Abstract
The activation of the anticancer prodrug CPT-11, to its active metabolite SN-38, is primarily mediated by carboxylesterases (CE). In humans, three CEs have been identified, of which human liver CE (hCE1; CES1) and human intestinal CE (hiCE; CES2) demonstrate significant ability to hydrolyze the drug. However, while the kinetic parameters of CPT-11 hydrolysis have been measured, the actual contribution of each enzyme to activate the drug in biological samples has not been addressed. Hence, we have used a combination of specific CE inhibition and conventional chromatographic techniques to determine the amounts, and hydrolytic activity, of CEs present within human liver, kidney, intestinal and lung specimens. These studies confirm that hiCE demonstrates the most efficient kinetic parameters for CPT-11 activation, however, due to the high levels of hCE1 that are expressed in liver, the latter enzyme can contribute up to 50% of the total of drug hydrolysis in this tissue. Conversely, in human duodenum, jejunum, ileum and kidney, where hCE1 expression is very low, greater than 99% of the conversion of CPT-11 to SN-38 was mediated by hiCE. Furthermore, analysis of lung microsomal extracts indicated that CPT-11 activation was more proficient in samples obtained from smokers. Overall, our studies demonstrate that hCE1 plays a significant role in CPT-11 hydrolysis even though it is up to 100-fold less efficient at drug activation than hiCE, and that drug activation in the intestine and kidney are likely major contributors to SN-38 production in vivo.
Keywords: Carboxylesterase, CPT-11, SN-38, drug activation
1. INTRODUCTION
The anticancer drug CPT-111 (irinotecan, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin), is a prodrug that is activated by esterases to yield SN-38 (7-ethyl-10-hydroxycamptothecin), a potent topoisomerase I poison [1]. The majority of biochemical studies have demonstrated that this is achieved by carboxylesterases (CE) [2–6], however butyrylcholinesterases (BChE) can also effect this process, albeit with poor efficiency [7–10]. In humans, three CEs have so far been identified. The human liver CE, hCE1 (CES1), is predominantly expressed in the liver and demonstrates a preference for small, non-bulky substrates [11–13]. The human intestinal CE, hiCE (CES2), is expressed in the gut and the liver, and can hydrolyze much larger, more complex molecules. This is likely due to flexible domains present within the active site of the enzyme that allows for accommodation of these esters [14,15]. The human brain CE, hBr3 (CES3), is believed to be expressed in the epithelia that form part of the blood brain barrier [16], although this protein has not been exhaustively tested for its substrate specificity [17]. However, all of these enzymes have been compared for their ability to activate CPT-11 [4,5,15,17].
Results from these studies indicate that the hiCE is 64- to 100-fold more efficient than hCE1 at CPT-11 hydrolysis, with hBr3 being 20-fold poorer than the latter enzyme. Hence, due to the poor kinetic parameters for hBr3 with the drug (~2000-fold less efficient than hiCE), and its very limited pattern of expression, it is unlikely that this CE significantly contributes to drug activation in vivo. Based upon this biochemical and enzyme kinetic evidence, we and others have assumed that hiCE would be the major esterase responsible for CPT-11 hydrolysis in cancer patients [4,5,17]. We hypothesized therefore that using selective hiCE inhibitors [18,19], it would be possible to determine the amount of this enzyme present in biological samples using a simple substrate such as o-NPA. Simply, the difference in the enzyme activity assays in the presence and absence of the inhibitor should represent the amount of hiCE in the preparation. This could then be used as a measure of the ability of the sample to hydrolyze CPT-11. Such an approach would obviate the need for expensive and time consuming assays (HPLC with fluorescence detection) to monitor drug hydrolysis.
The studies described here sought to validate this approach by examining the ability of selective hiCE inhibitors [18,19] to prevent the conversion of CPT-11 to SN-38 in a series of human microsomal samples. However, we were unable to dramatically reduce drug activation in these specimens using these specific inhibitors, suggesting that other proteins within the extracts could mediate the hydrolysis of CPT-11. Therefore, we have used a combination of chromatography and biochemical assays using CE inhibitors (both specific and non-specific), to determine the contribution of other enzymes towards CPT-11 activation. These studies demonstrate that hCE1, while demonstrating poor kinetic parameters for this substrate, can significantly contribute to drug hydrolysis. Furthermore, our studies identify the kidney as a source of CPT-11 activation and demonstrate that drug hydrolysis is more proficient in lung tissue isolated from smokers.
2. MATERIALS AND METHODS
2.1 Chemicals, CPT-11 and chromatographic media
Reagents for biochemical assays and HPLC were provided by Sigma Aldrich (St. Louis, MO). CPT-11 was provided as a kind gift by Dr. J. P. McGovren. Sephacryl S-200 high resolution resin was purchased from Amersham Biosciences Inc. (Piscataway, NJ).
2.2 Enzymes, antibodies and inhibitors
Pure recombinant hiCE and hCE1, used as markers for western analyses and controls for biochemical assays, were prepared as previously described [20]. Anti-rabbit hiCE- and hCE1-specific antibodies were generated by repeated injection of animals with recombinant protein and subsequent purification from serum using IgG-based affinity chromatography. Benzil, a pan CE inhibitor [21], was purchased from Sigma Aldrich and the selective hiCE inhibitor 876 (N,N'-(2,3,5,6-tetrafluoro-1,4-phenylene)bis(4-bromobenzenesulfonamide)) was synthesized as previously reported [18].
2.3 Human microsomal samples
All human microsomal samples were obtained from commercial sources with no individual identifying markers. Liver microsomes were purchased from BD Biosciences (Woburn, MA) and CellzDirect (Durham, NC); intestinal samples were obtained from Celsis In Vitro Inc. (Baltimore, MA); and renal and pulmonary microsomes were purchased from Xeno Tech (Lenexa, KS).
