Abstract
BACKGROUND
This study aims to determine the role of antibodies (Abs) to donor mismatched HLA developed during the post-transplant period in inducing defensins and their synergistic role in the pathogenesis of chronic rejection, Bronchiolitis Obliterans Syndrome (BOS), following human lung transplantation (LTx).
METHODS
Bronchoalveolar Lavage (BAL) and serum from twenty-one BOS+ LTx patients were assayed for β-defensins HNP1-3 (ELISA) and Anti-HLA Abs (Luminex). Human Airway Epithelial Cells (AEC) were treated with anti-HLA Abs, HNP-1/2 or both and the levels of β-defensin was measured by ELISA. Using a mouse model of obliterative airway disease induced by anti-MHC class-I Abs, we quantitatively and qualitatively determined neutrophil infiltration (Myeloperoxidase (MPO) staining) and activity (MPO assay) and defensin levels in the BAL.
RESULTS
In human LTx patients, higher defensin levels correlated with presence of circulating anti-HLA Abs (p<0.05). AEC treated with anti-HLA Abs or HNP-1/2, produced β-defensin with synergistic effects in combination (612±06 vs. 520±23 anti-HLA Ab or 590±10 pg/ml for HNP treatment, p<0.05).Neutrophil numbers (6 fold) and activity (5.5 folds) was higher in the lungs of mice treated with anti-MHC Abs compared to control. Two-fold increase in α-defensin and β-defensin levels was also present in BAL on day 5 following anti-MHC administrations.
CONCLUSIONS
Anti-HLA Abs developed during the post-transplant period and α-defensins stimulate β-defensin production by epithelial cells leading to increased cellular infiltration and inflammation. Chronic stimulation of epithelium by Abs to MHC and resulting increased levels of defensins induce growth factor production and epithelial proliferation contributing towards development of chronic rejection following LTx.
Keywords: Human Neutrophil Peptide, Bronchiolitis Obliterans Syndrome, Bronchoalveolar Lavage, Human Beta Defensin 2, Airway Epithelial Cells
Introduction
Chronic allograft rejection following human lung transplantation (LTx) manifesting as bronchiolitis obliterans syndrome (BOS) is the primary reason for adverse long-term survival outcomes in -LTx recipients (1). Although immunologic and non-immunologic causes of BOS pathogenesis are postulated to play a role, mechanisms leading to BOS are still elusive. Alloimmune responses to mismatched donor histocompatibility antigens play an important role in the pathogenesis of BOS. Antibodies (Abs) specific for donor HLA class I have been shown to precede the development of BOS (2) and can stimulate growth factor production, proliferation and apoptosis of epithelial cells (EC) (3, 4).
Defensins are anti-microbial peptides produced by neutrophils (α-defensins) and EC (β-defensins) which have been implicated in immune modulation, inflammation and wound healing. Nelsestuen et.al., using proteomic approach have reported increased levels of human neutrophil α-defensins in the Bronchoalveolar Lavage (BAL) of lung allograft recipients with chronic rejection (5). Human β-defensins (HBD) produced by the epithelium act as chemokines via CCR6 receptor providing a link between innate and adaptive immunity(6).
In this study we tested the hypothesis that specific immune responses against donor HLA can induce production of not only Abs to HLA but also defensins and both synergistically lead to the epithelial changes seen during chronic lung allograft rejection. Towards this, we determined the development of Abs to donor HLA (DSA), quantitated the levels of defensins in BAL and sera of BOS+ LTx recipients. In addition, using a mouse model of anti-MHC class I induced obliterative airway disease (OAD), we determined the role of neutrophils infiltrating the lung and its production of α- and β-defensin in development of OAD. Our results using both human LTx recipients with BOS and animal model of OAD demonstrated that Abs to HLA as well as α-defensins stimulate airway epithelial cells (AEC) to produce HBD2 and induce morphological changes in the epithelium. Therefore, anti-HLA Abs developed post-transplant stimulate EC to augment the production of defensin and DSA as well as defensins synergistically activate EC, leading to sustained production of growth factors resulting in EC proliferation, fibrosis and remodeling, the cardinal features of BOS.
