Abstract
Neuregulin-1 (Nrg1) provides a key axonal signal that regulates Schwann cell proliferation, migration and myelination through binding to ErbB2/3 receptors. The analysis of a number of genetic models has unmasked fundamental mechanisms underlying the specificity of the Nrg1/ErbB signaling axis. Differential expression of Nrg1 isoforms, Nrg1 processing, and ErbB receptor localization and trafficking represent important regulatory themes in the control of Nrg1/ErbB function. Nrg1 binding to ErbB2/3 receptors results in the activation of intracellular signal transduction pathways that initiate changes in Schwann cell behavior. Here, we review data that has defined the role of key Nrg1/ErbB signaling components like Shp2, ERK1/2, FAK, Rac1/Cdc42 and calcineurin in development of the Schwann cell lineage in vivo. Many of these regulators receive converging signals from other cues that are provided by Notch, integrin or G-protein coupled receptors. Signaling by multiple extracellular factors may act as key modifiers and allow Schwann cells at different developmental stages to respond in distinct manners to the Nrg1/ErbB signal.
Keywords: Neuregulin, Shp2, Erk, Erbin, myelination
Introduction
The ErbB receptors were originally identified by virtue of their oncogenic potential [1–3]. Due to their important role in cancer, the structure and activity of ErbB receptors was extensively studied, and consequently the mechanism of ErbB receptor signaling is well understood today [4, 5]. Ligand binding to the extracellular domain of ErbB receptors promotes receptor dimerization and activation of the intracellular tyrosine kinase domain. Activated receptors phosphorylate each other on a number of tyrosine residues, which serve as docking sites for the downstream enzymes or adaptor proteins that mediate further intracellular signal transduction [6, 7]. ErbB receptors activate various signaling cascades, among them the Ras/Extracellular Signal Regulated Kinase 1/2 (Erk1/2) and Phosphatidylinositol-3-Kinase (PI3K)/Akt pathways, the mobilization of Ca2+, and the regulation of Ca2+-dependent Protein Kinase C (PKC) and NFAT activity (Fig. 1) [4]. Activation of these pathways elicits, alone or in concert, cellular responses like proliferation, differentiation, motility and cell survival. The signaling cascades that act downstream of ErbB receptors have been primarily analyzed in cultured fibroblasts or epithelial and carcinoma cells, and the results obtained provide a paradigm for ErbB signaling in other cell types.
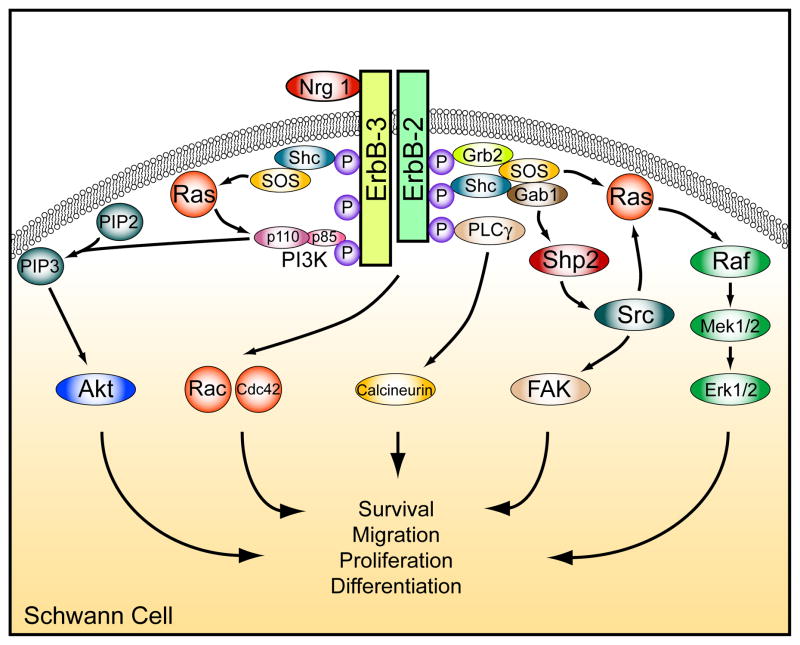
Figure 1. Nrg1/ErbB signaling.
Following Nrg1 binding, heteromeric ErbB2/3 receptors auto- and trans-phosphorylate specific tyrosine residues, and to these phosphorylated residues specific adaptor proteins and enzymes are recruited. This results in the activation of a number of downstream signaling pathways, including PI3K/Akt, Erk1/2, FAK, and Rac/Cdc42, and Ca2+ regulated pathways downstream of PLCγ lead to activation of calcineurin. Shp2 and Src are thought to regulate Erk1/2 signaling by modulating Ras, although other mechanisms also have been observed. These pathways regulate the activity of cytoplasmic and nuclear signal transduction molecules that mediate specific Schwann cell responses to the Nrg1 signal.
