Abstract
Poliomyelitis has appeared in epidemic form, become endemic on a global scale, and been reduced to near-elimination, all within the span of documented medical history. Epidemics of the disease appeared in the late 19th century in many European countries and North America, following which polio became a global disease with annual epidemics. During the period of its epidemicity, 1900–1950, the age distribution of poliomyelitis cases increased gradually. Beginning in 1955, the creation of poliovirus vaccines led to a stepwise reduction in poliomyelitis, culminating in the unpredicted elimination of wild polioviruses in the United States by 1972. Global expansion of polio immunization resulted in a reduction of paralytic disease from an estimated annual prevaccine level of at least 600,000 cases to fewer than 1,000 cases in 2000. Indigenous wild type 2 poliovirus was eradicated in 1999, but unbroken localized circulation of poliovirus types 1 and 3 continues in 4 countries in Asia and Africa. Current challenges to the final eradication of paralytic poliomyelitis include the continued transmission of wild polioviruses in endemic reservoirs, reinfection of polio-free areas, outbreaks due to circulating vaccine-derived polioviruses, and persistent excretion of vaccine-derived poliovirus by a few vaccinees with B-cell immunodeficiencies. Beyond the current efforts to eradicate the last remaining wild polioviruses, global eradication efforts must safely navigate through an unprecedented series of endgame challenges to assure the permanent cessation of all human poliovirus infections.
Keywords: epidemiology, history of medicine, poliomyelitis, poliovirus, vaccines
From the viewpoint of medical history, the epidemiology of poliomyelitis provides an intriguing and instructive case study. Each new stage in the history of poliomyelitis was unpredicted at the time of its occurrence. First, polio is one of the few major diseases whose appearance in epidemic guise was so recent that it was very well documented, together with its emergence as a worldwide scourge. Second, the application of 2 different vaccines—inactivated poliovirus vaccine (IPV) or Salk vaccine (1) and oral poliovirus vaccine (OPV) or Sabin vaccine (2)—has resulted in a dramatic reduction in paralytic poliomyelitis, constituting one of the most successful public health programs ever conducted on a global scale (3). Third, the “endgame” in polio eradication has offered some unexpected challenges that are so difficult it is hard to foresee the eventual outcome. In this review, we highlight these phenomena, offer some speculative epidemiologic interpretations, and update an earlier review of poliomyelitis epidemiology published about 30 years ago (4), before the launch of international polio eradication initiatives (5, 6).
BACKGROUND: HUMAN INFECTION WITH POLIOVIRUSES
Biology of poliovirus
Polioviruses are enteroviruses that are transmitted from person to person following excretion in feces and pharyngeal secretions, mainly via the hand-to-hand-to-mouth route. Because the poliovirus receptor is only expressed on cells of humans and a few subhuman primate species, there are no known extrahuman reservoirs (7, 8). Following infection, the virus replicates in the gastrointestinal tract and may cause viremia (9, 10). Occasionally, the virus then invades the central nervous system and destroys lower motor neurons, causing a clinically distinctive flaccid paralysis without permanent sensory loss (11). The average incubation period for paralysis is approximately 10 days (range, 5–25 days) (12, 13). Only 1 in 150 primary poliovirus infections causes paralytic poliomyelitis; since most infections are subclinical, paralytic cases represent only the “tip of the epidemiologic iceberg” (4). Polioviruses can be sorted into 3 different antigenic types (types 1, 2, and 3) that are based on their ability to induce protection against second paralytic attacks (14) and are confirmed by neutralization tests (15). In the prevaccine era in the United States (16), it was observed that the 3 poliovirus types varied substantially in their paralytogenicity (Table 1); type 1 accounted for approximately 80% of paralytic cases (17, 18).
Table 1.
Ratio of Paralytic Poliomyelitis Cases to Number of Infections for the 3 Serotypes of Poliovirus, Assuming an Overall Ratio of 1:150a
| Poliovirus Serotype | % of Paralytic Cases | No. of Paralytic Cases per 100 Infections | No. of Infections per Paralytic Case |
| 1 | 79 | 0.526 | 190 |
| 2 | 8 | 0.053 | 1,886 |
| 3 | 13 | 0.087 | 1,149 |
| Total | 100 | 0.667 | 150 |
The distribution of paralytic cases by serotype was based on unpublished laboratory studies on typing of poliovirus isolates for the United States for 1955, as reported by the Centers for Disease Control and Prevention (17). The ratio of 0.667 paralytic cases per 100 infections is the median of values from the 15 studies cited in Table 9 (4). The breakdown by serotype was computed from these data.
IPV is formulated as a trivalent product containing a representative virus isolate of each antigenic type. OPV is usually formulated as a trivalent product, but monovalent OPV (mOPV) formulations for each serotype (mOPV1, mOPV2, and mOPV3) were used in the United States from 1961 to 1964, and both monovalent (primarily mOPV1) and bivalent formulations have been used in other countries since the 1960s (19). Trivalent OPV is used in many countries for routine immunization and supplemental immunization activities (mass campaigns); mOPV1 and mOPV3 were licensed in 2005 and bivalent OPV (types 1 and 3) was licensed in 2009 for use in supplemental immunization activities in polio-endemic countries (20, 21). The properties of both vaccines have been described in detail (22, 23).
Elimination and eradication of poliovirus
Various definitions have been used to describe the stages of prevention of infectious diseases, ranging from local control of the disease to elimination of disease or infection within a defined geographic area to global eradication of the infectious agent (24). It has been possible to certify the disappearance of indigenous wild polioviruses in entire countries and large geographic regions (25–27) because the nucleotide sequences of wild polioviruses indigenous to different parts of the world are readily distinguishable (28). In this review, we have adopted these general definitions (24); we use “elimination” to mean the absence of circulating indigenous wild polioviruses and “eradication” to mean the absence of circulating vaccine-derived poliovirus (VDPV) as well as wild polioviruses.
POLIOMYELITIS EMERGES: ANCIENT HISTORY AND THE EARLY OUTBREAKS, THROUGH 1916
Early history
Although the historical record is very fragmentary and must be interpreted with caution, there is a general consensus that isolated cases of poliomyelitis have been occurring for many millennia (18). The most compelling ancient case is pictured on an Egyptian stele dating from the 18th dynasty (1580–1350 BCE) showing an adult with a withered, flaccid leg and crutch; the image is strikingly similar to a modern image of a young man with paralytic poliomyelitis (29). Another convincing example is the description by Walter Scott of his attack of acute “infantile paralysis” at age 18 months in 1773, which left him with a permanent limp (18). The disease's striking presentation, in which previously healthy infants underwent an acute febrile illness followed by localized paralysis, would have made outbreaks conspicuous. However, few if any cases were reported until late in the 19th century.
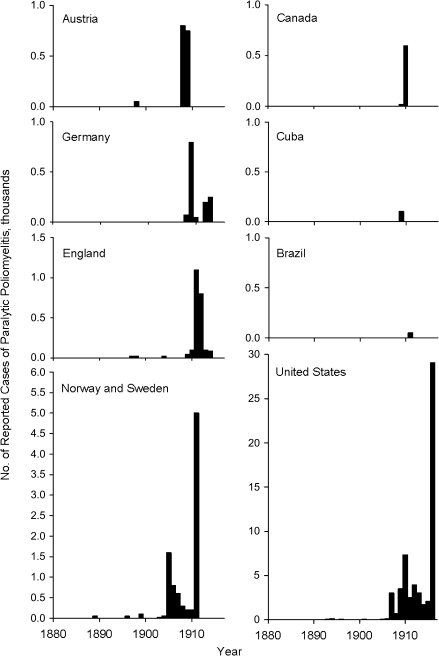
Beginning around 1880, a series of outbreaks of infantile paralysis were reported from several Scandinavian countries and the United States. The abrupt appearance of epidemic poliomyelitis is illustrated in Figure 1, which shows data from the countries where the first outbreaks were recorded (30). Most remarkable is the almost simultaneous appearance of outbreaks in European countries and the United States. Also notable is the absence of outbreaks in the rest of the world, as illustrated by Cuba and Brazil.
Figure 1.
Reported numbers of paralytic cases in poliomyelitis epidemics occurring between 1880 and 1916, by country and year, including the countries where large outbreaks were first observed. Data were obtained from chart 1 in the article by Lavinder et al. (30).
Polio deconstructed: the appearance of epidemics
What accounts for this striking phenomenon? The most probable hypothesis is that outbreaks were associated with an increase in the age at which poliovirus infection was occurring (4). In the pre-epidemic era, enteric infections were so ubiquitous that most infants were infected within 6–12 months, at a time when they had circulating antibodies passively derived from their nursing mothers. Although serum antibodies did not prevent enteric infection, they were sufficient to preclude viremia, thereby avoiding invasion of the central nervous system and paralysis. The result was the acquisition of active immunity under the cover of passive protection. However, with the advent of improved personal hygiene and public sanitation, the transmission of enteric infections was delayed so that some infants were first infected after 12 months of age, when levels of passive antibodies had waned, reducing the barrier against invasion of the central nervous system. Consistent with this hypothesis, all of the early outbreaks occurred among very young children, and the disease was known as “infantile paralysis.”
What is the evidence for this hypothetical explanation? Although the evidence is circumstantial, there are a number of observations that support the hypothesis. First, the earliest outbreaks occurred in countries where hygiene and sanitation were most advanced. As public health improved in less developed countries, outbreaks followed, over a period of 60 years from about 1890 to 1950 (18, 31). Second, once polio became established, the age distribution gradually increased over many years, consistent with an increasing delay in initial infections (discussed further below). Third, in pre-epidemic countries, a high proportion of infants transitioned from passive antibodies to active antibodies without a seronegative gap (32).
Additional evidence for the hypothesis is provided by the classic study by Paul and Horstmann (32) in Casablanca, Morocco (Table 2). In the 1950s, Casablanca had 2 sizeable populations, native Moroccans and Europeans. During the period 1947–1953, there were cases of paralytic poliomyelitis in both populations, but the attack rate was 20-fold higher in the European sector. Furthermore, the Moroccan cases occurred mainly in infants, while many of the European cases appeared in older children and young adults. It appears that Casablanca was a microcosm of the dynamics that led to the appearance of poliomyelitis as an epidemic disease. In 2010, the age distribution of paralytic poliomyelitis in Nigeria (33) and India (34) is similar to that seen in the native population of Casablanca in the 1950s.