Methods
Human Subjects
LTx patients at Washington University Medical Center/Barnes-Jewish Hospital were enrolled in this study with informed consent according to protocol approved by Institutional Review Board. Standard immunosuppression consisted of cyclosporine, azathioprine and prednisone. After BOS diagnosis, immunotherapy was modified to FK506 (tacrolimus), mycophenolate mofetil and prednisone. BOS diagnosis was according to ISHLT standard criteria (7), forced expiratory volume in 1s (FEV1) was measured at <80% of baseline established in their stable post-operative period, or there was histological evidence of BOS. Serum and BAL samples from twenty one BOS+ patients and nine BOS- patients were collected 6 (± 2.3) months after the clinical diagnosis (for BOS+), processed on the day of collection and stored at −70°C.
Anti-HLA testing
Anti-HLA Abs and their donor specificity were detected in patient sera by a solid phase assay (Luminex) using reagents from One Lambda, Canoga Park, CA.
Human Neutrophil Peptide (HNP (1-3)) and HBD2 ELISA
HNP1-3 and HBD2 levels were determined using ELISA test kits purchased from Cell Sciences, (Canton, MA) and Phoenix Pharmaceuticals (Burlingame, CA), respectively.
Cell lines
SAECs were cultured in small airway growth medium (Cambrex BioScience, Rockland, ME). Normal human bronchial epithelial cells (BEC) were obtained from ATCC (American Type Culture Collection, Manassas, VA) and cultured in bronchial epithelial growth medium (Cambrex Bio Science, Rockland, ME).
Treatment of AECs with anti-HLA Abs (W6/32)
In order to test the effect of anti-HLA Abs on defensin production, AEC/BEC were serum starved for 16 hrs and then incubated for 24 hrs with various concentrations (2.5, 5 or 10μg/ml) of anti-HLA class I Ab, W6/32. The supernatants from the treated cells were tested for HBD2.
Treatment of AECs with HNPs
In order to determine the mechanism by which increased defensin levels affect EC, human AEC/BEC were serum starved for 16 hrs and treated for 24 hrs with HNP1/HNP2 (Bachem, Torrance, CA) at 10 μg/ml concentrations. The supernatants from the treated cells were tested for HBD2.
Intrabronchial administration of anti-MHC Abs into murine lungs
Murine mAb (IgG2a) or its isotype control C1.18.4 with no detectable endotoxin (LAL assay) was given at a dose of 200 μg per administration intrabronchially into the lungs of H2Kd (BALB/c) test and control mice as detailed in our publication respectively (8). All experiments were performed in compliance with guidelines of the Institutional Laboratory Animal Care and Use Committee of Washington University School of Medicine (protocol 20070121).
Staining for Myeloperoxidase (MPO) in mouse lungs administered with anti-MHC Abs
Frozen lung samples were embedded in freeze tissue matrix (OCT), and sections were cut at 5 μm thickness. The sections were fixed in cold alcohol for 2 min (−20°C) and air dried. The presence of positive cells was detected with the Myeloperoxidase kit (Sigma, Saint Louis), counter-stained with hematoxylin, and examined using a light microscope. Positive cells were counted by random sampling.
MPO assay
Lung extracts were prepared from 100 mg frozen mouse lung samples by sonication and freeze thaw in K-phosphate buffer. MPO standard (Sigma, Saint Louis) was used at various dilutions. To all wells, MPO reaction mixture containing 0.167mg/ml of O-dianisidine dihydrochloride in K-phosphate buffer with 0.05% H2O2 was added to make 150 μl total reaction mixture. The reaction was stopped after 5 minutes with 25 μl of 1% sodium azide prepared in K-phosphate buffer and read on ELISA reader at 460nm.
Statistical analysis
Data are expressed as means ± SD. The minimum number of replicates for all measurements was at least three. For ELISA and Luminex differences between control and samples were compared by t test. Significance was assigned at p < 0.05.
