Abstract
Objective:
To investigate the hepatoprotective activity of L-ornithine-L-aspartate against thioacetamide (TAA)-induced hepataopathy in rats.
Materials and Methods:
The hepatoprotective activity of L-ornithine-L-aspartate (OA) at a dose of 2 g/kg, p.o. for 10 days was evaluated against TAA (250 mg/kg, i.p. for 2 days) induced hepatopathy in rats. Biochemical parameters such as serum aspartate transaminase, alanine transaminase, alkaline phosphatase, bilirubin and glutathione, thiobarbituric acid reactive substances, and protein in liver tissues were estimated to assess the liver function.
Results:
TAA-induced pathogenic changes in the levels of the above indices were significantly (P < 0.01) reversed by the OA treatment. OA treatment also exhibited significant restoration of the hepatic architecture and lobular structure in histological evaluation of the rat liver sections.
Conclusion:
Ornithine aspartate exhibited significant hepatoprotective activity against TAA-induced hepatic damage in rats.
Keywords: Hepatopathy, ornithine aspartate, oxidative stress, thioacetamide
Introduction
L-ornithine-L-aspartate (OA) is a stable salt of natural amino acids ornithine and aspartic acid. It has been studied extensively and proven to be effective in both experimental as well as clinical set up of hepatic encephalopathy (HE).[1,2] HE is caused by hyperammonemia[3,4] that may be caused by insufficient removal of ammonia from blood by liver due to its compromised functional status[5] or portacaval shunting.[6] Hyperammonemia leads to increased levels of ammonia in brain, which is responsible for the development of HE.[6,7] OA produces relief in the symptoms of HE by reducing elevated levels of ammonia both peripherally and centrally.[8,9] Although a number of studies are available for the action of OA on HE, data on its direct action on the liver are lacking.[10]
The present investigation was designed to evaluate the effects of OA on thioacetamide (TAA)-induced hepatopathy in rats. The rats with hepatic damage caused by TAA were chosen as an animal model for this study, because TAA is very frequently used to induce HE in rats.[11,12]
Materials and Methods
Animals
Adult Wistar strain albino rats (weighing, 150–200 g) were used for the study. Animals were supplied by Central Animal House Facility of Hamdard University and kept under standard laboratory conditions in polypropylene cages in 12-h light/dark cycle at 25 ± 2°C. Animals were provided with standard pellet diet (Lipton, India) and water ad libitum. All the procedures carried out on animals were approved by Institutional Animal Ethics Committee (JHAEC).
Drugs and chemicals
OA was purchased from Systopic Laboratories, New Delhi, India. TAA and all other biochemicals and chemicals used were of analytical grade.
Experimental protocol
Rats were randomly divided into three groups of six animals each. Groups II and III were administered two injections of TAA (250 mg/kg, i.p.) at 24 h interval on the first and second day. Group I served as normal control and received equivalent volume of vehicle. From the second day onward, Groups I and II received normal saline (1 mL/kg, p.o.) for 10 days. Group III was administered ornithine aspartate (OA, 2 g/kg, p.o.) for 10 days. On the twelfth day, blood was collected under light ether anesthesia and animals were killed by high dose of ether. Liver samples were also collected in ice-cold normal saline for histopathological and biochemical evaluation.
Biochemical analysis
Serum alanine transaminase (ALT),[13] aspartate transaminase (AST),[13] alkaline phosphatase (ALKP),[14] bilirubin (BRN)[15] and liver tissue glutathione (GSH),[16] thiobarbituric acid reactive substances (TBARS),[17] and protein[18] were estimated according to the reported procedures.
Histological studies
Histological liver sections were prepared by modified method of Luna et al.[19] as described previously.[20]
Statistical analysis
The data presented as mean ± SEM were analyzed by one-way ANOVA followed by Dunnett’s post-test. The results were considered statistically significant if the P-value was 0.05 or less.
Results
A significant (P < 0.01) increase in serum AST, ALT, ALKP, BRN, and tissue TBARS levels was observed in animals treated with TAA (Group II) as compared to normal control group (Group I). Levels of AST, ALT, ALKP, BRN, and tissue TBARS concentration increased by TAA treatment were decreased significantly (P < 0.01) by post-treatment of animals with OA (2 g/kg, p.o.) for 10 days. Moreover, the significant decrease (P < 0.01) in tissue GSH, which was observed in animals with TAA treatment (Group II) as compared to normal control (Group I), was significantly (P < 0.01) reversed by OA post-treatment. Blood GSH and liver protein concentrations were not altered significantly (P > 0.05) by any of the above treatments [Tables 1 and 2].