Table 2.
Incidence of Paralytic Poliomyelitis in European and Native Moroccan Populations, Casablanca, Morocco, 1947–1953a
| Cultural Background |
||
| European | Moroccan | |
| Total population | 125,000 | 530,000 |
| No. of paralytic poliomyelitis cases | 117 | 25 |
| Average annual attack rate per 100,000 population | 13.4 | 0.7 |
| Age group of patients from 1953, years | ||
| <2 | 8 | 9 |
| 2–9 | 15 | 2 |
| 10–39 | 5 | 0 |
Data were obtained from Paul and Horstmann (32).
POLIOMYELITIS ASCENDANT: ANNUAL EPIDEMICS IN THE UNITED STATES, 1916–1954
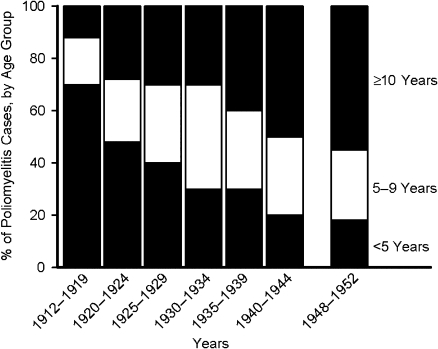
In the United States, beginning in the early 1900s, annual epidemics of poliomyelitis occurred with regularity until the introduction of IPV in 1955. During these years, the age distribution gradually increased, as illustrated in Figure 2 (35). Again, this suggests that gradual improvements in sanitation and hygiene reduced the circulation of enteric infections. However, in the instance of poliomyelitis, these advances did not abate the disease but merely deferred it to older age groups.
Figure 2.
Age distribution of patients with poliomyelitis (paralytic and nonparalytic) in Massachusetts, 1912–1952. Data were obtained from Dauer (35).
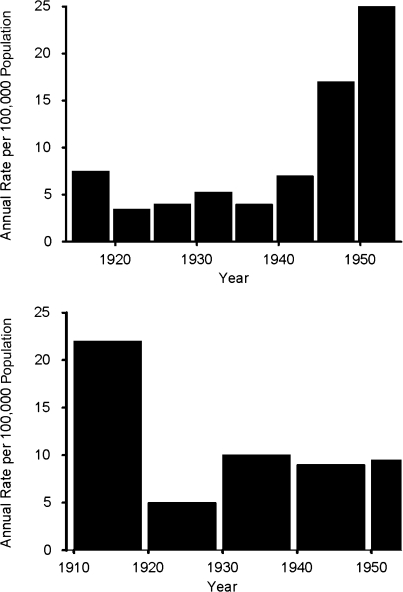
More disputable is the question of whether the increasing age of poliovirus infection was accompanied by an increase in disease burden. Figure 3 shows 2 contrasting data sets (36–40). The upper panel sets forth data for the United States in which average incidence appears to be quite constant from 1915 to 1944, with an increase during the period 1945–1954. However, during the 1945–1954 time period, the numbers are approximately doubled by the inclusion of nonparalytic cases, which were not reported prior to 1945. Furthermore, another large data set for New York City, shown in the lower panel of Figure 3, failed to show any increase in average annual incidence during the period 1910–1954.
Figure 3.
Annual poliomyelitis attack rates per 100,000 population in the United States (top) and New York City (bottom) during the first half of the 20th century. Upper panel: poliomyelitis incidence by 5-year period, United States, 1915–1954. Reports for 1915–1944 were almost entirely on cases of paralytic poliomyelitis, while reports for 1945–1954 comprised approximately equal numbers of paralytic and nonparalytic cases. Data were obtained from Serfling and Sherman (36) Sabin (37), and the Centers for Disease Control and Prevention (40). Lower panel: poliomyelitis incidence by 10-year period, New York City, 1910–1954. Data were obtained from Sabin (37), Greenberg et al. (38), and Siegel et al. (39).
Polio deconstructed: does the case:infection ratio increase with age?
Another perspective on this question is provided by prospective studies of the age-specific case:infection ratio. Table 3 shows data from a 1952 study in which the population was sampled for antibodies before and after an outbreak, to estimate the number of seroconverters in different age groups (41). The number of paralytic cases per 100 seroconverters was then computed for each age group. Although the case:infection ratio may have been somewhat lower for infants, there was no evidence of an increased ratio from age 3 years to age 14 years. This study is consistent with the New York City data, which failed to show an increase in incidence during a period when the age distribution gradually rose (38, 39).
Table 3.
Age-Specific Case:Infection Ratios Observed During a Poliomyelitis Outbreak Among Children Under Age 15 Years, North Carolina, 1948a
| Age Group, years | Total Population | Proportion of Seroconverters | No. of Seroconverters | No. of Paralytic Cases | No. of Cases Per 100 Seroconverters |
| <1 | 1,800 | 5/20 | 450 | 3 | 0.66 |
| 1–2 | 3,900 | 10/39 | 1,000 | 10 | 1.00 |
| 3–4 | 3,600 | 7/34 | 741 | 12 | 1.62 |
| 5–9 | 7,300 | 8/56 | 1,042 | 25 | 2.40 |
| 10–14 | 6,300 | 5/44 | 716 | 13 | 1.82 |
The proportion of seroconverters is the ratio of the number of seroconverters to the total number of subjects tested and was multiplied by the age-specific population for estimation of the number of seroconverters in the population. Data were obtained from Melnick and Ledinko (41).
In addition to the case:infection ratio, there are other ways to ask whether there is an age-specific increase in the severity of paralytic poliomyelitis. The case-fatality rate is the proportion of paralytic patients who die during the acute phase of the disease (within 1–2 months of onset, in contrast to the postpolio syndrome, which has onset many years after the acute illness). Another perspective is the distribution of sites of paralysis among paralytic cases, which reflects the severity of the acute illness, the risk of death, and the degree of long-term disability. One of the best studies of these parameters is the report by Olin (42), who analyzed data for Sweden for the years 1905–1950, a period during which there was a stepwise increase in the age distribution. Table 4 shows the case-fatality rates in Sweden during this period, which were remarkably stable over a 40-year interval. However, when the same data set is analyzed on an age-specific basis (Table 5), a quite different picture emerges. The case-fatality rate increases very substantially with age, and this reflects the age-specific differences in the distribution of paralysis. At the youngest age (<3 years), only 19% of cases have involvement of either both arms and legs or the respiratory system (the most severe paralytic categories), while among patients aged 25 years or older, 55% of cases fall into this category. Increased case-fatality rates in older age groups were observed in many countries in the prevaccine era (43) and are also being observed in the recent polio outbreak in Tajikistan (44).
Table 4.
Chronologic Trends in the Poliomyelitis Case-Fatality Rate in Sweden, 1905–1944a
| 1905 | 1911–1913 | 1925–1934 | 1935–1944 | |
| No. of paralytic cases | 868 | 6,775 | 4,156 | 11,455 |
| No. of deaths | 145 | 1,239 | 624 | 1,594 |
| Case-fatality rate, % | 16.7 | 18.3 | 15.0 | 13.9 |
Data were obtained from Olin (42).
Table 5.
Age-Specific Poliomyelitis Case-Fatality Rates and Age-Specific Sites of Paralysis in Sweden, by Age Group, 1925–1944a
| Age Group, years |
|||||
| <3 | 3–6 | 7–14 | 15–24 | ≥25 | |
| Case-fatality rate (n = 15,611), % | 4.5 | 6 | 11 | 18 | 23.5 |
| Location of paralysis (n = 15,303), % | |||||
| Leg(s) only | 58 | 40 | 34 | 23 | 20 |
| Arm(s) only | 10 | 9.5 | 10 | 10.5 | 10 |
| Arm(s) plus leg(s) | 17 | 27 | 33 | 38 | 37 |
| Respiratory system | 2 | 4 | 7 | 15 | 18 |
| Other sites | 13 | 19.5 | 16 | 13.5 | 15 |
| Total | 100 | 100 | 100 | 100 | 100 |
Data were obtained from Olin (42).
In summary, while the data fail to provide convincing evidence of an age-specific increase in the ratio of paralytic cases to infections, there is substantial evidence that among paralytic cases, the probability of severe paralysis increases markedly from infancy to adulthood.
Virulence of poliovirus serotypes
During the period when poliomyelitis was pandemic in the United States, there were marked year-to-year differences in the incidence of disease in any 1 city or region and also marked region-to-region differences in any 1 year. This phenomenon is illustrated in Table 6, showing a study conducted in the United States during 1952, a high-incidence year for the whole country (16). The data were broken down by reporting region, and the regions were grouped according to attack rate. The rates in high-incidence regions exceeded those in low-incidence regions by at least 10-fold. Table 6 also shows the distributions of the 3 serotypes of poliovirus isolates for regions with different incidences. Strikingly, in the highest-incidence regions, 94% of isolates were type 1, while only 6% were types 2 and 3 combined. In the lowest-incidence regions, 59% of isolates were type 1 and 41% were types 2 and 3 combined. Similar observations were subsequently made in polio-endemic developing countries (45–48). These data suggest considerable differences in the average virulence (paralytogenicity) of the 3 poliovirus types. In turn, this implies that virulence differences were probably significant contributors to the incidence variations described above.
Table 6.
Distribution of Poliovirus Isolates by Serotype in Areas With Different Attack Rates, United States, 1952a
| Attack Rate Per 100,000 Populationfor Each Quartile | No. of Isolates | % of Isolates for Each Regional Group |
||
| Type 1 | Type 2 | Type 3 | ||
| 103–445 | 270 | 94 | 4 | 2 |
| 43–94 | 156 | 77 | 10 | 13 |
| 16–40 | 235 | 77 | 20 | 3 |
| 4–15 | 133 | 59 | 28 | 13 |
The country was divided into 32 geographic regions that were grouped into quartiles according to their attack rates; the virus isolation data were then collated for each quartile. Data were obtained from Shelokov et al. (16).
