.
In summary, these results indicate the SNS input to the spleen is necessary for entraining and synchronizing the expression of cytokines and cytolytic factors in immune spleen cells. Additionally, the functionality of the immune cells in the spleen may be under regulation, in part, by the SCN via NE neural input. In other peripheral tissues, SCN lesions cause a loss of tissue NE rhythms and functionality (Cailotto et al., 2005; Terazono et al., 2003). It is likely the circadian regulation by rhythmic NE input from the SNS to the spleen acts in an integrative manner with other signals oscillating in a circadian rhythm, including glucocorticoids, prolactin, melatonin, and pro-opiomelanocortin-derived peptides (Boyadjieva et al., 2001;Currier et al., 2000; Gan et al., 2002; Sun et al., 2003; Zhou et al., 1997), to regulate the circadian expression and function of cytokines and cytolytic factors. Further studies would be necessary to determine how humoral signaling factors and neural inputs to the spleen and other peripheral tissues integrate timing information originating from the SCN.
Research Highlight.
Both animal and human research indicates circadian disruption or desynchrony promotes the progression and severity of certain types of cancers. The immune response by lymphoid tissues, particularly natural killer (NK) cells, is important for the destruction of malignant cells. Previous research in our laboratory has demonstrated robust circadian variations of cytokines and cytolytic factors in enriched NK cells from rat spleen, strongly suggesting these functions may be subject to circadian regulation. We have shown in this manuscript for the first time the evidence for the existence of rhythmic noradrenergic sympathetic input to the spleen. Furthermore, we have shown here that chemically induced local splenic sympathectomy altered the circadian rhythms of cytokines and cytolytic factors in NK cells and splenocytes. These data strongly suggest that rhythmic sympathetic noradrenergic neural input to the spleen entrains the molecular clock in NK cells and other lymphocytes, in turn regulating the daily rhythms of specific cytokines and cytolytic factors in the spleen. The present data provide novel information on the central control of circadian immune function.
Acknowledgments
This investigation was supported by National Institutes of Health Grant R01 HL088041-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht U, Oster H. The circadian clock and behavior. Behav Brain Res. 2001;125:89–91. doi: 10.1016/s0166-4328(01)00288-1. [DOI] [PubMed] [Google Scholar]
- Arjona A, Boyadjieva N, Sarkar DK. Circadian rhythms of granzyme B, perforin, IFN-gamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J Immunol. 2004;172:2811–2817. doi: 10.4049/jimmunol.172.5.2811. [DOI] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20:469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- Baxevanis CN, Voutsas IF, Tsitsilonis OE, Tsiatas ML, Gritzapis AD, Papamichail M. Compromised anti-tumor responses in tumor necrosis factor-alpha knockout mice. Eur J Immunol. 2000;30:1957–1966. doi: 10.1002/1521-4141(200007)30:7<1957::AID-IMMU1957>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- Boyadjieva N, Dokur M, Advis JP, Meadows GG, Sarkar DK. Chronic ethanol inhibits NK cell cytolytic activity: role of opioid peptide beta-endorphin. J Immunol. 2001;167:5645–5652. doi: 10.4049/jimmunol.167.10.5645. [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Evans B, Gannon BJ, Heath JW, James V. A new method of destroying adrenergic nerves in adult animals using guanethidine. Br J Pharmacol. 1971;43:295–301. [PMC free article] [PubMed] [Google Scholar]
- Cagampang FR, Okamura H, Inouye S. Circadian rhythms of norepinephrine in the rat suprachiasmatic nucleus. Neurosci Lett. 1994;173:185–188. doi: 10.1016/0304-3940(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- Chacon F, Cano P, Lopez-Varela S, Jimenez V, Marcos A, Esquifino AI. Chronobiological features of the immune system. Effect of calorie restriction. Eur J Clin Nutr. 2002;56(Suppl 3):S69–72. doi: 10.1038/sj.ejcn.1601491. [DOI] [PubMed] [Google Scholar]
- Cifone MG, D’Alo S, Parroni R, Millimaggi D, Biordi L, Martinotti S, Santoni A. Interleukin-2-activated rat natural killer cells express inducible nitric oxide synthase that contributes to cytotoxic function and interferon-gamma production. Blood. 1999;93:3876–3884. [PubMed] [Google Scholar]
- De Boer SF, Van der Gugten J. Daily variations in plasma noradrenaline, adrenaline and corticosterone concentrations in rats. Physiol Behav. 1987;40:323–328. doi: 10.1016/0031-9384(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Demas GE, Bartness TJ. Novel method for localized, functional sympathetic nervous system denervation of peripheral tissue using guanethidine. J Neurosci Methods. 2001;112:21–28. doi: 10.1016/s0165-0270(01)00452-6. [DOI] [PubMed] [Google Scholar]
