Abstract
Anaemia in pregnancy defined as haemoglobin (Hb) level of < 10 gm/dL, is a qualitative or quantitative deficiency of Hb or red blood cells in circulation resulting in reduced oxygen (O2)- carrying capacity of the blood. Compensatory mechanisms in the form of increase in cardiac output (CO), PaO2, 2,3 diphosphoglycerate levels, rightward shift in the oxygen dissociation curve (ODC), decrease in blood viscosity and release of renal erythropoietin, get activated to variable degrees to maintain tissue oxygenation and offset the decreases in arterial O2 content. Parturients with concomitant medical diseases or those with acute ongoing blood losses may get decompensated, leading to serious consequences like right heart failure, angina or tissue hypoxemia in severe anaemia. Preoperative evaluation is aimed at assessing the severity and cause of anaemia. The concept of an acceptable Hb level varies with the underlying medical condition, extent of physiological compensation, the threat of bleeding and ongoing blood losses. The main anaesthetic considerations are to minimize factors interfering with O2 delivery, prevent any increase in oxygen consumption and to optimize the partial pressure of O2 in the arterial blood. Both general anaesthesia and regional anaesthesia can be employed judiciously. Monitoring should focus mainly on the adequacy of perfusion and oxygenation of vital organs. Hypoxia, hyperventilation, hypothermia, acidosis and other conditions that shift the ODC to left should be avoided. Any decrease in CO should be averted and aggressively treated.
Keywords: Anaemia, anaesthetic considerations, compensatory mechanisms, pregnancy
INTRODUCTION
WHO estimates indicate a 65-75% prevalence of anaemia in pregnant women in India.[1,2] Nearly half of the global maternal deaths due to anaemia occur in South Asian countries with 80% of these being contributed by India.[1,3]
DEFINITION OF ANAEMIA
Anaemia is a qualitative or quantitative deficiency of Hb or red blood cells (RBC) in circulation resulting in a reduced oxygen (O2)-carrying capacity of the blood to organs and tissues.[4] Anaemia in pregnancy is defined as an Hb concentration of < 11 gm/dL or a haematocrit < 0.33 in first and third trimesters, while in the second trimester a fall of 0.5 gm/dL is adjusted for an increase in plasma volume and a value of 10.5 gm/dL is used.[5,6] However, in India and most of the other developing countries a lower limit of 10 gm/dL is often accepted.[7]
CLASSIFICATION OF ANAEMIA
Anaemia during pregnancy may be classified based on etiology as
Physiological anaemia of pregnancy
-
Acquired:
Nutritional- Iron deficiency, folate deficiency, B-12 deficiency, etc.
Infections- Malaria, hookworm infestation, etc
Haemorrhagic- Acute or chronic blood loss
Bone marrow suppression- Aplastic anaemia, drugs, etc.
