Abstract
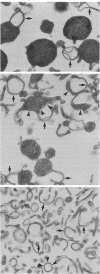
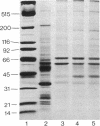
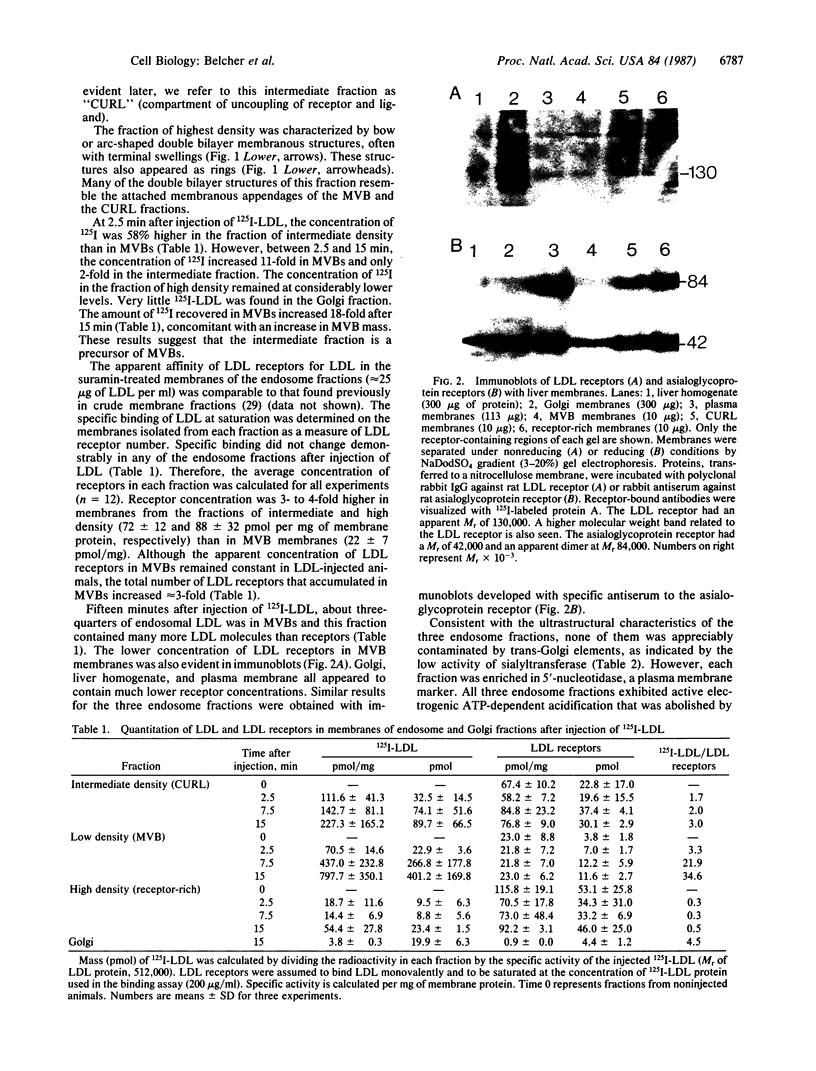
Three distinct endosomal fractions were isolated in high purity from livers of estradiol-treated rats. Each fraction had characteristic physical and ultrastructural properties, but the lipid composition and major proteins of their membranes were similar and differed from those derived from the Golgi apparatus. Injected radioiodinated low density lipoproteins accumulated first in the fraction of intermediate density and later in the low density fraction. The latter was composed almost exclusively of lipoprotein-filled multivesicular bodies, most of which had a single membranous appendage. The fraction of intermediate density was composed of lipoprotein-filled vesicles that were smaller than multivesicular bodies and also had membranous appendages. The high density fraction was composed of membranes resembling the appendages of the two vesicular fractions. All three fractions were enriched in receptors for low density lipoproteins and asialoglycoproteins, but receptor concentrations were considerably reduced in multivesicular bodies. The fraction of intermediate density may represent the compartment of uncoupling of receptor and ligand (CURL) described by Geuze et al. [Geuze, H. J., Slot, J. W., Strous, G. J. A. M., Lodish, H. F. & Schwartz, A. L. (1983) Cell 32, 277-287]. CURL vesicles may lose some of their appendage as multivesicular bodies are formed. The high density fraction then may represent a receptor-recycling compartment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Separation of two populations of endocytic vesicles involved in receptor-ligand sorting in rat hepatocytes. J Biol Chem. 1986 Jun 5;261(16):7445–7454. [PubMed] [Google Scholar]
- Beisiegel U., Schneider W. J., Brown M. S., Goldstein J. L. Immunoblot analysis of low density lipoprotein receptors in fibroblasts from subjects with familial hypercholesterolemia. J Biol Chem. 1982 Nov 10;257(21):13150–13156. [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y. S., Jones A. L., Hradek G. T., Windler E. E., Havel R. J. Autoradiographic localization of the sites of uptake, cellular transport, and catabolism of low density lipoproteins in the liver of normal and estrogen-treated rats. Proc Natl Acad Sci U S A. 1981 Jan;78(1):597–601. doi: 10.1073/pnas.78.1.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Evans W. H., Flint N. Subfractionation of hepatic endosomes in Nycodenz gradients and by free-flow electrophoresis. Separation of ligand-transporting and receptor-enriched membranes. Biochem J. 1985 Nov 15;232(1):25–32. doi: 10.1042/bj2320025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hardison W. G. Phospholipid, cholesterol, polypeptide and glycoprotein composition of hepatic endosome subfractions. Biochem J. 1985 Nov 15;232(1):33–36. doi: 10.1042/bj2320033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick C. A., Hamilton R. L., Spaziani E., Enders G. H., Havel R. J. Isolation and characterization of multivesicular bodies from rat hepatocytes: an organelle distinct from secretory vesicles of the Golgi apparatus. J Cell Biol. 1985 May;100(5):1558–1569. doi: 10.1083/jcb.100.