Abstract
Background
Atopic dermatitis (AD) is frequently associated with food allergies. In addition to the skin prick test (SPT) and serum-specific IgE, the atopy patch test (APT) has been introduced as a diagnostic procedure for food allergies.
Objective
Our aim was to evaluate the diagnostic value of the APT, the SPT and the serum-specific IgE levels compared with that of oral food challenge test against milk and egg in AD patients.
Methods
We conducted the SPT and APT, and determined the serum-specific IgE levels against milk and egg antigens for 101 patients. Oral food challenge tests were conducted for 86 out of 101 AD patients. The sensitivity, specificity and positive and negative predictable values were calculated for all the tests.
Results
Twenty-five patients were positive to oral food challenges. The sensitivity of the APT for milk was 66.7%, while the figures for the SPT and the serum-specific IgE were 35.5% and 14.2%. The sensitivity of the APT for egg was 50%, while that for the SPT and serum-specific IgE were 21.4% and 6.7%.
Conclusion
We were able to conclude that the APT test seems to be a valuable additional tool for the diagnostic method of food allergies in AD.
Keywords: Atopic, Dermatitis, Food hypersensitivity, Predictive value of tests, Sensitivity and specificity, Skin tests
INTRODUCTION
Atopic dermatitis (AD) is a chronic and recurrent inflammatory cutaneous pruritic disorder with world-wide morbidity rates of 10~20% in children and 1~3% in adults1. Although the etiology of AD is still not clear, it is known that there are very complicated relationships among immunological mechanisms, genetic factors and environmental factors, including food antigens2. It has been reported that about 10~40% of patients with AD showed allergic reactions against food antigens3-5, which are frequently caused by eggs, milk, beans, wheat and peanuts6. Both AD and food allergies frequently occur in children, and especially within several years after birth7,8. As a method for assessing food antigens, the double-blind, placebo-controlled food challenge (DBPCFC) is known to be the most informative method to date9. However, there are many difficulties for conducting a DBPCFC on the food allergies of AD patients. Therefore, it is necessary to find an easier, safer and more informative method of diagnosis.
Immediate hypersensitivity against a particular food antigen may be diagnosed relatively easily by history taking, the serum-specific IgE level, the skin prick test (SPT) and the food challenge test. However, in terms of delayed hypersensitivity, the causal relationship between the symptoms and the food antigen is sometimes obscure. Accordingly, there has been active research on the patch test as a means of explaining delayed hypersensitivity. In 1982, Mitchell et al.10 conducted the patch test using house dust mite Dermatophagoides to evaluate the usefulness of the atopy patch test (APT) for diagnosing AD patients. Since then, several authors have studied the APT to evaluate whether it is a useful diagnostic method to prove clinically related antigens11,12.
We measured the serum-specific IgE levels and we conducted the SPT, APT and the oral food challenge test against typical food allergens (cow's milk and hen's eggs) in AD patients under the age of 6 years in order to evaluate the accuracy and usefulness of each test for diagnosing the food allergies of these patients.
MATERIALS AND METHODS
Subjects
We studied 101 AD patients (58 males and 43 females) under the age of 6 years (mean age: 3.1±1.4) as subjects, on the basis of Hanifin and Rajka's guideline13, for the evaluation of suspected food allergy. Suspicion was defined as the feeling of the parents that food might have contributed to the clinical symptoms of their AD children. The patients with a clear history of a severe allergic reaction to an isolated food were not included. The mean age of the patients at onset was 0.8±1.2 years and the mean prevalence period was 2.4±2.9 years. Sixteen of the 101 patients (15.8%) had a personal history of bronchial asthma and allergic rhinitis besides AD, and 22 (21.8%) of them had a family history of one or more of these 3 atopy diseases above mentioned.