Results
Human LTx recipients with Anti-HLA DSA and BOS diagnosis have higher defensin levels
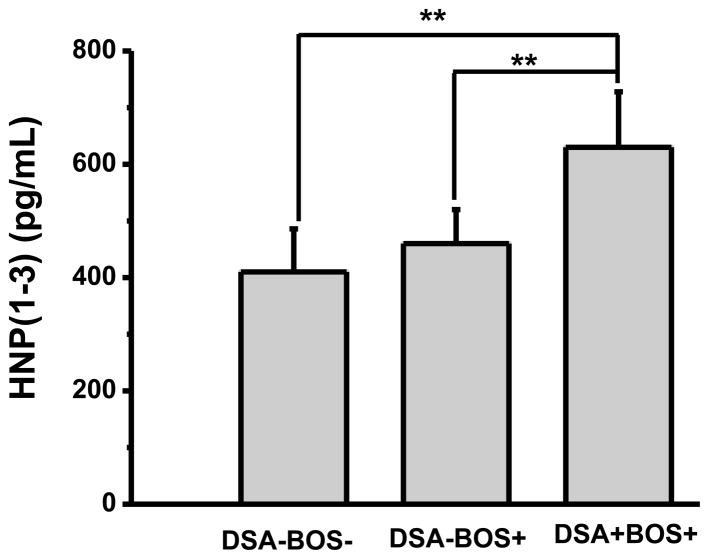
HNP (1-3) levels were measured by ELISA in the BAL fluid of LTx recipients who developed BOS. Development of anti-HLA Abs DSA was determined in serum using Luminex assay. Twelve out of 21 BOS+ patients had DSA. When compared with separate cohorts (nine patients in each cohort) of BOS+ and BOS− patients without DSA, HNP (1-3) levels are higher in the DSA+ patients (630 ± 98 pg/ml vs. 460 ± 60 and 410 ± 76 in BOS+DSA− and BOS−DSA−, respectively) as shown in Figure 1. These results demonstrate that defensins levels are significantly increased in BOS+ LTx recipients (p<0.05) and correlate strongly with development of DSA.
Figure 1. Defensin levels are higher in DSA+ BOS+ (11/21) patients.
ELISA was done to measure HNP (1-3) for BOS+ LTx recipients. HNP (1-3) levels were higher in the BOS+ recipients who also had DSA compared to BOS+ recipients who did not develop DSA, (**) p<0.05.
Stimulation of AECs by anti-HLA class I or HNPs induces β-defensin production
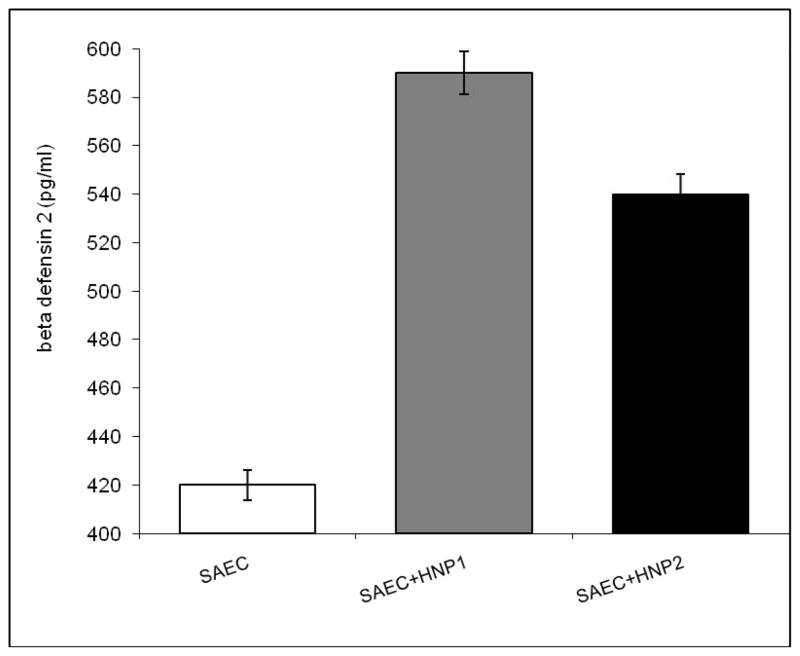
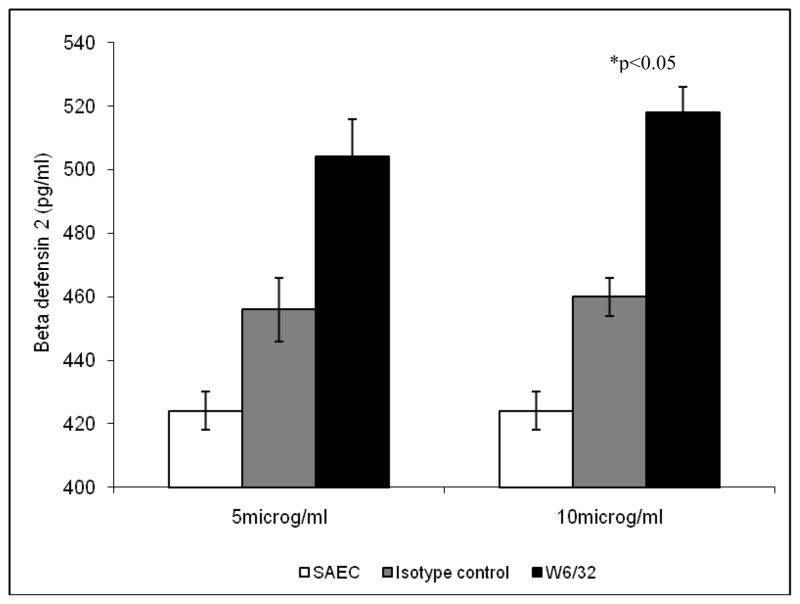
Supernatants from small airway epithelial cells (SAEC) incubated with HNP 1/2 for 24 hrs were tested for HBD2 production by ELISA. Figure 2 demonstrates significant increases in HBD2 levels in response to HNP1 or HNP2 treatment (590 ± 10 for HNP-1 and 540 ±8 for HNP-2 treatment vs. 420+12 for untreated cells, p<0.05). To test the effect of anti-HLA class I on defensin production by AECs, SAEC were treated with various concentrations of W6/32 (anti-HLA class I mAb) for 24 hrs. Results presented in Figure 3 demonstrate increase in HBD2 levels in response to W6/32 treatment compared with isotype control. (509± 8 for 5μg/ml and 520 ± 23 for 10μg/ml W6/32 Ab treatment vs. 420+12 for untreated cells, p<0.05). Further, SAECs treated with monoclonal and polyclonal anti-keratin Abs produced similar response as isotype control (data not shown). Anti-HLA