Abstract
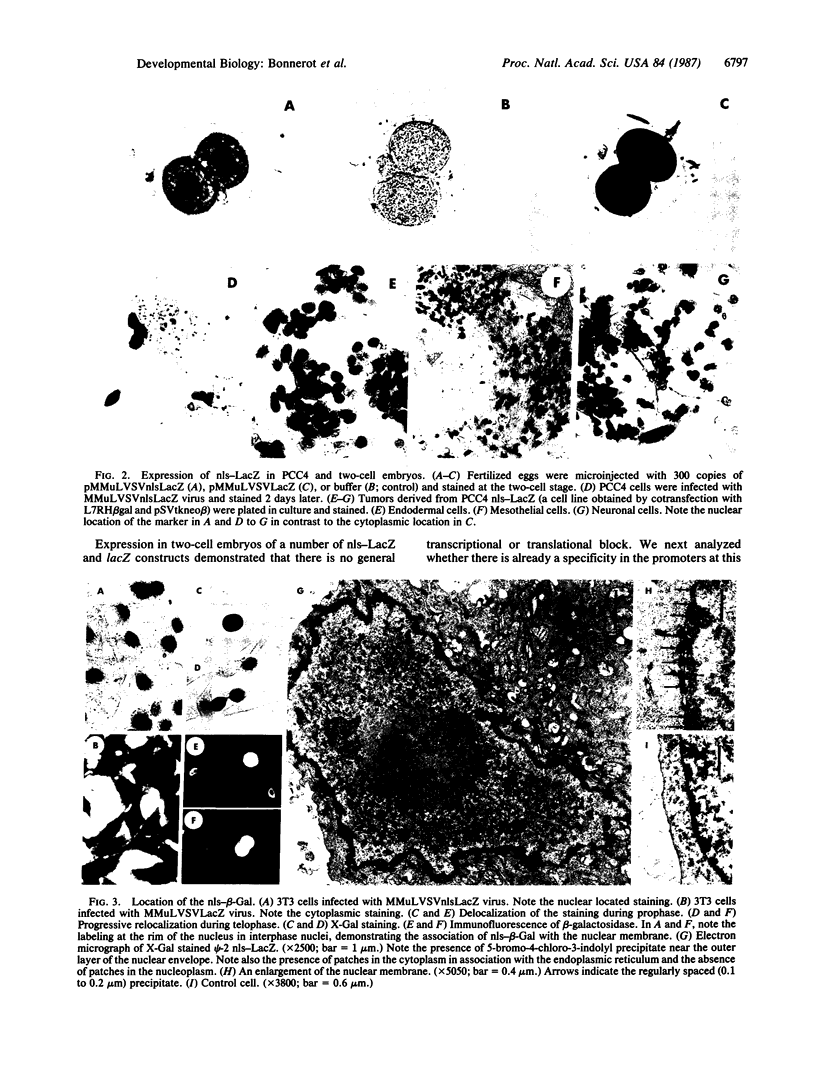
The Escherichia coli lacZ gene has been used as an indicator gene for the study of cell lineage in vivo. To adapt this marker for gene expression studies, a sequence encoding a modified beta-galactosidase and including the simian virus 40 large tumor nuclear location signal (nls-beta-Gal) has been introduced into vectors. In differentiated cells, multipotential cells, and embryos, the constructs led to the expression of an enzymatically active protein. Its location was examined by its beta-galactosidase activity or by using antibodies and electron microscopy. The results show that the nls-beta-Gal protein remains mainly located at the nuclear periphery (probably at the nuclear pores) but does not reach the nucleoplasm. It suggests that an interaction with the nuclear membrane is necessary but not sufficient for protein uptake into the nucleus. In multipotential cells, the expression of nuclear location signal LacZ (nls-LacZ) interferes neither with cell growth nor with differentiation. Using various lacZ constructs, the transcriptional activity of embryos was studied. At the two-cell stage, the promoters of the Rous sarcoma virus, simian virus 40, and the beta-actin gene are functional but the Moloney murine leukemia virus long terminal repeat is not. Thus, transcriptional specificity must already be present at the stage of activation of the embryonic genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton V. N., Oades P. J., Johnson M. H. The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J Embryol Exp Morphol. 1984 Feb;79:139–163. [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Flach G., Johnson M. H., Braude P. R., Taylor R. A., Bolton V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1(6):681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Jakob H., Nicolas J. F. Mouse teratocarcinoma cells. Methods Enzymol. 1987;151:66–81. doi: 10.1016/s0076-6879(87)51009-6. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Kanda P., Kennedy R. C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986 Aug 15;46(4):575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- Lo C. W. Localization of low abundance DNA sequences in tissue sections by in situ hybridization. J Cell Sci. 1986 Mar;81:143–162. doi: 10.1242/jcs.81.1.143. [DOI] [PubMed] [Google Scholar]
- Mann R., Baltimore D. Varying the position of a retrovirus packaging sequence results in the encapsidation of both unspliced and spliced RNAs. J Virol. 1985 May;54(2):401–407. doi: 10.1128/jvi.54.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J., Solter D. Inability of mouse blastomere nuclei transferred to enucleated zygotes to support development in vitro. Science. 1984 Dec 14;226(4680):1317–1319. doi: 10.1126/science.6542249. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Rubenstein J. L., Bonnerot C., Jacob F. Introduction of genes into embryonal carcinoma cells and preimplantation embryos by retroviral vectors. Cold Spring Harb Symp Quant Biol. 1985;50:713–720. doi: 10.1101/sqb.1985.050.01.088. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Duboule D., Chambon P. Viral enhancer activity in teratocarcinoma cells. Cold Spring Harb Symp Quant Biol. 1985;50:747–752. doi: 10.1101/sqb.1985.050.01.092. [DOI] [PubMed] [Google Scholar]
- Soriano P., Jaenisch R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell. 1986 Jul 4;46(1):19–29. doi: 10.1016/0092-8674(86)90856-1. [DOI] [PubMed] [Google Scholar]
- van der Putten H., Botteri F. M., Miller A. D., Rosenfeld M. G., Fan H., Evans R. M., Verma I. M. Efficient insertion of genes into the mouse germ line via retroviral vectors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6148–6152. doi: 10.1073/pnas.82.18.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]