2.4 Determination of CE activity
CE activity was measured by following the hydrolysis of o-nitrophenyl acetate (o-NPA) at 420nm as previously described [6,22]. Data were expressed as nmoles of o-nitrophenol (o-NP) produced per min per mg of protein (nmol/min/mg). One unit of enzyme activity is equivalent to 1nmol of nitrophenol produced per min. For enzymatic assays containing either 876 or benzil, bovine serum albumin was added to the samples to achieve a final protein concentration of 1.4mg/ml. This concentration was comparable to the levels of protein used for the CPT-11 activation assays, and eliminated any artifacts due to non-specific binding of the inhibitor to proteins present in the microsomal preparations.
2.5 Quantitation of CPT-11 and SN-38
To monitor enzymatic hydrolysis of CPT-11, samples were incubated with 5μM drug in 50mM Hepes pH7.4 for 60min at 37°C and the reactions terminated by the addition of an equal volume of acidified methanol (100μl 1M HCl/20ml methanol). Following centrifugation to remove any particulate matter, the concentrations of CPT-11 and SN-38 were determined in the supernatant using an HPLC separation and fluorescence detection system [23,24]. In all assays, less that 10% of the CPT-11 was hydrolyzed and sensitivity of detection was 20pg/μl and 1.5pg/μl for CPT-11 and SN-38, respectively.
2.6 Chromatographic separation of esterases
Esterases present within microsomal samples were separated on a Sephacryl S-200 HR gel (Bio-Rad Laboratories, Hercules, CA) filtration column (1.5 × 170 cm) equilibrated with 50mM Hepes, pH 7.4 containing 150mM NaCl, 150mM sucrose, and 0.5% Triton X-100 (buffer A). Briefly, microsomal samples were mixed with an equal volume, typically 500μl, of buffer A and incubated on ice for 10 minutes. Following clarification by centrifugation, the supernatant was applied to the column at a flow rate of 5ml/hr. After 100ml of buffer A had passed through the column, 3ml fractions were collected. These fractions were then subjected to CE and CPT-11 activity assays, as well as protein determinations and western analyses.
2.7 Western analysis of CEs
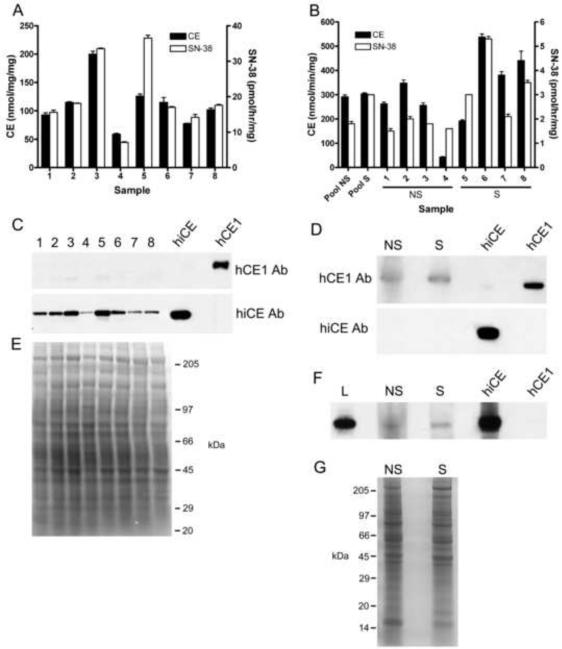
Detection of CEs on nylon membranes was accomplished using horseradish peroxidase-conjugated anti-rabbit secondary antibodies and visualized using enhanced ECL reagents and exposure to film. Since microsomal extracts were used for these assays and these samples lack (or have low levels of) suitable control proteins (e.g. β-tubulin, actin, TFIID), comparable gel loading for all westerns was verified by duplicate SDS-PAGE analysis and Coomassie blue staining. An example of this is shown in Figure 4. Levels of immunoreactive protein were quantitated using AlphaEase FC software (Alpha Innotech, San Leandro, CA).
Figure 4.
Analysis of CE expression in human lung and kidney microsomes.
A - Graph indicating the levels of CE activity (black bars) and SN-38 production (white bars) from individual kidney microsomal samples.
B – Graph indicating the levels of CE activity (black bars) and SN-38 production (white bars) by both pooled and individual lung microsomal samples. NS – non smokers; S – smokers.
C – Western analysis of hiCE and hCE1 expression in individual human kidney microsomal extracts. Purified hiCE and hCE1 protein (50ng) were included as positive controls.
D – Western analysis of hiCE and hCE1 expression in pooled human lung microsomal extracts isolated from 4 individuals. Purified hiCE and hCE1 protein (50ng) were included as positive controls. As before, NS – non smokers; S – smokers.
E – Coomassie blue stained gel demonstrating equal loading of the samples analyzed in panel C.
F – Prolonged exposure of a western analysis using larger amounts (200μg) of lung microsomal extracts following immunoblotting with hiCE antibody. L - human liver microsomes.
G – Coomassie blue stained gel demonstrating equal loading of the samples analyzed in the western analyses. As above, NS – non smokers; S – smokers.
3. RESULTS
3.1 Use of selective CE inhibitors to monitor enzyme levels in microsomal samples
Since human tissues can contain up to three different CEs (hiCE, hCE1 and hBr3), of which the former is the most efficient at activating CPT-11, we hypothesized that determining substrate hydrolysis in the presence or absence of selective hiCE inhibitors, might allow us to generate accurate estimates of the amounts of this protein in biological samples. The advantage of such an approach would be that the assay could be performed using a substrate other than CPT-11 (e.g. o-NPA), thereby making the procedure much more facile. Therefore, we assessed the amounts of o-NPA and CPT-11 that were hydrolyzed by liver microsomal extracts either in the presence or absence of the selective hiCE inhibitor 876, (N,N'-(2,3,5,6-tetrafluoro-1,4-phenylene)bis(4-bromobenzenesulfonamide); [18]). As indicated in Table 1, the actual loss (as well as the percentage loss) in drug hydrolyzing activities was not comparable for each sample. In addition, correlation coefficients for the loss in CE activity due to inhibition with 876 with the levels of CPT-11 hydrolyzed were poor, with an r2 value of 0.04 (data not shown).