ErbB receptors take over the essential functions of many cell types and organs during development. Here, we review an extensively studied and well-understood example of ErbB receptor function in vivo, their role during Schwann cell development [8]. Both ErbB2 and ErbB3 receptors are required, but the two receptors exhibit dissimilar roles: ErbB3, but not ErbB2, binds extracellular ligands with high affinity, however ErbB3 is catalytically inactive, and it is ErbB2 that contributes the tyrosine kinase activity essential for signaling [9]. A neuronally-produced ligand, Nrg1, is recognized by ErbB2/3 during Schwann cell development [10]. Data obtained in cell culture indicate that peripheral axons present Nrg1 to the accompanying Schwann cells [11]. During early Schwann cell development, the Nrg1/ErbB signal promotes proliferation of the Schwann cell precursor pool, stimulates migration of Schwann cell precursors along the axon, and during late stages controls myelination. Thus, depending on the developmental stage, Schwann cells respond in distinct manners to Nrg1/ErbB signals.
Nrg1 in Schwann cell development
Schwann cells are generated from neural crest cells, and undergo extensive migration, proliferation and maturation before they terminally differentiate [12]. During these events, Schwann cells are in continuous contact with axons, and axonal cues, especially Nrg1, are driving forces for Schwann cell development. In cell culture, many aspects of Schwann cell biology are affected by Nrg1: (i) Nrg1 suppresses neuronal differentiation of neural crest stem cells while promoting or allowing glial differentiation; (ii) Nrg1 is required for survival of cultured Schwann cell progenitors; (iii) Nrg1 promotes proliferation and migration of Schwann cell precursors; (iv) Nrg1 provides signals essential for myelination [11, 13–15]. In vivo, early stages of Schwann cell development depend upon Nrg1/ErbB signaling, as evidenced by the near complete absence of Schwann cell progenitors in the developing peripheral nerve of ErbB2, ErbB3, or Nrg1 mutant mice [16–20]. This phenotype is specific to the Schwann cell lineage since the formation of the satellite glia within dorsal root ganglia is not impaired. Dissection of the cellular defects in ErbB2/3 mutant mice and zebrafish suggests that the underlying cellular mechanism involves restriction of the migratory and proliferative abilities of Schwann cell progenitors [21, 22].
Shortly after birth, immature Schwann cells have a choice of two fates which is dictated by the type and diameter of the axons they ensheath: (i) Schwann cells myelinate a single, large caliber axon or (ii) they ensheath multiple, small caliber axons to form a Remak bundle. Myelination insulates the axonal membrane and increases the speed at which electrical nerve impulses propagate, whereas unmyelinated axons lack this insulation and propagate action potentials at much slower rates. Interestingly, the level of axon-derived Nrg1-type III has been linked to Schwann cell commitment to a myelinating fate in vitro [11, 23]. Several transcription factors are known to control Schwann cell myelination, and Nrg1 promotes the expression of some of these, for example Oct6/SCIP and Egr2/Krox-20 [23, 24]. Thus, Nrg1 controls Schwann cell differentiation during the early postnatal period.
The subsequent extent of myelination is also dependent upon the precise levels of Nrg1. Schwann cell-specific disruption of ErbB2 leads to hypomyelination (Fig. 2A,B). Similarly, heterozygous Nrg1 deletion results in significant hypo-myelination, while neuronal overexpression of Nrg1 has the converse effect and leads to hypermyelination [25–27] . How axonal diameter is linked to Nrg1 expression levels remains open. Myelinated and non-myelinated sensory neurons might express different levels of Nrg1, or they might differ in their ability to process and present Nrg1 in the axonal membranes. It should be noted that the volume of myelin produced by a Schwann cell correlates tightly with the surface area of the associated axon [28]. Thus, Nrg1 density on different axons might be similar, and a doubling of the axonal surface area would therefore double the amount of Nrg1 presented to the Schwann cell. Lastly, Nrg1 signaling coordinates the interaction between non-myelinating Schwann cells and small diameter axons in Remak bundles [29, 30]. These data indicate that axonal Nrg1 signals functionally modulate both myelinating and non-myelinating Schwann cells.
Figure 2. Hypomyelination of ErbB2 and Shp2 mutant peripheral nerves.

Electron microscopic analysis of peripheral nerves from control mice (A), and from mice that carry Schwann cell-specific mutations of ErbB2 (B) or Shp2 (C) that were introduced using a Krox20cre allele. Note that the conditional ErbB2 and Shp2 mutant mice display a marked hypomyelination in the adult. (D) Quantification of myelin thickness in adult control (blue bars), conditional ErbB2 (red bars) and Shp2 (yellow bars) mutant mice. Displayed is a plot of the myelin thickness versus axon diameter. Note the similarity of the effects of the ErbB2 and Shp2 mutations.
