Abstract
Background
CD4+CD25high+regulatory T cells (Tregs) are considered to be of vital importance for maintaining immunologic self-tolerance and preventing autoimmune diseases. These cells have been found to be deficient in skin lesions and in the peripheral blood of patients with psoriasis.
Objective
To investigate the role of Tregs in the pathogenesis of psoriasis and to evaluate the changes in Tregs in relation to the severity and the clinical course of psoriasis.
Methods
Immunohistochemistry (CD3, 4, 8, 79 and FOXP3) was performed in 22 psoriatic patients compared to 5 normal controls. Flow cytometry (CD3, 4, 8, 25 and FOXP3) was performed in 18 psoriatic patients and 8 normal volunteers and reverse transcriptase polymerase chain reaction (foxp3 mRNA) was performed in 8 psoriasis patients.
Results
An increase in the FOXP3+ cell fraction was detected in the lesional psoriatic skin irrespective of the severity of psoriasis as compared with the normal skin. However, a decrease in FOXP3+ cells was observed in the samples obtained from psoriasis of 'acute course'. FOXP3+ Treg populations in the blood of the 'acute course' psoriasis was not different compared to that of 'chronic course' psoriasis and normal controls.
Conclusion
The deficiency of FOXP3+ Tregs in the lesional psoriatic skin might be responsible for the exacerbation of psoriasis.
Keywords: CD4+CD25high+regulatory T cells, FOXP3, Psoriasis
INTRODUCTION
CD4+CD25high+regulatory T cells (Tregs) were identified in lesional psoriatic plaques. Tregs are a recognized subset of regulatory T cells which actively suppress self-reactive T cells and maintain immunologic self-tolerance. Phenotypically, Tregs express high levels of FOXP3, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), certain members of toll-like receptors, CD103 (αEβ7 integrin) and glucocorticoid-induced TNF receptor family-related gene (GITR)1-4. Tregs also express lymphocyte activation gene-3, programmed death receptor-1 and neurophilin on their surface5. Among these, Tregs were seen to be controlled by the transcription factor, FOXP3, thus it is considered to be most crucial.
Tregs have been reported to be decreased in a variety of diseases with immune dysregulation, including transplantation rejection6 and graft versus host disease7,8, neoplastic conditions including breast adenocarcinoma, adenocarcinoma of the colon and lung cancer9-11, and various other autoimmune diseases.
The purpose of this study was to investigate the role of Tregs in the pathogenesis of psoriasis and to evaluate the changes in Tregs in relation to the severity and the clinical course of psoriasis.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Asan Medical Center. From April 2004 to June 2006, in the dermatology clinic of Asan Medical Center, 4-mm punch biopsies were taken from the untreated lesional skin of 22 patients with psoriasis (10 males, 12 females, mean age 41.0 years). In psoriasis patients, biopsies were taken from the center of the lesion. For controls, 5 skin biopsy samples were obtained from the normal skin of the abdomen (1 male, 4 females, mean age 33.2 years). Review of clinical records, medical photographs and biopsy slides was done and clinicopathologic correlation was performed by two independent dermatologists. In the psoriatic patients, psoriasis area and severity index (PASI) scores were recorded (Table 1).
Table 1.
Results of studies on skin lesions in psoriasis patients
Dur: dutation, PASI: psoriasis area and severity index, Sev: severe, Mod: moderate, ND: not done.
For flow cytometry, peripheral venous blood was drawn from 18 other patients with mild to moderate psoriasis (10 males, 8 females, mean age 39.6 years, 6 acute course and 12 with chronic course) and 8 healthy volunteers (6 males, 2 females, mean age 26.9 years). From these, the available peripheral blood samples of 8 psoriasis patients (4 males, 4 females, mean age 45.2 years; 4 with acute course and 4 with chronic course) was used to perform reverse transcriptase polymerase chain reaction (RT-PCR).
