Abstract
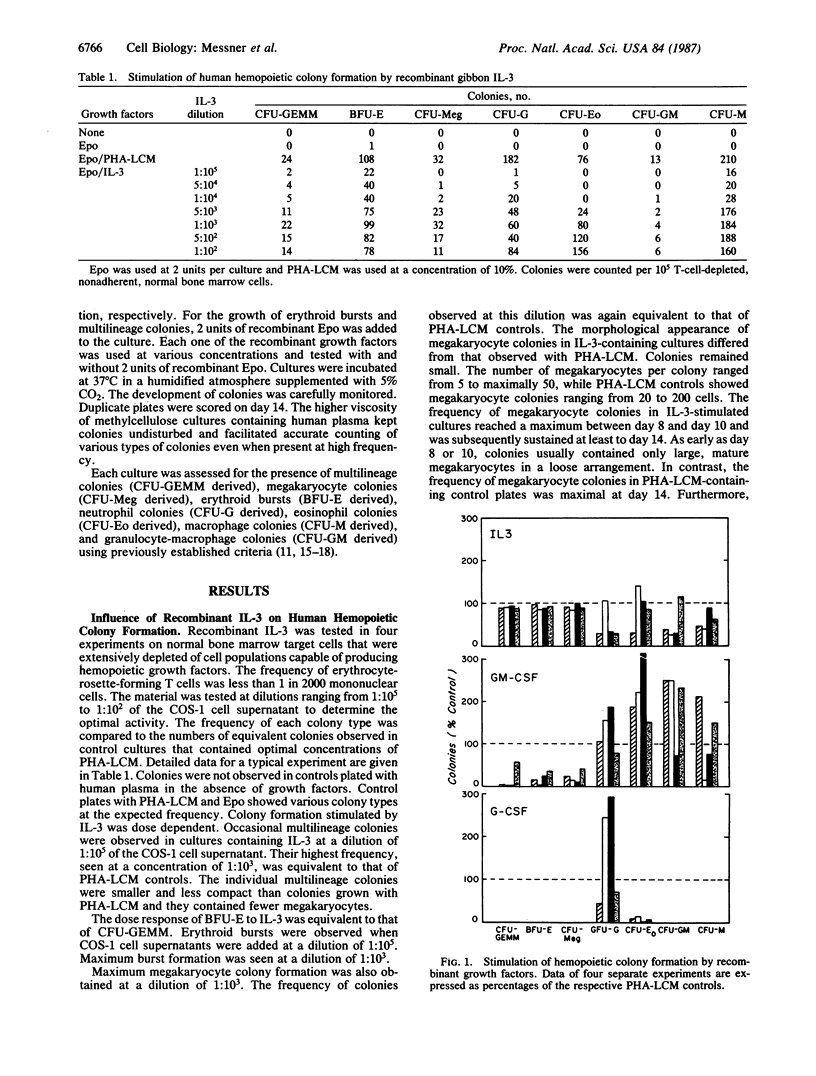
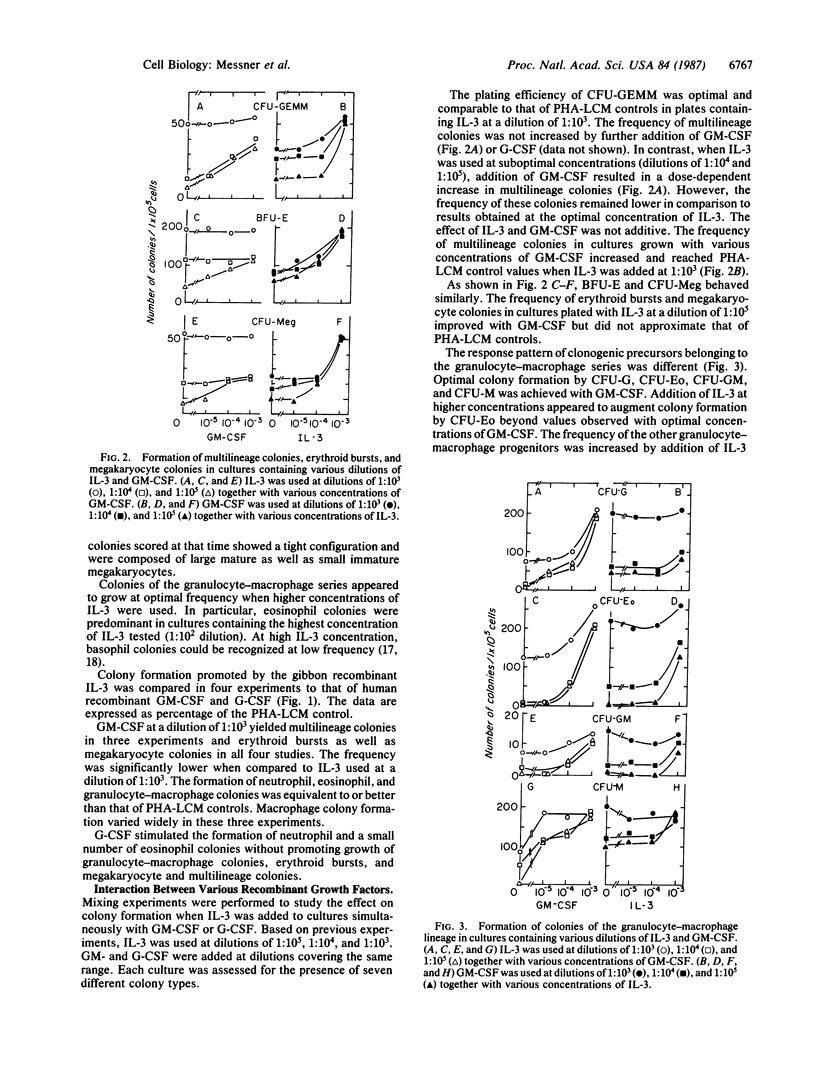
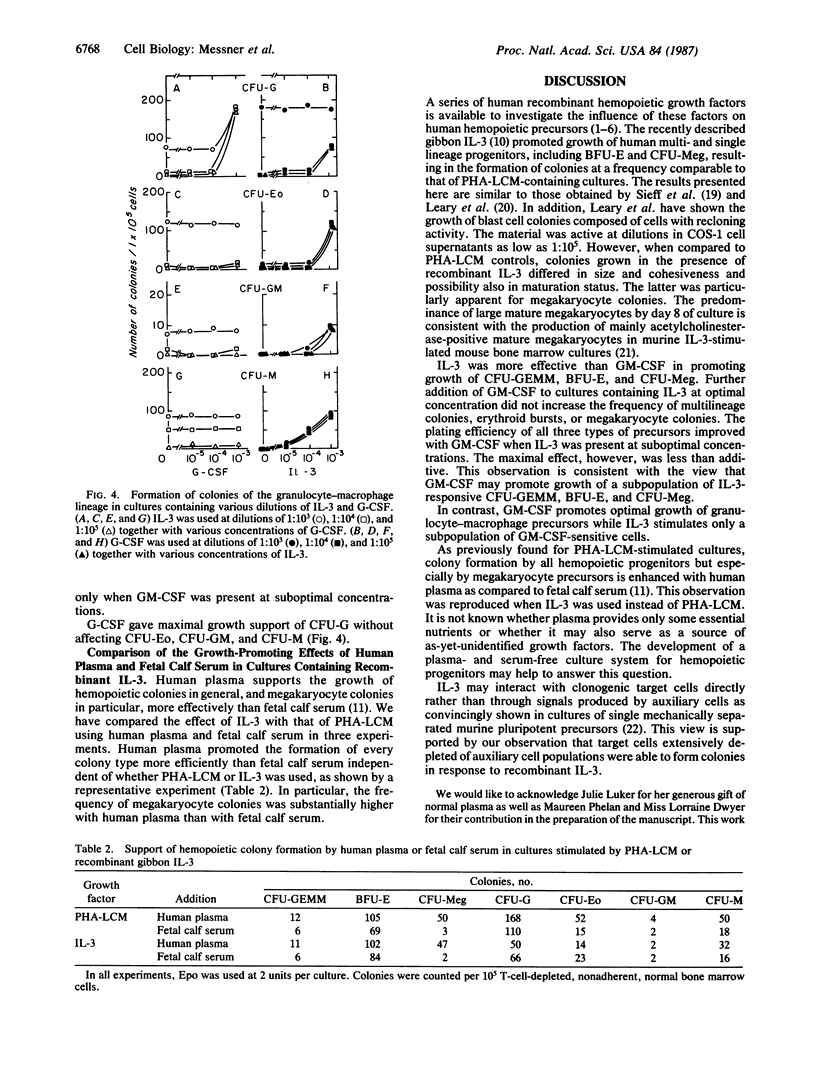
Supernatants of COS-1 cells transfected with gibbon cDNA encoding interleukin 3 (IL-3) with homology to sequences for human IL-3 were tested for ability to promote growth of various human hemopoietic progenitors. The effect of these supernatants as a source of recombinant IL-3 was compared to that of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) as well as to that of medium conditioned by phytohemagglutinin-stimulated leukocytes. The frequency of multilineage colonies, erythroid bursts, and megakaryocyte colonies in cultures containing the COS-1 cell supernatant was equivalent to the frequency observed in the controls and significantly higher than found in cultures plated with recombinant GM-CSF. G-CSF did not support the formation of multilineage colonies, erythroid bursts, and megakaryocyte colonies. In contrast, growth of granulocyte-macrophage colonies was best supported with GM-CSF, while recombinant IL-3 yielded colonies at lower or at best equivalent frequency. The simultaneous addition of higher concentrations of GM-CSF to cultures containing IL-3 in optimal amounts did not enhance the formation of multilineage colonies, erythroid bursts, and megakaryocyte colonies. However, the frequency of such colonies and bursts increased with GM-CSF when cultures were plated with suboptimal concentrations of IL-3. Growth of colonies within the granulocyte-macrophage lineage is optimally supported by GM-CSF and does not increase with further addition of IL-3.