Nrg1 and ErbB2/3 are expressed well into adulthood, suggesting a potential role in mature Schwann cell function [31, 32]. Indeed, early work on axotomized nerves indicated exogenous Nrg1 rescues apoptosis of Schwann cell associated with axons or neuromuscular nerve terminals [33, 34]. Furthermore, overexpression of a transdominant ErbB4 receptor in non-myelinating Schwann cells was reported to affect Schwann cell survival [30], whereas the inducible loss of Nrg1 in sensory neurons did not impair Schwann cell survival, but affected Remak bundle morphology and the thickness of the myelin sheath [29]. The inducible loss of ErbB2 in adult myelinating glia appears to have a limited effect on the maintenance of peripheral myelin or injury-induced Schwann cell proliferation and survival; if Nrg1 is required for re-myelination following injury has yet to be tested [35]. Interestingly, leprosy bacteria bind to and activate ErbB2 in myelinating Schwann cells, which causes demyelination [36].
Nrg1 isoforms and proteolytic processing
The Nrg1 gene spans more than 1.2 megabases of DNA, and it is thus one of the largest genes in the mammalian genome. Many different mRNA isoforms are produced from the gene by the use of distinct promoters and by alternative splicing. These messenger variants encode protein isoforms of distinct structure [8]. Each of the major isoform classes are encoded by mRNAs with distinct 5′ ends, indicating that they are generated by the use of different promoters. In accordance, a comparative expression analysis in the developing rat and mouse showed that the major isoform classes are expressed in different temporal and spatial patterns, and genetic experiments show that they take over distinct functions [10, 21]. All of these isoforms contain an EGF-like domain that alone is sufficient to bind and activate the receptor. The raison d'être for the isoform diversity is starting to emerge: genetic evidence suggests that different isoforms have distinct functions and, possibly, that they are also presented in a distinct manner to target cells.
Sensory neurons project to the periphery, convey nociceptive, mechanoreceptive, and proprioceptive information, and their axons are ensheathed by Schwann cells. Sensory neurons produce types I and III Nrg1. Type III Nrg1 is expressed by most, if not all sensory neurons, whereas type I is mainly expressed by proprioceptive neurons [10]. Analysis of isoform-specific Nrg1 mutations in the mouse demonstrated that the presence of the type III isoform suffices to prevent the early deficits in Schwann cell development present in Nrg1 null mutant mice, suggesting that it is type III Nrg1 that drives proliferation and survival of Schwann cell precursors [10]. Furthermore, hypermyelination of peripheral nerves can be induced by neuronal overexpression of type III Nrg1, whereas overexpression of type I Nrg1 does not affect peripheral myelination [26]. Thus, type III Nrg1 plays an important role during Schwann cell development and myelination, indicating this isoform is presented by the axon to the Schwann cells. In contrast, Nrg1 null mutation in sensory neurons, but not type III Nrg1-specific mutations, interferes with muscle spindle formation, indicating that the type I isoform induces formation of muscle spindles [37]. Type I and type III Nrg1 might locate to specific sub-cellular membrane compartments and therefore be accessible to the two distinct target cell types, Schwann cells and skeletal muscle. Alternatively, the release of the two isoforms by proteolysis might be controlled in such a manner that one isoform preferentially signals to glia, and the other to muscle. Indeed, proteolytic processing is an important aspect for Nrg1 function.
Bace1, a type I transmembrane aspartyl protease, is required for processing of the beta-site of amyloid precursor protein, and is essential for the generation of amyloid beta peptide in Alzheimer’s disease. Bace1 is preferentially expressed in neurons, and Bace1 activity can release protein fragments from neuronal membranes [38]. The major phenotype observed in Bace1 mutant mice is a hypomyelination of peripheral nerves and aberrant sorting of sensory axons, very similar to the changes seen in mice with heterozygous mutations in Nrg1 or Schwann cell-specific ErbB2 mutations [39]. In fact, Bace1 processes Nrg1 fragments in the stalk region in cell culture, and unprocessed Nrg1 accumulates in the brains of Bace1 mutant mice [39, 40]. Neural crest migration and heart development, two further Nrg1-dependent developmental processes, are apparently not impaired in Bace1 mutant mice. Also, despite the pronounced hypomyelination, early Schwann cell development appears to be normal. Bace1 therefore might not be the only protease that processes Nrg1 or alternatively, neural crest migration and heart development might not depend on the presence of cleaved Nrg1. Plausible too, is that reduced levels of processed Nrg1 can still drive these developmental events but later not suffice for normal myelination.