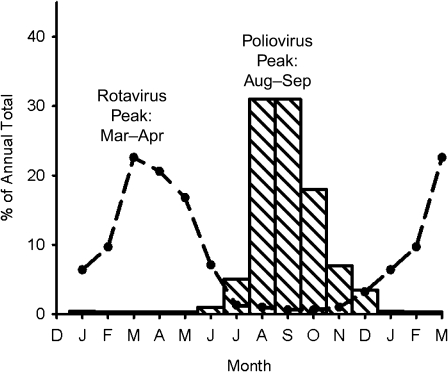
Seasonality
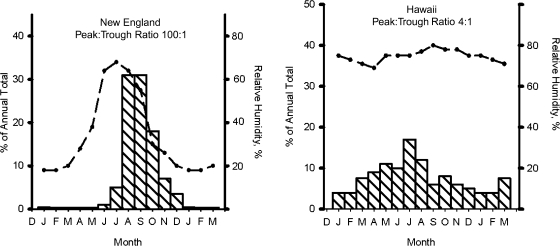
Prior to the vaccine era, poliomyelitis was a very seasonal disease in temperate zones, as shown in Figure 4 (36, 49). Furthermore, there was a hierarchy in the degree of seasonality, which was most marked in colder climates and gradually decreased toward the equator, so that it was almost absent in the tropics (Figure 5) (36, 50, 51).
Figure 4.
Seasonal variation in reported poliovirus (striped bars) in New England during 1942–1951 and in isolation of rotavirus (dashed line) in the United States during 1991–1997. Data were obtained from Serfling and Sherman (36) and Török et al. (49).
Figure 5.
Seasonal variation in poliomyelitis incidence (striped bars) and relative humidity (dashed line) in New England during 1942–1951 and in Hawaii during 1938–1952. Ratios show the degree of seasonal variation in poliomyelitis incidence. Data were obtained from Serfling and Sherman (36), Enright (50), and the National Oceanic and Atmospheric Administration (51).
Polio deconstructed: the mechanism of seasonality
Although there is no definitive explanation for polio seasonality, there are some data worth pondering (4). One hypothesis is that seasonality reflects seasonal variations in human activity, which in turn influences the probability of person-to-person transmission of enteric infections. A comparison of poliomyelitis with other human enteric infections provides a test of this idea. Figure 4 compares the seasonal variation in rotavirus isolations with the seasonality of poliomyelitis cases. Both viruses show marked seasonal variation but—strikingly—the 2 curves are almost mirror images, with peaks about 6 months apart. This suggests that seasonality is not a reflection of variations in human activity but perhaps reflects the biologic properties of different viruses.
Another set of relevant data is provided by a comparison of temperate and tropical regions, as shown in Figure 5. For poliomyelitis in New England, the peak-to-trough ratio was approximately 100-fold, while it was only 4-fold in Hawaii. Furthermore, when seasonal variation in relative humidity is plotted for the same regions, the relative humidity shows marked seasonality in New England but is almost constant throughout the year in Hawaii. Could extrahuman survival of poliovirus vary according to relative humidity and thereby influence the probability of transmission? Actually, there are some old data that suggest that poliovirus survives poorly below 40% relative humidity—exactly when poliomyelitis transmission is infrequent in New England (52). It is interesting that a similar hypothesis has been suggested for the seasonality of influenza, except that the parameters are reversed, in that influenza—which peaks in the winter—survives better under cold and dry conditions (53). However, until further studies are done, this hypothesis will remain speculative.
Seasonality has been an important enabling factor for the eradication of wild polioviruses in developing countries. Mass immunization campaigns in the cooler months, when the transmission chains of poliovirus (and potentially competing enteroviruses) are at their seasonal low (54), have been a mainstay of eradication efforts (45, 55, 56). In the northern Andean region, polio circulation ceased in cities in the temperate highlands years before it ceased in cities of the tropical coastlands (57). In temperate Bolivia, OPV coverage rates of only 50% were sufficient to eradicate polio (58). Genetic data showed that wild poliovirus was imported from coastal Peru into the Bolivian highlands (28). In Brazil, polio was rapidly controlled in the south, but reservoirs persisted in the northeast, which has a more tropical climate as well as poor sanitation. Polio had already been eradicated from the temperate southern cone of South America (Argentina, Chile, Paraguay, and Uruguay) before the launch of the Polio Eradication Initiative in the Americas (5). In China, polio persisted in the provinces of the southeast but not in the coastal northeast (59–61). One caveat is that the level of immunization is usually higher in temperate zones than in tropical zones.
POLIOMYELITIS IN RETREAT: FROM IPV TO ERADICATION OF INDIGENOUS WILD POLIOVIRUSES IN THE UNITED STATES, 1955–1973
Impact of polio vaccine
In the United States, IPV was introduced in 1955 and OPV in 1961. Both vaccines were designed to protect immunized recipients. However, it was recognized that OPV might also spread from vaccinees to their unvaccinated close contacts, thereby increasing the level of immunity in the population (62). In the United States, experience with other vaccines had shown that it was difficult to immunize more than 80%–90% of the children in the population, particularly if 2 or 3 doses were required for maximum immune responses. Consistent with that view, serosurveys conducted in the 1960s indicated that 5%–10% of children lacked antibodies to each poliovirus type (63, 64). With a calculated susceptible population of approximately 10 million for each poliovirus type, it was assumed that wild polioviruses would circulate indefinitely. Reduction in paralytic poliomyelitis was the goal of public health programs, and no one considered the possibility of eradication.
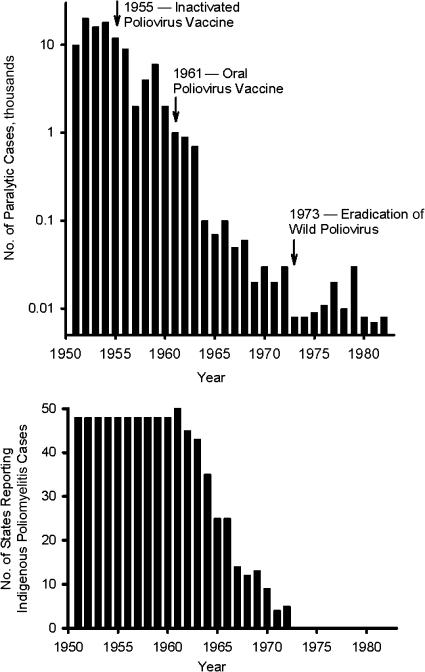
Following the introduction of IPV and OPV in the United States, annual incidence fell exponentially, beginning in 1955 (Figure 6, upper panel) (65). The date of the last indigenous wild poliovirus case in the United States is uncertain. Wild poliovirus type 1 isolates associated with outbreaks in southern Texas (1970) and Connecticut (1972) were closely related to viruses indigenous to Mexico (66), but sustained poliovirus circulation on both sides of the United States-Mexico border could not be ruled out. Without much fanfare, the last cases of poliomyelitis due to possibly indigenous wild polioviruses occurred in 1972. From this date onward, all residual cases were either vaccine-associated paralytic poliomyelitis (VAPP) or cases imported from Mexico or acquired by persons traveling abroad. The last US polio outbreak occurred in 1979 in an underimmunized Amish population (67, 68), caused by a wild poliovirus imported from Turkey via the Netherlands and Canada (66).
Figure 6.
Upper panel: annual numbers of reported cases of poliomyelitis in the United States, 1951–1982. For the years 1973–1982, cases were either imported cases or cases of vaccine-associated paralytic poliomyelitis, with the exception of an outbreak that occurred among the Amish population in 1979. Data were obtained from the Centers for Disease Control and Prevention (65). Lower panel: number of US states reporting indigenous poliomyelitis due to wild polioviruses, 1951–1982. Data were obtained from the Centers for Disease Control and Prevention (69).
Polio deconstructed: the mechanism of wild poliovirus eradication in the United States
Why did wild polioviruses disappear from the United States in spite of a substantial population of susceptible children and adults? One hypothesis focuses on the seasonality of poliovirus transmission (67, 68). The generation time (interval between 2 successive infections) for polio infections is about 10 days. During the seasonal trough in New England, in February and March, approximately 0.1% of the total annual cases (and infections) occur in 1 generation period. If one makes a calculation for the prevaccine era, for a hypothetical population of 1 million persons, it can be estimated that—of the annual total of 20,000 poliovirus infections—only about 20 occurred during a trough generation period. If the vaccine reduced the number of susceptible persons by 90%–95%, then there would only be 1–2 infections during the trough. Under these circumstances, polioviruses might “fade out” altogether. This reconstruction could explain the temporary disappearance of wild polioviruses from local areas.
For this hypothesis to explain the stepwise eradication of wild polioviruses, it would be necessary to assume that, following its disappearance, poliovirus was not readily reintroduced from neighboring areas. The lower panel in Figure 6 (69) provides some relevant data—namely, the number of states reporting any cases of poliomyelitis in each year during the period 1951–1980. Prior to the introduction of poliovirus vaccines, each state reported some cases of poliomyelitis every year. However, beginning with the introduction of OPV around 1961, the number of states reporting cases of polio due to wild polioviruses gradually dropped, reaching zero in 1973. This suggests that not only was poliovirus fading out of individual regions but it was not being reintroduced or, if reintroduced, it was not able to spread sufficiently to cause cases of paralytic poliomyelitis.
POLIOMYELITIS ON THE RUN: STEPS TOWARD GLOBAL ERADICATION, 1973–2000
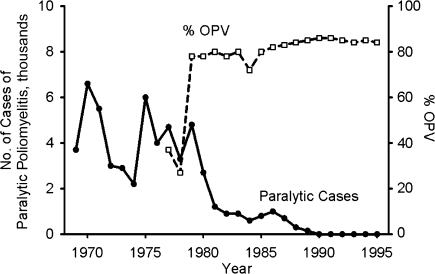
The disappearance of wild poliovirus in the United States and other developed countries demonstrated the plausibility of eradicating indigenous wild polioviruses in other regions. Inspired by these examples and the successful control programs in Cuba (the first country to successfully eradicate wild poliovirus) and Brazil (55, 56, 70), the Pan American Health Organization, under the leadership of Dr. Ciro de Quadros, undertook a vigorous immunization campaign with the goal of eradicating all wild polioviruses in the Americas. As Figure 7 shows, this program was very effective, leading to eradication of all indigenous wild polioviruses by 1991 (25). In this instance, routine pediatric immunization, supplemented with mass immunization campaigns in the form of national and subnational immunization days and mop-up campaigns in outbreak areas, provided at least 3 doses of trivalent OPV to approximately 80% of children by 12 months of age.
Figure 7.