Abs treatment of AECs in combination with defensins (HNP1/2 or HBD2) showed a further increase in HBD2 production (612±6 vs. 520±23 and 590±10 for anti-HLA or HNP alone, p<0.05 Table 1) demonstrating a synergestic effect. Since treatment of SAEC with HNP 1 or 2 and/or anti-HLA class I results in significant increase in HBD2 production, we propose that higher production of α-defensins by neutrophils may result in higher β-defensin production from EC.
Figure 2. Increased HBD2 levels in response to HNP1 or HNP2 treatment compared to untreated cells.
SAEC were treated with HNP1 or HNP2 for 24 hrs. HBD2 production was measured by ELISA in the culture supernatant.
Figure 3. Increased HBD2 levels in response to various concentrations of anti-HLA class I Ab as compared to isotype control Ab.
SAEC were treated with various concentrations of anti-HLA class I Ab as mentioned in methods for 24 hrs. HBD2 production was measured by ELISA in the culture supernatant.
Table 1.
HBD2 production from SAECs increases synergistically after treatment with both anti-HLA Ab and HNP
| SAE Cells | β-defensin levels (HBD2) (pg/ml) |
|---|---|
| Untreated | 420 ± 12 |
| With anti-HLA Ab (W6/32) | 520 ± 23 |
| With α-defensins (HNP1/2) | 590 ± 10 |
| With anti-HLA Ab(W6/32) α-defensins (HNP1/2) | 612 ± 06 |
Endobronchial administration of anti-MHC class I Abs lead to increased neutrophil activity (MPO assay) in lungs
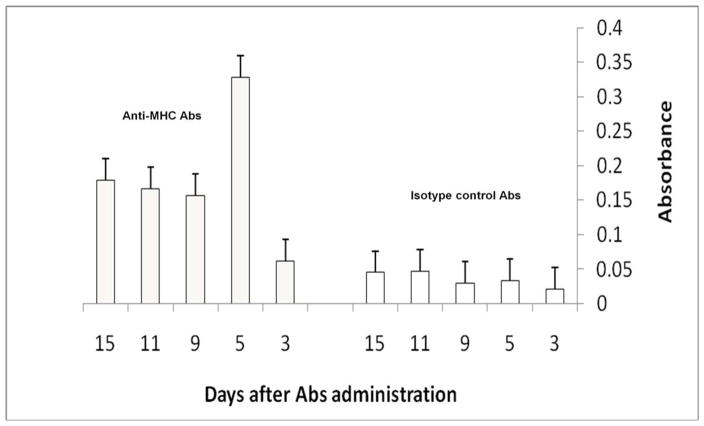
To determine that anti-HLA Abs can induce defensin production from lung epithelium, a mouse model of OAD was used wherein anti-HLA class I Abs were administered endobronchially to native lungs. Neutrophil activity was assayed by MPO assay in the lung lysate following administration of anti-MHC Abs/isotype control. As shown in Figure 4, lungs of mice treated with anti-MHC class I show higher neutrophil activity (6 folds at day 5, p< 0.01), and remained elevated at least 4 folds upto day 15 after Ab administration) compared to lungs of mice administered isotype control.