A large family of membrane-anchored zinc-dependent proteases, known as 'a disintegrin and metalloprotease' (ADAM) family, are key components in the ectodomain shedding of membrane-anchored proteins. ADAMs process several members of the EGF ligand family, like EGF itself, TGFalpha, amphiregulin, and HB-EGF [8, 41]. ADAMs are expressed in complex spatiotemporally controlled patterns in the organism, and are furthermore regulated post-transcriptionally, for instance by G-protein coupled receptor (GPCR) signaling [42]. Several lines of evidence that rely on co-transfection of Nrg1 and ADAM cDNAs indicate that ADAMs participate in Nrg1 processing [43, 44]. Different proteases might preferentially process different Nrg1 isoforms, for instance PMA (phorbol-12-myristate-13-acetate), an activator of PKC, increases the proteolysis only of type I, but not of type III in COS cells [45]. Furthermore, mutation of Nardilysin, which encodes a zinc-peptidase that cleaves selectively dibasic sites, causes hypomyelination. The underlying mechanism was attributed to an enhanced Bace1- or ADAM-dependent ectodomain shedding of Nrg1 [46] .
Receptor trafficking and localization: Erbin and ErbB2 signaling
Myelination is a complex morphogenetic event, and requires the spiral wrapping of the Schwann cell membrane around the axon. It is accompanied by a huge increase in the size of a Schwann cell, particular of its membrane area. During this process, only a small portion of the Schwann cell, the adaxonal membrane, contacts the axon, and only in that small area axonal signals can be received. Therefore, mechanisms must exist that ensure that ErbB receptors are present in the adaxonal membrane. The mechanism used to locate ErbB receptor to a particular membrane compartment has been extensively studied in C. elegans, where appropriate receptor localization is essential for signaling.
Signaling of the EGF receptor during development of C. elegans depends on a ternary complex consisting of three PDZ domain proteins. The complex (LIN-7, LIN-2, LIN-10) localizes the EGF receptor to the baso-lateral compartment of the epithelial vulval precursor cells [47]. The neighboring anchor cell provides the EGF-like ligand, and receptor activation depends on the location of the receptor in the membrane that faces the anchor cell. Many PDZ proteins exist in the mammalian genome, and one of these, Erbin, interacts with ErbB2 but not other ErbB receptors, and co-localizes with ErbB2 in the baso-lateral surface of epithelial cells [48]. In vitro studies suggested that Erbin may have a broad range of interaction factors, binding not only to ErbB2, but also to other proteins, like integrin-beta4, SMADs, ion channels or delta-catenin [49-52]. Early data emphasized the role of Erbin in locating the receptor to the baso-lateral membrane compartment in epithelial cells, but recent data implicate Erbin in stabilization and internalization of the ErbB2 protein. The first genetic analysis of Erbin revealed a role for Erbin in the myelination of peripheral nerves. Erbin null mutant mice displayed hypomyelination of peripheral nerves and aberrant axonal segregation of small-diameter afferent fibers, very similar to that seen in mice with mutations in type III Nrg1 or Schwann cell-specific ErbB2 knockouts [53]. When the PDZ domain of Erbin is selectively removed, precluding the interaction between Erbin and ErbB2, identical changes were observed. ErbB2 protein, but not mRNA levels were reduced in the peripheral nerves of such mutant mice, indicating that ErbB2 is unstable and that the reduced ErbB2 levels compromise Nrg1 signaling.
Nrg1 signal transduction in Schwann cells
Nrg1 activation of ErbB2/3 triggers a complex sequence of molecular interactions that result in the recruitment of adaptor proteins and enzymes to phospho-tyrosine residues on the receptors. Among these are Grb2, Shc, Sos, PLCγ, PI3K, and Src, and their binding and/or phosphorylation results in the activation of canonical signaling cascades, such as PI3K/Akt, Ras/Erk1/2, PLCγ, and focal adhesion kinase (FAK), which in turn direct changes in cytoplasmic processes and gene expression (Fig. 1) [54–59]. Progress continues to be made in identifying the signal transduction elements that mediate the effects of Nrg1 in Schwann cells. A link between Nrg1, PLCγ, and calcineurin/NFAT signaling in the control of myelination was recently identified: neural crest specific mutation of calcineurin caused a severe disruption of myelination without affecting early Schwann cell development (Fig. 1) [59]. FAK lies downstream of Nrg1 activation in Schwann cells [58] and conditional mutation of FAK in Schwann cells resulted in impaired axonal sorting and myelination [60]. Recent analyses implicate the cytoplasmic tyrosine kinase Src and Erk1/2 as major players downstream of Nrg1-ErbB in Schwann cells (see below).