Immunohistochemistry of the skin
We performed immunohistochemical stainings in formalin-fixed paraffin-embedded sections. Standard streptavidin-biotin peroxidase method was used. Primary antibodies included CD3 (Dako, Glostrup, Denmark), CD4 (Dako), CD8 (Dako), CD79 (Dako), and FOXP3 (Mouse IgG1, Abcam, Cambridge, UK). In brief, 5-µm transverse sections of paraffin-embedded tissue were cut, deparaffinized and then immersed in 3% hydrogen peroxide in methanol for 30 minutes to quench endogenous peroxidase activity. Antigen retrieval was achieved by microwaving the slides for 18 minutes (8 minutes to boiling and 10 minutes at boiling) in 0.01 M citrate buffer (pH 6.0). Sections were incubated with the primary monoclonal antibodies mentioned above. After washing in Tris-buffered saline, pH 7.6, incubation with a secondary biotinylated antibody (Vectastain Universal Elite ABC kit; Vector Laboratories, Peterborough, England) was carried out for 30 minutes at room temperature. A tertiary peroxidase-labeled avidin-biotin complex was applied at room temperature for 30 minutes before developing with diaminobenzidine (Dako). All slides were counterstained with Hematoxylin (Dako) and dehydrated in a series of alcohols before being mounted with a nonaqueous mounting media. The results were evaluated by two dermatologists and one pathologist. Cells were counted in three fields per high power field (magnification ×200).
Flow cytometry of blood
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood using density gradient centrifugation on Ficoll. The antibodies included CD3 PE (Dako), CD4 PerCP, CD8 FITC, CD25 FITC (all from BD-Pharmingen) and FOXP3 (Abcam). Appropriate isotype controls were included. Two-color flow cytometry was performed by incubation of 5×105 peripheral blood mononuclear cells with the indicated monoclonal antibodies (mAb). FOXP3+ cells or CD4+CD25high+ cells were analyzed. Differences were analyzed using ANOVA and rank sum test and p<0.05 was considered to be statistically significant.
RT-PCR for detection of FOXP3+ mRNA in peripheral blood
Total RNA was isolated from whole blood using RNAeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and reverse-transcribed. To exclude the possibility of DNA contamination, mRNA was treated with DNAse (Qiagen). The PCR primers (Qiagen) were: 5'-ACACCACCCACCACCGCCACT-3' for forward foxp3 and 5'-TCGGATGATGCCACAGATGAAGC-3' for reverse foxp3. Thermal cycling conditions were as follows: Initial template denaturation at 94℃ for 5 minutes followed by primer annealing for 35 cycles of amplification at 57℃, extension at 72℃ for 1 min and melting at 95℃ for 1 min. PCR products were analyzed by electrophoresis in a 2% agarose gel and visualized by ethidium bromide staining.
RESULTS
The population of FOXP3+Treg in psoriatic skin lesions and in normal skin
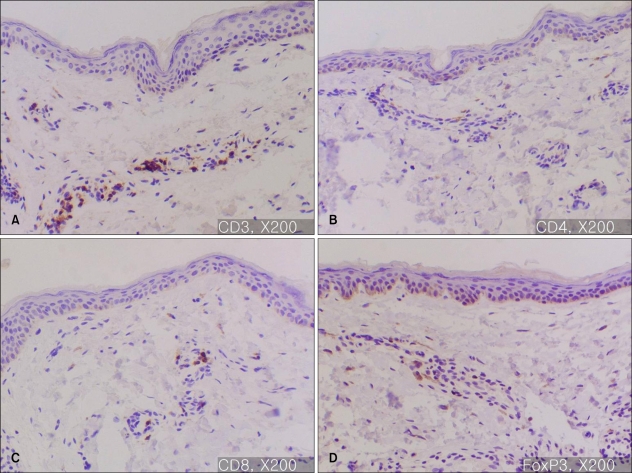
In skin biopsy samples from the normal skin, the percentage of FOXP3+Tregs among the dermal mononuclear cells was about 10~20% (Fig. 1).
Fig. 1.
Immunohistochemistry of normal skin (A: CD3, ×200, B: CD4, ×200, C: CD8, ×200, D: FOXP3, ×200). The percentage of FOXP3+ mononuclear cells in normal skin is 10~20%; the mononuclear cells are CD3, CD4 and CD8+.