Figure 4. Increased neutrophil activity (MPO assay) in lung lysates of mice treated with anti-MHC Abs.
Balb/c mice were administered anti-MHC (H2kd) Abs endobronchially as described in methods. Neutrophil activity at day 3, 5, 9, 11 and 15 post-Ab administration was measured in lung lysates of mice treated with anti-MHC Abs using MPO assay and compared to mice treated with isotype control Abs.
To determine neutrophil infiltration in the lungs of mice administered with anti-MHC Abs, MPO staining was done on lung sections after administration of anti-MHC Abs/isotype control. As shown in Figure 5, lungs of mice treated with anti-MHC Abs show increased neutrophil numbers (5.5 folds at day 5, p< 0.01) compared to lungs of mice administered isotype control.
Figure 5. Increased neutrophils (MPO staining) in lungs of mice treated with anti-MHC Abs.
Frozen sections of lungs from mice treated with anti-MHC Abs at day 5 were stained for MPO and neutrophil infiltration was compared to mice administered isotype control Abs.
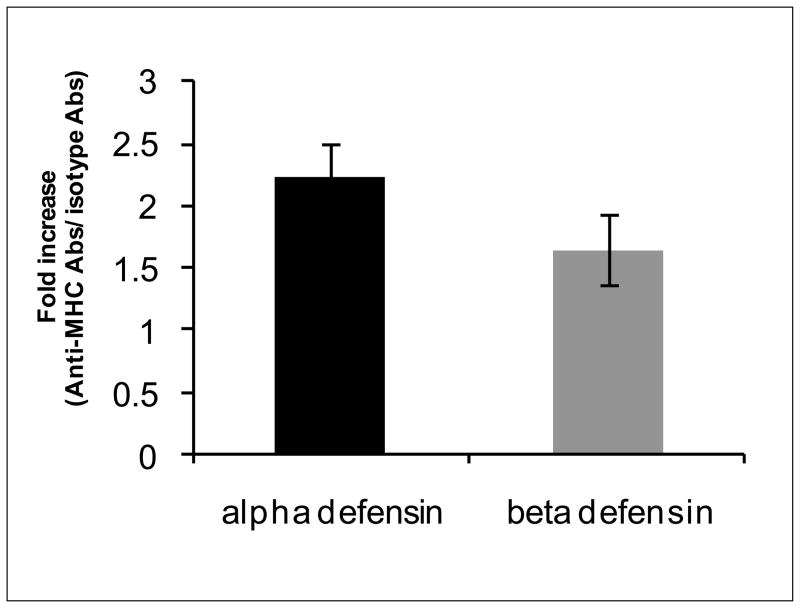
α and β-defensin levels are higher in BAL of mice administered with anti-MHC Abs
α-defensin and β-defensin levels were measured by Western blotting using anti-mouse α-defensin and β-defensin Abs (Santacruz Biotechnology) in BAL of mice treated with anti-MHC Abs/isotype control. α-defensin as well as β-defensin levels in BAL fluid of mice administered with anti-MHC Abs were 1.7 to 2.5 folds higher than in BAL of mice treated with isotype control (Figure 6). These results demonstrate that chronic stimulation of lung AECs both by defensins and anti-HLA class I can lead to increased cellular infiltration in the lungs, and secretion of cytokines and growth factors contributing to the fibroproliferative changes seen in BOS.
Figure 6. α and β-defensin levels are higher in BAL of mice administered anti-MHC Abs.
BAL fluid of mice administered with anti-MHC Abs was tested for α and β-defensins by western blotting and compared to mice treated with isotype control Abs.
Discussion
Improvements in surgical procedure and peri-/post-operative management have led to significant improvement in quality of life after LTx. But long term outcomes remain poor due to chronic rejection manifesting as BOS. An important role for immune response to mismatched donor HLA antigens in the pathogenesis of BOS has been demonstrated in earlier studies from our laboratory (9, 10) and others (11, 12). Development of Abs to donor HLA mismatches (13, 14), as well as increased precursor frequency of CD4+ T-cells specific for the mismatched HLA class I and II antigens (2, 9, 15) have been demonstrated to be important risk factors for BOS. Development of anti-HLA Abs precedes the development of BOS (2, 4, 15) giving rise to its possible pathogenic role in the development of BOS. HLA Abs developed during post-transplant have been shown to result in complement mediated cell death, proliferation of AEC leading to both apoptosis of AEC and production of growth factors (3, 4).