Despite its enzymatic activity, the protein tyrosine phosphatase Shp2 functions as an activator of signaling, and this molecule is of particular importance for the regulation of the Ras/Erk1/2 pathway [61]. Mutation of the Drosophila homologue of Shp2, corkscrew, reproduces phenotypes observed in flies mutant for genes encoding tyrosine kinase receptors like torso or sevenless [62, 63]. In the mouse, null-mutations of Shp2 cause early embryonic lethality, and have been linked to deficits in FGF receptor signaling [64]. In humans, germline mutations in Shp2 have been found in patients suffering from Noonan and LEOPARD syndromes, two multisymptomatic developmental disorders, and also occur in several types of malignancies, such as the most common type of juvenile leukaemia, JMML [61, 65]. Neural crest specific mutation of Shp2 in mice results in a striking loss of Schwann cell progenitors in the peripheral nerve, while Shp2 inactivation in immature Schwann cells results in major deficits in myelination (Fig. 2C)[66]. These phenotypes are very similar to those observed in Nrg1/ErbB2/3 mutant mice (Fig. 2). Indeed, Nrg1-evoked cellular responses like proliferation and migration were virtually abolished in cultured Schwann cells lacking Shp2 [66].
Biochemical analyses demonstrated that Shp2 mutation in Schwann cells results in impaired sustained Erk1/2 activity in vivo and in vitro, but leaves PI3K/Akt activation unchanged [66]. Previous studies using pharmacological inhibitors implicated PI3K/Akt signaling as key in Schwann cell development and myelination, and suggested that Erk1/2 played a limited role [40, 56, 57, 67]. Recent genetic evidence that relies on the conditional mutation of Erk1/2 indicates that this notion has to be revisited [68]. Similar to the Shp2 mutation, inactivation of Erk1/2 in neural crest results in a lack of Schwann cell progenitors in the peripheral nerve, while Schwann cell specific mutation inhibits myelination (Newbern and Snider, unpublished data). These results suggest that Shp2-dependent, sustained activation of Erk1/2 is critical for the Nrg1-evoked effects in vivo. It will be interesting to further delineate the mechanisms by which the Shp2-Erk1/2 module controls Schwann cell myelination.
Further molecular analysis of cultured Shp2 mutant Schwann cells revealed a reduction in Src and FAK activity in response to Nrg1 [66]. Pharmacological inhibition of Src was able to reproduce the effects of the loss of Shp2 on Erk1/2 and FAK activity. Similarly, FGF receptor signaling was previously shown to depend on Shp2 for Src and sustained Erk1/2 activation [64]. The precise mechanism by which Shp2 regulates Src and sustained Erk1/2 activity remains unclear. The second kinase that is regulated via Nrg1/Shp2, FAK, is known to be required for Schwann cell proliferation in vivo [60]. In sum, it appears that Shp2 regulates the output of several signaling molecules, including FAK, Src, and Erk1/2, to elicit the proliferation, migration and myelination of Schwann cells in vivo (Fig. 1).
In Schwann cells, Nrg1 also regulates the activity of members of the Rho-GTPase family, Cdc42 and Rac1, which have been shown to control Schwann cell migration and proliferation in vivo [69–71]. Activation of Rac1 and Cdc42 has been linked to the c-Jun N-terminal kinase (JNK) and the p38 mitogen-activated protein kinase pathways (MAPK) [72, 73]. This connection is interesting since inhibition of JNK or p38 MAPK interferes with myelination in vitro [74–76]. Thus, ErbB signaling through the Rho GTPase family may directly modulate Schwann cell differentiation.
Network dynamics and the control of myelination: A summary and outlook
During Schwann cell development, axonal Nrg1 signals regulate the distribution of progenitors along the nerve, the size of the progenitor pool and the generation of myelinating or non-myelinating cells with a high degree of fidelity. Recent genetic analyses have correlated particular Nrg1/ErbB signaling cascades with Schwann cell growth, migration and myelination. However, the intrinsic mechanisms that couple Nrg1/ErbB signaling to proliferation and migration during early development, and to myelination at later stages remain open.