Reported numbers of confirmed cases of paralytic poliomyelitis (solid line) in Latin America and the Caribbean region and percentages of children aged 12 months given at least 3 doses of oral poliovirus vaccine (OPV) (dashed line), 1969–1995. Data were obtained from de Quadros et al. (5).
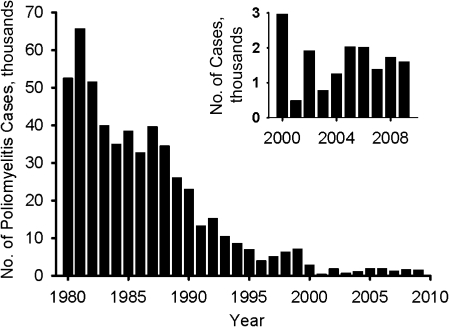
In 1988, the experience in the Americas emboldened the World Health Assembly to set a goal for global eradication of wild polioviruses (71). This campaign has been very successful, as shown in Figure 8. Reported numbers of confirmed polio cases worldwide have been reduced from an annual level of about 50,000 in 1980 to fewer than 1,000 in 2001. Because of gross underreporting of polio cases in developing countries (72), the number of polio cases prevented annually by vaccination is estimated at approximately 600,000 (6, 19, 23), so the current case count represents a >99% reduction in the potential global polio burden. Furthermore, paralytic cases due to type 2 wild poliovirus were last reported in 1999 in Uttar Pradesh, India, reinforcing the credibility of global eradication (73). By 2001, the number of countries where wild polioviruses were endemic had shrunk from over 100 to fewer than 10; the main residual foci are in Nigeria and a belt of Asian countries extending from Afghanistan across Pakistan to northern India (74).
Figure 8.
Global incidence of poliomyelitis, reported as virologically confirmed cases of paralytic poliomyelitis, during the period 1980–2009. Cases for 2000–2009 have been replotted in the inset to demonstrate recent incidence. It is estimated that during the period from 1980 to the late 1990s, virologically confirmed cases represented only a modest proportion (15%–25%) of all cases of paralytic poliomyelitis. Data were obtained from the World Health Organization (100).
POLIOMYELITIS FIGHTS BACK: POCKETS THAT DEFY ERADICATION, 2000–2010
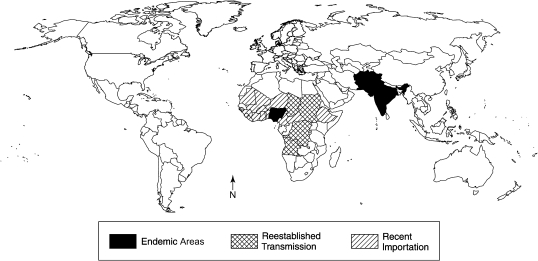
In the years leading up to 2000, there was an expectation that global eradication of wild poliovirus was imminent. However, that hope has been dashed by several unwanted developments. As Figure 8 shows, since 2000, 500–2,000 cases have been reported each year, and incidence appears to have reached a plateau. Wild polioviruses have continued to be endemic in Nigeria and in northern India, as well as Pakistan and Afghanistan. Furthermore, there has been periodic seeding of polioviruses from these endemic sources to other countries in Africa, Southeast and Central Asia, and Europe (Figure 9) (33, 44, 75, 76).
Figure 9.
Transmission of wild polioviruses worldwide in 2009. Countries with wild polioviruses are classified into 3 categories: those with endemic polioviruses, those with imported viruses that have reestablished transmission, and those with recently imported viruses. Data were obtained from the World Health Organization (33).
Polio deconstructed: persistence of wild polioviruses
Why has it been so difficult to complete the eradication of poliomyelitis, considering past successes in so many countries, including tropical nations, where all of the risk factors prevail and resources are often limited? Explanations fall into 3 main categories: failure to vaccinate, failure of the vaccine, and viral epidemiology. Each mechanism appears to play a role to varying degrees in different polio-endemic countries.
Failure to vaccinate
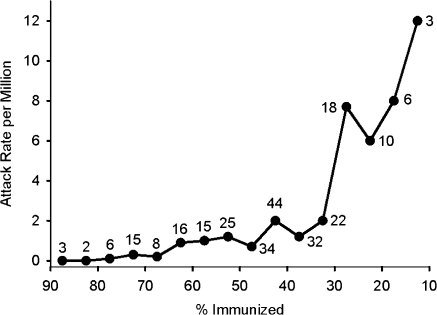
In Nigeria, underutilization of vaccine has been well documented (77). A recent study (Figure 10) showed a correlation between the attack rate for virologically confirmed cases of paralytic polio and the percentage of children under age 5 years who had received at least 3 doses of OPV. Nigeria reported 541 wild poliovirus cases in 2009, but only 6 wild poliovirus cases as of June 2010. Greatly improved vaccine coverage in door-to-door immunization campaigns in the northern states, where previously less than 40% of young children had been well immunized, has apparently brought the country to the verge of eradication of indigenous wild polioviruses (78). These recent observations indicate that if all of the states in Nigeria reached the immunization level achieved by those states with the most effective programs, it would be possible to stop all poliovirus circulation in Nigeria.
Figure 10.
Annual attack rate of type 1 paralytic poliomyelitis in Nigeria according to the percentage of children under age 5 years who were immunized with oral poliovirus vaccine (≥3 doses), 2001–2007. Data for 37 individual states for each of the 7 years were sorted according to attack rate and the proportion of children immunized. The data were then collated to construct each point on the graph. Shown next to each point is the number of instances with the parameters for that point. Data were obtained from Jenkins et al. (77).
Insecure areas in Afghanistan and Pakistan present a different set of challenges. Afghanistan and Pakistan constitute a single epidemiologic block, with ongoing cross-border transmission accompanying the large population movements between the 2 countries. Polio has been quickly controlled by mass trivalent OPV campaigns in the accessible areas of Afghanistan, but transmission has continued in 12 insecure districts in southern Afghanistan and along the border with Pakistan. Northern Punjab is frequently reseeded by wild polioviruses from neighboring endemic areas (79). The feasibility of eradication from both countries has been demonstrated by the absence of locally indigenous polioviruses in highly populous northern Punjab, where OPV coverage rates have been highest.
Another related issue is the failure to immunize children at a sufficiently early age. Where enteroviruses are circulating with high frequency, poliomyelitis manifests as “infantile paralysis,” and infection often takes place in babies aged 6–12 months. Under these circumstances, it is critical to immunize very young infants before they are exposed to wild polioviruses. This can be difficult to achieve and may require continued house-to-house campaigns that are highly labor-intensive. However, recent data from both Nigeria and India on the age distribution of paralytic cases indicate that most infections with wild polioviruses are occurring in infants over 12 months of age (Table 7). This observation provides a “window” for OPV immunization at ages 6–12 months.
Table 7.
Age Distribution of Paralytic Poliomyelitis Cases Caused by Wild Polioviruses in Nigeria, 2008 and 2009a
| Age Group, months | % of Cases |
|
| 2008 (n = 782 Cases) | 2009 (n = 388 Cases) | |
| 0–11 | 6 | 6 |
| 12–23 | 34 | 28 |
| 24–35 | 33 | 34 |
| 36–47 | 18 | 15 |
| 48–59 | 5 | 9 |
| ≥60 | 3 | 8 |
| Total | 99 | 100 |
Data were obtained from the World Health Organization (33).
Vaccine failure
During the development of OPV in the late 1950s, it was observed that a number of variables influenced efficacy, including the ages of infants and the titer of passively acquired maternal antibody, the season of year and the geographic locale, and the titer of OPV and the number of doses administered (80). Another factor in developing countries is the high prevalence of diarrheal disease, which can reduce the efficacy of orally administered vaccines, including OPV (81).
A phenomenon called “interference” was recognized in these early studies. When OPV is administered to children who are concurrently infected with other enteroviruses, the proportion of vaccinees who become OPV-infected and develop an immune response against poliovirus is reduced (45). Furthermore, the 3 types of poliovirus in the vaccine can interfere with each other. For this reason, when OPV was first introduced, it was formulated as monovalent OPV, given in the sequence type 1, type 3, and type 2. In the late 1950s, many comparisons of monovalent OPV and trivalent OPV were made; all studies showed that the “take rate” was greater with monovalent OPV. However, conversion rates were relatively high with trivalent OPV (50%–90%) in comparison with monovalent OPV (65%–95%). Even in tropical areas with very high rates of intercurrent enteric virus infections, conversion rates for trivalent OPV were rarely below 50%. These observations were reflected in the evolving vaccine policy in the United States. Monovalent OPV was launched in the United States in 1961 and was used until 1964, when trivalent OPV was substituted, since the advantages of a single vaccine were thought to outweigh the slightly higher conversion rates with monovalent OPV (23).
Because of recent problems with persistence of wild polioviruses, vaccine failure has been restudied in Nigeria and India (77, 82). In contrast with historical data, trivalent OPV has conferred much lower levels of protection (77, 82, 83). Table 8 summarizes data from Nigeria, in which a single dose of trivalent OPV was estimated to provide only 16%–18% protection against paralytic poliomyelitis. Subsequent serologic studies have found substantially higher, but still suboptimal, seroconversion rates in India (20, 21).
Table 8.
Estimated Protective Efficacy of Trivalent and Monovalent Oral Poliovirus Vaccines Against Type 1 and Type 3 Paralytic Poliomyelitis, Nigeria, 2001–2007a
| Poliovirus Type | No. of Case-Control Matches | Efficacy of Trivalent OPV, % | 95% CI | Efficacy of Monovalent OPV, % | 95% CI |
| 1 | 1,174 | 16 | 10, 21 | 67 | 39, 82 |
| 3 | 1,092 | 18 | 9, 26 | —b |
Abbreviations: CI, confidence interval; OPV, oral poliovirus vaccine.
Estimates were based on a case-control study design. Data were obtained from Jenkins et al. (77).
There was insufficient use of monovalent type 3 OPV for reliable estimation of efficacy.
Viral epidemiology
Undoubtedly, the epidemiologic context has also contributed to the difficulty of eradication. As discussed above, in tropical climates it is harder to interrupt transmission of polioviruses because of the relative lack of seasonality and the higher prevalence of enteric infections (81). Furthermore, transmission is enhanced in locales with high population densities and poor sanitation, which occurs in both Uttar Pradesh and Bihar in India, as well as in Nigeria and Pakistan. These epidemiologic parameters conspire both to enhance the circulation of wild polioviruses in very young infants and to reduce the effectiveness of OPV.