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Wall D. A., Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983 Jan;96(1):217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost-Vu E., Hamilton R. L., Hornick C. A., Belcher J. D., Havel R. J. Multivesicular bodies isolated from rat hepatocytes. Cytochemical evidence for transformation into secondary lysosomes by fusion with primary lysosomes. Histochemistry. 1986;85(6):457–466. doi: 10.1007/BF00508427. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J. Phospholipid class and fatty acid composition of golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry. 1970 Jan 6;9(1):19–25. doi: 10.1021/bi00803a003. [DOI] [PubMed] [Google Scholar]
- Khan M. N., Savoie S., Bergeron J. J., Posner B. I. Characterization of rat liver endosomal fractions. In vivo activation of insulin-stimulable receptor kinase in these structures. J Biol Chem. 1986 Jun 25;261(18):8462–8472. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Stanley K. K. The isolation of endosome-derived vesicles from rat hepatocytes. Biochem J. 1983 Oct 15;216(1):27–36. doi: 10.1042/bj2160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Griffiths G., Dean G. E., Mellman I., Helenius A. Three-dimensional structure of endosomes in BHK-21 cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2899–2903. doi: 10.1073/pnas.83.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat hepatocytes: receptor-positive and receptor-negative endosomes. J Cell Biol. 1986 Mar;102(3):932–942. doi: 10.1083/jcb.102.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Lucocq J. M., Weinstein J., Paulson J. C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985 Nov;43(1):287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Schneider W. J., Beisiegel U., Goldstein J. L., Brown M. S. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem. 1982 Mar 10;257(5):2664–2673. [PubMed] [Google Scholar]
- Schneider W. J., Goldstein J. L., Brown M. S. Purification of the LDL receptor. Methods Enzymol. 1985;109:405–417. doi: 10.1016/0076-6879(85)09106-6. [DOI] [PubMed] [Google Scholar]
- Sigurdsson G., Noel S. P., Havel R. J. Catabolism of the apoprotein of low density lipoproteins by the isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):628–634. [PubMed] [Google Scholar]
- Stewart C. P., Hendry E. B. The phospholipins of blood. Biochem J. 1935 Jul;29(7):1683–1689. doi: 10.1042/bj0291683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke R. W., Hornick C. A., Belcher J., Scharschmidt B. F., Havel R. J. Identification and characterization of ATP-dependent proton transport by rat liver multivesicular bodies. J Biol Chem. 1985 Sep 15;260(20):11021–11026. [PubMed] [Google Scholar]
- Wall D. A., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat liver hepatocytes: biochemical characterization of the endosomal compartments. J Cell Biol. 1985 Dec;101(6):2104–2112. doi: 10.1083/jcb.101.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]