Abstract
An atypical clinical form of pityriasis versicolor has been infrequently reported, in which cutaneous atrophy is associated with individual pityriasis versicolor lesions. The pathogenesis of this atrophy remains unclear, but is believed to be a delayed-type hypersensitivity reaction to antigens derived from the Malassezia species. A 60-year-old man presented with multiple, slightly scaly, and depressed maculopatches or plaques on the trunk and extremities. Our microscopic examination of the skin scrapings on a KOH preparation revealed numerous short hyphae and spores. The patient was treated daily with 200 mg of itraconazole in combination with topical antifungals, achieving clinical improvement and mycological recovery, which was confirmed upon follow-up 1 month later. This is the first case report of atrophying pityriasis versicolor in Korea. It needs to be differentiated from other atrophying disorders of the skin.
Keywords: Atrophy, Pityriasis versicolor
INTRODUCTION
Pityriasis versicolor is a chronic superficial fungal infection caused by the Malassezia species in the horny layer. It is characterized by hypopigmented or hyperpigmented scaly maculopatches of various sizes, which develop principally in the trunk1. The lesions of pityriasis versicolor can be accompanied by skin atrophy, although this is quite rare. To describe this, Crowson and Magro2 coined the term 'atrophying pityriasis versicolor'.
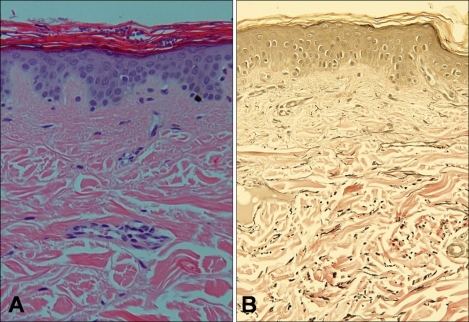
Fig. 2.
(A) Multiple roundish spores with some hyphae of Malassezia in the horny layer and focal thinning of the epidermis (H&E, ×200). (B) Focal decreased and fragmented elastic fibers (Verhoff-van Gieson, ×200).
We experienced a case of atrophying pityriasis versicolor. To the best of our knowledge, this has never been reported in the Korean literature. Here, we report our case with a review of the literature.
CASE REPORT
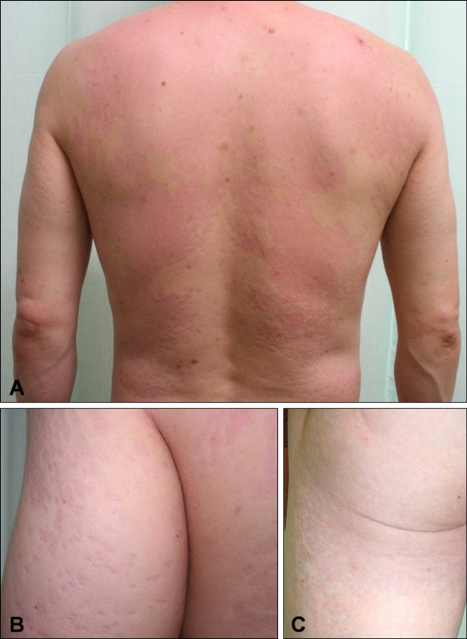
A 50-year-old Korean man presented with a 1-month history of multiple scaly erythematous atrophic macules and plaques. The patient was treated topically with hydrocortisone 1% lotion and diflucortolone valerate 0.3% ointment for essential pruritus. The patient had had a 2-year history of this disorder prior to visiting our hospital. We noted the presence of multiple bean-sized or coin-sized erythematous scaly depressed plaques, which were scattered or confluent in the trunk and upper arm (Fig. 1). On the KOH test, the presence of multiple short hyphae and spores were noted. However, cultures on Leeming and Notman media (LN media) were contaminated, and thus the Malassezia strains could not be identified.
Fig. 1.
Numerous coin-sized confluent erythematous atrophic patches or plaques with scales on the back (A), upper arm (B), and buttock and thigh (C).
A skin biopsy of the back lesion showed the presence of multiple short hyphae and spores in the horny layer, as well as partial atrophy of the epidermis. A comparison with normal areas could not be made, but we noted the presence of multiple fragmented elastic fibers (Fig. 2).
Itraconazole was administered orally for 2 weeks at a dosage of 200 mg/day. Concurrently, flutrimazole ointment was applied topically. One month after the outpatient visit, KOH test results were negative and atrophied lesions improved. To date, which is approximately 3 months thereafter, follow-up observations indicate that there were no episodes of recurrence.
DISCUSSION
The finding that pityriasis versicolor lesions are accompanied by skin atrophy was first reported by De Graciansky and Mery3 in 1971. Since then, this finding has been described in only 13 reports, and remains a very rare condition2-14. With regard to the pathogenesis of the skin atrophy, previous studies suggested that it may be associated with long-term topical steroid use3,4,9-12. Tatnall and Rycroft9 discontinued the use of topical steroids and then compared the non-treated lesions with lesions treated with topical anti-fungal agents. These authors suggested that topical steroid use plays a pivotal role in the development of atrophy, based on the finding that the atrophy disappeared spontaneously in both lesions, but the brown scales remained only in the non-treated lesions. It was hypothesized that long-term use of topical steroids impairs collagen synthesis and reduces mitosis in keratinocytes15. Also, decreased function of the skin barrier due to fungal infection may contribute to increased topical steroid penetration16. Therefore, the finding that the atrophy occurred only in the pityriasis versicolor lesions, although topical steroids were applied to the whole body, can be explained by this mechanism. Our patient applied topical steroids to his entire body, but skin atrophy was noted only in the pityriasis versicolor regions.