Table 1.
CE and CPT-11 activities of human liver microsomal extracts in the presence or absence of 10μM of the hiCE specific inhibitor 876 ((N,N'-(2,3,5,6-tetrafluoro-1,4-phenylene)bis(4-bromobenzenesulfonamide)), or 10μM benzil.
| Sample | CE activity (nmol/mg/min) | CE activity + 876 (nmol/mg/min) | Loss in CE activity + 876 (nmol/mg/min) [%] | CE activity + benzil (nmol/mg/min) | Loss in CE activity + benzil (nmol/mg/min) [%] | CPT-11 hydrolysis (pmol/mg/hr) | CPT-11 hydrolysis + 876 (pmol/mg/hr) | Loss in CPT-11 hydrolysis + 876 (pmol/mg/hr) [%] | CPT-11 hydrolysis + benzil (pmol/mg/hr) | Loss in CPT-11 hydrolysis + benzil (pmol/mg/hr) [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| HH13 | 893 ± 113 | 672 ± 47 | 221 [24.8] | 389 ± 41 | 504 [56.4] | 33.9 ± 2.0 | 18.5 ± 0.14 | 15.4 [45.4] | 3.94 ± 0.12 | 30.0 [88.4] |
| HH61 | 5878 ± 65 | 3925 ± 125 | 1953 [33.2] | 406 ± 21 | 5472 [93.1] | 22.2 ± 0.5 | 13.4 ± 0.07 | 8.80 [39.7] | 2.80 ± 0.007 | 19.4 [87.4] |
| SD136 | 3196 ± 107 | 1719 ± 243 | 1477 [46.2] | 314 ± 35 | 2882 [90.1] | 55.3 ± 0.71 | 33.6 ± 0.64 | 21.8 [39.3] | 7.76 ± 0.18 | 47.6 [86.0] |
| SD004 | 5622 ± 165 | 3460 ± 234 | 2162 [38.5] | 552 ± 35 | 5070 [90.2] | 33.9 ± 0.21 | 19.3 ± 1.0 | 14.6 [43.0] | 4.38 ± 0.07 | 29.5 [87.1] |
| SD124 | 3408 ± 81 | 1997 ± 189 | 1411 [41.4] | 268 ± 11 | 3140 [92.1] | 27.1 ± 0.28 | 15.8 ± 0.28 | 11.3 [41.7] | 5.04 ± 0.09 | 22.1 [81.4] |
| SD128 | 6313 ± 443 | 4144 ± 401 | 2169 [34.4] | 713 ± 2.7 | 5600 [88.7] | 29.6 ± 1.2 | 16.5 ± 0.35 | 13.1 [44.3] | 4.03 ± 0.01 | 25.5 [86.4] |
| SD132 | 8194 ± 359 | 5018 ± 209 | 3176 [38.8] | 935 ± 79 | 7259 [88.6] | 26.6 ± 0.28 | 14.1 ± 0.50 | 12.6 [47.2] | 4.30 ± 0.01 | 22.3 [83.9] |
| HG03 | 6575 ± 262 | 3759 ± 84 | 2816 [42.8] | 640 ± 97 | 5935 [90.3] | 44.2 ± 0.14 | 29.6 ± 0.64 | 14.7 [33.1] | 6.62 ± 0.16 | 37.6 [85] |
| HK37 | 6332 ± 382 | 4052 ± 120 | 2280 [36] | 476 ± 58 | 5856 [92.5] | 35.4 ± 0.64 | 17.0 ± 0.57 | 18.4 [51.9] | 4.31 ± 0.02 | 31.0 [87.8] |
| HG93 | 4728 ± 361 | 2938 ± 87 | 1790 [37.9] | 521 ± 86 | 4207 [89.0] | 30.6 ± 0.14 | 18.2 ± 0.42 | 12.4 [40.5] | 4.58 ± 0.10 | 26.0 [85.0] |
| SD123 | 5945 ± 164 | 3874 ± 101 | 2071 [34.8] | 544 ± 29 | 5401 [90.9] | 30.0 ± 0.07 | 17 ± 0.14 | 13.0 [43.2] | 4.00 ± 0.09 | 30.0 [86.6] |
| SD104 | 5388 ± 139 | 3493 ± 146 | 1895 [35.2] | 507 ± 48 | 4881 [90.6] | 19.5 ± 0.78 | 13.7 ± 0.07 | 5.80 [29.8] | 3.89 ± 0.32 | 15.6 [80.0] |
| SD003 | 5243 ± 239 | 3434 ± 224 | 1809 [34.5] | 439 ± 42 | 4804 [91.6] | 54.6 ± 0.42 | 34.2 ± 0.92 | 20.5 [37.5] | 8.00 ± 0.01 | 46.6 [85.4] |
| HG74 | 7207 ± 636 | 4341 ± 152 | 2866 [39.8] | 854 ± 97 | 6353 [88.2] | 39.8 ± 0.57 | 25.8 ± 1.1 | 14.1 [35.3] | 6.25 ± 0.13 | 33.6 [84.3] |
| HG95 | 5125 ± 101 | 3285 ± 242 | 1840 [35.9] | 469 ± 45 | 4656 [90.9] | 24.5 ± 0.64 | 16.2 ± 0.21 | 8.30 [33.9] | 5.39 ± 0.09 | 19.1 [78.0] |
| SD120 | 4495 ± 163 | 2610 ± 102 | 1885 [41.9] | 367 ± 27 | 4128 [91.8] | 43.8 ± 0.50 | 18.5 ± 0.14 | 22.3 [57.7] | 6.00 ± 0.20 | 37.8 [86.4] |
| HH74 | 3706 ± 211 | 2235 ± 203 | 1471 [39.7] | 377 ± 7.8 | 3329 [89.8] | 15.3 ± 0.35 | 7.14 ± 0.23 | 8.22 [53.5] | 2.49 ± 0.04 | 12.9 [83.8] |
| HG103 | 3706 ± 126 | 2224 ± 202 | 1482 [40.0] | 448 ± 25 | 3258 [87.9] | 32.6 ± 0.35 | 19.4 ± 0.14 | 13.2 [40.1] | 4.22 ± 0.10 | 28.3 [87.0] |