Renal disease
Genetic - haemoglobinopathies – sickle cell disease, thalassaemia, etc
Anaemia in pregnancy can also be classified as mild, moderate or severe, with WHO classifying mild anaemia as Hb level of 10.0-10.9 gm/dL, moderate anaemia as 7-9.9 gm/dL and < 7gm/dL as severe anaemia.[8]
PHYSIOLOGICAL HAEMATOLOGICAL CHANGES IN PREGNANCY PERTINENT TO ANAEMIA
Maternal blood volume begins to increase early at 6th week and continues to rise by 45-50% till 34 weeks of gestation, returning to normal by 10-14 days postpartum.[9–13] This adaptive physiological hypervolemia helps to maintain blood pressure in presence of decreased vascular tone[9,14,15], facilitates maternal and fetal exchange of respiratory gases, nutrients and metabolites and protects the mother from hypotension, by reducing the risks associated with haemorrhage at delivery.[10]
Increased fetal and maternal production of estrogen and progesterone contribute to the rise in plasma volume.[10,16] Progesterone enhances aldosterone production. Both esterogen and aldosterone increase plasma renin activity, enhancing renal sodium absorption to 900 mEq and water retention to 8.5 L approximately, via the renin-angiotensinaldosterone system.[10,17] The concentration of plasma adrenomedullin, a potent vasodilating peptide, rises during pregnancy, and correlates significantly with blood volume.[10,18]
RBC volume decreases during the first 8 weeks, increases to the prepregnancy level by 16 weeks, and undergoes a further rise to 30% above the prepregnancy volume at term.[9,10,12,14,19] Elevated erythropoietin concentration[9,20] and the erythropoietin effects of progesterone, prolactin and placental lactogen[9] result in an increase in RBC volume.[9,14]
Hence the plasma volume expansion increase exceeds the rise in RBC volume, resulting in haemodilution and consequent physiological anaemia of pregnancy,[9–14] with an average Hb and haematocrit of 11.6 gm/dL and 35.5%, respectively.[21] This represents a 15% decrease from prepregnancy levels.[9] The decrease in blood viscosity from the lower haematocrit reduces resistance to blood flow, as a compensatory mechanism.[10] However, if the Hb concentration falls < 10 gm/dL, other causes of anaemia should be considered.[9]
PATHOPHYSIOLOGY OF ANAEMIA
The anaesthetic implications of anaemia in pregnancy stem from the adverse effects of decreased tissue O2 delivery. Let us briefly review the normal and compensatory O2 delivery mechanisms in anaemia.
Oxygen is carried in the blood in two forms as:
Physical solution in plasma (dissolved form)
Reversible chemical combination with haemoglobin (Oxyhaemoglobin)
Arterial blood contains only 0.3 mL of O2, in each 100 mL of blood at a PO2 of 100 mm Hg and temperature of 37°C.[22] This small quantity reflects tension of O2 in the blood and acts as a pathway for the supply of O2 to Hb and for the transfer of O2 to cells.
Majority of the O2 carried in blood is in combination with Hb. As blood leaves the lung, Hb reversibly binds to four molecules of O2 which equals to 1.37-1.39 mL/g of Hb.[22,23]
Therefore, the O2 content of the blood is the quantity of O2 contained in the red cell added to the quantity dissolved in plasma, defined as the volume of O2 in milliliters carried in 1 dL of blood. It is calculated from the equation:[22]
[a-arterial sample; CaO2- arterial O2 content in mL/dL of blood, Hb-concentration of Hb in gm/dL; 1.37 is the volume of O2 in milliliters carried by 1 g of fully saturated Hb; SaO2-fractional Hb saturation defined as the ratio of oxyHb to total Hb, hence SaO2 =HbO2 / (HbO2 + reducedHb + methHb + COHb); 0.0034 are the solubility coefficients of O2 in plasma (mL of O2/dL plasma in mm Hg); PaO2-arterial O2 tension measured in mm Hg.]