Strategies to reduce circulation of endemic wild polioviruses
Several strategies are available for enhancing the control of polio in endemic locales, including the use of monovalent OPV, the use of bivalent OPV, the employment of enhanced immunization programs, and the possible inclusion of IPV (84). Recent experience with OPV (77, 83, 85) is quite different from the early observations, in 2 respects: a much lower conversion rate with trivalent OPV and a much greater advantage of monovalent OPV. Particularly impressive is the study by Jenkins et al. (77), which compared the effectiveness of different vaccines for the prevention of virologically confirmed paralytic poliomyelitis in Nigeria, a critical test site for OPV (Table 8). Trivalent OPV showed effectiveness rates of 16% and 18%, respectively, against type 1 and type 3 poliomyelitis, while mOPV1 showed 67% effectiveness against type 1 disease.
Bivalent OPV containing both Sabin type 1 and Sabin type 3 has been introduced into all 4 persistently endemic countries, beginning in 2009 with Afghanistan (20, 21, 79). Bivalent OPV has the advantage of eliminating interference from the robust type 2 OPV component, while inducing immunity comparable to that of mOPV1 and mOPV3. Currently, the Global Polio Eradication Initiative uses 4 different OPV formulations: 1) trivalent OPV in routine immunization, during national immunization days, and to respond to circulating VDPV2 outbreaks; 2) bivalent OPV in mass campaigns in areas endemic for wild polioviruses 1 and 3; and 3) mOPV1 or 4) mOPV3 in some mass campaigns and in mop-up campaigns implemented in response to cases and to induce type-specific population immunity (20, 21).
POLIO FIGHTS BACK: THE DARK SIDE OF OPV, 2000–2010 (AND BEYOND)
During the early development of OPV, it was recognized that candidate vaccine strains of poliovirus were excreted by vaccinees and that some of these excreted viruses exhibited increased paralytogenicity. Dr. George Dick summarized the problem in his epigram, “in like a lamb, out like a lion” (86). Dr. Philip Minor sequenced isolates of OPV excreted by vaccinees and, in collaboration with other investigators, identified the point mutations that were associated with reversion to enhanced virulence (11, 87). Following the introduction of OPV immunization in the United States, meticulous surveillance documented rare cases of VAPP occurring in both vaccinees and their close contacts (88–92) (Marjorie Pollack, Centers for Disease Control and Prevention, personal communication, 1979). During the period 1961–1999, there were 1–2 cases of VAPP per million primary immunizations with OPV. For the period 1961–1999, similar numbers of cases were reported in vaccinees and in contacts. However, the relative distribution was influenced by vaccine policy: When all family members were immunized, almost all cases occurred in vaccinees (1961–1964); when immunization was limited to children, the number of cases in their parents and other contacts approached the number of cases in vaccinees (1965–1999). Approximately 20% of cases in vaccine recipients occurred in children with hypo- or agammaglobulinemia, and some of these patients failed to clear their infections and excreted immunodeficiency-associated VDPVs for many years (93–96). The relative risk of VAPP varies by serotype: Immunologically normal recipients are at highest risk from type 3 virus, immunodeficient recipients are at highest risk from type 2, and contacts are at highest risk from type 2. During the period 1997–1999, IPV was given prior to OPV, and in 2000 an exclusive IPV policy was adopted. Subsequently, no cases of VAPP have been reported in the United States (92).
These observations established the plausibility of community-wide spread of OPV leading to outbreaks of poliomyelitis. However, since the introduction of OPV in the United States in 1961, there have not been any reports of such outbreaks. Outbreaks associated with circulating VDPVs (cVDPVs) were first recognized by sequence characterization of isolates from polio cases in Hispaniola (Haiti and the Dominican Republic) in 2000–2001 (97). Since then, global surveillance has identified a number of such outbreaks, summarized in Table 9 (96, 98–100).
Table 9.
Reported Numbers of Virologically Confirmed Cases of Paralytic Poliomyelitis Associated With Vaccine-Derived Polioviruses, 1988–June 2010a
| Country | Poliovirus Type | 1988–1998 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
| Egypt | 2 | 30 | |||||||||||
| Hispaniola (Haiti and the Dominican Republic) | 1 | 12 | 9 | ||||||||||
| Philippines | 1 | 3 | |||||||||||
| Madagascar | 2 | 4 | |||||||||||
| China | 1 | 3 | |||||||||||
| Madagascar | 2 | 5 | |||||||||||
| Indonesia | 1 | 46 | |||||||||||
| Cambodia | 3 | 1 | 1 | ||||||||||
| Nigeria | 2 | 1 | 21 | 68 | 63 | 153 | 9 | ||||||
| Niger | 2 | 2 | |||||||||||
| Myanmar | 1 | 1 | 4 | ||||||||||
| Democratic Republic of the Congo | 2 | 14 | 2 | 3 | |||||||||
| Guinea | 2 | 1 | |||||||||||
| India | 2 | 11 | 1 | ||||||||||
| Ethiopia | 3 | 1 | 5 |
Abbreviation: VDPV, vaccine-derived poliovirus.
This table does not include 21 polio-compatible cases in Hispaniola and 10 polio-compatible cases in Indonesia that were not virologically confirmed. Both the Niger VDPVs and the Guinea VDPV were linked to the Nigerian VDPV2 outbreak. Data were obtained from Wringe et al. (99) and the World Health Organization (100).
Polio deconstructed: cVDPV outbreaks
Why were cVDPV-associated outbreaks first identified only in 2000, 40 years after the introduction of OPV? Several factors contributed to the late recognition of this phenomenon. First, the development of methods for rapid sequencing of polioviruses made it possible to definitely distinguish OPV-revertant viruses from wild polioviruses (101). Second, the gradual establishment of an extraordinary global network for the isolation and characterization of polioviruses (102) during the period 1990–2000 facilitated reconstruction of the molecular epidemiology of such outbreaks (97, 98). However, the most important reason for the recent recognition of cVDPV-associated outbreaks is that most cVDPV outbreaks have occurred when immunization programs are very incomplete, with less than 50% of children receiving 3 doses of OPV. In such situations, there are enough susceptible children to sustain widespread infection, leading to outbreaks of paralytic disease. This observation explains the relative infrequency of such outbreaks and their absence in most regions where OPV campaigns have reached a large proportion of the population. For instance, in Cuba, OPV has been administered during annual national immunization days that have saturated the population (55, 103, 104). After such national immunization days, surveillance of sewage has detected OPV-related virus for about 3 months, following which it disappears. Cuba, unlike nearby Haiti, has not experienced cVDPV-associated outbreaks, and the probability of such outbreaks is apparently low under these conditions.
Furthermore, the Global Polio Eradication Initiative has fundamentally altered poliovirus ecology. In all but a few districts in the world, immunity to poliovirus is no longer conferred by natural infection but depends solely upon vaccination. In areas free of wild polioviruses, cVDPVs can emerge if immunity gaps are allowed to develop. The magnitude of the risk depends on the extent of immunization gaps, the intensity of other environmental risks (sanitation, hygiene, population density), and the poliovirus serotype (type 2 > type 1 > type 3). It has become essential to maintain high population immunity and sensitive surveillance for acute flaccid paralysis and poliovirus to avoid outbreaks caused by cVDPVs or introduced wild polioviruses.
How extensive are VDPV outbreaks and how can they best be controlled or prevented?
Table 9 summarizes the recognized confirmed cVDPV outbreaks, which appear not to require an immunodeficiency-associated VDPV precursor infection (101). With the exception of the type 2 outbreak in Nigeria, most outbreaks have been relatively modest in extent, with fewer than 50 reported confirmed paralytic cases. Of course, for each case there are many more subclinical infections; depending upon the case:infection ratio, there could be between 100 and >1,000 infections for each paralytic case. One recent analysis estimated the number of cVDPV infections in the millions (99). The number of outbreaks, particularly the ongoing outbreak in Nigeria (96), indicates that cVDPVs present a significant obstacle to the eradication of virulent polioviruses. In addition, the 1968 outbreak of almost 500 paralytic cases in Poland, which was caused by a candidate attenuated type 3 vaccine strain (USOL-D-bac), underlines the potential problem (105, 106).
Of the cVDPV outbreaks listed in Table 9, most were ended by vigorous OPV immunization responses that closed the immunity gap among susceptible children, followed by a fadeout of cVDPVs. However, a strategy of indefinite use of OPV is obviously fraught with danger, since it is unlikely to lead to a world free of potentially dangerous polioviruses. Eventually, it will be necessary to terminate the distribution of OPV, requiring an “endgame” strategy for the global eradication of circulating polioviruses.
THE “ENDGAME” IN POLIOVIRUS ERADICATION
Elimination of wild polioviruses
Once a country or continent has eliminated wild polioviruses through the use of OPV, public health authorities have a choice of at least 3 strategies: indefinite continuation of OPV immunization, transition from OPV to IPV, or carefully coordinated termination of OPV without IPV replacement. In the United States, OPV immunization was maintained for about 20 years following the elimination of wild polioviruses in 1973. In the late 1990s, there was a transition to IPV, via an intermediate stage of sequential IPV–OPV, with IPV being the recommended vaccine since 2000 (92, 107). This transition was made primarily for 2 reasons: 1) in the absence of indigenous wild polioviruses in the Americas, the continuing burden of VAPP was considered unacceptable, even though the frequency was only about 2 cases per million primary immunizations; and 2) the risk of acquisition of wild polioviruses, either through importation or during international travel, mandated continued immunization.
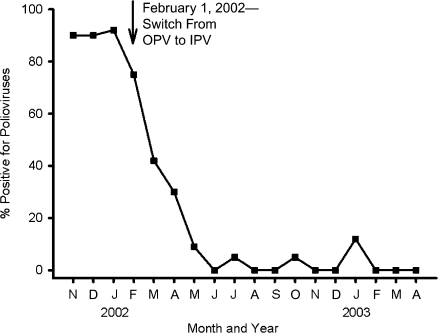
Many other industrialized countries have followed a similar strategy. An instructive example is the experience of New Zealand, shown in Figure 11 (108). In February 2002, New Zealand switched from OPV to IPV. During the transition, sewage was sampled periodically to determine the frequency of OPV-related viruses. Prior to the switch, polioviruses were isolated from approximately 90% of sewage samples. Following the switch, the frequency fell in a stepwise fashion, reaching 0% in about 4 months. Although there were occasional isolations of poliovirus thereafter, it was concluded that these were OPV-related viruses shed by visitors to New Zealand. This conclusion was based on the small number of mutations and the known evolution rate for polioviruses (57), implying that these isolates were derived from OPV administered elsewhere after the February 2002 cessation of OPV immunization in New Zealand.