| SD134 | 542 ± 26 | 345 ± 28 | 197 [36.4] | 322 ± 43 | 220 [40.6] | 17.5 ± 0.21 | 8.49 ± 0.18 | 8.96 [51.3] | 2.57 ± 0.03 | 14.9 [85.3] |
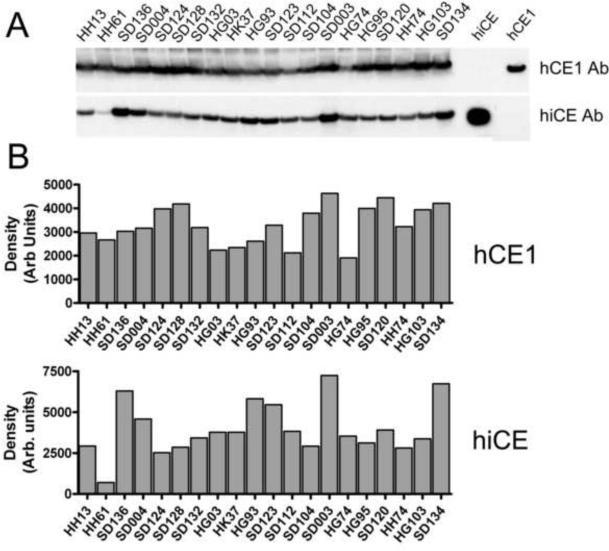
Furthermore, attempts to correlate the levels of SN-38 produced by these liver microsomal extracts, to the amounts of hiCE that could be detected by western analyses in these same samples (Figure 1, panel A and Figure 2, panel B), also yielded poor correlation coefficients (r2 = 0.22). Interestingly, the levels of hCE1 were relatively consistent amongst these liver microsomal preparations (maximal variation was seen between samples HG74 and SD003 of ~2.4-fold), whereas the levels of hiCE varied as much as 10.5-fold between extracts HH61 and SD003 (Figure 1, panels A and B). Overall however, these studies suggested that enzymes other than hiCE were present within these extracts that could activate CPT-11.
Figure 1.
Analysis of CE expression in human liver microsomes
A – Western analysis of hCE1 and hiCE expression in human liver microsomal extracts. Purified proteins (50ng of each) were used as positive controls and are indicated on the right hand side of the image.
B – Densitometric quantitation of the western analyses depicted in panel A.
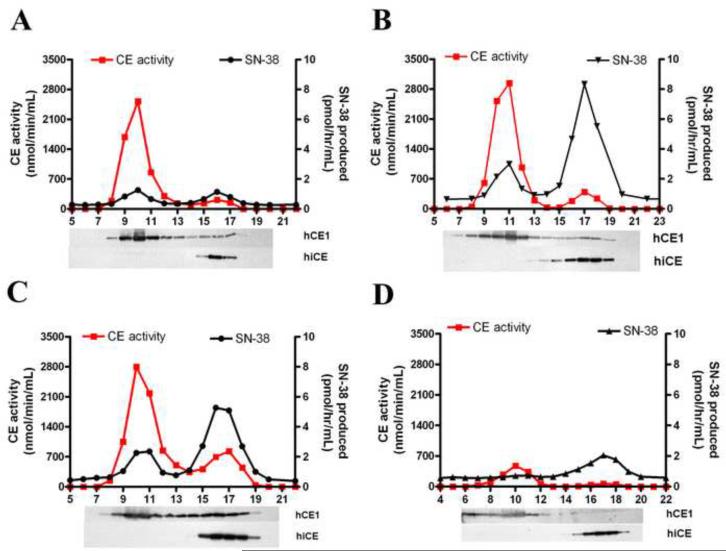
Figure 2.
Chromatographic separation of CEs expressed in human liver microsomes.
Four individual microsomal extracts (HH61, SD136, SD132 and HH13) were run on Sephacryl S-200HR and fractions were assayed for both CE activity using o-NPA and CPT-11 as substrates, and immuno-reactive protein. Each panel represents the elution profile for a different extract, with expression levels ranging from high hCE1, low hiCE (HH61; panel A); moderate hCE1, high hiCE (SD136; panel B); moderate hCE1, moderate hiCE (SD132; panel C); to low hCE1, moderate hiCE (HH13; panel D). CE activity and CPT-11 hydrolysis are indicated by the red and the black lines, respectively. Western analysis of the individual fractions, designed to identify hCE1 and hiCE, are aligned and indicated below each graph.
To evaluate the possibility that hCE1 may contribute to drug hydrolysis, we performed similar enzymatic assays in the presence of benzil (a potent pan inhibitor of mammalian carboxylesterases). Under these conditions, both the levels of CE activity and the yields of SN-38 produced were markedly reduced, typically by ~80–90% (Table 1). These results are consistent with the hypothesis that CEs are primarily responsible for CPT-11 activation in these extracts. However, since the levels of SN-38 produced by the same samples were considerably lower that that seen when using 876, our results indicate that hCE1 significantly contributed to CPT-11 hydrolysis.
3.2 Separation and analysis of esterases present in liver microsomal samples
Since our results indicated that other enzymes within the liver microsomal samples could hydrolyze CPT-11, we sought to identify these using chromatographic techniques. Therefore, we separated the proteins present within 4 extracts (HH61 (Figure 2, panel A), SD136 (panel B), SD132 (panel C) and HH13 (panel D)) using Sephacryl S-200HR resin. These particular samples were chosen since they demonstrated varying levels of drug hydrolysis and differing amounts of hiCE and hCE1 (see Table 1 and Figure 1).