Hence, the O2 content of the blood where PO2 is 100 mm Hg, SaO2 is normal and Hb concentration is 15 gm/ dL, is 20 ml. In anaemia when Hb concentration falls by 50% (7.5 gm/dL), O2 content decreases to 10 ml/dL. Thus, Hb and SaO2 are the primary determinants of arterial O2 content.[22,23]
This total quantity of O2 in arterial blood delivered to tissues is a function of cardiac output (CO). Therefore, Oxygen delivery= CaO2 × Cardiac Index × 10 mL/ min/m2
Whenever anaemia occurs, i.e., CaO2 decreases, CO increases as a compensatory mechanism to maintain O2 delivery to tissues.[22]
Oxygen consumption (VO2), an important determinant of adequacy of tissue oxygenation, is determined primarily by CO and arterio-venous O2 content difference C (a-v)O2.[7,22,23]
Hence, O2 extraction = 250/1000 mL O2 = 25%
In chronic anaemia, CO increases to maintain a constant arterial venous O2 content difference and the O2 extraction ratio. However, in an anaemic parturient, whenever the O2 demand rises acutely, the SvO2 falls to enhance O2 extraction along with a further increase in CO as compensatory mechanisms. Acute cardiac failure may result due to excessive strain on myocardium, hence increases in CO should be < 10 L/min.[23]
Adequacy of tissue oxygenation (tissue PO2) is judged by mixed venous PO2 (PvO2).[22,23] Normal values of PvO2 is 35-45 mm Hg (5-6 KPa), which corresponds to a normal mixed venous O2 content difference CvO2 of 12-15 vol% and a mixed venous Hb saturation SvO2 of 72-78%.[23–25] Thus, we can deduct that as PvO2 falls from 40 - 47 in normal patients to 26 mm Hg in severe anaemia [Figure 1], tissue hypoxia may occur. In an attempt to prevent tissue hypoxia the body decreases the amount of O2 released per 100 mL of blood and effectively moves the mixed venous point, by increasing the tissue blood flow and CO.[7,22,23] The cardiovascular system of the patient should be healthy enough to compensate for increases in CO. Hence, the risk of anaemia will depend both on the magnitude of fall in tissue O2 content and on the nature and severity of coexisting medical diseases.
Figure 1.
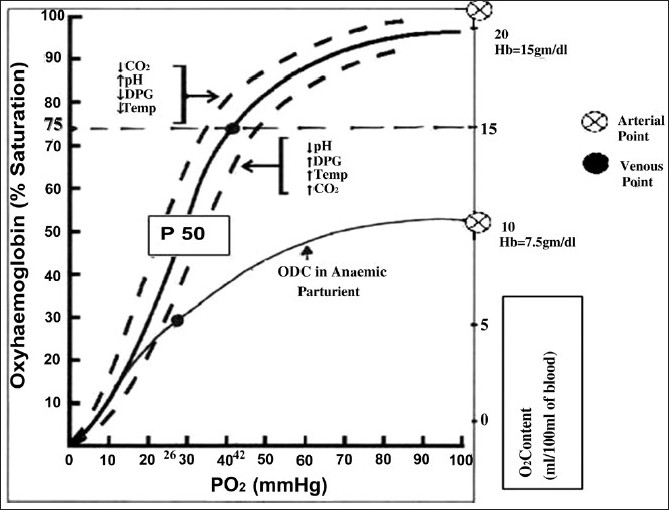
Oxygen dissociation curve
Apart from an increase in CO and O2 extraction, the O2 delivery is greatly affected by the relationship between the saturation of Hb with O2 (SaO2) and the partial pressure of O2 in the blood, best described by the oxygen dissociation curve (ODC)[23] [Figure 1]. The Hb molecule has specific characteristics which allows the oxygenation of one subunit of Hb molecule to facilitate 300 times greater O2 affinity of the other subunits. Similarly release of O2 from the first Hb subunit will facilitate the release of further O2. “This enhancement of O2 uptake and release is the cause of the sigmoidal shape of ODC.”[22] The sigmoid shape is important as the rapid descent allows a large fraction of O2 to be released to the tissues with a modest drop in the partial pressure of O2 [22,23] [Figure 1]. The partial pressure of O2 in the blood at which the Hb is 50% saturated, known as P50 is 26.6 mm Hg at a pH of 7.4.[22]
The P50 is a conventional measure of Hb affinity for O2. Increased temperature and rise in hydrogen ion [H+] and 2,3- diphosphoglycerate (DPG) concentrations reduce the affinity of Hb for O2, leading to an increase in the P50 and rightward shift of the curve thus facilitating the unloading of O2 at peripheral tissues. A decrease in P50 indicates a left shift in the ODC and an increased affinity of Hb for O2, so that a lowerthan- normal O2 tension saturates Hb in the lung and the subsequent release of O2 to the tissues occurs at a lower-than-normal capillary O2 tension[22,23] [Figure 1].