Table 1.
Effects of ornithine aspartate post-treatment on serum parameters in thioacetamide-induced hepatic damage in rats
| Group | Treatment | ALT (IU/mL) | AST (IU/mL) | ALKP (IU/mL) | Bilirubin (mg%) |
|---|---|---|---|---|---|
| I | NS | 56.0 ± 2.463 | 111.33 ± 9.670 | 20.719 ± 2.185 | 0.473 ± 0.038 |
| II | TAA + NS | 158.5 ± 14.080** | 257.0 ± 35.409** | 220.203 ± 6.425** | 1.233 ± 0.142** |
| III | TAA + OA | 84.0 ± 17.390** | 123.90 ± 9.757* | 36.215 ± 1.908** | 0.590 ± 0.086** |
| F-ratio | 11.811 | 8.340 | 62.86 | 13.187 |
Data expressed as mean ± SEM, n = 6; Significance of difference was evaluated with respect to Group II by one-way ANOVA followed by Dunnett’s post hoc test
(*P < 0.05,
P < 0.01, ns = nonsignificant); NS = Normal saline; TAA = thioacetamide; OA = ornithine aspartate; ALT = Alanine transaminase; AST = aspartate transaminase; ALKP = alkaline phosphatase.
Table 2.
Effects of ornithine aspartate post-treatment on liver tissue biochemicals in thioacetamide-induced hepatic encephalopathy in rats
| Group | Treatment | Blood GSH (mg%) | Tissue GSH (μmol/g of liver) | Tissue TBARS (nmol MDA/mg protein) | Protein (mg/g wet tissue) |
|---|---|---|---|---|---|
| I | NS | 1.197 ± 0.130 | 29.141 ± 1.228 | 0.126 ± 0.023 | 19.66 ± 1.253 |
| II | TAA + NS | 1.017 ± 0.133ns | 9.841 ± 1.561** | 0.676 ± 0.048** | 16.26 ± 1.305ns |
| III | TAA + OA | 1.306 ± 0.130ns | 20.522 ± 2.191** | 0.189 ± 0.026** | 15.2 ± 0.935ns |
| F-ratio | 0.868 | 25.247 | 57.665 | 4.390 |
Data expressed as mean ± SEM, n = 6; Significance of difference was evaluated with respect to Group II by one-way ANOVA followed by Dunnett’s post hoc test
(*P < 0.05,
P < 0.01,
ns = non significant); NS, normal saline; TAA, thioacetamide; OA, ornithine aspartate; GSH, glutathione; TBARS, thiobarbituric acid reactive substances; MDA, malondialdehyde.
Histopathological observations
Histology of the liver sections of normal control animals (Group I) showed normal hepatic architecture and normal liver lobular structure with well-preserved cytoplasm, prominent nucleus, and nucleolus [Figure 1]. The liver sections of TAA-treated animals (Group II) showed hepatic cells with severe toxicity characterized by centrilobular necrosis, periportal hepatocytic vacoulation with clearing of cytoplasm, heavy pigmentation around central veins, scattered inflammation, and giant cell transformation [Figure 2]. OA post-treatment (Group III) appeared to significantly reduce the TAA-induced toxicity as evidenced by less inflammatory changes, less pigmentation, and no necrosis [Figure 3].
Figure 1.
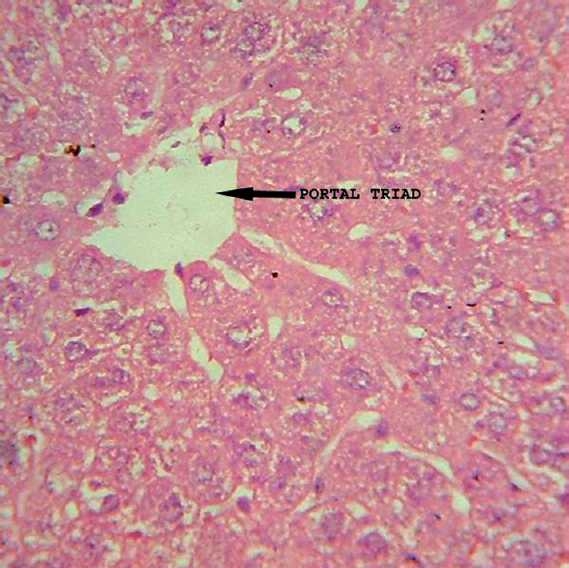
Group I: Liver section of normal control rats showing normal hepatic architecture and lobular structure (H and E, ×400).