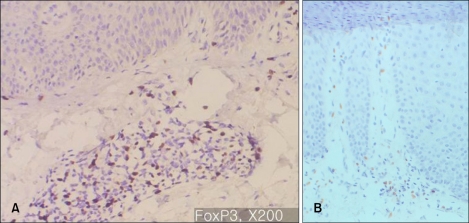
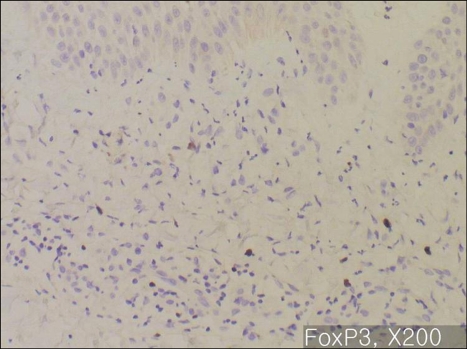
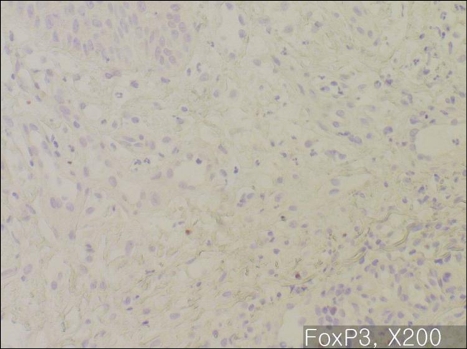
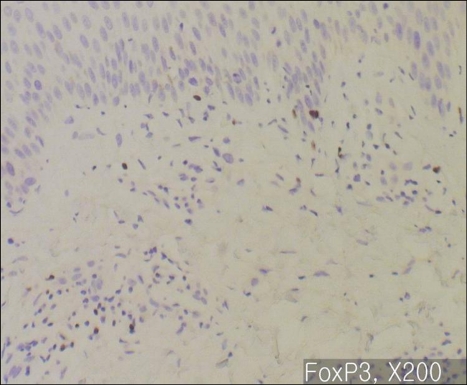
In majority of the psoriatic skin lesions, FOXP3+ cells were mainly observed in the papillary dermis, consisting of about 20~40% of the mononuclear cellular infiltration (Fig. 2A). There was no difference in the population of FOXP3+Treg within solitary plaque (case No. 1, 10) and in palmoplantar pustular psoriasis (case No. 13, 15) as compared to that in plaque-type psoriasis (Fig. 2B). In seven psoriasis patients (31.8%), the percentage of FOXP3+ Tregs decreased to less than 10% of T cell infiltrates in the papillary dermis (Fig. 3~5). Due to initiation or acute exacerbation of psoriatic skin lesions in these patients at the time of skin biopsy, the clinical characteristics of these seven cases were categorized into the 'acute course'. They comprised of guttate type (n=3, case No. 7, 14 and 19 of Table 1), acute severe plaque type (n=1, case No. 4 of Table 1), acute erythrodermic type (n=1, case No. 18 of Table 1), and acute moderate to severe plaque type (n=2, case No. 8 and 20 of Table 1). Among these, in one case of acute severe plaque type (case No. 4 of Table 1), in three cases of acute guttate type (case No. 7, 14 and 19 of Table 1), and in one case of acute erythrodermic type (case No. 18 of Table 1), the percentage of FOXP3+Tregs was less than 5% of the papillary dermal mononuclear cells.
Fig. 2.
(A) Immunohistochemistry for FOXP3, ×200: Typical plaque type psoriasis (Case No. 3). In psoriatic skin lesions, FOXP3+ cells are mainly observed in papillary dermis, consisting of about 20~40% of the mononuclear cellular infiltration. (B) Immunohistochemistry for FOXP3, ×200: Palmoplantar pustular psoriasis (Case No. 15). FOXP3+ cells consists of about 20~30% of the mononuclear cellular infiltration.
Fig. 3.
Immunohistochemistry for FOXP3, ×200: Acute severe plaque type psoriasis (Case No. 4). FOXP3+ T cells are relatively deficient.
Fig. 5.
Immunohistochemistry for FOXP3, ×200: Acute exacerbating erythrodermic psoriasis (Case No. 18). FOXP3+ cells are almost absent.
RT-PCR and flow cytometry in peripheral blood of psoriasis patients and normal controls
In RT-PCR for FOXP3 mRNA in peripheral blood, no difference was observed between the cases with 'acute course' psoriasis (n=4), and the cases with 'chronic course' psoriasis (n=4) and the normal controls (n=5).