Methods
1) Measurement of AD severity
For the measurement of AD severity, we used the standardized technique of the Severity Scoring of Atopic Dermatitis (SCORAD) index14. To begin with, we measured the area of the skin lesions and then we assessed them for 5 items (erythema, edema/papulation, oozing/crusts, excoriation and lichenification) from 0 (none) to 3 (severe). Dryness was evaluated on the noninflammatory skin from 0 (none) to 3 (severe). Finally, using 2 visual analogue scales for the degree of itching and sleep deprivation, the patients were subjectively checked from 0 to 10. These measures were used for calculating scores in the equation below, and the SCORAD index varies from 0 to 103; it can be classified into mild: 1~15, moderate: 16~40 and severe: 41~103. The equation is as follows: SCORAD=(0.2×area)+{3.5×(erythema+edema/papulation+oozing/crusts+excoriations+lichenification+dry skin)}+subjective scores.
2) Oral food challenge test
We started the oral food challenge test with 5 ml, 10 ml, 50 ml and 100 ml of milk in 20 minute intervals. After 2 days, we conducted the egg challenge test. We started with 1/4 of a boiled egg, and then we gradually increased this amount to a whole boiled egg in 20 minute intervals15,16. The provocation was stopped if clinical symptoms or signs such as urticaria, angioedema, asthma attack, vomiting, diarrhea, shock and exacerbation of pre-existing eczema lesions (at least a 10 point increase of the SCORAD score) were observed. The outcome of the food challenge test was divided into immediate and delayed reactions. Immediate reactions were defined as those occurring within 20 min after the last dose and delayed reactions were defined when the reactions were observed between 2 and 24 hr after the last dose of the food challenge17. All the subjects were restricted as to the intake of milk and egg for at least 2 weeks prior to the food challenge test. Those subjects were also restricted as to intake of oral antihistamines and topical steroid applications for at least 1 week prior to the food challenge test.
3) Measurement of serum-total IgE and serum-specific IgE levels
We collected blood samples from the subjects and we measured the serum-total IgE levels using the paper radioimmunosorbent test (PRIST) kit (Behring, Marbug, Germany) and we measured the specific IgE levels using the radioallergosorbent test (RAST) kit (Pharmacia, Uppsala, Sweden) for milk and egg antigens. A positive response was defined when the antigen-specific IgE level was over class 3 (6.9 kUA/L) for milk and over class 3 (5.45 kUA/L) for egg, respectively.
4) SPT
The antigens used in our study were whole cow's milk and hen's egg (Allergopharma, Reinbek, Germany) with histamine as a positive control and saline solution as a negative control. We cleaned the normal skin on the subject's back with alcohol and then prick tests were done at 2 cm intervals using a lancet. Skin responses were observed after 20 min. If the erythema was less than 15 mm, it was marked +, if the erythema was 15 mm or more and the wheal was 3 mm or less, then it was marked ++, and if the wheal was 3~5 mm, then it was marked +++18. Skin reactions were considered positive when they were marked above +++. All the subjects were also restricted as to intake of oral antihistamines and topical steroid applications for at least 1 week prior to the prick test.
5) APT
APTs were done with cow's milk (100% and 10%), boiled hen's egg yolk (100% and 10%), and boiled hen's egg white (100% and 10%) using small a Finn chamber® (Epitest Ltd Oy, Tuusula, Finland) with Scanpor tape (Alpharma AS, Vennesla, Norway) on the back skin of patients. We read the skin reactions 48 hr after the application of patches in order to evaluate the delayed reactions at the patched sites. Skin reactions were considered positive when they were marked + or more according to the International Contact Dermatitis Research Group standards19. All the subjects were also restricted as to intake of oral antihistamines and topical steroid applications for at least 1 week prior to the APT.