Changes in 2,3-DPG are most important and often seen in chronic anaemia especially sickle cell anaemia, and chronic hypoxemia. 2,3-DPG is a metabolic product of anaerobic metabolism with a normal intraerythrocyte concentration of 15 μmol/gm Hb. It is able to bind with β-chains when Hb is deoxygenated, thus increasing O2 availability[7,22,23,26–28].
To summarize, in an anaemic pregnant patient various compensatory mechanisms get activated:[7,22,23,24,28]
Increase in CO
Rightward shift of ODC
Decrease in blood viscosity
Increase in 2,3-DPG concentration in RBC
Release of renal erythropoietin leading to stimulation of erythroid precursors in bone marrow
Thus, though tissue oxygenation is not impaired during physiological, or chronic anaemia as a result of compensatory mechanisms, these may be compromised in severe or acute onset anaemia leading to serious consequences like right heart failure, angina, tissue hypoxemia, etc.[7,28]
ANAESTHETIC CONSIDERATIONS
Preoperative assessment
Clinical assessment should focus at assessment of the cause, type and severity of anaemia and adequacy of compensatory mechanisms.[7,28]
History suggestive of poor tissue perfusion can manifest as tiredness, easy fatiguability in mild anaemia to breathlessnesss/dyspnea, palpitations, angina in moderate to severe anaemia.[7,28]
Signs of high CO like tachycardia, wide pulse pressure and systolic ejection murmur are essential for planning the mode of anaesthetic management.
Investigations should include a complete haemogram, reticulocyte count, peripheral smears and blood grouping. Other investigations include stool and urine analysis, ESR, blood urea nitrogen levels, S. creatinine, bilirubin levels, S. proteins S. Iron, total iron-binding capacity, B12 and folate levels, Hb electrophoresis and ECG for any evidence of myocardial ischaemia, etc.[7,28]
Minimal acceptable level of Hb and need for preoperative transfusion
A ‘minimum acceptable haemoglobin level’ does not exist.[29] A healthy myocardium compensates for the low Hb or Hct levels (7-8 gm/dL of Hb or 21-24% Hct) in order to optimize O2 delivery. In patients with overt or silent episodes of myocardial ischaemia (diabetic parturient), a level of < 10 gm/dL carries risk of decompensation.[29,30]
Many task force guidelines recommend that RBC transfusions should not be dictated by a single Hb “trigger”; instead, it should be based on the patient’s needs and risks of developing complications of inadequate oxygenation.[31–34] The decision to perform RBC transfusion should be made on both clinical and haematological grounds. Transfusion is rarely indicated in the stable patient when Hb is > 10 gm/dL and is almost always indicated when < 6 gm/dL.[32,33] If the Hb is < 7-8 gm/dL in labour or in the postpartum period, the decision to transfuse should be made on an informed basis according to the symptoms, coexisting medical conditions, continuing blood loss or threat of bleeding. There is little evidence of the benefit of blood transfusion in asymptomatic parturients.[30,33,34]
Benefits from replenishing O2-carrying capacity by transfusion must always be balanced against transfusion-associated risks like pulmonary oedema, immune suppression, etc.[35,36] In a large randomized controlled trial (RCT), Hebert established that there was no difference in mortality rates between restrictive and liberal transfusion strategies in noncardiac, critically ill patients who were able to tolerate lower levels of Hb.[36,37] Reiles and Linden[38] indicated in a study that the maintenance of a higher Hb concentration with RBC transfusion in an attempt to increase tissue O2 delivery is not associated with clinical benefit, as transfusion-related increased blood viscosity can result in a reduction in blood flow and incipient cardiac failure. Also the storage process affects the ability of RBCs to transport and deliver O2 to the tissues, due to decreases in erythrocyte concentrations of 2,3-DPG to 1 μmol/g of Hb or less at 21 days of storage.[27,39] This point, however, remains controversial.[27]
If transfusion is necessary for severe cases of chronic anaemia, leukoreduced red cells given carefully under strict monitoring have beneficial effects.[38] Further research should be done to evaluate symptomatic transfusion strategy to a Hb-based strategy on the outcome of high-risk parturients.