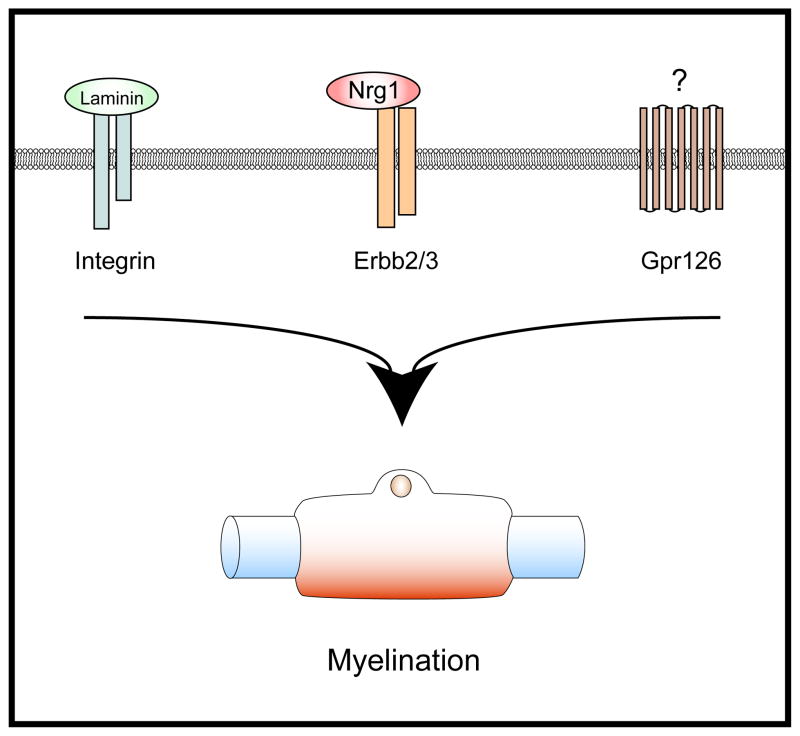
Input from multiple neuronal cues may modulate the outcome of Nrg1 signaling in Schwann cells. Among these are signals derived from Notch receptors, integrins or other growth factor receptors [77–80]. Notch signaling elevates levels of ErbB2 receptors and responsiveness to Nrg1 [77]. FAK, Src, Cdc42, and Rac1 have prominent roles in the signal transduction pathways downstream of integrins, which might alter convergent input arising from Nrg1. cAMP has long been known to drive myelination [81, 82], but the ligand/receptor complex that raises cAMP levels at onset of myelination was unknown. Work performed in zebrafish has recently identified a key role for a GPCR, Gpr126, in myelination. In Gpr126 mutant fish, Schwann cell development was arrested at a promyelinating state, and myelination was restored by the addition of the adenylate cyclase activator forskolin [83]. Thus, zebrafish genetics has finally identified the elusive receptor that drives myelination by raising cAMP levels, but the molecular nature of the ligand that activates Gpr126 remains unknown. In summary, Nrg1, cell adhesion components, and ligands of GPCRs act in conjunction to control Schwann cell development and myelination (Fig. 3). Much remains to be learned about the integration of the signals provided by these distinct molecules, and about the dynamic interaction of the downstream signaling cascades that shape development and differentiation of Schwann cells.
Figure 3.
Nrg1/ErbB signaling acts cooperatively with other extracellular cues to control myelination. Amongst many potentially important ligand/receptor complexes, key roles have been attributed to laminin/integrin and Gpr126 signaling in Schwann cell myelination in vivo.
The Schwann cell lineage provides an example of a cell type that strongly depends on Nrg1/ErbB signaling. Oligodendrocytes, the myelinating glia of the central nervous system, express ErbB receptors and respond to Nrg1 in vitro and in vivo. Recent experiments that relied on conditional mutations of all receptors known to bind Neuregulins did not reveal myelination deficits in the oligodendrocyte lineage, suggesting that the axonal control of myelination in vivo is different in the central and peripheral nervous system [84]. Nrg1/ErbBs also take over important roles in the physiology and pathophysiology of other organs like the heart or skeletal muscle [37, 85, 86]. Particularly interesting is recent work that correlates certain haplotypes of Nrg1 and the ErbB4 receptor with schizophrenia, a common mental disease in man [87, 88]. These findings triggered a wide interest for Nrg1 functions in the central nervous system [89]. Evidence emerged that assigns a role to Nrg1/ErbB4 in the migration of cortical inhibitory neurons and the formation of thalamocortical projections during development, as well as in synaptic functions of inhibitory and excitatory neurons [90–92]. Thus, understanding functions of Nrg1/ErbB signaling in the central nervous system is expected to define one mechanism responsible for schizophrenia, and might provide new avenues for therapeutic intervention.
Acknowledgments
We gratefully acknowledge Michael Strehle (MDC, Berlin) for the help with the manuscript and the drawings, Carola Griffel (MDC, Berlin) for providing the electron microscopic pictures used in Fig. 2, Alistair Garratt and Dominique Bröhl (MDC, Berlin) for critically reading the manuscript, and William Snider for his support and discussions. J.N. is supported by NRSA award F32 NS061591 from the NIH. Work in the laboratory of C.B on Nrg1/ErbB is supported by the DFG (Developmental Disturbances of the Nervous System, SFB 665) and the Helmholtz Association (HelMA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason Newbern, Email: jason_newbern@med.unc.edu.
Carmen Birchmeier, Email: cbirch@mdc-berlin.de.