BAL obtained from normal lungs show that the predominant cell is alveolar macrophage (85%), followed by lymphocytes (7–12%). Eosinophils, basophils (1%) and neutrophils (1– 2%) are minimal in normal lung BAL (16). In contrast, significantly elevated numbers of neutrophils have been reported in the BAL of LTx recipients with BOS and are described as a hallmark of obliterative bronchiolitis (17–19). Defensins, produced by neutrophils, are a part of the innate immune system (20). These are small molecules released at the site of injury that promote inflammation and resistance to infections. They also affect various immune functions and can mediate wound repair. Exposure to neutrophil defensins results in more chemotaxis and proliferation of inflammatory and EC and fibroblasts (21). Hence, defensins functions as a link between innate and adaptive immune mechanisms (22, 23).
Studies have shown that the levels of defensins are higher in serum and BAL fluids of BOS+ LTx recipients compared to the BOS− patients (24). It has also been reported that higher HNP levels can predate the clinical onset of disease up to 15 months (5). In our study, BAL samples from LTx patients, collected post-transplant demonstrated significantly higher levels of α and β-defensins and furthermore, development of DSA correlated with higher defensins levels in BOS+ LTx patients (Figure 1).
The mechanisms involved in recruitment and activation of neutrophils in the airways of patients with BOS is not fully understood. The finding that neutrophilia independent of infection has been thought to play an important role in the pathogenesis of BOS (25, 26) has been reported. Data presented here, both using human LTx recipients with chronic rejection (Figure 1) as well as the animal model of OAD induced by anti-MHC class I, clearly demonstrates that anti-HLA Abs produced post-transplant can activate AECs to produce β-defensins which is an important chemoattractant for neutrophils and macrophages. This can further increase production of defensins (HNP1-3 by the infiltrating neutrophils as well as HBD2 from EC). We have also shown the production of HBD2 in vitro by AECs in response to anti-HLA Ab (W6/32) (Figure 3). It is significant that in vitro treatment of AEC with HNP and anti-HLA Abs further increased HBD2 expression as shown in Table 1. All of these results clearly support our hypothesis that an immune response to mismatched donor HLA can activate AEC both directly by ligation of HLA molecules and can also induce activation indirectly by production of defensins. Most of the Abs to donor HLA may also have the capacity to activate complement which can induce infiltration of neutrophils and its activation (27, 28). In addition, studies from our laboratory (8) and others (18, 29) have shown that IL-17 may play an important role in the pathogenesis of BOS. It is likely that IL-17 produced following alloimmune responses can also play an important role in attracting neutrophils to the transplanted lungs.
The contribution of pro-inflammatory cytokines towards LTx rejection has been reported by us (30, 31) and others (32, 33). HBD2 with CD14 can complex with “toll-like receptors” in the bronchiolar epithelium and can activate NF-kB pathway, leading to increase in cytokine gene expression. HBD2 has been shown to induce recruitment of immature CD34+ dendritic cells and memory (CD4+/CD45RO+) T-lymphocytes through chemokine receptor CCR6 (24). DiGiovine et al. (34) first reported increased levels of IL-8 in conjunction with elevated neutrophil numbers in BAL from patients with BOS and found that the IL-8 in BAL of patients with BOS was biologically active as a neutrophil chemoattractant. Thus, release of IL-8 by bronchial EC locally may also significantly contribute in attracting and activating neutrophils in the allograft.
MPO activity is an indicator of oxidative stress due to neutrophil activation. MPO was found to be significantly elevated in patients with BOS compared to patients after LTx without BOS and healthy controls (35). Riise et al observed the increase of MPO in BAL fluid preceding the clinical diagnosis of BOS by several months (36). Neutrophil MPO activity also induces the generation of reactive nitrogen species using nitrite and hydrogen peroxide or hypochlorite (37). This is in agreement with our results presented in Figure 4 demonstrating higher MPO activity in the lungs of mice treated with anti-MHC Abs.
In conclusion, results presented here show that anti-HLA Abs developed during the post-transplant can result in the activation of α-defensins from neutrophils which can stimulate β-defensin production by EC leading to cellular infiltration and inflammation. Chronic stimulation of EC both by defensins and anti-HLA developed during the post-transplant can synergistically increase MPO activity, reactive oxygen and nitrogen species, pro-inflammatory cytokines, and growth factor production contributing towards BOS.