Figure 11.
Environmental surveillance for poliovirus excretion following the transition from oral poliovirus vaccine (OPV) to inactivated poliovirus vaccine (IPV) in New Zealand, 2001–2003. Sewage samples were collected weekly from 3 different sewage treatment plants before and after the termination of OPV utilization. Routine use of OPV ended on February 1, 2002, and the prevalence of OPV in sewage fell from approximately 90% to 0% by June 2002 (4 months later). During the following 10 months (July 2002–April 2003), there were 5 isolates of OPV; on the basis of sequence analysis, all of these isolates were determined to be from children recently immunized with OPV, suggesting that they represented imported OPV. Data were obtained from Huang et al. (108).
Polio deconstructed: the posteradication strategy
Let us assume for purposes of discussion that it is feasible to eliminate wild polioviruses from those countries where it is still endemic. There has been extensive discussion among experts regarding alternative strategies for the posteradication endgame (109–116). Indefinite continuation of OPV carries several liabilities: continued cases of VAPP (including new prolonged excretion of immunodeficiency-associated VDPVs by immunodeficient vaccinees); the potential for new outbreaks caused by cVDPVs; and public health “fatigue,” leading to reduced OPV coverage and its attendant dangers. Termination of OPV without transition to IPV carries the risk of initiating new outbreaks caused by cVDPVs, because of either spread of cVDPV during the termination phase or unregulated importation of OPV from other countries. Additionally, this option carries the ethical liability of a double standard for high- and low-income countries. For these reasons, there is an evolving (but not necessarily total) consensus that, in principle, the optimal choice is a transition from OPV to IPV (109, 113–115, 117).
The most important concerns about the IPV option have focused on practical issues regarding the cost and global coverage levels attainable with an injected vaccine as compared with an oral vaccine (118). However, most middle- and low-income countries are already using injected vaccines for other diseases, so they could adopt IPV as either an additional or incorporated product. IPV is more expensive than OPV, and it has been estimated that it would cost at least $1 billion to produce sufficient IPV for developing countries. In addition, there are issues pertaining to formulation (a single product vs. incorporation of IPV with other immunogens), dosage regimens, and recruitment of collaborating manufacturers with sufficient production capacity (119). Recently, the Global Polio Eradication Initiative undertook to fund a research program for creation of an affordable IPV for developing countries (21, 118, 120), including exploration of the efficacy of fractional intradermal IPV doses (121, 122). A key research question, which is the subject of several ongoing and proposed studies, is the efficacy of IPV in preventing poliovirus circulation in the highest-risk settings (21, 123).
CONCLUSIONS
There is an epidemiologic imperative to eradicate polioviruses, for several reasons: the recurring spread of wild polioviruses from endemic sites to other countries; the potential dangers of vaccination “fatigue” in countries that have already eliminated wild polioviruses; and the documented dangers of cVDPV. It has also been argued that eradication of polioviruses would cost less than control programs (124) and that there is an ethical imperative for eradication (125).
If polioviruses are to be eradicated globally, several requirements must be met. First, in countries where wild polioviruses still circulate, there must be a massive effort to immunize a high proportion of very young infants with OPV—perhaps followed by 1 or more doses of IPV. Second, in countries where wild polioviruses are no longer circulating, there must be a transition from OPV to IPV to eliminate VAPP and reduce the dangers of outbreaks associated with cVDPVs. Finally, there must be continuation of rigorous surveillance efforts to inform these programs, with a plan for emergency intervention whenever polio outbreaks occur. This strategy will require the coordinated effort of international and national public health programs, supported with sufficient resources to produce and administer both OPV and IPV. Only time will tell whether this strategy will be implemented and, if implemented, whether it will succeed.
Acknowledgments
Author affiliations: Departments of Microbiology and Neurology, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania (Neal Nathanson); and Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia (Olen M. Kew).
Conflict of interest: none declared.
Glossary
Abbreviations
- cVDPV
circulating vaccine-derived poliovirus
- IPV
inactivated poliovirus vaccine
- mOPV
monovalent oral poliovirus vaccine
- OPV
oral poliovirus vaccine
- VAPP
vaccine-associated paralytic poliomyelitis
- VDPV
vaccine-derived poliovirus
References
- 1.Salk JE, Krech U, Youngner JS, et al. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. Am J Public Health Nations Health. 1954;44(5):563–570. doi: 10.2105/ajph.44.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabin AB. Present position of immunization against poliomyelitis with live virus vaccines. Br Med J. 1959;1(5123):663–680. doi: 10.1136/bmj.1.5123.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Progress towards interruption of wild poliovirus transmission worldwide, 2009. Wkly Epidemiol Rec. 2010;85(20):178–184. [PubMed] [Google Scholar]
- 4.Nathanson N, Martin JR. The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance. Am J Epidemiol. 1979;110(6):672–692. doi: 10.1093/oxfordjournals.aje.a112848. [DOI] [PubMed] [Google Scholar]
- 5.de Quadros CA, Hersh BS, Olivé JM, et al. Eradication of wild poliovirus from the Americas: acute flaccid paralysis surveillance, 1988–1995. J Infect Dis. 1997;175(suppl 1):S37–S42. doi: 10.1093/infdis/175.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 6.Aylward RB, Hull HF, Cochi SL, et al. Disease eradication as a public health strategy: a case study of poliomyelitis eradication. Bull World Health Organ. 2000;78(3):285–297. [PMC free article] [PubMed] [Google Scholar]
- 7.Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Mueller S, Wimmer E, Cello J. Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event. Virus Res. 2005;111(2):175–193. doi: 10.1016/j.virusres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Bodian D. Emerging concept of poliomyelitis infection. Science. 1955;122(3159):105–108. doi: 10.1126/science.122.3159.105. [DOI] [PubMed] [Google Scholar]
- 10.Sabin AB. Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science. 1956;123(3209):1151–1157. doi: 10.1126/science.123.3209.1151. [DOI] [PubMed] [Google Scholar]
- 11.Nathanson N. The pathogenesis of poliomyelitis: what we don't know. Adv Virus Res. 2008;71:1–50. doi: 10.1016/S0065-3527(08)00001-8. [DOI] [PubMed] [Google Scholar]
- 12.Sartwell PE. The incubation period of poliomyelitis. Am J Public Health Nations Health. 1952;42(11):1403–1408. doi: 10.2105/ajph.42.11.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horstmann DM, Paul JR. The incubation period in human poliomyelitis and its implications. J Am Med Assoc. 1947;135(1):11–14. doi: 10.1001/jama.1947.02890010013004. [DOI] [PubMed] [Google Scholar]
- 14.Bodian D. Differentiation of types of poliomyelitis viruses; reinfection experiments in monkeys (second attacks) Am J Hyg. 1949;49(2):200–223. doi: 10.1093/oxfordjournals.aje.a119271. [DOI] [PubMed] [Google Scholar]
- 15.Bodian D. Immunologic classification of poliomyelitis viruses. I. A cooperative program for the typing of one hundred strains. Am J Hyg. 1951;54(2):191–204. [PubMed] [Google Scholar]
- 16.Shelokov A, Habel K, McKinstry DW. Relation of poliomyelitis virus types to clinical disease and geographic distribution: a preliminary report. Ann N Y Acad Sci. 1955;61(4):998–1004. doi: 10.1111/j.1749-6632.1955.tb42558.x. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Poliomyelitis Surveillance Report, Number 65. Atlanta, GA: Centers for Disease Control and Prevention; 1956. [Google Scholar]
- 18.Paul JR. A History of Poliomyelitis. New Haven, CT: Yale University Press; 1971. [Google Scholar]
- 19.Sabin AB. Vaccination against poliomyelitis in economically underdeveloped countries. Bull World Health Organ. 1980;58(1):141–157. [PMC free article] [PubMed] [Google Scholar]
- 20.Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Wkly Epidemiol Rec. 2010;85(23):213–228. [PubMed] [Google Scholar]
- 21.Polio Research Committee, World Health Organization. PolioPipeline. Geneva, Switzerland: World Health Organization; 2010. 1–5. ( http://wwwpolioeradication.org/content/poliopipeline/PolioPipeline_05.pdf). (Accessed May 3, 2010) [Google Scholar]
- 22.Plotkin SA, Vidor E. Poliovirus vaccine—inactivated. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia, PA: WB Saunders Company; 2008. pp. 605–629. [Google Scholar]
- 23.Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine—live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia, PA: WB Saunders Company; 2008. pp. 631–685. [Google Scholar]
- 24.Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76(suppl 2):22–25. [PMC free article] [PubMed] [Google Scholar]
- 25.Certification of poliomyelitis eradication—the Americas, 1994. MMWR Morb Mortal Wkly Rep. 1994;43(39):720–722. [PubMed] [Google Scholar]
- 26.Certification of poliomyelitis eradication. WHO Western Pacific region, October 2000. Wkly Epidemiol Rec. 2000;75(49):399–400. [PubMed] [Google Scholar]