References
- 1.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319(6050):226–30. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 2.Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, et al. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984;307(5951):521–7. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117(8):2051–8. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 5.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12(3):541–52. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 6.Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116(2):191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- 7.Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439(7073):168–74. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 8.Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56(14):1491–7. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- 9.Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284(1):54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 10.Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, et al. Isoform-specific expression and function of neuregulin. Development. 1997;124(18):3575–86. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 11.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47(5):681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 13.Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77(3):349–60. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 14.Aquino JB, Hjerling-Leffler J, Koltzenburg M, Edlund T, Villar MJ, Ernfors P. In vitro and in vivo differentiation of boundary cap neural crest stem cells into mature Schwann cells. Exp Neurol. 2006;198(2):438–49. doi: 10.1016/j.expneurol.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Sinanan A, Parkinson D, Parmantier E, Mirsky R, Jessen KR. Schwann cell development in embryonic mouse nerves. J Neurosci Res. 1999;56(4):334–48. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378(6555):386–90. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 17.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389(6652):725–30. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 18.Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23(2):273–83. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 19.Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, et al. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13(19):2538–48. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin W, Sanchez HB, Deerinck T, Morris JK, Ellisman M, Lee KF. Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc Natl Acad Sci U S A. 2000;97(3):1299–304. doi: 10.1073/pnas.97.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, et al. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12(12):1825–36. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, et al. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15(6):513–24. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Leimeroth R, Lobsiger C, Lussi A, Taylor V, Suter U, Sommer L. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent Progenitor cells. Dev Biol. 2002;246(2):245–58. doi: 10.1006/dbio.2002.0670. [DOI] [PubMed] [Google Scholar]
- 24.Murphy P, Topilko P, Schneider-Maunoury S, Seitanidou T, Baron-Van Evercooren A, Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122(9):2847–57. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- 25.Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol. 2000;148(5):1035–46. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304(5671):700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, et al. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26(12):3079–86. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith KJ, Blakemore WF, Murray JA, Patterson RC. Internodal myelin volume and axon surface area. A relationship determining myelin thickness? J Neurol Sci. 1982;55(2):231–46. doi: 10.1016/0022-510x(82)90103-4. [DOI] [PubMed] [Google Scholar]
- 29.Fricker FR, Zhu N, Tsantoulas C, Abrahamsen B, Nassar MA, Thakur M, et al. Sensory axon-derived neuregulin-1 is required for axoglial signaling and normal sensory function but not for long-term axon maintenance. J Neurosci. 2009;29(24):7667–78. doi: 10.1523/JNEUROSCI.6053-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, et al. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6(11):1186–93. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 31.Cohen JA, Yachnis AT, Arai M, Davis JG, Scherer SS. Expression of the neu proto-oncogene by Schwann cells during peripheral nerve development and Wallerian degeneration. J Neurosci Res. 1992;31(4):622–34. doi: 10.1002/jnr.490310406. [DOI] [PubMed] [Google Scholar]
- 32.Chen MS, Bermingham-McDonogh O, Danehy FT, Jr, Nolan C, Scherer SS, Lucas J, et al. Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol. 1994;349(3):389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- 33.Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16(19):6107–18. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trachtenberg JT, Thompson WJ. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379(6561):174–7. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- 35.Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, et al. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci. 2006;26(7):2124–31. doi: 10.1523/JNEUROSCI.4594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12(8):961–6. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 37.Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36(6):1035–49. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 38.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 39.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314(5799):664–6. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9(12):1520–5. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 41.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 42.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379(6565):557–60. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 43.Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol Cell Neurosci. 2000;16(5):631–48. doi: 10.1006/mcne.2000.0896. [DOI] [PubMed] [Google Scholar]
- 44.Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A. Roles of Meltrin beta /ADAM19 in the processing of neuregulin. J Biol Chem. 2001;276(12):9352–8. doi: 10.1074/jbc.M007913200. [DOI] [PubMed] [Google Scholar]
- 45.Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem. 2001;276(4):2841–51. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- 46.Ohno M, Hiraoka Y, Matsuoka T, Tomimoto H, Takao K, Miyakawa T, et al. Nardilysin regulates axonal maturation and myelination in the central and peripheral nervous system. Nat Neurosci. 2009;12(12):1506–13. doi: 10.1038/nn.2438. [DOI] [PubMed] [Google Scholar]