Acknowledgments
This work was supported in part by NIH/NHLBI ARRA Award HL056643 (TM), and ISHLT Research Fellowship (DS). The authors thank Ms. Billie Glasscock for assistance in preparing and submitting this manuscript.
Footnotes
There is no financial or professional conflict of interest by any of the authors of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, et al. Actuarial survival of heart-lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15:371–383. [PubMed] [Google Scholar]
- 2.Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock E, Lynch J, et al. Temporal relationship between the development of anti-HLA antibodies and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplant Proc. 1999;31(1–2):185–186. doi: 10.1016/s0041-1345(98)01495-x. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo A, Zhang L, Mohanakumar T. Binding of anti-HLA class I antibodies to airway epithelial cells induces activation and growth factor production and indirectly upregulates lung fibroblast proliferation. J Heart Lung Transplant. 2001;20(2):166. doi: 10.1016/s1053-2498(00)00304-1. [DOI] [PubMed] [Google Scholar]
- 4.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64(5):521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 5.Nelsestuen GL, Martinez MB, Hertz MI, Savik K, Wendt CH. Proteomic identification of human neutrophil alpha-defensins in chronic lung allograft rejection. Proteomics. 2005;5(6):1705–1713. doi: 10.1002/pmic.200401036. [DOI] [PubMed] [Google Scholar]
- 6.Schutte BC, McCray PB., Jr [beta]-defensins in lung host defense. Annu Rev Physiol. 2002;64:709–748. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- 7.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH. Revisions of the 1990 working formulation for the classification of pulmonary allograft. Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 8.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundaresan S, Mohanakumar T, Smith MA, Trulock EP, Lynch J, Phelan D, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65(5):648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama T, Jaramillo A, Narayanan K, Higuchi T, Mohanakumar T. Induction of obliterative airway disease by anti-HLA class I antibodies. Am J Transplant. 2005;5(9):2126–2134. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 11.Girnita AL, Duquesnoy R, Yousem SA, Iacono AT, Corcoran TE, Buzoianu M, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5(1):131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 12.Reinsmoen NL, Nelson K, Zeevi A. Anti-HLA antibody analysis and crossmatching in heart and lung transplantation. Transpl Immunol. 2004;13(1):63–71. doi: 10.1016/j.trim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Reznik SI, Jaramillo A, SivaSai KS, Womer KL, Sayegh MH, Trulock EP, et al. Indirect allorecognition of mismatched donor HLA class II peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Am J Transplant. 2001;1(3):228–235. doi: 10.1034/j.1600-6143.2001.001003228.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu KC, Jaramillo A, Mendeloff EN, Huddleston CB, Sweet SC, Patterson GA, et al. Concomitant allorecognition of mismatched donor HLA class I- and class II-derived peptides in pediatric lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22(1):35–43. doi: 10.1016/s1053-2498(02)00478-3. [DOI] [PubMed] [Google Scholar]
- 15.Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock EP, Lynch JP, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67(8):1155–1161. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 16.Reynaud-Gaubert M, Marin V, Thirion X, Farnarier C, Thomas P, Badier M, et al. Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart and Lung Transplantation. 2002;21 (7):721–730. doi: 10.1016/s1053-2498(02)00392-3. [DOI] [PubMed] [Google Scholar]