Figure 2.
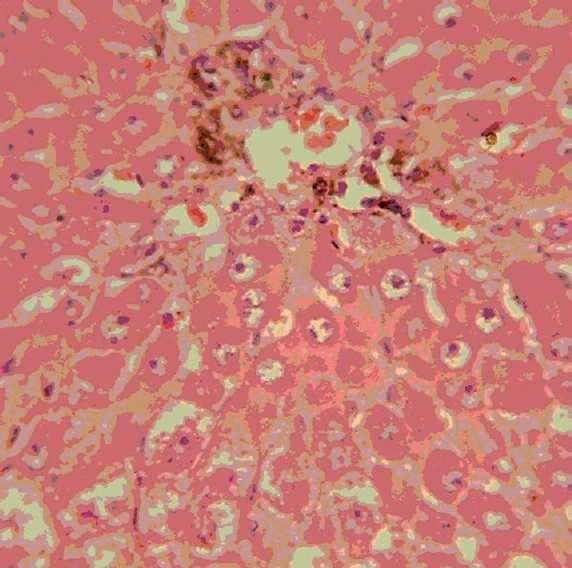
Group II: Liver section of thioacetamide-treated rats, showing periportal hepatocytic vacoulation, clearing of cytoplasm, pigmentation and scattered inflammation, necrosis, and giant cell transformation (H and E, ×400).
Figure 3.
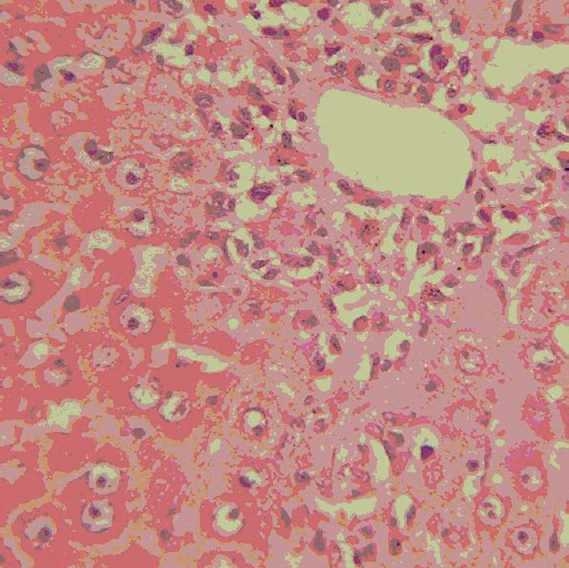
Group III: Liver section of rats treated with thioacetamide + ornithine aspartate showing only mild inflammation and pigmentation, but no necrosis (H and E, ×400).
Discussion
TAA was originally used as a fungicide to protect oranges from decay. In the aftermath of its inadvertent use, it was soon recognized as a potent hepatotoxin and carcinogen in rats.[21] The compound has also been reported toxic to kidney and thymus.[22] It is also reported to induce symptoms comparable to experimental partial hepatectomy. Cyt-P450 system is known to metabolize TAA in rat liver. Mechanism of toxicity is postulated to be due the formation of thioacetamide-S-oxide which causes change in cell permeability, increases in intracellular Ca++ concentration, increases in nuclear volume and enlargement of nucleoli, and inhibition of mitochondrial activity which leads to cell death.[23] TAA also increases the BRN levels in serum,[24] but decreases the protein contents in the liver by inhibiting incorporation of amino acids into liver protein.[25]
In a number of animal models, TAA-induced cirrhosis seems to resemble the important features of human disease.[26] Elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of the cell membrane in liver.[27] Damage to liver cells cause leakage of cellular enzymes into serum. Significant rise in levels of AST, ALT, and ALKP could be taken as an index of liver damage. TAA is known to cause changes in cell permeability by its metabolite thioacetamide-S-oxide.[23] In our study, the rise in AST, ALT, and ALKP levels induced by TAA administration was significantly reduced by OA post-treatment, suggesting that its protective activity might be due its effect against cellular leakage and loss of functional integrity of the cell membrane in hepatocytes. TAA is also reported to elevate BRN concentration[24] by damaging hepatic cells. In this study also, TAA treatment increased serum BRN concentration significantly, which was reversed significantly in rats treated with OA.