- 47.Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94(6):761–71. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, et al. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol. 2000;2(7):407–14. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- 49.Arikkath J, Israely I, Tao Y, Mei L, Liu X, Reichardt LF. Erbin controls dendritic morphogenesis by regulating localization of delta-catenin. J Neurosci. 2008;28(28):7047–56. doi: 10.1523/JNEUROSCI.0451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai F, Chang C, Lin X, Dai P, Mei L, Feng XH. Erbin inhibits transforming growth factor beta signaling through a novel Smad-interacting domain. Mol Cell Biol. 2007;27(17):6183–94. doi: 10.1128/MCB.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calin-Jageman I, Yu K, Hall RA, Mei L, Lee A. Erbin enhances voltage-dependent facilitation of Ca(v)1.3 Ca2+ channels through relief of an autoinhibitory domain in the Ca(v)1.3 alpha1 subunit. J Neurosci. 2007;27(6):1374–85. doi: 10.1523/JNEUROSCI.5191-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favre B, Fontao L, Koster J, Shafaatian R, Jaunin F, Saurat JH, et al. The hemidesmosomal protein bullous pemphigoid antigen 1 and the integrin beta 4 subunit bind to ERBIN. Molecular cloning of multiple alternative splice variants of ERBIN and analysis of their tissue expression. J Biol Chem. 2001;276(35):32427–36. doi: 10.1074/jbc.M011005200. [DOI] [PubMed] [Google Scholar]
- 53.Tao Y, Dai P, Liu Y, Marchetto S, Xiong WC, Borg JP, et al. Erbin regulates NRG1 signaling and myelination. Proc Natl Acad Sci U S A. 2009;106(23):9477–82. doi: 10.1073/pnas.0901844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HA, DeClue JE, Ratner N. cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res. 1997;49(2):236–47. [PubMed] [Google Scholar]
- 55.Li Y, Tennekoon GI, Birnbaum M, Marchionni MA, Rutkowski JL. Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival. Mol Cell Neurosci. 2001;17(4):761–7. doi: 10.1006/mcne.2000.0967. [DOI] [PubMed] [Google Scholar]
- 56.Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, et al. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24(30):6724–32. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci. 2000;20(12):4635–45. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vartanian T, Goodearl A, Lefebvre S, Park SK, Fischbach G. Neuregulin induces the rapid association of focal adhesion kinase with the erbB2-erbB3 receptor complex in schwann cells. Biochem Biophys Res Commun. 2000;271(2):414–7. doi: 10.1006/bbrc.2000.2624. [DOI] [PubMed] [Google Scholar]
- 59.Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, et al. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323(5914):651–4. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176(3):277–82. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer. Curr Opin Genet Dev. 2007;17(1):23–30. doi: 10.1016/j.gde.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Perkins LA, Larsen I, Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70(2):225–36. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- 63.Herbst R, Carroll PM, Allard JD, Schilling J, Raabe T, Simon MA. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell. 1996;85(6):899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 64.Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, et al. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell. 2006;10(3):317–27. doi: 10.1016/j.devcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–8. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 66.Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, et al. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci U S A. 2009;106(39):16704–9. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. Embo J. 2004;23(15):3061–71. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newbern J, Zhong J, Wickramasinghe RS, Li X, Wu Y, Samuels I, et al. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proc Natl Acad Sci U S A. 2008;105(44):17115–20. doi: 10.1073/pnas.0805239105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamauchi J, Miyamoto Y, Chan JR, Tanoue A. ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration. J Cell Biol. 2008;181(2):351–65. doi: 10.1083/jcb.200709033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, et al. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177(6):1051–61. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, et al. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177(6):1063–75. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81(7):1137–46. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 73.Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81(7):1147–57. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 74.Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181(4):625–37. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fragoso G, Robertson J, Athlan E, Tam E, Almazan G, Mushynski WE. Inhibition of p38 mitogen-activated protein kinase interferes with cell shape changes and gene expression associated with Schwann cell myelination. Exp Neurol. 2003;183(1):34–46. doi: 10.1016/s0014-4886(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 76.Haines JD, Fragoso G, Hossain S, Mushynski WE, Almazan G. p38 Mitogen-activated protein kinase regulates myelination. J Mol Neurosci. 2008;35(1):23–33. doi: 10.1007/s12031-007-9011-0. [DOI] [PubMed] [Google Scholar]
- 77.Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D'Antonio M, Parkinson DB, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12(7):839–47. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43(2):183–91. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23(2):561–7. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pietri T, Eder O, Breau MA, Topilko P, Blanche M, Brakebusch C, et al. Conditional beta1-integrin gene deletion in neural crest cells causes severe developmental alterations of the peripheral nervous system. Development. 2004;131(16):3871–83. doi: 10.1242/dev.01264. [DOI] [PubMed] [Google Scholar]
- 81.Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112(3):457–67. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salzer JL, Bunge RP. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980;84(3):739–52. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, et al. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325(5946):1402–5. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59(4):581–95. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garratt AN, Ozcelik C, Birchmeier C. ErbB2 pathways in heart and neural diseases. Trends Cardiovasc Med. 2003;13(2):80–6. doi: 10.1016/s1050-1738(02)00231-1. [DOI] [PubMed] [Google Scholar]
- 86.Leu M, Bellmunt E, Schwander M, Farinas I, Brenner HR, Muller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130(11):2291–301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- 87.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–43. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 88.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71(4):877–92. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–52. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125(1):127–42. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44(2):251–61. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 92.Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107(3):1211–6. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]