The profiles of CE and CPT-11 hydrolysis obtained from the fractionated samples, as well as the levels of hiCE and hCE1 in the fractions are indicated in Figure 2. As can be seen, two peaks of CE activity are discernable in all samples. The majority of hCE1 elutes in fractions ranging from 8–13 and this represent the trimeric form of the protein. Smaller amounts elute in fractions 15–19 and represent monomeric hCE1. hiCE exclusively elutes in fractions 15–19 since it does not form oligomers. Examination of the ability of these fractions to hydrolyze CPT-11 indicated that while hiCE was clearly the most efficient enzyme at drug activation, appreciable amounts of SN-38 were produced by hCE1 (Figure 2). For example in HH61 (Figure 2, panel A), the yields of SN-38 were similar for both enzymes peaks indicating that for this sample, the contribution of hCE1 and hiCE towards CPT-11 activation was approximately equal. In contrast, with extract HH13 (Figure 2, panel D), which contains relatively little hCE1, the vast majority of the SN-38 produced was due to hydrolysis by hiCE. These results indicate that in individuals with relatively low hepatic expression of hiCE, hCE1 significantly contributes to CPT-11 activation.
3.3 CPT-11 hydrolysis by microsomes isolated from the intestine
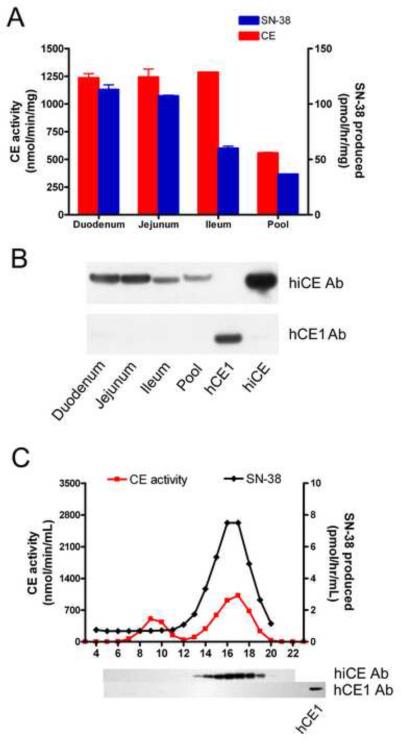
Since hiCE is known to be principally expressed in the intestine, we assessed the levels of CEs and CPT-11 hydrolysis in a pooled microsomal sample prepared from several individuals. As expected, high levels of CE were detected and this sample was proficient at drug activation (Figure 3, panels A and B). To determine in which compartment of the intestine CPT-11 hydrolysis was maximal, we obtained microsomal extracts isolated from the duodenum, jejunum and ileum from one individual, and determined the levels of CE activity, protein and drug activation by these different regions of the small intestine. As indicated in Figure 3, panel A, the overall levels of CE activity in these samples were high and were essentially comparable, however, the ileum demonstrated ~50% less CPT-11 hydrolysis than either the duodenum or the jejunum. This is consistent with the differences in the levels of hiCE in these samples as detected by western analyses (Figure 3, panel B). Furthermore, the levels of CPT-11 activation in these extracts were up to 7-fold higher than that seen in the liver microsomes (see Table 1 and Figure 3). This suggests that the small intestine is likely to be a major contributor towards CPT-11 activation in vivo.
Figure 3.
Analysis of CE expression in human intestinal microsomes.
A – CE and CPT-11 hydrolytic activities of microsomal extracts isolated from different regions of the small intestine. The duodenum, jejunum and ileum samples are isolated from the same individual, and the extract labeled `Pool' represents small intestinal microsomes mixed from 8 donors.
B – Western analysis of the samples described in A. Samples were probed with either an antibody recognizing hiCE (upper panel) or hCE1 (lower panel). As positive controls for these experiments, 50ng of purified proteins were included on the membrane.
C – Chromatographic profile of the CEs present within the duodenum microsomal extract. The extract was separated on Sephacryl S-200 and fractions were assayed in an identical fashion to that described in the legend for Figure 2. Western analyses for hiCE and hCE1 are indicated under the respective fractions. A positive control is included for hCE1 to confirm reactivity of the antibody.
To confirm that CPT-11 hydrolysis was mediated by hiCE in the duodenal sample, we separated the CEs by chromatography (as described for the liver extracts above) and assessed levels of enzymatic activity and protein by both biochemical and western analyses. As indicated in Figure 3, panel C, only hiCE was detectable within this sample, and this corresponded to the peak production of SN-38. However it should be noticed that a second esterase activity was seen in this sample (fractions 8–11). Whether this represents low levels of hCE1 that were not detected in the western analyses, or another esterase present within this tissue is unclear. Overall however, CPT-11 hydrolysis by the duodenum is exclusively mediated by hiCE.
3.4 CPT-11 hydrolysis by microsomes isolated from kidney and lung
Having observed efficient hydrolysis of CPT-11 by both hCE1 and hiCE in liver and intestinal microsomal samples, we examined the ability of microsomes from other tissues, including those made from normal human kidney, as well as lung tissue derived from both smokers and non-smokers. As indicated in Figure 4, panel A, the CE activity of the kidney specimens was significantly lower than that of the lung with activities ranging from 70–200 nmol/min/mg. In contrast, the lung samples demonstrated generally higher CE activities (Figure 4, panel B), with one sample exceeding 500 nmol/min/mg. However, when CPT-11 was used as a substrate, all of the kidney samples were more proficient at hydrolyzing the drug, with at least two samples being as active as liver microsomes. However, it should be noted that the kidney samples were considerably less efficient at CPT-11 activation as compared to the intestinal extracts.