Choice of anaesthesia
Choice of anaesthesia will depend on the severity and type of anaemia, extent of physiological compensation, concomitant medical conditions, type and nature of procedure and anticipated blood loss. The main anaesthetic considerations in chronic anaemia are to minimize factors interfering with O2 delivery, prevent any increase in O2 consumption and to optimize the partial pressure of O2 in the arterial blood. The following measures need to be diligently adhered to in the perioperative period, while giving either General Anaesthesia or Regional anaesthesia:[7,10,28]
-
Avoidance of hypoxia
Preoxygenation is mandatory with 100% O2.
Oxygen supplementation should be given in the peri- and postoperative period.
Maintenance of airway is important to prevent fall in FiO2 due to airway obstruction, difficult intubation, etc. Hence measures and expertise to secure a definitive airway should be available immediately.
Spontaneous ventilation technique is suitable only for short procedures. High FiO2 (40-50%) is administered to overcome effects of hypoventilation. High concentration of volatile agents depresses both the myocardium as well as ventilation resulting in an undesirable decrease in O2 flux.
Aggressively treat and avoid conditions that increase the O2 demands like fever, shivering, acute massive blood losses leading to an acute drop of Hb below 7 gm/dL.
Nitrous oxide should be used cautiously in patients with folate and Vitamin B-12 deficiency.
-
Minimize drug-induced decreases in CO
Intravenous induction of anaesthesia should be slowly titrated to prevent precipitous fall in CO.
Careful positioning of the patient to minimize position associated volume shifts.
Mild tachycardia and wide pulse pressure may be physiological and should not be confused with light anaesthesia
-
Factors leading to left shift of ODC should be avoided
Avoid hyperventilation to minimize respiratory alkalosis. Hypocapnia also decreases CO. Maintain normocapnia.
-
Hypothermia should be avoided –
Take all measures to ensure normal core body temperatures
IV fluids and blood products if any should be warmed
Monitoring should be aimed at assessing the adequacy of perfusion and oxygenation of vital organs.[34] It should include routine monitors like ECG, NIBP, EtCO2, Temperature monitoring, Pulse oximetry, urine output and may include CVP, invasive arterial blood pressure monitoring, ABG analysis and measurement of mixed venous PvO2 in severe anaemia wherein major blood losses are anticipated like in placenta previa or acccreta etc. Serial Hb and Haematocrit values can guide us to monitor ongoing blood losses.[27]
Regional anaesthesia is preferred for peripheral limb surgery as they are associated with reduced blood loss.