6) Statistics
The mean values of the acquired data were compared using SPSS 10.0 for Windows (SPSS Inc., Chicago, IL, USA) in order to examine the difference between the severity and serum IgE level according to descriptive statistical analysis, gender and the food challenge test. We analyzed the correlations among the specific serum IgE level, the patch and prick test and the open-oral challenge test based on severity. We also performed correlation analysis between the size of the wheal and the serum-specific IgE level on the prick test, with a p-value of 0.05 being statistically significant. Then, based on the results of the oral food challenge test, we studied the sensitivity, specificity and the positive and negative predictive values of the serum-specific IgE level and the prick and the patch tests.
RESULTS
Severity of AD
Of the 101 AD patients whose average SCORAD index was 48.3±17.8, 37 patients (36.6%) had a moderate degree of AD (SCORAD score: 16~40 points), and 64 (63.4%) had severe AD (SCORAD score: 41~103 points). There was no significant difference in severity between the male and female patients (p=0.619). The correlation between the severity of AD and positive results from the serum-specific IgE level test, the APT, the SPT and the oral food challenge test was not significant. However, the severity of AD displayed a positive correlation with the serum total IgE level (r=0.458, p=0.00).
Clinical outcomes of the oral food challenge test
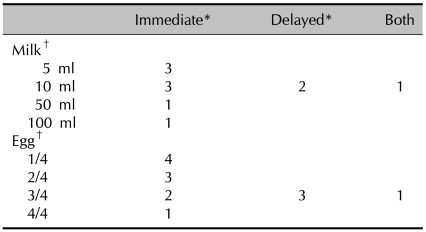
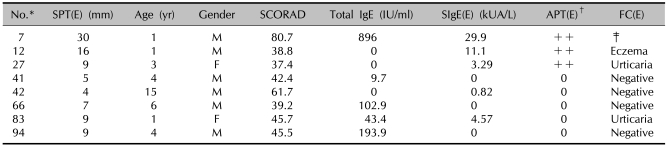
Oral food challenge tests with cow's milk and hen's egg were performed with cow's milk in 86 children with AD and with hen's egg in 84 children with AD. There were positive reactions in 11 (12.8%) of 86 patients who underwent the oral food challenge test with cow's milk. Immediate reactions occurred in 8 children and delayed reactions were observed in 2 children; 1 patient showed both immediate and delayed reactions after the oral food challenge test. Among 8 patients who showed immediate reaction; 3 displayed urticaria 10, 13 and 18 min after drinking 5 ml of milk, 3 displayed urticaria 9, 12 and 15 min after drinking 10 ml of milk, 1 showed signs of urticaria, nausea and vomiting 15 min after drinking 50 ml of milk and 1 displayed urticaria and nausea 10 min after drinking 100 ml of milk. In the 2 patients who displayed delayed reactions, 1 showed severe symptoms of itching with darkening erythema and oozing on the flexura of the arms and legs 8 hr after drinking 100 ml of milk, and 1 developed erythema with itching in the neck and face 12 hr after drinking 100 ml of milk. One patient who displayed both immediate and delayed reactions showed urticaria 10 min after drinking 10 ml of milk, followed by severe itching, erythema, scale and excoriation on the flexura of both arms 12 hr later (Tables 1, 2). There were positive reactions in 14 (16.7%) of the 84 patients who underwent the oral food challenge test with hen's egg. Immediate reactions occurred in 10 children and delayed reactions were observed in 3 children; 1 patient showed both immediate and delayed reactions after the oral food challenge test. Among the 10 patients who showed immediate reactions, 2 developed urticaria 10 min after eating 1/4 of an egg, 2 developed rhinorrhea 15 and 18 min after eating 1/4 of an egg, 2 showed signs of urticaria, nausea and vomiting 15 min after eating 1/2 of an egg, 1 developed urticaria 10 min after eating 1/2 of an egg, 1 developed urticaria 8 min after eating 3/4 of an egg, 1 showed symptoms of urticaria and nausea 16 min after eating 3/4 of an egg and 1 developed urticaria 20 min after eating a whole egg. Among the 3 patients who showed delayed reactions, 1 developed intensifying itching on the flexura of both arms and legs with darkening erythema and discharge 10 hr after eating a whole egg, 1 developed severe itching with erythema and oozing on both legs 11 hrs after eating a whole egg and 1 developed itching and erythema on the neck and the flexura of the arms and legs with erythema 16 hr after eating a whole egg. One patient who displayed both immediate and delayed reactions developed urticaria 15 min after eating 3/4 of an egg, followed by intensifying itching on the trunk and face with erythema, excoriation and oozing 13 hr later (Tables 1, 3).