Analysis of the different CEs expressed in the kidney and lung microsomes by western analysis indicated that greater than 95% of the enzymatic activity expressed in the kidney was due to hiCE (Figure 4, panels C and D). Under normal detection conditions, virtually no hiCE was present in the lung samples, and the majority of the CE activity was due to hCE1. However, when significantly larger amounts of microsomal extracts were used (200 vs 80μg), and the exposure time for the western analysis was prolonged, low but detectable levels of hiCE could be visualized (Figure 4F). While the difference was small, more hiCE was present in the smoker samples vs the non-smoker lung microsomes. Overall, the outcome of these studies is entirely consistent with the biochemical results described above.
3.5 Differential activation of CPT-11 by lung microsomes isolated from smokers and non-smokers
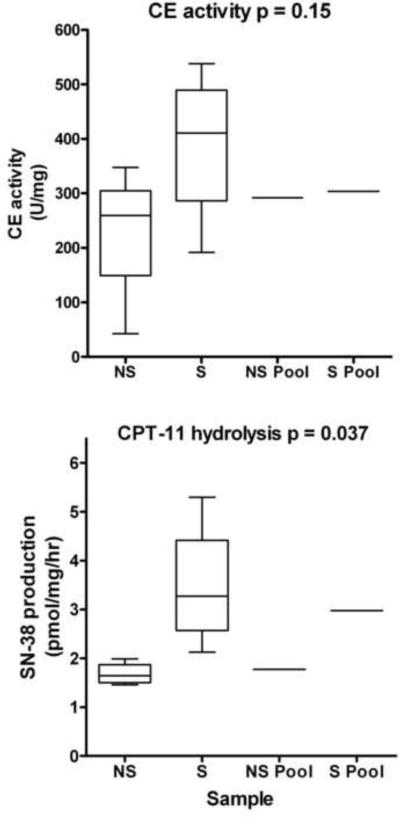
Analysis of the levels of CE activity and CPT-11 activation in microsomal samples isolated from smokers and non-smokers indicated that the former demonstrated ~2-fold greater production of SN-38 (Figure 4, panel B, and Figure 5). Indeed, even within this small sample size, these results were statistically significant (p=0.037). However, analysis of the CE activities indicated that while the mean values were different (227 vs 388 for non-smokers vs smokers), these results were not statistically significant (p=0.15). These results suggest that exposure of lung epithelia to compounds present in smoke induces the expression of hiCE, resulting in increased CPT-11 hydrolysis. We presume that this is due to transcriptional upregulation of expression, mediated by xenobiotics present in smoke.
Figure 5.
CE expression and CPT-11-converting activities of human lung microsomes.
Graphs comparing the levels of CE activity (upper panel) and CPT-11 hydrolysis (lower panel) in microsomal extracts isolated from lung tissue of both smokers (S) and non-smokers (NS). Data for individual samples were analyzed using the unpaired T-test, with p values for each dataset indicated on the graph. Samples marked as `Pool' represent mixed extracts obtained from 4 individuals.
4. DISCUSSION
We and others have previously reported that hiCE is the most efficient carboxylesterase at activating CPT-11 in humans [4,5,17,20]. This has been based entirely upon in vitro studies using either CEs purified from human liver specimens, or recombinant protein expressed in mammalian or insect cells. Based upon the poor kinetic parameters demonstrated by hCE1 (~25-fold higher Km and 90-fold lower kcat/Km than hiCE [4,5,17]), it has been assumed that this enzyme is unlikely to significantly contribute to CPT-11 activation. However, here we demonstrate that drug activation by hCE1 is significant, and in tissues exhibiting low levels of hiCE, the former may represent the major contributing enzyme towards drug activation.
This is exemplified by our studies that indicate that a selective hiCE inhibitor (876; N,N'-(2,3,5,6-tetrafluoro-1,4-phenylene)bis(4-bromobenzenesulfonamide); [18]) was unable to reduce the levels of CPT-11 activation in liver microsomal samples to zero (Table 1 and Figure 2). In addition, poor correlations were observed between the levels of enzyme activity and drug hydrolysis. In contrast, studies using benzil, an inhibitor of both hiCE and hCE1 [21], resulted in marked reductions both in CPT-11 hydrolysis, and CE activity (Table 1), for all liver microsomal extracts. This indicated that in these samples, CPT-11 activation was not entirely due to hiCE. Based upon these results, we sought to examine the contribution of different CEs towards drug hydrolysis in a panel of human tissues. Commercially available microsomal extracts were obtained (prepared in the absence of PMSF since this can inhibit esterases) and the levels of CE activity, drug activation and protein expression were determined. These results indicated that for samples rich in hiCE (e.g. intestine, kidney, and some liver specimens), these preparations tended to have higher levels of SN-38 production. This was exemplified by both pooled and individual sections, of the small intestine that demonstrated the highest levels of CPT-11 activation of any of the samples that we assayed. However in some liver samples, the overall levels of enzymatic activity did not match the levels observed on the Western analyses (e.g., SD134). Whether this is a result of the production of modified proteins via alternative splicing or SNPs, through posttranslational modification of the mature enzyme, or via another mechanism is unclear. We originally determined that a CE expressed in the human intestine could efficiently activate CPT-11 [5], and here we demonstrate that this enzyme (hiCE) is expressed throughout the small intestine. Indeed, for the duodenum and jejunum, the levels of drug hydrolysis ranged from 2- to 6-fold greater than that observed with liver microsomal samples. hiCE was detected in all three compartments of this section of the gut, and since the small intestine is approximately 6m long, has an absorptive area of ~250m2, and is rich in this enzyme, potentially, this organ represents the major site of CPT-11 activation in vivo.