GSH is an important endogenous antioxidant system that is found in particularly high concentration in liver, and it is known to have key functions in protective processes. The reduced form of GSH becomes readily oxidized to glutathione disulfide (GSSG) on interacting with free radicals. Excessive production of free radicals resulted in the oxidative stress, which leads to damage of macromolecules, e.g. lipids and can induce lipid peroxidation in vivo.[28] In our study, TAA depleted tissue GSH and increased tissue TBARS significantly. The post-treatment of animals with OA significantly reversed the TAA-induced changes of these oxidative stress markers.
Histologically, TAA toxicity leads to the prominent changes in the liver tissue architecture including the appearance of necrotic cells and inflammatory cells, mostly macrophages around the central vein. TAA produces centrilobular necrosis with marked accumulation of lipids on acute exposure.[23] In our study, TAA administration produced severe periportal inflammation with pigment deposition and also scattered inflammatory cell infiltration in the hepatic lobule. The post-treatment of animals with OA, reversed significantly TAA-induced pathogenic changes in liver. A mild periportal inflammation with no pigmentation or necrosis was observed in the liver sections of animals treated with TAA and OA.
OA therapy is one of the key treatments of the HE. A number of mechanisms have been attributed to its action in HE. It has been proven to lower blood and brain ammonia concentration in both clinical and experimental set up.[1,8,9] Hyperammonemia is considered to be a major player in the pathogenesis of HE.[4] The infusion of OA has also been shown to reduce the plasma levels of aromatic amino acids such as phenyalanine, tyrosine, etc., in a dose-dependent manner.[29] Increased aromatic amino acid concentration in CNS has been implicated in the synthesis of false neurotransmitters such as phenyl ethanolamine, octopamine, etc. (instead of dopamine, norepinephrine, etc.), which leads to disturbance in normal physiological neurotransmission during HE.[30]
This study suggests that OA-induced alleviation in the symptoms of HE may be due to the overall improvement in the efficiency of liver functions. Most of the hepatic damage caused by TAA in rats is due to the oxidative stress it produces in the liver. Our results also suggest that OA treatment could control the TAA-induced oxidative stress by (i) strengthening the endogenous antioxidant defense and reducing thiobarbituric acid reactive species and (ii) stabilizing hepatic cell membrane leading to maintenance of cell membrane integrity, which further assists in reducing oxidative stress.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Feher J, Lang I, Gogl A, Varga L, Tampos G, Pronia L. Effect of ornithine aspartate infusion on elevated serum ammonia concentration in cirrhotic patients- results of randomized placebo-controlled double blind multicentre trial. Med Sci Monit. 1997;3:669–73. [Google Scholar]
- 2.Rose C, Michalak A, Pannunzio P, Therein G, Quack G, Kircheis G. L-ornithine-L-aspartate in experimental portal systemic encephalopathy: Therapeutic efficacy and mechanism of action. Metab Brain Dis. 1998;13:147–57. doi: 10.1023/a:1020613314572. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth RF. Hepatic encephalopathy. Neurologist. 1995;1:95–104. [Google Scholar]
- 4.Jalan R, Shawcross D, Daveis N. The molecular pathogenesis of hepatic encephalopathy. Int J Biochem Cell Biol. 2003;35:1175–81. doi: 10.1016/s1357-2725(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 5.Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990;70:701–48. doi: 10.1152/physrev.1990.70.3.701. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth RF, Giguere JF, Michaud J, Lavoie J, Pomeir-Layrargues G. Ammonia: Key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1–12. doi: 10.1007/BF02833598. [DOI] [PubMed] [Google Scholar]
- 7.Lizardi-Carvera L, Almeda P, Guevara L, Uribe M. Hepatic encephalopathy: A review. Ann Hepatol. 2003;2:122–30. [PubMed] [Google Scholar]
- 8.Gebhardt R, Beckers G, Guanitz F, Haupt W, Jonitza D, Klein S. Treatment of cirrhotic rats with L-ornithine-L-aspartate enhances urea synthesis and lowers serum ammonia levels. J Pharmacol Exp Ther. 1997;283:1–6. [PubMed] [Google Scholar]