Table 1.
Oral food challenge tests with milk and egg
*Immediate reactions were defined as the reactions within 20 minutes after the test was done, and delayed reactions were defined when the reactions occurred between 2 hours and 24 hours after the test was done. †The number of subjects tested with milk was 86 and that for egg was 84.
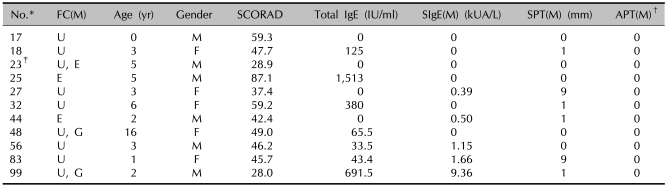
Table 2.
Results of the SCORAD score, the total IgE, the specific IgE, the SPT and the APT in the patients showing a positive FC reaction with milk
*ID number of the children used in the study. †Scored from + to +++. ‡One patient (ID number 23) showed a combined reaction on the food challenge test with egg. SPT: skin prick test, APT: atopy patch test, FC: food challenge test, (M): milk, SIgE: specific IgE, U: urticaria, E: eczema, G: gastrointestinal symptom.
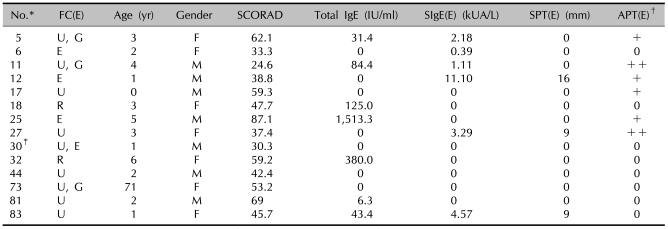
Table 3.
Results of the SCORAD score, the total IgE, the specific IgE, the SPT and the APT in the patients showing a positive FC reaction with egg
*ID number of the children used in the study. †Scored from + to +++. ‡One patient (ID number 30) showed a combined reaction on the food challenge test with milk. SPT: skin prick test, APT: atopy patch test, FC: food challenge test, (E): egg, SIgE: specific IgE, U: urticaria, G: gastrointestinal symptom, E: eczema, R: rhinitis.
Among the 25 food challenge positive reactions with milk and egg, immediate reactions were observed in 20 patients. No severe anaphylactic reactions were observed during the food challenge test. The 20 immediate reactions were comprised of urticaria (n=19), nausea/vomiting (n=5) and rhinitis (n=2). Five patients among the 20 patients who developed urticaria also had gastrointestinal symptoms (nausea/vomiting). Delayed reactions were observed in 7 patients among the 25 food challenge positive reactions with milk and egg. All the delayed reactions were deterioration of eczema as a flare up of pre-existing lesions. There were no gastrointestinal or respiratory reactions. In 2 patients, both immediate and delayed reactions after the oral food challenge test were observed.