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denburg J. A., Messner H., Lim B., Jamal N., Telizyn S., Bienenstock J. Clonal origin of human basophil/mast cells from circulating multipotent hemopoietic progenitors. Exp Hematol. 1985 Mar;13(3):185–188. [PubMed] [Google Scholar]
- Emerson S. G., Sieff C. A., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Purification of fetal hematopoietic progenitors and demonstration of recombinant multipotential colony-stimulating activity. J Clin Invest. 1985 Sep;76(3):1286–1290. doi: 10.1172/JCI112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Berkner K., Segal G. M., Hagen F. S., Adamson J. W. Genomic cloning, characterization, and multilineage growth-promoting activity of human granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3101–3105. doi: 10.1073/pnas.83.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Ogawa M. Identification of pure and mixed basophil colonies in culture of human peripheral blood and marrow cells. Blood. 1984 Jul;64(1):78–83. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Gemmell L., Larson N., Luh J., Arai K., Rennick D. Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4360–4364. doi: 10.1073/pnas.82.13.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B., Jamal N., Messner H. A. Flexible association of hemopoietic differentiation programs in multilineage colonies. J Cell Physiol. 1984 Nov;121(2):291–297. doi: 10.1002/jcp.1041210205. [DOI] [PubMed] [Google Scholar]