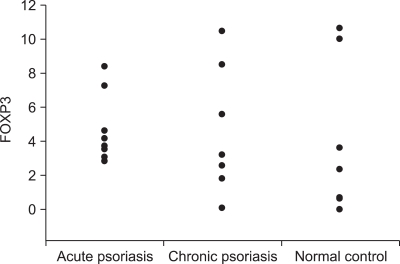
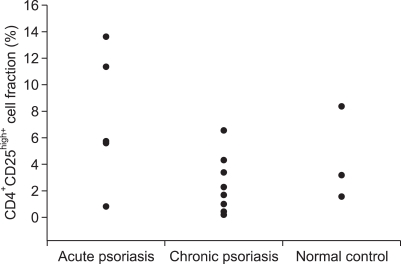
In flow cytometry, about 5% of FOXP3+ T cells were observed among the PBMCs of the normal control group (n=8) and the result was similar in the cases with 'acute course' psoriasis (n=6), and in the cases with 'chronic course' psoriasis (n=12). FOXP3+ or CD4+CD25high+ cell fraction ranged between 3~10% (Fig. 6, 7) of the PBMC in all the subsets of patients and no statistically significant difference was found in all the subsets of patients using the ANOVA and rank sum test (p>0.05) (Fig. 6, 7).
Fig. 6.
Result of flow-cytometry for FOXP3. In all samples, the percentage of FOXP3+ was 3~10% among the peripheral blood mononuclear cells of the normal control group (n=8) and the results were similar between the cases of acute psoriasis (n=6), and chronic psoriasis (n=12).
Fig. 7.
Results of flow-cytometry for CD4 and CD25. CD4+CD25high+cell fraction ranged between 3~10% of the peripheral blood mononuclear cell in all subsets of the patients.
DISCUSSION
'Regulatory T cells' are recognized as cells that have potent immune-regulatory function, especially the inhibition of T-cell activation and proliferation. Three subsets of regulatory T cells are discerned by surface molecule expression and mechanism of immunosuppression. These encompass Tregs, T-helper-3 cells (Th3), and type 1 regulatory T cells. Among these subsets, Tregs, the primary area of this study, are the most intriguing12,13. On their surface, they have the interleukin-2 receptor chain (CD25), which plays a vital role in maintenance of tolerance to self antigen and induction of non-responsiveness to alloantigen and prevents the activation and proliferation of auto- and allo-antigen-reactive T cells5. The role of Tregs in suppression of CD8+ T-cell activation as well as in activation of allergen-specific Th1 and Th2 cells has been widely reported14. Tregs are believed to act through interaction with other T-cells via both cell-to-cell contact mechanism and indirect cytokine-dependent mechanism14-16. FOXP3 gene is mutated in X-linked autoimmunity-allergic dysregulation syndrome in humans and in 'scurfy' mice. Overexpression of FOXP3 has been shown to lead to a hypoactive immune state suggesting that it is a central regulator of T cell activity. FOXP3 has significance for several reasons. Firstly, FOXP3 is more specific marker of Tregs than most cell surface markers such as CD4 and CD25. Secondly, FOXP3 plays an important role in the development of Tregs. It has been observed that the transduced expression of FOXP3 in CD4+CD25- T cells has been shown to induce GITR, CD103 and CTLA-4, which are the surface molecules expressed on Tregs. Thirdly, the expression of FOXP3 reflects the activity of Tregs1-4.
Our results indicate that the percentage of FOXP3+Tregs among T lymphocytes increased in the skin lesions of patients with chronic plaque type psoriasis, but the percentage decreased in the skin lesions of patients with psoriasis with acute course. It seems that it is not the absolute number of FOXP3+Tregs, but it is their relative proportion which is more important. The degree of inflammatory cell infiltrate in the lesional psoriatic skin varies, thus the increase in the absolute number of FOXP3+Tregs may be the result of non-specific inflammation of unknown significance. This may explain the variable and inconsistent results of previous studies on FOXP3+Treg population in the lesional psoriatic skin ranging from increment to total absence of FOXP3+Tregs. We suggest that lesional variation of FOXP3+Treg population according to biopsied sites in psoriatic skin should be considered in those studies, given that the localization of most FOXP3+Tregs in the center of the psoriatic plaque rather than in the periphery17. In the present study, we took skin biopsies from the center of the lesional psoriatic skin to avoid sampling error.