Serum-total IgE and serum-specific IgE levels: There was no statistically significant difference in the serum-total IgE and serum-specific IgE levels between the groups that were positive and negative on the oral food challenge test
On the basis of a normal range of serum-total IgE levels (1~5 years: <60, 6~9 years: <90, 10~15 years: <200), 36 (35.6%) out of the 101 patients showed higher levels of serum-total IgE than the normal range (the mean value of the serum-total IgE level was 601.8 IU/ml). The mean level of serum-total IgE was 261.1±275.5 IU/ml in the food challenge positive group with cow's milk and 327.3±677.2 IU/ml in the food challenge negative group with cow's milk. Those values in the hen's egg test positive group were 312.0±544.5 IU/ml and they were 318.5±676.5 IU/ml in the hen's egg negative group. There were no significant differences in the serum-total IgE levels between the oral food challenge positive and negative groups with using milk and egg antigen (p>0.05).
For the results of the serum-specific IgE level, 22 (21.8%) out of 101 patients showed higher levels of specific IgE against milk than the normal range and 19 (18.8%) out of 101 patients showed higher levels of specific IgE against egg than the normal range. The mean level of specific IgE against egg was 1.5±3.2 kUA/L in the food challenge positive group with cow's milk and 0.3±1.4 kUA/L in the food challenge negative group with cow's milk. Those in the hen's egg test were 1.7±3.2 kUA/L and 0.56±2.1 kUA/L in the positive and negative groups, respectively. There were no significant differences in the specific IgE levels between the oral food challenge positive group and negative group with using milk and egg antigen (p>0.05).
SPT: There was a positive correlation between the size of the wheals developed during SPT and the serum-specific IgE level
There were positive responses in 7 (6.9%) out of 101 patients on the SPT against milk, and in 8 (7.9%) out of 101 patients on the SPT against egg (Tables 4, 5). For the SPT against egg, there was a high and significant correlation between the size of the wheal and the serum-specific IgE level (r=0.853, p=0.00). For the SPT against milk, the correlation was moderate and nonsignificant (r=0.051, p=0.36).
Table 4.
Results of the SCORAD score, the total IgE, the specific IgE, the SPT and the FC in the patients showing a positive SPT with milk
*ID number of the children used in the study. †Scored from + to +++. ‡Food challenge test not performed. SPT: skin prick test, FC: food challenge test, (M): milk, SIgE: specific IgE, APT: atopy patch test.
Table 5.
Results of the SCORAD score, the total IgE, the specific IgE, the SPT and the FC in the patients showing a positive SPT with egg
*ID number of the children used in the study. †Scored from + to +++. ‡Food challenge test not performed. SPT: skin prick test, FC: food challenge test, (E): egg, SIgE: specific IgE, APT: atopy patch test, U: urticaria.
APT: the oral food challenge, serum-specific IgE and SPT results of the APT positive group
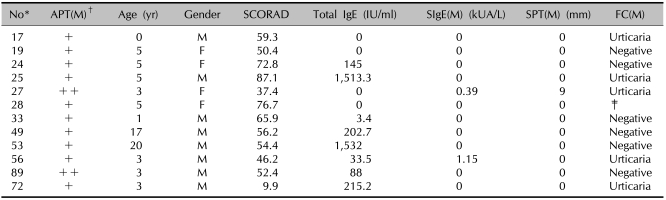
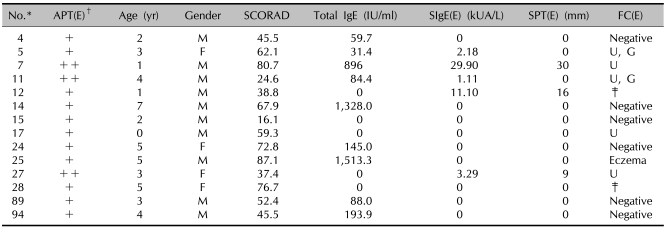
There were positive reactions in 26 patients (25.7%) out of 101 on the APT; 12 patients had positive reactions to milk and 14 patients had positive reactions to boiled egg (Tables 6, 7). All the patients who were positive on the APT with milk demonstrated negative serum-specific IgE (sIgE) levels and one of them showed a positive reaction on the SPT with milk. Six patients of these showed negative reactions on the food challenge test to milk. Five of these patients showed positive immediate reactions such as urticaria. Among the patients showing positivity to egg, 1 patient had a positive sIgE level and 3 patients showed positive reactions on the SPT with egg. Six patients showed negative reactions to egg on the food challenge test; the 6 patients displayed positive reactions with immediate reactions in five cases and delayed reactions in one case.