- 9.Kircheis G, Wettstein M, Dahl S, Haussinger D. Clinical efficacy of L-ornithine-L-aspartate in the management of hepatic encephalopathy. Metab Brain Dis. 2002;17:453–62. doi: 10.1023/a:1021934607762. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Q, Jiang XH, Zheng MH, Chen YP. l-ornitine-l-aspartate in the management of hepatic encephalopathy: A meta analysis. J Gastroenterol Hepatol. 2009;24:9–14. doi: 10.1111/j.1440-1746.2008.05582.x. [DOI] [PubMed] [Google Scholar]
- 11.Sathyasaikumar KV, Swapna I, Reddy PV, Murthy CR, Roy KR, Gupta A, et al. Co-administration of C-Phycocyamin ameliorates thioacetamide induced hepatic encephalopathy in wistar rats. J Neurol Sci. 2007;252:67–75. doi: 10.1016/j.jns.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Zarros A, Theocharis S, Skandali N, Tsakiris S. Effects of fulminant hepatic encephalopathy on the adult rat brain antioxidant status and the activities of acetylcholinestrase, Na(+), K(+)- and Mg(2+) -ATPase: Comparison of the enzymes response to in vitro treatment with ammonia. Metab Brain Dis. 2008;23:255–64. doi: 10.1007/s11011-008-9091-8. [DOI] [PubMed] [Google Scholar]
- 13.Reitman S, Frankel AS. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:53–6. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 14.King PR, King EJ. Estimation of plasma phosphatase by determination of hydrolyzed phenol with amino antipyrine. J Clin Pathol. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varley H. London: William Heinemann Medical books Ltd; 1980. Practical clinical Biochemistry. [Google Scholar]
- 16.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animals tissues by thioabarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Forr AL, Ramdall RJ. Protein measurement with the Folins phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 19.Luna LG. 3rd ed. New York: McGraw Hill Book Co; 1968. Manual of Histology, Staining Methods of Armed Forces Institute of Pathology. [Google Scholar]
- 20.Aftab A, Pillai KK, Najmi AK, Ahmad SJ, Pal SN, Balani DK. Evaluation of hepatoprotective potential of jigrine post treatment against thioacetamide induced hepatic damage. J Ethnopharmacol. 2002;79:35–41. doi: 10.1016/s0378-8741(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 21.Fitzhugh OG, Nelson AA. Liver tumours in rats fed thiourea or thioacetamide. Science. 1948;108:626–8. doi: 10.1126/science.108.2814.626. [DOI] [PubMed] [Google Scholar]
- 22.Barker EA, Smucklear EA. Non-hepatic thioacetamide injury, the morphological features of proximal tubular injury. Am J Pathol. 1994;74:575–90. [PMC free article] [PubMed] [Google Scholar]
- 23.Neal RA, Halpert J. Toxicity of thionosulfur compounds. Ann Rev Pharmcol Toxicol. 1982;22:321–9. doi: 10.1146/annurev.pa.22.040182.001541. [DOI] [PubMed] [Google Scholar]
- 24.Kumar G, Banu GS, Pappa PV, Sundararajan M, Pandian MR. Hepatoprotective activity of Trianthema portulacastum L. against paracetamol and thioacetamide intoxication in albino rats. J Ethnopharmacol. 2004;92:37–40. doi: 10.1016/j.jep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Perez MJ, Sanchez-Medina F, Torres M, Gil A, Saurez A. Dietary nucleotides enhance the liver redox state and protein synthesis in cirrhotic rats. J Nutr. 2004;134:2504–8. doi: 10.1093/jn/134.10.2504. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Lopez MI, Fernandez I, Gil A, Rios A. Influence of dietary nucleotide on liver structural recovery and hepatocyte binuclearity in cirrhosis induced by thioacetamide. Gut. 1996;38:260–4. doi: 10.1136/gut.38.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drotman RB, Lawhorn GT. Serum enzymes are indicators of chemical induced liver damage. Drug Chem Tox. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair AJ, Barnett AH, Lunie J. Free radical and auto-oxidant systems in health and disease. J Appl Med. 1991;17:409. [Google Scholar]
- 29.Staedt U, Leweling H, Gladisch R, Kortsik C, Hagmuller E, Holm E. Effects of ornithine aspartate on plasma ammonia and amino acids in patients with cirrhosis. A double blind, randomized study using a four fold cross over design. J Hepatol. 1993;19:424–30. doi: 10.1016/s0168-8278(05)80553-7. [DOI] [PubMed] [Google Scholar]
- 30.Abou-Assi S, Vlahsevic ZR. Hepatic encephalopathy: Metabolic consequence of cirrhosis often is reversible. Postgrad Med. 2001;109:52–70. doi: 10.3810/pgm.2001.02.850. [DOI] [PubMed] [Google Scholar]