FOXP3 positivity can represent the activity of Tregs, but the possibility of reactive FOXP3+Treg increment with functional aberration should also be taken into consideration. Sugiyama et al.18 have reported that Tregs are numerically and functionally deficient in their ability to suppress the abnormally persistent psoriatic immune response, in both the lesional skin and in peripheral blood. Although psoriasis is considered to be a systemic disease with some immunologic features, alteration in the percentage of the population of FOXP3+Tregs in PBMC was not observed in the present study. Functional aberration in FOXP3+Tregs may explain the lack of difference between the acute psoriasis cases, the chronic psoriasis cases and the normal controls on flow cytometry of blood.
In psoriasis, the expression of specific transcription factor of Tregs, FOXP3, was found to be decreased or was aberrant in the results of previous studies18,19. Sugiyama et al.18 have shown the deficiency and dysfunction of Tregs in the psoriatic plaques. In their study, the number of Tregs in peripheral blood of psoriasis patients was normal, but were dysfunctional18. In another study19, Tregs were detected in the psoriatic skin lesions but not in the normal skin. In psoriasis, while a majority of dermal T-cell infiltrates were CD4+ and most of the T-cells in epidermis were CD8+, Tregs were seen predominantly in the upper dermis of the psoriatic plaques.
In this study, size of the lesions, itching and duration of the skin lesions had no effect on the FOXP3+Treg levels (Table 1). Also, neither the extent nor the severity of the disease were related to the FOXP3+Treg levels. Rather, FOXP3+Tregs were reduced in the lesional skin of acute onset psoriasis. Based on this finding, we can suggest that FOXP3+Tregs play a key role in acute exacerbation when the balance of immunosuppression and immune activation is just about to shift, but not in stable maintenance. It could be also inferred that the increase in Tregs in chronic plaque type psoriasis might be compensatory to the immune activation, and the consequential immunosuppression might contribute to the immune balance throughout the course of the disease.
Fig. 4.
Immunohistochemistry for FOXP3, ×200: Acute moderate to severe plaque type psoriasis (Case No. 8). The ratio of FOXP3+ Treg to mononuclear cells is decreased.
References
- 1.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 3.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, et al. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25-regulatory T cells. Proc Natl Acad Sci U S A. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Leung BP. CD4+CD25+ regulatory T cells in health and disease. Clin Exp Pharmacol Physiol. 2006;33:519–524. doi: 10.1111/j.1440-1681.2006.04401.x. [DOI] [PubMed] [Google Scholar]
- 6.Taams L, Vukmanovic-Stejic M, Salmon M, Akbar A. Immune regulation by CD4+CD25+ regulatory T cells: implications for transplantation tolerance. Transpl Immunol. 2003;11:277–285. doi: 10.1016/S0966-3274(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 7.Mutis T, Aarts-Riemens T, Verdonck LF. The association of CD25 expression on donor CD8+ and CD4+ T cells with graft-versus-host disease after donor lymphocyte infusions. Haematologica. 2005;90:1389–1395. [PubMed] [Google Scholar]
- 8.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 10.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 11.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 12.Kagen MH, McCormick TS, Cooper KD. Regulatory T cells in psoriasis. Ernst Schering Res Found Workshop. 2006:193–209. doi: 10.1007/3-540-37673-9_12. [DOI] [PubMed] [Google Scholar]
- 13.Veldman C, Nagel A, Hertl M. Type I regulatory T cells in autoimmunity and inflammatory diseases. Int Arch Allergy Immunol. 2006;140:174–183. doi: 10.1159/000092576. [DOI] [PubMed] [Google Scholar]
- 14.Chattopadhyay S, Mehrotra S, Chhabra A, Hegde U, Mukherji B, Chakraborty NG. Effect of CD4+CD25+ and CD4+CD25- T regulatory cells on the generation of cytolytic T cell response to a self but human tumor-associated epitope in vitro. J Immunol. 2006;176:984–990. doi: 10.4049/jimmunol.176.2.984. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 17.Vissers WH, Arndtz CH, Muys L, Van Erp PE, de Jong EM, van de Kerkhof PC. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late. Br J Dermatol. 2004;150:852–859. doi: 10.1111/j.1365-2133.2004.05863.x. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bovenschen HJ, van Vlijmen-Willems IM, van de Kerkhof PC, van Erp PE. Identification of lesional CD4+ CD25+ Foxp3+ regulatory T cells in Psoriasis. Dermatology. 2006;213:111–117. doi: 10.1159/000093849. [DOI] [PubMed] [Google Scholar]