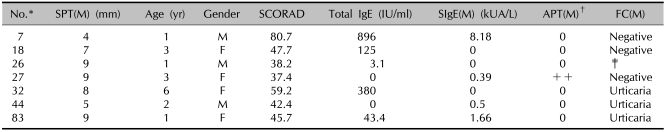
Table 6.
Results of the SCORAD score, the total IgE, the specific IgE, the SPT and the FC in the patients showing a positive APT with milk
*ID number of the children used in the study. †Scored from + to +++. ‡Food challenge test not performed. SPT: skin prick test, FC: food challenge test, APT: atopy patch test, (M): milk, SIgE: specific IgE.
Table 7.
Results of the SCORAD score, the total IgE, the specific IgE, the SPT and the FC in the patients showing a positive APT with egg
*ID number of the children used in the study. †Scored from + to +++. ‡Food challenge test not performed. SPT: skin prick test, FC: food challenge test, APT: atopy patch test, (E): egg, SIgE: specific IgE, U: urticaria, G: gastrointestinal symptom.
The sensitivity, specificity and positive and negative predictive values: On comparing the SPT, the specific IgE level and the APT with the FC results, the APT's sensitivity and positive predictive value were greatest
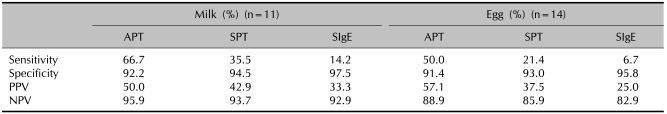
We compared the diagnostic accuracy of the SPT, APT and specific IgE level with the results of the food challenge tests. The sensitivities of the APT, SPT and specific IgE with milk were 66.7%, 35.5% and 14.2%, respectively. The positive predictive values of the APT, SPT and specific IgE with milk were 50%, 42.9% and 33.3%, respectively. The sensitivities of the APT, SPT and specific IgE with egg were 50%, 21.4% and 6.7%, respectively. The positive predictive values of the APT, SPT and specific IgE with egg were 57.1%, 37.5% and 25%, respectively. The APT showed relatively higher sensitivity and a higher positive predictive value than the other tests with both milk and egg (Table 8). The performance characteristics of the different diagnostic tests are listed in Table 8.
Table 8.
The diagnostic value of the APT, SPT and specific IgE level compared to the outcome of oral challenge
APT: atopy patch test, SPT: skin prick test, SIgE: serum specific IgE, PPV: positive predictive value, NPV: negative predictive value.
DISCUSSION
It is known that food antigens play a key role in the onset or exacerbation of AD and that 10~40% of AD patients have an allergic response to food antigens4-6. The most precise and confirmative method for diagnosing food allergy is the DBPCFC test. However, the limitations to use this as a practical trial are the effort and cost required for the test process, the difficulty in interpreting the results and the risk of anaphylaxis during the test. Therefore, it is necessary to find a new simple and accurate diagnostic method9,11,17. Isolauri and Turjanmaa19 suggested that the hypersensitivity to milk in AD infants is divided into a specific IgE antibody reaction and a T lymphocyte-mediated response, so the diagnosis of food allergy may be more accurate by conducting the prick and patch tests together. Roehr et al.16 asserted that the food challenge test may not be needed if both the serum-specific IgE level is above a particular value against food antigens (≥0.35 kU/L for milk, ≥17.5 kU/L for egg) and the positive result of the APT are used. Chang et al.11 suggested that the patch test for food antigens may be useful for diagnosing the food allergies of patients with AD, and especially if there is a delayed immune response. In 1982, Mitchell et al.10 performed the APT using inhalant allergens with house dust mite Dermatophagoides antigen to examine delayed immune reactions. Since then, a number of studies have conducted patch tests using the same antigen and they found positive reactions that were clinically and histopathologically similar to delayed hypersensitivity reactions 24~48 hrs after the test. Based on these results, it has been reported that AD lesions can be induced by not only IgE-mediated immediate reaction, but also by contact with various environmental antigens and cell-mediated allergic contact reactions10,20,21. AD is associated with both an immediate hypersensitivity reaction and a delayed immune reaction. The delayed response begins 3~4 hr after disappearance of the immediate response induced by IgE-mediated mast cell degranulation with increased expressions of IL-1, IL-3, IL-4, IL-5 and GM-CSF on the lesion, allowing the Th-2 lymphocytes to exacerbate the pre-existing eczematous lesion11.