Central neuraxial blocks can be judiciously employed using either a low-dose spinal anaesthesia along with adjuvants or an intermittent dosing, continuous epidural. These are advantageous in providing good analgesia, ability to provide supplemental O2, and decreased blood loss with stable haemodynamics. However, central neuraxial blocks are fraught with imminent dangers of hypotension, haemodilution and subsequent heart failure or pulmonary oedema on the return of vascular tone. It is advisable to use vasoconstrictors to sustain blood pressure.[7,28] Regional anaesthesia can also be implicated in the worsening of symptoms of subacute degeneration of spinal cord and hence should be avoided in parturients with overt Vitamin B12 deficiencies with neurological symptoms.[7,28]
Special situations
Sickle cell disease
Sickle cell disease is a congenital haemoglobinopathy.[40] Parturients with sickle cell anaemia have an increased incidence of preterm labour, placental abruption, placenta previa and hypertensive disorders of pregnancy.[41]
In Hb S, valine is substituted for glutamic acid at the 6th amino acid in the β-chains. This causes the Hb molecules to aggregate when deoxygenated.[24,40] HbS begins to aggregate at a PO2 of < 50 mm Hg, with all getting aggregated at a PO2 of 23 mm Hg.[24,42]
Dehydration, hypotension, hypothermia, acidosis and a high concentration of HbS, predispose the pregnant patient to sickling.[27] Sickle cell anaemia is a chronic anaemia. Marked ventricular hypertrophy secondary to increased CO, may lead to a deterioration in ventricular diastolic function.[24,43]
Blood transfusions need to be given only when they are specifically indicated (e.g., severe anaemia, hypoxemia, preeclampsia, septicemia, renal failure, acute chest pain syndrome, anticipated surgery, aplastic crisis). The goals of transfusion are to achieve a Hb concentration > 8 gm/dL and HbA > 40% of the total Hb.[24]
Anaesthetic management aims at avoidance of hypoxemia, hypovolemia, hypothermia and acidosis along with provision of good analgesia.[24,40] Both neuraxial and general anaesthesia are acceptable.[44]
Thalassaemia
Thalassaemias are a diverse group of microcytic, haemolytic anaemias wherein there is a reduced synthesis of one or more of the polypeptide globin chains.[24]
Advances in the management of β-thalassaemia major by extensive blood transfusions and chelation therapy have prolonged life expectancy.[45] Higher transfusion requirements in pregnancy worsen haemosiderosis and cardiac failure.[24]
The major anaesthetic considerations are due to chronic anaemia with resultant tissue hypoxia, multiple transfusions leading to increased iron load especially in the myocardial cells and concomitant difficult airway. Both general anaesthesia and central neuraxial anaesthesia[45] can be safely administered for cesarean delivery after estimation of the platelet count and after excluding history of spontaneous haemorrhage.[24]
CONCLUSION
The anaesthetic implications of anaemia in pregnancy are based on the understanding of the normal and compensatory mechanisms that optimize tissue oxygenation. The main aim is to maintain a fine balance between the compensatory mechanisms and adequate tissue oxygenation in these parturients. Both regional and general anaesthesia can be used judiciously. Monitoring should aim at assessing the adequacy of perfusion and oxygenation and the magnitude of ongoing losses. Deleterious effects of chronic tissue hypoxemia along with threat of major blood losses in the perioperative period need to be anticipated and treated adequately.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kalaivani K. Prevalence & consequences of anaemia in pregnancy. Indian J Med Res. 2009;130:627–33. [PubMed] [Google Scholar]
- 2.DeMaeyer E, Adiels-Tegman M. Prevalence of anaemia in the World. World Health Stat Q. 1998;38:302–16. [PubMed] [Google Scholar]