- 27.Certification of poliomyelitis eradication—European region, June 2002. Wkly Epidemiol Rec. 2002;77(27):221–223. [PubMed] [Google Scholar]
- 28.Kew OM, Mulders MN, Lipskaya GY, et al. Molecular epidemiology of polioviruses. Semin Virol. 1995;6(6):401–414. [Google Scholar]
- 29.Wikipedia. Poliomyelitis. San Francisco, CA: Wikimedia Foundation, Inc; 2010. ( http://en.wikipedia.org/wiki/Poliomyelitis). (Accessed April 29, 2010) [Google Scholar]
- 30.Lavinder CH, Freeman AW, Frost WH. Epidemiologic Studies of Poliomyelitis in New York City and the Northeastern United States During the Year 1916. Washington, DC: US GPO; 1918. [Google Scholar]
- 31.Paul JR. Poliomyelitis. Geneva, Switzerland: World Health Organization; 1955. Epidemiology of poliomyelitis. 9–29. (WHO Monograph Series, no. 26) [Google Scholar]
- 32.Paul JR, Horstmann DM. A survey of poliomyelitis virus antibodies in French Morocco. Am J Trop Med Hyg. 1955;4(3):512–524. doi: 10.4269/ajtmh.1955.4.512. [DOI] [PubMed] [Google Scholar]
- 33.Strategic Advisory Group of Experts on Immunization, World Health Organization. Global Polio Eradication: Progress and Current Epidemiological/Operational Risks. Geneva, Switzerland: World Health Organization; 2010. ( http://www.who.int/immunization/sage/Polio_1_epidemiology_S_Cochi_SAGE_April_2010.pdf). (Accessed April 12, 2010) [Google Scholar]
- 34.Jafari H. Public Health Grand Rounds, January 21, 2010 [Webcast] Atlanta, GA: Office of the Director, Centers for Disease Control and Prevention; 2010. Defining the challenges in India and refining the strategies and tools to achieve polio eradication. ( http://intranet.cdc.gov/od/odweb/about/grand-rounds/archive/2010/download/GR-012110.pdf). (Accessed April 12, 2010) [Google Scholar]
- 35.Dauer CC. The changing age distribution of paralytic poliomyelitis. Ann N Y Acad Sci. 1955;61(4):943–955. doi: 10.1111/j.1749-6632.1955.tb42553.x. [DOI] [PubMed] [Google Scholar]
- 36.Serfling RE, Sherman IL. Poliomyelitis distribution in the United States. Public Health Rep. 1953;68(5):453–466. [PMC free article] [PubMed] [Google Scholar]
- 37.Sabin AB. Poliomyelitis: Papers and Discussions Presented at the First International Poliomyelitis Conference. Philadelphia, PA: J B Lippincott & Company; 1949. Epidemiologic patterns of poliomyelitis in different parts of the world; pp. 3–33. [Google Scholar]
- 38.Greenberg M, Siegel M, Magee MC. Poliomyelitis in New York City, 1949. N Y State J Med. 1950;50(9):1119–1123. [PubMed] [Google Scholar]
- 39.Siegel M, Greenberg M, Stone P., Jr Risk of paralytic and nonparalytic forms of poliomyelitis to household contacts in nonepidemic years. N Engl J Med. 1955;252(18):752–756. doi: 10.1056/NEJM195505052521803. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Neurotropic Viral Diseases Surveillance: Annual Poliomyelitis Summary—1973. Atlanta, GA: Centers for Disease Control and Prevention; 1975. [Google Scholar]
- 41.Melnick JL, Ledinko N. Development of neutralizing antibodies against the three types of poliomyelitis virus during an epidemic period; the ratio of inapparent infection to clinical poliomyelitis. Am J Hyg. 1953;58(2):207–222. doi: 10.1093/oxfordjournals.aje.a119602. [DOI] [PubMed] [Google Scholar]
- 42.Olin G. The epidemiologic pattern of poliomyelitis in Sweden from 1905 to 1950. In: Fishbein M, editor. Poliomyelitis: Papers and Discussions Presented at the Second International Poliomyelitis Conference. Philadelphia, PA: J B Lippincott & Company; 1952. pp. 367–375. [Google Scholar]
- 43.Freyche M-J, Nielsen J. Poliomyelitis. Geneva, Switzerland: World Health Organization; 1955. Incidence of poliomyelitis since 1920. 59–106. (WHO Monograph Series, no. 26) [PubMed] [Google Scholar]
- 44.Poliomyelitis in Tajikistan: first importation since Europe certified polio-free. Wkly Epidemiol Rec. 2010;85(18):157–158. [PubMed] [Google Scholar]
- 45.Sabin AB, Ramos-Alvarez M, Alvarez-Amezquita J, et al. Live, orally given poliovirus vaccine. Effects of rapid mass immunization on population under conditions of massive enteric infection with other viruses. JAMA. 1960;173(14):1521–1526. doi: 10.1001/jama.1960.03020320001001. [DOI] [PubMed] [Google Scholar]
- 46.Cockburn WC, Drozdov SG. Poliomyelitis in the world. Bull World Health Organ. 1970;42(3):405–417. [PMC free article] [PubMed] [Google Scholar]
- 47.Basu RN. Magnitude of problem of poliomyelitis in India. Indian Pediatr. 1981;18(8):507–511. [PubMed] [Google Scholar]
- 48.Schonberger LB, Thaung U, Khi DK, et al. The epidemiology of poliomyelitis in Burma. Dev Biol Stand. 1981;47:283–292. [PubMed] [Google Scholar]
- 49.Török TJ, Kilgore PE, Clarke MJ, et al. Visualizing geographic and temporal trends in rotavirus activity in the United States, 1991 to 1996. National Respiratory and Enteric Virus Surveillance System Collaborating Laboratories. Pediatr Infect Dis J. 1997;16(10):941–946. doi: 10.1097/00006454-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Enright JR. The epidemiology of paralytic poliomyelitis in Hawaii. Hawaii Med J. 1954;13(5):350–354. [PubMed] [Google Scholar]
- 51.National Oceanic and Atmospheric Administration, US Department of Commerce. Local Climatological Data, Annual Summary With Comparative Data 1977. Washington, DC: National Oceanic and Atmospheric Administration; 1977. [Google Scholar]
- 52.Hemmes JH, Winkler KC, Kool SM. Virus survival as a seasonal factor in influenza and poliomyelitis. Antonie Van Leeuwenhoek. 1962;28(1):221–233. doi: 10.1007/BF02538737. [DOI] [PubMed] [Google Scholar]
- 53.Lowen AC, Mubareka S, Steel J, et al. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shulman LM, Handsher R, Yang CF, et al. Resolution of the pathways of poliovirus type 1 transmission during an outbreak. J Clin Microbiol. 2000;38(3):945–952. doi: 10.1128/jcm.38.3.945-952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodríguez Cruz R. Cuba: mass polio vaccination program, 1962–1982. Rev Infect Dis. 1984;6(suppl 2):S408–S412. [PubMed] [Google Scholar]
- 56.Baptista Risi J., Jr The control of poliomyelitis in Brazil. Rev Infect Dis. 1984;6(suppl 2):S400–S403. doi: 10.1093/clinids/6.supplement_2.s400. [DOI] [PubMed] [Google Scholar]
- 57.Jorba J, Campagnoli R, De L, et al. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol. 2008;82(9):4429–4440. doi: 10.1128/JVI.02354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan American Health Organization. EPI Newsletter Previous Issues [electronic archive]. (EPI Newsletter, 1985–1994, volumes and issues 7(3)–16(4)) Washington, DC: Pan American Health Organization; pp. 1985–1994. ( http://www.paho.org/english/ad/fch/im/prev_newsletter.htm). (Accessed May 3, 2010) [Google Scholar]
- 59.Wang K, Zhang LB, Otten MW, Jr, et al. Status of the eradication of indigenous wild poliomyelitis in the People's Republic of China. J Infect Dis. 1997;175(suppl 1):S105–S112. doi: 10.1093/infdis/175.supplement_1.s105. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Zhang LB, Otten MW, Jr, et al. Surveillance for polio eradication in the People's Republic of China. J Infect Dis. 1997;175(suppl 1):S122–S134. doi: 10.1093/infdis/175.supplement_1.s122. [DOI] [PubMed] [Google Scholar]
- 61.Liu HM, Zheng DP, Zhang LB, et al. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J Virol. 2000;74(23):11153–11161. doi: 10.1128/jvi.74.23.11153-11161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henry JL, Jaikaran ES, Davies JR, et al. A study of poliovaccination in infancy: excretion following challenge with live virus by children given killed or living poliovaccine. J Hyg (Lond) 1966;64(1):105–120. doi: 10.1017/s0022172400040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poland JD, Plexico K, Flynt JW, et al. Poliovirus neutralizing antibody levels among preschool children. Public Health Rep. 1968;83(6):507–512. [PMC free article] [PubMed] [Google Scholar]
- 64.Melnick JL, Burkhardt M, Taber LH, et al. Developing gap in immunity to poliomyelitis in an urban area. JAMA. 1969;209(8):1181–1185. [PubMed] [Google Scholar]
- 65.Poliomyelitis surveillance summary. MMWR Morb Mortal Wkly Rep. 1982;30(1):12–17. [Google Scholar]
- 66.Rico-Hesse R, Pallansch MA, Nottay BK, et al. Geographic distribution of wild poliovirus type 1 genotypes. Virology. 1987;160(2):311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 67.Nathanson N. Eradication of poliomyelitis in the United States. Rev Infect Dis. 1982;4(5):940–950. doi: 10.1093/clinids/4.5.940. [DOI] [PubMed] [Google Scholar]
- 68.Nathanson N. Epidemiologic aspects of poliomyelitis eradication. Rev Infect Dis. 1984;6(suppl 2):S308–S312. doi: 10.1093/clinids/6.supplement_2.s308. [DOI] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention. CDC Annual Report. (Annual poliomyelitis surveillance summaries for the years 1960–1973) Atlanta, GA: Centers for Disease Control and Prevention; pp. 1961–1974. [Google Scholar]
- 70.Dowdle WR, Cochi SL. Global eradication of poliovirus: history and rationale. In: Semler BL, Wimmer E, editors. Molecular Biology of Picornaviruses. Washington, DC: ASM Press; 2002. pp. 473–480. [Google Scholar]
- 71.World Health Assembly. Polio Eradication by the Year 2000. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 72.Poliomyelitis 1980, parts 1 and 2. Wkly Epidemiol Rec. 1981;56:329–332. 337–341. [Google Scholar]
- 73.Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep. 2001;50(12):222–224. [PubMed] [Google Scholar]
- 74.Nathanson N, Fine P. Virology. Poliomyelitis eradication—a dangerous endgame. Science. 2002;296(5566):269–270. doi: 10.1126/science.1071207. [DOI] [PubMed] [Google Scholar]
- 75.Resurgence of wild poliovirus type 1 transmission and consequences of importation—21 countries, 2002–2005. MMWR Morb Mortal Wkly Rep. 2006;55(6):145–150. [PubMed] [Google Scholar]