However, even in tissues that demonstrated very low levels of hiCE (e.g., lung extracts), appreciable amounts of CPT-11 were hydrolyzed. For example, one sample isolated from a smoker (#6 in Figure 4. panel C) demonstrated similar levels of drug activation to some of the kidney samples, and only 4-fold less than a liver extract. Western analysis of this lung microsomal extract using standard conditions did not indicate the presence of hiCE. However when using very large amounts of extract and prolonged exposure of the membranes, we were able to detect the presence of higher levels of hiCE in the smoker samples, as compared to the non-smoker microsomes (Figure 4F). This would account for the increase in drug hydrolysis by this sample. Since CPT-11 is currently in clinical trials for lung cancer [25,26], and patients who have higher levels of CEs that can activate the drug in the tumor tissue might be expected to demonstrate improved response to the drug, it will be interesting to document whether better responses are observed in individuals who used tobacco. We have not assayed lung tumor tissue for the presence of the different CEs, but we presume that they express a similar cadre of enzymes to that contained in normal tissue.
Interestingly however, we noted that lung microsomal samples that were isolated from smokers demonstrated greater levels of SN-38 production than those prepared from non-smokers (Figure 4, panel C and Figure 5). While we appreciate that this represents a small sample size, these results were significant (p=0.037) and suggests that exposure to smoke increases CE activity. In addition, the level of CPT-11 hydrolysis generated by the pooled lung microsomes isolated from smokers was significantly greater than that seen in similar samples obtained from non-smokers (Figure 4, panel C and Figure 5). Whether this represents an upregulation of gene expression via transcriptional or translational processes, or potentially, a stabilization of the proteins themselves, is not known. Since CEs are thought to be responsible for xenobiotic metabolism, and may well be induced in response to such agents in cigarette smoke, this is likely due to an increase in gene transcription. Furthermore, a recent article has indicated that the expression of human CEs can be upregulated in the presence of dexamethasone [27], suggesting that induction of transcription of these genes may be a common event in the presence of xenobiotics.
However, this raises the interesting possibility that smokers may actually respond better to chemotherapy for lung tumors than non-smokers. Presumably, present and forthcoming clinical trails with CPT-11 developed to treat this tumor type will assess any correlation between efficacy and smoking status. Currently, we are in the process of obtaining more lung samples, including tumor specimens, to confirm our results and to evaluate the mechanism of increased CE activity in these extracts.
In conclusion, our studies demonstrate that the human liver CE hCE1 can significantly contribute to CPT-11 activation, both in tissues that contain, and lack, hiCE. For organs that contain large amounts of the former enzyme, such as the liver and lung, it is likely that a substantial proportion of drug hydrolysis is mediated by hCE1. Therefore, approaches designed to ameliorate CPT-11 toxicity and to maximize drug activation based upon enzyme specificity will require careful evaluation. Furthermore, our data indicates that hiCE expression can be induced following exposure to xenobiotics. This suggests that improved CPT-11 hydrolysis may be achievable in tumor tissue that would presumably result in enhanced antitumor activity. We are currently assessing whether such approaches are feasible, with the ultimate goal of developing protocols that may allow improved chemotherapeutic efficacy with this agent.
Acknowledgements
We thank Dr. J.P. McGovren (Pfizer) for the gift of CPT-11. This work was supported in part by NIH Grants CA108775, an NIH Cancer Center Core Grant CA21765, and by the American Lebanese Syrian Associated Charities and St Jude Children's Research Hospital (SJCRH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: Abbreviations: BChE – butyrylcholinesterase; CE – carboxylesterase; CPT-11 – irinotecan, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin; hBr3 – human brain CE, CES3; hCE1 - human liver CE, CES1; hiCE human intestinal CE, CES2; HPLC - high performance liquid chromatography; o-NP - o-nitrophenol; o-NPA - o-nitrophenyl acetate; SN-38 - 7-ethyl-10-hydroxycamptothecin.
6. References
- [1].Tanizawa A, Fujimori A, Fujimori Y, Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994;86:836–42. doi: 10.1093/jnci/86.11.836. [DOI] [PubMed] [Google Scholar]
- [2].Danks MK, Morton CL, Krull EJ, Cheshire PJ, Richmond LB, Naeve CW, et al. Comparison of activation of CPT-11 by rabbit and human carboxylesterases for use in enzyme/prodrug therapy. Clin Cancer Res. 1999;5:917–24. [PubMed] [Google Scholar]
- [3].Danks MK, Morton CL, Pawlik CA, Potter PM. Overexpression of a rabbit liver carboxylesterase sensitizes human tumor cells to CPT-11. Cancer Res. 1998;58:20–2. [PubMed] [Google Scholar]
- [4].Humerickhouse R, Lohrbach K, Li L, Bosron W, Dolan M. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res. 2000;60:1189–92. [PubMed] [Google Scholar]
- [5].Khanna R, Morton CL, Danks MK, Potter PM. Proficient metabolism of CPT-11 by a human intestinal carboxylesterase. Cancer Res. 2000;60:4725–8. [PubMed] [Google Scholar]
- [6].Potter PM, Pawlik CA, Morton CL, Naeve CW, Danks MK. Isolation and partial characterization of a cDNA encoding a rabbit liver carboxylesterase that activates the prodrug Irinotecan (CPT-11) Cancer Res. 1998;52:2646–51. [PubMed] [Google Scholar]