- 3.Ezzati M, Lopus AD, Dogers A, Vander HS, Murray C. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 4. Available from: http://www.biology-online.org/dictionary/Anaemia [last accessed on 2010 Jul 20]
- 5.Nutritional anaemias. Report of a WHO Scientific Group. WHO Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 6.Centers for Disease Control (CDC). CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep. 1989;38:400–4. [PubMed] [Google Scholar]
- 7.Basu SM. Anaemia and Pregnancy. In: Gupta S, editor. Obstetric Anaesthesia. 1st ed. Delhi: Arya Publications; 2004. pp. 433–56. [Google Scholar]
- 8.Idowu OA, Mafiana CF, Sotiloye D. Anaemia in pregnancy: A survey of pregnant women in Abeokuta, Nigeria. Afr Health Sci. 2005;5:295–9. doi: 10.5555/afhs.2005.5.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaiser R. Physiologic Changes of Pregnancy. In: Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. Chestnut’s Obstetric Anesthesia. Principles and Practice. 4th ed. USA: Mosby Elsevier; 2009. pp. 21–3. [Google Scholar]
- 10.Birnbach DJ, Browne IM. Anesthesia for Obstetrics. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Miller’s Anesthesia. 7th ed. USA: Churchill Livinstone Elsevier; 2010. pp. 2204–6. [Google Scholar]
- 11.Bernstein IM, Ziegler W, Badger GJ. Plasma volume expansion in early pregnancy. Obstet Gynecol. 2001;97:669–72. doi: 10.1016/s0029-7844(00)01222-9. [DOI] [PubMed] [Google Scholar]
- 12.Lund CJ, Donovan JC. Blood volume during pregnancy: Significance of plasma and red cell volumes. Am J Obstet Gynecol. 1967;98:394–403. [PubMed] [Google Scholar]
- 13.Hytten FE, Paintin DB. Increase in plasma volume during normal pregnancy. J Obstet Gynaecol Br Emp. 1963;70:402–7. doi: 10.1111/j.1471-0528.1963.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 14.Taylor DJ, Lind T. Red cell mass during and after normal pregnancy. Br J Obstet Gynaecol. 1979;86:364–70. doi: 10.1111/j.1471-0528.1979.tb10611.x. [DOI] [PubMed] [Google Scholar]
- 15.Schrier RW, Berl T, Anderson RJ. Osmotic and nonosmotic control of vasopressin release. Am J Physiol. 1979;236:F321–32. doi: 10.1152/ajprenal.1979.236.4.F321. [DOI] [PubMed] [Google Scholar]
- 16.Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Peeters LH. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol. 1993;169:1382–92. doi: 10.1016/0002-9378(93)90405-8. [DOI] [PubMed] [Google Scholar]
- 17.Theunissen I, Parer J. Fluid and electrolytes in pregnancy. Clin Obstet Gynecol. 1994;37:3–15. doi: 10.1097/00003081-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi Y, Ueyama H, Mashimo T, Kangawa K, Minamino N. Circulating mature adrenomedullin is related to blood volume in full-term pregnancy. Anesth Analg. 2005;101:1816–20. doi: 10.1213/01.ANE.0000182329.02880.83. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393–9. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cotes PM, Canning CE, Lind T. Changes in serum immunoreactive erythropoietin during the menstrual cycle and normal pregnancy. Br J Obstet Gynaecol. 1983;90:304–11. doi: 10.1111/j.1471-0528.1983.tb08914.x. [DOI] [PubMed] [Google Scholar]
- 21.Conklin KA. Maternal physiological adaptations during gestation, labor and the puerperium. Semin Anesth. 1991;10:221–34. [Google Scholar]
- 22.Rutter TW, Tremper KK. The physiology of oxygen transport and red cell transfusion. In: Thomas EJ, Healy, Knight PR, editors. Wylie and Churchill-Davidson’s Anesthesia. 7th ed. London: Arnold; 2003. pp. 167–83. [Google Scholar]
- 23.Bailey K, Gwinnutt C. The physiology of red blood cells and haemoglobin variants. http://www.frca.co.uk/Documents/103-The%20physiology%20of%20red%20blood%20cells%20and%20haemaglobin%20variants.pdf [last accessed on 2010 Aug 4] [Google Scholar]
- 24.Sharma SK. Hematologic and coagulation disorders. In: Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. Chestnut’s Obstetric Anesthesia. Principles and Practice. 4th ed. USA: Mosby Elsevier; 2009. pp. 943–7. [Google Scholar]