- 17.Neurohr C, Huppmann P, Samweber B, Leuschner S, Zimmermann G, Leuchte H, et al. Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transplant. 2009;28(5):468–474. doi: 10.1016/j.healun.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8(9):1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 19.Slebos DJ, Postma DS, Koeter GH, Van Der Bij W, Boezen M, Kauffman HF. Bronchoalveolar lavage fluid characteristics in acute and chronic lung transplant rejection. J Heart Lung Transplant. 2004;23(5):532–540. doi: 10.1016/j.healun.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23(2):327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 21.van Wetering S, Tjabringa GS, Hiemstra PS. Interactions between neutrophil-derived antimicrobial peptides and airway epithelial cells. J Leukoc Biol. 2005;77(4):444–450. doi: 10.1189/jlb.0604367. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci. 2001;58(7):978–989. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang D, Liu ZH, Tewary P, Chen Q, de la Rosa G, Oppenheim JJ. Defensin participation in innate and adaptive immunity. Curr Pharm Des. 2007;13(30):3131–3139. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- 24.Ross DJ, Cole AM, Yoshioka D, Park AK, Belperio JA, Laks H, Strieter RM, Lynch JP, Kubak B, Ardehali A, Ganz T. Increased bronchoalveolar lavage human beta-defensin type 2 in bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;78(8):1222–1224. doi: 10.1097/01.tp.0000137265.18491.75. [DOI] [PubMed] [Google Scholar]
- 25.Nagarkar DR, Wang Q, Shim J, Zhao Y, Tsai WC, Lukacs NW, et al. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J Immunol. 2009;183(10):6698–6707. doi: 10.4049/jimmunol.0900298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teske S, Bohn AA, Regal JF, Neumiller JJ, Lawrence BP. Activation of the aryl hydrocarbon receptor increases pulmonary neutrophilia and diminishes host resistance to influenza A virus. American journal of physiology. 2005;289(1):L111–124. doi: 10.1152/ajplung.00318.2004. [DOI] [PubMed] [Google Scholar]
- 27.Girnita AL, Lee TM, McCurry KR, Baldwin WM, 3rd, Yousem SA, Detrick B, et al. Anti-human leukocyte antigen antibodies, vascular C4d deposition and increased soluble c4d in broncho-alveolar lavage of lung allografts. Transplantation. 2008;86(2):342–347. doi: 10.1097/TP.0b013e31817cf2e2. [DOI] [PubMed] [Google Scholar]
- 28.Ionescu DN, Girnita AL, Zeevi A, Duquesnoy R, Pilewski J, Johnson B, et al. C4d deposition in lung allografts is associated with circulating anti-HLA alloantibody. Transpl Immunol. 2005;15(1):63–68. doi: 10.1016/j.trim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Vanaudenaerde BM, Wuyts WA, Dupont LJ, Van Raemdonck DE, Demedts MM, Verleden GM. Interleukin-17 stimulates release of interleukin-8 by human airway smooth muscle cells in vitro: A potential role for interleukin-17 and airway smooth muscle cells in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22:1280–1283. doi: 10.1016/s1053-2498(02)01234-2. [DOI] [PubMed] [Google Scholar]
- 30.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 31.Rizzo M, SivaSai KSR, Smith MA, Trulock EP, Lynch JP, Patterson GA, et al. Increased expression of inflammatory cytokines and adhesion molecules by alveolar macrophages of human lung allograft recipients with acute rejection: Decline with resolution of rejection. J Heart and Lung Transplantation. 2000;19(9):858–865. doi: 10.1016/s1053-2498(00)00165-0. [DOI] [PubMed] [Google Scholar]
- 32.Elssner A, Jaumann F, Dobmann S, Behr J, Schwaiblmair M, Reichenspurner H, et al. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group Transplantation. 2000;70(2):362–367. doi: 10.1097/00007890-200007270-00022. [DOI] [PubMed] [Google Scholar]
- 33.Tosi MF, Stark JM, Smith CW, Hamedani A, Gruenert DC, Infeld MD. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. American Journal Respiratory Cell Molecular Biology. 1992;7(2):214–221. doi: 10.1165/ajrcmb/7.2.214. [DOI] [PubMed] [Google Scholar]
- 34.DiGiovine B, Lynch JP, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol. 1996;157(9):4194–4202. [PubMed] [Google Scholar]
- 35.Hirsch J, Elssner A, Mazur G, Maier KL, Bittmann I, Behr J, et al. Bronchiolitis obliterans syndrome after (heart-)lung transplantation. Impaired antiprotease defense and increased oxidant activity. American journal of respiratory and critical care medicine. 1999;160(5 Pt 1):1640–1646. doi: 10.1164/ajrccm.160.5.9902012. [DOI] [PubMed] [Google Scholar]
- 36.Riise GC, Andersson BA, Kjellstrom C, Martensson G, Nilsson FN, Ryd W, et al. Persistent high BAL fluid granulocyte activation marker levels as early indicators of bronchiolitis obliterans after lung transplant. Eur Respir J. 1999;14(5):1123–1130. doi: 10.1183/09031936.99.14511239. [DOI] [PubMed] [Google Scholar]
- 37.de Andrade JA, Christie JD, Alexander CB, Young KR, McGiffin DC, Zorn GL, et al. Association of reactive nitrogen species metabolites, myeloperoxidase, and airway inflammation in lung transplants. J Investig Med. 2001;49(2):166–172. doi: 10.2310/6650.2001.34043. [DOI] [PubMed] [Google Scholar]