- [7].Guemei AA, Cottrell J, Band R, Hehman H, Prudhomme M, Pavlov MV, et al. Human plasma carboxylesterase and butyrylcholinesterase enzyme activity: correlations with SN-38 pharmacokinetics during a prolonged infusion of irinotecan. Cancer Chemother Pharmacol. 2001;47:283–90. doi: 10.1007/s002800000258. [DOI] [PubMed] [Google Scholar]
- [8].Morton CL, Wadkins RM, Danks MK, Potter PM. CPT-11 is a potent inhibitor of acetylcholinesterase but is rapidly catalyzed to SN-38 by butyrylcholinesterase. Cancer Res. 1999;59:1458–63. [PubMed] [Google Scholar]
- [9].Wierdl M, Morton CL, Danks MK, Potter PM. Isolation and characterization of a cDNA encoding a horse liver butyrylcholinesterase: evidence for CPT-11 drug activation. Biochemical Pharmacology. 2000;59:773–81. doi: 10.1016/s0006-2952(99)00389-5. [DOI] [PubMed] [Google Scholar]
- [10].Dodds HM, Rivory LP. The mechanism for the inhibition of acetylcholinesterases by irinotecan (CPT-11) Mol Pharmacol. 1999;56:1346–53. doi: 10.1124/mol.56.6.1346. [DOI] [PubMed] [Google Scholar]
- [11].Munger JS, Shi GP, Mark EA, Chin DT, Gerard C, Chapman HA. A serine esterase released by human alveolar macrophages is closely related to liver microsomal carboxylesterases. J Biol Chem. 1991;266:18832–8. [PubMed] [Google Scholar]
- [12].Kroetz DL, McBride OW, Gonzalez FJ. Glycosylation-dependent activity of baculovirus-expressed human liver carboxylesterases: cDNA cloning and characterization of two highly similar enzyme forms. Biochemistry. 1993;32:11606–17. doi: 10.1021/bi00094a018. [DOI] [PubMed] [Google Scholar]
- [13].Wadkins RM, Morton CL, Weeks JK, Oliver L, Wierdl M, Danks MK, et al. Structural constraints affect the metabolism of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (CPT-11) by carboxylesterases. Mol Pharmacol. 2001;60:355–62. doi: 10.1124/mol.60.2.355. [DOI] [PubMed] [Google Scholar]
- [14].Potter PM, Wadkins RM. Carboxylesterases – detoxifying enzymes and targets for drug therapy. Curr Med Chem. 2006;13:1045–54. doi: 10.2174/092986706776360969. [DOI] [PubMed] [Google Scholar]
- [15].Wierdl M, Tsurkan L, Hyatt JL, Hatfield MJ, Edwards CC, Danks MK, et al. An improved human carboxylesterase for use in enzyme/prodrug therapy with CPT-11. Cancer Gene Therap. 2008;15:183–92. doi: 10.1038/sj.cgt.7701112. [DOI] [PubMed] [Google Scholar]
- [16].Mori M, Hosokawa M, Ogasawara Y, Tsukada E, Chiba K. cDNA cloning, characterization and stable expression of novel human brain carboxylesterase. FEBS Lett. 1999;458:17–22. doi: 10.1016/s0014-5793(99)01111-4. [DOI] [PubMed] [Google Scholar]
- [17].Sanghani SP, Quinney SK, Fredenburg TB, Davis WI, Murry DJ, Bosron WF. Hydrolysis of irinotecan and its oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin and 7-ethyl-10-[4-(1-piperidino)-1-amino]-carbonyloxycamptothecin, by human carboxylesterases CES1A1, CES2, and a newly expressed carboxylesterase isoenzyme, CES3. Drug Metab Dispos. 2004;32:505–11. doi: 10.1124/dmd.32.5.505. [DOI] [PubMed] [Google Scholar]
- [18].Hicks LD, Hyatt JL, Stoddard S, Tsurkan L, Edwards CC, Wadkins RM, et al. Improved, selective, human intestinal carboxylesterase inhibitors designed to modulate 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (Irinotecan; CPT-11) toxicity. J Med Chem. 2009;52:3742–52. doi: 10.1021/jm9001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wadkins RM, Hyatt JL, Yoon KJ, Morton CL, Lee RE, Damodaran K, et al. Identification of novel selective human intestinal carboxylesterase inhibitors for the amelioration of irinotecan-induced diarrhea: Synthesis, quantitative structure-activity relationship analysis, and biological activity. Mol Pharmacol. 2004;65:1336–43. doi: 10.1124/mol.65.6.1336. [DOI] [PubMed] [Google Scholar]
- [20].Morton CL, Potter PM. Comparison of Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, Spodoptera frugiperda and COS7 cells for recombinant gene expression: Application to a rabbit liver carboxylesterase. Mol Biotechnol. 2000;16:193–202. doi: 10.1385/MB:16:3:193. [DOI] [PubMed] [Google Scholar]
- [21].Wadkins RM, Hyatt JL, Wei X, Yoon KJ, Wierdl M, Edwards CC, et al. Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J Med Chem. 2005;48:2905–15. doi: 10.1021/jm049011j. [DOI] [PubMed] [Google Scholar]
- [22].Beaufay H, Amar-Costesec A, Feytmans E, Thines-Sempoux D, Wibo M, Robbi M, et al. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974;61:188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guichard S, Morton CL, Krull EJ, Stewart CF, Danks MK, Potter PM. Conversion of the CPT-11 metabolite APC to SN-38 by rabbit liver carboxylesterase. Clin Cancer Res. 1998;4:3089–94. [PubMed] [Google Scholar]
- [24].Morton CL, Iacono L, Hyatt JL, Taylor KR, Cheshire PJ, Houghton PJ, et al. Metabolism and antitumor activity of CPT-11 in plasma esterase-deficient mice. Cancer Chemother Pharmacol. 2005;56:629–36. doi: 10.1007/s00280-005-1027-y. [DOI] [PubMed] [Google Scholar]
- [25].Jazieh AR, Komrokji R, Gupta A, Patil S, Flora D, Knapp M, et al. Phase II trial of thalidomide, irinotecan and gemcitabine in chemonaive patients with advanced non-small cell lung cancer. Cancer Invest. 2009;27:932–6. doi: 10.3109/07357900801944856. [DOI] [PubMed] [Google Scholar]
- [26].Spigel DR, Greco FA, Zubkus JD, Murphy PB, Saez RA, Farley C, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol. 2009;4:1555–60. doi: 10.1097/JTO.0b013e3181bbc540. [DOI] [PubMed] [Google Scholar]
- [27].Zhu WZ, Song L, Zhang H, Matoney L, Lecluyse E, Yan BF. Dexamethasone differentially regulates expression of carboxylesterase genes in humans and rats. Drug Metab Dispos. 2000;28:186–91. [PubMed] [Google Scholar]