- Demas GE, Bartness TJ, Nelson RJ, Drazen DL. Photoperiod modulates the effects of norepinephrine on lymphocyte proliferation in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2003;285:R873–879. doi: 10.1152/ajpregu.00209.2003. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Fehm HL, Born J. A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain Behav Immun. 2004;18:368–374. doi: 10.1016/j.bbi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Dokur M, Boyadjieva N, Sarkar DK. Catecholaminergic control of NK cell cytolytic activity regulatory factors in the spleen. J Neuroimmunol. 2004;151:148–157. doi: 10.1016/j.jneuroim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Drouyer E, LeSauter J, Hernandez AL, Silver R. Specializations of gastrin-releasing peptide cells of the mouse suprachiasmatic nucleus. J Comp Neurol. 518:1249–1263. doi: 10.1002/cne.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Kim YI, Bouskila Y. Electrophysiology of the suprachiasmatic nucleus: synaptic transmission, membrane properties, and neuronal synchronization. J Biol Rhythms. 1993;8(Suppl):S33–37. [PubMed] [Google Scholar]
- Elenkov IJ, Kovacs K, Duda E, Stark E, Vizi ES. Presynaptic inhibitory effect of TNF-alpha on the release of noradrenaline in isolated median eminence. J Neuroimmunol. 1992;41:117–120. doi: 10.1016/0165-5728(92)90203-w. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Selgas L, Arce A, Maggiore VD, Cardinali DP. Twenty-four-hour rhythms in immune responses in rat submaxillary lymph nodes and spleen: effect of cyclosporine. Brain Behav Immun. 1996;10:92–102. doi: 10.1006/brbi.1996.0010. [DOI] [PubMed] [Google Scholar]
- Evans BK, Heath JW, Burnstock G. Reinnervation following guanethidine-induced sympathectomy of adult rats. J Neurocytol. 1979;8:381–400. doi: 10.1007/BF01236127. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Carandente F, Halberg E, Halberg F, Good RA. Circadian rhythm in activity of lympholytic natural killer cells from spleens of Fischer rats. J Immunol. 1979;123:622–625. [PubMed] [Google Scholar]
- Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci U S A. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Garabette M, King VM, Chahad-Ehlers S, O’Brien J, Maywood ES. Expression of clock gene products in the suprachiasmatic nucleus in relation to circadian behaviour. Novartis Found Symp. 2003;253:203–217. doi: 10.1002/0470090839.ch15. discussion 102–209, 218–222, 281–204. [DOI] [PubMed] [Google Scholar]
- Heath JW, Evans BK, Gannon BJ, Burnstock G, James VB. Degeneration of adrenergic neurons following guanethidine treatment: an ultrastructural study. Virchows Arch B Cell Pathol. 1972;11:182–197. doi: 10.1007/BF02889397. [DOI] [PubMed] [Google Scholar]
- Hermann C, von Aulock S, Dehus O, Keller M, Okigami H, Gantner F, Wendel A, Hartung T. Endogenous cortisol determines the circadian rhythm of lipopolysaccharide-- but not lipoteichoic acid--inducible cytokine release. Eur J Immunol. 2006;36:371–379. doi: 10.1002/eji.200535470. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Hohagen F, Timmer J, Weyerbrock A, Fritsch-Montero R, Ganter U, Krieger S, Berger M, Bauer J. Cytokine production during sleep and wakefulness and its relationship to cortisol in healthy humans. Neuropsychobiology. 1993;28:9–16. doi: 10.1159/000118993. [DOI] [PubMed] [Google Scholar]
- Honma S, Shirakawa T, Katsuno Y, Namihira M, Honma K. Circadian periods of single suprachiasmatic neurons in rats. Neurosci Lett. 1998;250:157–160. doi: 10.1016/s0304-3940(98)00464-9. [DOI] [PubMed] [Google Scholar]
- Jewett A, Bonavida B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996;156:907–915. [PubMed] [Google Scholar]
- Jilg A, Moek J, Weaver DR, Korf HW, Stehle JH, von Gall C. Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur J Neurosci. 2005;22:2845–2854. doi: 10.1111/j.1460-9568.2005.04485.x. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Garcia RM, Heitkemper M, Helton WS. Polymyxin B prevents increased sympathetic activity and alveolar macrophage tumor necrosis factor release in parenterally fed rats. Arch Surg. 1995;130:1294–1299. doi: 10.1001/archsurg.1995.01430120048007. discussion 1299–1300. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Ichijo T, Take S, Hori T. Hypothalamic modulation of splenic natural killer cell activity in rats. J Physiol. 1993;471:209–221. doi: 10.1113/jphysiol.1993.sp019898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol. 2003;145:77–85. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kent J, Meredith AL. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLoS One. 2008;3:e3884. doi: 10.1371/journal.pone.0003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewska B, Felten DL, Stevens SY, Moynihan JA. Sympathectomy-induced immune changes are not abrogated by the glucocorticoid receptor blocker RU-486. Brain Behav Immun. 1998;12:181–200. doi: 10.1006/brbi.1998.0527. [DOI] [PubMed] [Google Scholar]