- 25.Physiology of Perfusion. http://www.emcrit.org/1-resus/physioperfusion.htm [last accessed on 2010 Aug 7] [Google Scholar]
- 26.Hamasaki N, Asakura T, Minakami S. Effect of oxygen tension of glycolysis in human erythrocytes. J Biochem (Tokyo) 1970;68:157–61. doi: 10.1093/oxfordjournals.jbchem.a129341. [DOI] [PubMed] [Google Scholar]
- 27.Weiskopf RB, Feiner J, Hopf H, Lieberman J, Finlay HE, Quah C, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–20. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Christine S, Rinder . Hematologic disorders. In: Paul AK (adapting editor), Hines RL, Marschall KE, editors. Stoelting’s Anesthesia and Co-existing diseases. 5th ed. India: Elsevier; 2010. pp. 448–56. [Google Scholar]
- 29.Lundsgaard-Hansen P, Doran JE, Blauhut B. Is there a generally valid, minimum acceptable hemoglobin level? Infusionstherapie. 1989;16:167–75. doi: 10.1159/000222372. [DOI] [PubMed] [Google Scholar]
- 30.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–47. [PubMed] [Google Scholar]
- 31.Hardy JF. Should we reconsider triggers for red blood cell transfusion? Acta Anaesthesiol Belg. 2003;54:287–95. [PubMed] [Google Scholar]
- 32.Murphy MF, Wallington TB, Kelsey P, Boulton F, Bruce M, Cohen H, et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 33.Blood Transfusions in Obstetrics. RCOG Green-top Guideline. No. 47. December. 2007 http://www.rcog.org.uk/files/rcog-corp/uploaded-files/GT47BloodTransfusions1207amended.pdf [last accessed on 2010 Aug 4] [Google Scholar]
- 34.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 2006;105:198–208. [PubMed] [Google Scholar]
- 35.Kubanek B. The critical hemoglobin value in the therapy of chronic anemia. Beitr Infusionsther. 1992;30:224–7. [PubMed] [Google Scholar]
- 36.Alvarez G, Hébert PC, Szick S. Debate: transfusing to normal haemoglobin levels will not improve outcome. Crit Care. 2001;5:56–63. doi: 10.1186/cc987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. The Transfusion Requirements in Critical Care Investigators: A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 38.Reiles E, Van der Linden P. Transfusion trigger in critically ill patients: has the puzzle been completed? Crit Care. 2007;11:142. doi: 10.1186/cc5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valeri CR, Valeri DA, Gray A, Melaragno A, Dennis RC, Emerson CP. Viability and function of red blood cell concentrates stored at 4 degrees C for 35 days in CPDA-1, CPDA-2, or CPDA-3. Transfusion. 1982;22:210–6. doi: 10.1046/j.1537-2995.1982.22382224943.x. [DOI] [PubMed] [Google Scholar]
- 40.Firth PG. Anaesthesia for peculiar cells-a century of sickle cell disease. Br J Anaesth. 2005;95:287–99. doi: 10.1093/bja/aei129. [DOI] [PubMed] [Google Scholar]
- 41.Koshy M, Burd L, Wallace D, Moawad A, Baron J. Prophylactic red-cell transfusions in pregnant patients with sickle cell disease: A randomized cooperative study. N Engl J Med. 1988;319:1447–52. doi: 10.1056/NEJM198812013192204. [DOI] [PubMed] [Google Scholar]
- 42.Solanki DL, McCurdy PR, Cuttitta FF, Schechter GP. Hemolysis in sickle cell disease as measured by endogenous carbon monoxide production: A preliminary report. Am J Clin Pathol. 1988;89:221–5. doi: 10.1093/ajcp/89.2.221. [DOI] [PubMed] [Google Scholar]
- 43.Veille JC, Hanson R. Left ventricular systolic and diastolic function in pregnant patients with sickle cell disease. Am J Obstet Gynecol. 1994;170:107–10. doi: 10.1016/s0002-9378(94)70393-0. [DOI] [PubMed] [Google Scholar]
- 44.Firth PG, Head CA. Sickle cell disease and anesthesia. Anesthesiology. 2004;101:766–85. doi: 10.1097/00000542-200409000-00027. [DOI] [PubMed] [Google Scholar]
- 45.Butwick A, Findley I, Wonke B. Management of pregnancy in a patient with β thalassaemia major. Int J Obstet Anesth. 2005;14:351–4. doi: 10.1016/j.ijoa.2005.02.002. [DOI] [PubMed] [Google Scholar]