The APT can be used to test delayed immune reactions. However, the APT methods and interpretation of the results have yet to be standardized. Niggemann et al.9,22 suggested the use of tests with both crude and 1:10 diluted solutions in order to exclude a false positive result by an irritant reaction, along with the use of a 12 mm size Finn chamber in order to reduce a false negative result, and to keep the patches on for 48 hours and interpret the result at 72 hr. In Korea, Yim et al.20 conducted three patching methods on lesional skin and non-lesional skin: the patch tests without pre-treatment, the patch tests after barrier disruption by scratching 2 times using a needle, and patch tests after mixing allergens with 10% DMSO. They reported that there was no significant difference in the positive reaction rate among these methods. In this study, we used undiluted food antigen and a solution diluted at a ratio of 1:10. The size of the Finn chamber was 12 mm, the patch was applied for 48 hr and reading was done 48 and 96 hr after testing. In order for the APT to be effectively used as a means of identifying antigens related to a delayed immune reaction in AD patients, it is essential to standardize the method and procedures of APT, including the test apparatus and the concentration of the antigen used in the test.
In this study, we measured the serum-specific IgE and we conducted the SPT, APT and the oral food challenge test against cow's milk and hen's egg, which are typical food allergens, in AD patients under the age of 6 years in order to evaluate the accuracy and usefulness of each test for making the diagnosis of food allergy in AD patients. The APT had the highest specificity and positive predictive value among those diagnostic tests. Therefore, it could be considered that the APT may be useful when food allergy is suspected as a causative or etiologic factor of AD. With such results, although we cannot conclude that the APT is more accurate than the food challenge test, it may be added to other tests or used as a safe and convenient alternative diagnostic method when severe hypersensitivity reactions are expected on the food challenge test.
In conclusion, we aimed to examine the accuracy and usefulness of each diagnostic test for food allergy by assessing the serum-specific IgE level for milk and egg and performing the SPT, APT and food challenge test for milk and egg. There were no significant relationships between the AD severity and the results of the serum-specific IgE, the SPT and the APT. Among these 3 diagnostic tests, the APT presented the highest sensitivity and positive predictive value. Accordingly, we believe that the APT can be used as both a screening test and an auxiliary method for diagnosing food allergy in AD patients.