- 76.Wild poliovirus type 1 and type 3 importations—15 countries, Africa, 2008–2009. MMWR Morb Mortal Wkly Rep. 2009;58(14):357–362. [PubMed] [Google Scholar]
- 77.Jenkins HE, Aylward RB, Gasasira A, et al. Effectiveness of immunization against paralytic poliomyelitis in Nigeria. N Engl J Med. 2008;359(16):1666–1674. doi: 10.1056/NEJMoa0803259. [DOI] [PubMed] [Google Scholar]
- 78.Progress towards eradicating poliomyelitis in Nigeria, January 2009–June 2010. Wkly Epidemiol Rec. 2010;85(28):273–280. [PubMed] [Google Scholar]
- 79.Progress towards eradicating poliomyelitis in Afghanistan and Pakistan, 2009. Wkly Epidemiol Rec. 2010;85(11):93–100. [PubMed] [Google Scholar]
- 80.Pan American Health Organization. Second International Conference on Live Poliovirus Vaccines. Washington, DC: Pan American Health Organization; 1960. (PAHO Scientific Publication no. 50) [Google Scholar]
- 81.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13(5):926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 82.Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science. 2006;314(5802):1150–1153. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 83.el-Sayed N, el-Gamal Y, Abbassy AA, et al. Monovalent type 1 oral poliovirus vaccine in newborns. N Engl J Med. 2008;359(16):1655–1665. doi: 10.1056/NEJMoa0800390. [DOI] [PubMed] [Google Scholar]
- 84.Aylward RB, Maher C. Interrupting poliovirus transmission—new solutions to an old problem. Biologicals. 2006;34(2):133–139. doi: 10.1016/j.biologicals.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 85.Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet. 2007;369(9570):1356–1362. doi: 10.1016/S0140-6736(07)60531-5. [DOI] [PubMed] [Google Scholar]
- 86.Dick G. Immunity to poliomyelitis [letter] Br Med J. 1963;2(5370):1468–1469. doi: 10.1136/bmj.2.5370.1468-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minor PD. The molecular biology of poliovaccines. J Gen Virol. 1992;73(12):3065–3077. doi: 10.1099/0022-1317-73-12-3065. [DOI] [PubMed] [Google Scholar]
- 88.Henderson DA, Witte JJ, Morris L, et al. Paralytic disease associated with oral polio vaccines. JAMA. 1964;190(1):41–48. doi: 10.1001/jama.1964.03070140047006. [DOI] [PubMed] [Google Scholar]
- 89.Schonberger LB, McGowan JE, Jr, Gregg MB. Vaccine-associated poliomyelitis in the United States, 1961–1972. Am J Epidemiol. 1976;104(2):202–211. doi: 10.1093/oxfordjournals.aje.a112290. [DOI] [PubMed] [Google Scholar]
- 90.Nkowane BM, Wassilak SG, Orenstein WA, et al. Vaccine-associated paralytic poliomyelitis. United States: 1973 through 1984. JAMA. 1987;257(10):1335–1340. [PubMed] [Google Scholar]
- 91.Strebel PM, Sutter RW, Cochi SL, et al. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin Infect Dis. 1992;14(2):568–579. doi: 10.1093/clinids/14.2.568. [DOI] [PubMed] [Google Scholar]
- 92.Alexander LN, Seward JF, Santibanez TA, et al. Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA. 2004;292(14):1696–1701. doi: 10.1001/jama.292.14.1696. [DOI] [PubMed] [Google Scholar]
- 93.Yoneyama T, Hagiwara A, Hara M, et al. Alteration in oligonucleotide fingerprint patterns of the viral genome in poliovirus type 2 isolated from paralytic patients. Infect Immun. 1982;37(1):46–53. doi: 10.1128/iai.37.1.46-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kew OM, Sutter RW, Nottay BK, et al. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J Clin Microbiol. 1998;36(10):2893–2899. doi: 10.1128/jcm.36.10.2893-2899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacLennan C, Dunn G, Huissoon AP, et al. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet. 2004;363(9420):1509–1513. doi: 10.1016/S0140-6736(04)16150-3. [DOI] [PubMed] [Google Scholar]
- 96.Update on vaccine-derived polioviruses—worldwide, January 2008–June 2009. MMWR Morb Mortal Wkly Rep. 2009;58(36):1002–1006. [PubMed] [Google Scholar]
- 97.Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–359. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 98.Kew OM, Wright PF, Agol VI, et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull World Health Organ. 2004;82(1):16–23. [PMC free article] [PubMed] [Google Scholar]
- 99.Wringe A, Fine PE, Sutter RW, et al. Estimating the extent of vaccine-derived poliovirus infection. PLoS ONE. 2008;3(10):e3433. doi: 10.1371/journal.pone.0003433. (doi: 10.1371/journal.pone.0003433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.World Health Organization. Data, Statistics and Graphics by Subject [database] Geneva, Switzerland: World Health Organization; 2010. ( http://www.who.int/immunization_monitoring/data/data_subject/en/#b). (Accessed April 12, 2010) [Google Scholar]
- 101.Kew OM, Sutter RW, de Gourville EM, et al. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 102.Laboratory surveillance for wild and vaccine-derived polioviruses—worldwide, January 2008–June 2009. MMWR Morb Mortal Wkly Rep. 2009;58(34):950–954. [PubMed] [Google Scholar]
- 103.Más Lago P, Cáceres VM, Galindo MA, et al. Persistence of vaccine-derived poliovirus following a mass vaccination campaign in Cuba: implications for stopping polio vaccination after global eradication. Int J Epidemiol. 2001;30(5):1029–1034. doi: 10.1093/ije/30.5.1029. [DOI] [PubMed] [Google Scholar]
- 104.Más Lago P, Gary HE, Jr, Pérez LS, et al. Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. Int J Epidemiol. 2003;32(5):772–777. doi: 10.1093/ije/dyg185. [DOI] [PubMed] [Google Scholar]
- 105.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol. 1999;150(10):1001–1021. doi: 10.1093/oxfordjournals.aje.a009924. [DOI] [PubMed] [Google Scholar]
- 106.Martín J, Ferguson GL, Wood DJ, et al. The vaccine origin of the 1968 epidemic of type 3 poliomyelitis in Poland. Virology. 2000;278(1):42–49. doi: 10.1006/viro.2000.0614. [DOI] [PubMed] [Google Scholar]
- 107.Cochi SL, Kew O. Polio today: are we on the verge of global eradication? JAMA. 2008;300(7):839–841. doi: 10.1001/jama.300.7.839. [DOI] [PubMed] [Google Scholar]
- 108.Huang QS, Greening G, Baker MG, et al. Persistence of oral polio vaccine virus after its removal from the immunisation schedule in New Zealand. Lancet. 2005;366(9483):394–396. doi: 10.1016/S0140-6736(05)66386-6. [DOI] [PubMed] [Google Scholar]
- 109.Fine PE, Oblapenko G, Sutter RW. Polio control after certification: major issues outstanding. Bull World Health Organ. 2004;82(1):47–52. [PMC free article] [PubMed] [Google Scholar]
- 110.Sutter RW, Cáceres VM, Más Lago P. The role of routine polio immunization in the post-certification era. Bull World Health Organ. 2004;82(1):31–39. [PMC free article] [PubMed] [Google Scholar]
- 111.Agol VI. Vaccine-derived polioviruses. Biologicals. 2006;34(2):103–108. doi: 10.1016/j.biologicals.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 112.Dowdle W, van der Avoort H, de Gourville E, et al. Containment of polioviruses after eradication and OPV cessation: characterizing risks to improve management. Risk Anal. 2006;26(6):1449–1469. doi: 10.1111/j.1539-6924.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 113.Chumakov K, Ehrenfeld E, Wimmer E, et al. Vaccination against polio should not be stopped. Nat Rev Microbiol. 2007;5(12):952–958. doi: 10.1038/nrmicro1769. [DOI] [PubMed] [Google Scholar]
- 114.Ehrenfeld E, Glass RI, Agol VI, et al. Immunisation against poliomyelitis: moving forward. Lancet. 2008;371(9621):1385–1387. doi: 10.1016/S0140-6736(08)60597-8. [DOI] [PubMed] [Google Scholar]
- 115.Thompson KM, Tebbens RJ, Pallansch MA, et al. The risks, costs, and benefits of possible future global policies for managing polioviruses. Am J Public Health. 2008;98(7):1322–1330. doi: 10.2105/AJPH.2007.122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heymann DL, Sutter RW, Aylward RB. A vision of a world without polio: the OPV cessation strategy. Biologicals. 2006;34(2):75–79. doi: 10.1016/j.biologicals.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 117.Fine PE, Ritchie S. Perspective: determinants of the severity of poliovirus outbreaks in the post eradication era. Risk Anal. 2006;26(6):1533–1540. doi: 10.1111/j.1539-6924.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 118.Inactivated poliovirus vaccine following oral poliovirus vaccine cessation. Wkly Epidemiol Rec. 2006;81(15):137–144. [PubMed] [Google Scholar]
- 119.Chumakov K, Ehrenfeld E. New generation of inactivated poliovirus vaccines for universal immunization after eradication of poliomyelitis. Clin Infect Dis. 2008;47(12):1587–1592. doi: 10.1086/593310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roberts L. Polio eradication. Looking for a little luck. Science. 2009;323(5915):702–705. doi: 10.1126/science.323.5915.702. [DOI] [PubMed] [Google Scholar]
- 121.Mohammed AJ, Al Awaidy S, Bawikar S, et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med. 2010;362(25):2351–2359. doi: 10.1056/NEJMoa0909383. [DOI] [PubMed] [Google Scholar]
- 122.Resik S, Tejeda A, Más Lago P, et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis. 2010;201(9):1344–1352. doi: 10.1086/651611. [DOI] [PubMed] [Google Scholar]
- 123.Polio Research Committee, World Health Organization. PolioPipeline. Geneva, Switzerland: World Health Organization; 2010. 1–6. ( http://wwwpolioeradication.org/content/poliopipeline/PolioPipeline_06.pdf). (Accessed May 3, 2010) [Google Scholar]
- 124.Thompson KM, Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet. 2007;369(9570):1363–1371. doi: 10.1016/S0140-6736(07)60532-7. [DOI] [PubMed] [Google Scholar]
- 125.Emerson CI, Singer PA. Is there an ethical obligation to complete polio eradication? Lancet. 2010;375(9723):1340–1341. doi: 10.1016/s0140-6736(10)60565-x. [DOI] [PubMed] [Google Scholar]