- Levi F, Canon C, Dipalma M, Florentin I, Misset JL. When should the immune clock be reset? From circadian pharmacodynamics to temporally optimized drug delivery. Ann N Y Acad Sci. 1991;618:312–329. doi: 10.1111/j.1749-6632.1991.tb27251.x. [DOI] [PubMed] [Google Scholar]
- Madden KS, Stevens SY, Felten DL, Bellinger DL. Alterations in T lymphocyte activity following chemical sympathectomy in young and old Fischer 344 rats. J Neuroimmunol. 2000;103:131–145. doi: 10.1016/s0165-5728(99)00243-x. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A. Melatonin and the immune-hematopoietic system therapeutic and adverse pharmacological correlates. Neuroimmunomodulation. 1996;3:325–332. doi: 10.1159/000097292. [DOI] [PubMed] [Google Scholar]
- Manshardt J, Wurtman RJ. Daily rhythm in the noradrenaline content of rat hypothalamus. Nature. 1968;217:574–575. doi: 10.1038/217574a0. [DOI] [PubMed] [Google Scholar]
- Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, Nance DM. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun. 2004;18:262–273. doi: 10.1016/j.bbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Molina PE. Noradrenergic inhibition of TNF upregulation in hemorrhagic shock. Neuroimmunomodulation. 2001;9:125–133. doi: 10.1159/000049016. [DOI] [PubMed] [Google Scholar]
- Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989;3:281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Peng YP, Qiu YH, Jiang JL, Wang JJ. Effect of catecholamines on IL-2 production and NK cytotoxicity of rats in vitro. Acta Pharmacol Sin. 2004;25:1354–1360. [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Edgar DM, Heller HC, Miller JD. A possible glial role in the mammalian circadian clock. Brain Res. 1994;643:296–301. doi: 10.1016/0006-8993(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Reder A, Checinski M, Chelmicka-Schorr E. The effect of chemical sympathectomy on natural killer cells in mice. Brain Behav Immun. 1989;3:110–118. doi: 10.1016/0889-1591(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Roberts JE. Light and immunomodulation. Ann N Y Acad Sci. 2000;917:435–445. doi: 10.1111/j.1749-6632.2000.tb05408.x. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Bar-Ziv I, Ben-Eliyahu S. Diurnal changes in lung tumor clearance and their relation to NK cell cytotoxicity in the blood and spleen. Int J Cancer. 2001;94:401–406. doi: 10.1002/ijc.1477. [DOI] [PubMed] [Google Scholar]
- Shresta S, Heusel JW, Macivor DM, Wesselschmidt RL, Russell JH, Ley TJ. Granzyme B plays a critical role in cytotoxic lymphocyte-induced apoptosis. Immunol Rev. 1995;146:211–221. doi: 10.1111/j.1600-065x.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Poirel VJ, Garidou ML, Nguyen D, Diaz-Rodriguez E, Pevet P. Daily rhythm and regulation of clock gene expression in the rat pineal gland. Brain Res Mol Brain Res. 2004;120:164–172. doi: 10.1016/j.molbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145:1430–1434. [PubMed] [Google Scholar]
- Straub RH, Gluck T, Cutolo M, Georgi J, Helmke K, Scholmerich J, Vaith P, Lang B. The adrenal steroid status in relation to inflammatory cytokines (interleukin-6 and tumour necrosis factor) in polymyalgia rheumatica. Rheumatology (Oxford) 2000;39:624–631. doi: 10.1093/rheumatology/39.6.624. [DOI] [PubMed] [Google Scholar]
- Takekida S, Yan L, Maywood ES, Hastings MH, Okamura H. Differential adrenergic regulation of the circadian expression of the clock genes Period1 and Period2 in the rat pineal gland. Eur J Neurosci. 2000;12:4557–4561. doi: 10.1046/j.0953-816x.2000.01324.x. [DOI] [PubMed] [Google Scholar]
- Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci U S A. 2003;100:6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen KM, Vaage JT, Levy FO, Hansson V, Rolstad B, Tasken K. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem. 1997;272:5495–5500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- Torres-Farfan C, Seron-Ferre M, Dinet V, Korf HW. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2006;40:64–70. doi: 10.1111/j.1600-079X.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Yang S, Koo DJ, Ornan DA, Chaudry IH, Wang P. The role of Kupffer cell alpha(2)-adrenoceptors in norepinephrine-induced TNF-alpha production. Biochim Biophys Acta. 2001;1537:49–57. doi: 10.1016/s0925-4439(01)00055-2. [DOI] [PubMed] [Google Scholar]