References
- 1.Schultz Larsen F, Hanifin JM. Epidemiology of atopic dermatitis. Immunol Allergy Clin North Am. 2002;22:1–24. [Google Scholar]
- 2.Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088–1095. doi: 10.1016/s0190-9622(03)02539-8. [DOI] [PubMed] [Google Scholar]
- 3.Burks AW, Mallory SB, Williams LW, Shirrell MA. Atopic dermatitis: clinical relevance of food hypersensitivity reactions. J Pediatr. 1988;113:447–451. doi: 10.1016/s0022-3476(88)80626-7. [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–728. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis: pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104:S114–S122. doi: 10.1016/s0091-6749(99)70053-9. [DOI] [PubMed] [Google Scholar]
- 6.Burks AW, James JM, Hiegel A, Wilson G, Wheeler JG, Jones SM, et al. Atopic dermatitis and food hypersensitivity reactions. J Pediatr. 1998;132:132–136. doi: 10.1016/s0022-3476(98)70498-6. [DOI] [PubMed] [Google Scholar]
- 7.Host A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990;45:587–596. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 8.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987;79:683–688. [PubMed] [Google Scholar]
- 9.Niggemann B, Sielaff B, Beyer K, Binder C, Wahn U. Outcome of double-blind, placebo-controlled food challenge tests in 107 children with atopic dermatitis. Clin Exp Allergy. 1999;29:91–96. doi: 10.1046/j.1365-2222.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell EB, Crow J, Chapman MD, Jouhal SS, Pope FM, Platts-Mills TA. Basophils in allergen-induced patch test sites in atopic dermatitis. Lancet. 1982;1:127–130. doi: 10.1016/s0140-6736(82)90379-8. [DOI] [PubMed] [Google Scholar]
- 11.Chang DS, Seo SJ, Hong CK. Patch test and specific IgE level with food antigens in atopic dermatitis patients. Korean J Dermatol. 2002;40:1028–1034. [Google Scholar]
- 12.Leung DYM, Eichenfield LF, Boguniewicz M. Atopic dermatitis. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Karz SI, editors. Fitzpatrick's dermatology in general medicine. 6th ed. New York: McGraw-Hill; 2003. pp. 1180–1194. [Google Scholar]
- 13.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92(Suppl):44–47. [Google Scholar]
- 14.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 15.Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Standardization of food challenges in patients with immediate reactions to foods-position paper from the European Academy of Allergology and Clinical Immunology. Allergy. 2004;59:690–697. doi: 10.1111/j.1398-9995.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 16.Roehr CC, Reibel S, Ziegert M, Sommerfeld C, Wahn U, Niggemann B. Atopy patch tests, together with determination of specific IgE levels, reduce the need for oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2001;107:548–553. doi: 10.1067/mai.2001.112849. [DOI] [PubMed] [Google Scholar]
- 17.Osterballe M, Andersen KE, Bindslev-Jensen C. The diagnostic accuracy of the atopy patch test in diagnosing hypersensitivity to cow's milk and hen's egg in unselected children with and without atopic dermatitis. J Am Acad Dermatol. 2004;51:556–562. doi: 10.1016/j.jaad.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Saxon A. Immediate hypersensitivity: approach to diagnosis. In: Lawlor GJ, Fischer TJ, editors. Manual of allergy and immunology: diagnosis and therapy. 2nd ed. Boston: Little-Brown; 1988. pp. 29–30. [Google Scholar]
- 19.Isolauri E, Turjanmaa K. Combined skin prick and patch testing enhances identification of food allergy in infants with atopic dermatitis. J Allergy Clin Immunol. 1996;97:9–15. doi: 10.1016/s0091-6749(96)70277-4. [DOI] [PubMed] [Google Scholar]
- 20.Yim YS, Park CW, Lee CH. A comparative study of atopy patch test using house dust mite antigens with skin prick test and specific serum IgE level in atopic dermatitis. Korean J Dermatol. 2001;39:1072–1079. [Google Scholar]
- 21.Rawle FC, Mitchell EB, Platts-Mills TA. T cell responses to the major allergen from the house dust mite Dermatophagoides pteronyssinus, Antigen P1: comparison of patients with asthma, atopic dermatitis, and perennial rhinitis. J Immunol. 1984;133:195–201. [PubMed] [Google Scholar]
- 22.Niggemann B, Ziegert M, Reibel S. Importance of chamber size for the outcome of atopy patch testing in children with atopic dermatitis and food allergy. J Allergy Clin Immunol. 2002;110:515–516. doi: 10.1067/mai.2002.126659. [DOI] [PubMed] [Google Scholar]