- Lin F. K., Suggs S., Lin C. H., Browne J. K., Smalling R., Egrie J. C., Chen K. K., Fox G. M., Martin F., Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner H. A., Jamal N., Izaguirre C. The growth of large megakaryocyte colonies from human bone marrow. J Cell Physiol Suppl. 1982;1:45–51. doi: 10.1002/jcp.1041130410. [DOI] [PubMed] [Google Scholar]
- Messner H. A., McCulloch E. A. Interacting cell populations affecting granulopoietic colony formation by normal and leukemic human marrow cells. Blood. 1973 Nov;42(5):701–710. [PubMed] [Google Scholar]
- Messner H., Till J. E., McCulloch E. A. Density distributions of marrow cells from mouse and man. Ser Haematol. 1972;5(2):22–36. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Vadas M. A., Lopez A. F., Williamson D. J., Wong G. G., Clark S. C., Wang E. A. Biologic properties in vitro of a recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1986 Jan;67(1):37–45. [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Sieff C. A., Emerson S. G., Donahue R. E., Nathan D. G., Wang E. A., Wong G. G., Clark S. C. Human recombinant granulocyte-macrophage colony-stimulating factor: a multilineage hematopoietin. Science. 1985 Dec 6;230(4730):1171–1173. doi: 10.1126/science.3877981. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M., Ihle J. N. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J Cell Physiol. 1985 Aug;124(2):182–190. doi: 10.1002/jcp.1041240203. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]