If there were no past history of topical steroid use, however, the above pathogenesis for skin atrophy could not be explained. Crowson and Magro2 conducted clinical and histological studies in 12 patients with atrophying pityriasis versicolor. However, only one used topical steroids chronically. Therefore, these authors maintained that the skin atrophy had occurred due to mechanisms such as delayed-type hypersensitivity reactions, or to the direct effect of Malassezia on NF-κB signaling in the remaining 11 patients. Also, the degeneration of elastic fibers was histologically detected in two of these patients. These findings show that histiocytes stimulated by inflammatory responses release elastase, and this leads to elastolysis. Furthermore, stimulation of Malassezia in the horny layer increased the synthesis of pro-inflammatory cytokines such as IL-1β and TNF-α. Regarding these inflammatory mediators, it has been explained that epidermal atrophy occurs as a result of the apoptosis and impaired proliferation of keratinocytes caused by TNF-α. Based on these results, Crowson and Magro2 coined the term 'atrophying pityriasis versicolor'. These authors also maintained that this should be considered one of the rare variants of pityriasis versicolor.
Thereafter, there was one case report which was not associated with topical steroid use13. In this case, the numbers of elastic fibers were reduced, and the fibers were fragmented. These findings show that the degeneration of elastic fiber contributes to the occurrence of skin atrophy. In particular, it has been noted that the degeneration of elastic fiber is well developed in older lesions. In our current case, the lesions occurred one month prior to the outpatient visit. Therefore, the lesions were not old lesions, but there were findings that the elastic fibers were reduced in number and fragmented. According to previous studies, these findings were present in cases which were not associated with topical steroid use. Conversely, in the current case, these findings could also be observed in patients whose symptoms might have originated from the use of topical steroids. Generally, the histopathological findings reported in cases of skin atrophy resulting from topical steroid use are characterized by epidermal atrophy, a loss of epidermal function, and the thinning and fragmentation of collagen fibers in the dermis. However, thinning and fragmentation of elastic fibers, and vasodilation in the upper dermis can also be observed17,18.
Accordingly, in this case, it cannot be determined whether the degenerative changes of elastic fiber occurred as the result of topical steroid use or immune responses to Malassezia. As topical steroids provoke the inhibition of collagen synthesis, however, degenerative changes in the collagen fibers are more commonly encountered. The lack of degenerative changes in the collagen fibers noted in the current case implies that immune responses to Malassezia may have been involved. However, further studies are needed to elucidate the exact mechanism of atrophy, which cannot be clearly determined at this point, owing to the small number of cases.
It is essential to make a differential diagnosis of atrophying pityriasis versicolor from other diseases that cause skin atrophy, such as anetoderma, atrophoderma of Pasini and Pierini, morphea, parapsoriasis, mycosis fungoides, and sarcoidosis2. On the basis of such clinical findings as the concurrent presence of scales and a positive KOH test, however, a differential diagnosis can indeed be made. Accordingly, in cases in which scales exist concurrently with atrophic lesions, it would be wise to ascertain whether atrophying pityriasis versicolor might be the cause.
For the treatment of atrophying pityriasis versicolor, conventional treatment methods for pityriasis versicolor should be utilized, following the discontinued use of topical steroids if the patients have had a past history of topical steroid use.1 In most cases, however, extensive lesions are present. Thus, as compared with conventional treatments for pityriasis versicolor, a longer treatment period might be required for atrophying pityriasis versicolor. Additionally, in our case a mycological recovery was achieved and the atrophic lesions disappeared within approximately one month after the initiation of treatment. This shows that atrophying pityriasis versicolor has a relatively good prognosis compared with other diseases that cause skin atrophy.
References
- 1.Gupta AK, Batra R, Bluhm R, Faergemann J. Pityriasis versicolor. Dermatol Clin. 2003;21:413–429. v–vi. doi: 10.1016/s0733-8635(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 2.Crowson AN, Magro CM. Atrophying tinea versicolor: a clinical and histological study of 12 patients. Int J Dermatol. 2003;42:928–932. doi: 10.1111/j.1365-4632.2003.02110.x. [DOI] [PubMed] [Google Scholar]
- 3.De Graciansky P, Mery F. Atrophie sur pityriasis versicolor apres corticotherapie locale prolongee. Bull Soc Fr Dermatol Syphiligr. 1971;78:295. [Google Scholar]
- 4.Schöpf E. Adverse effects of external corticosteroid therapy. Hautarzt. 1972;23:295–301. [PubMed] [Google Scholar]
- 5.Runne U. Pseudo-Anetodermie im Bereich einer mit Corticosteroidexterna behandelten Pityriasis versicolor (2 Falle) Z Hautkr. 1975;50:449–451. [Google Scholar]
- 6.Mascaro JM, Torres V. Pitiriasis versicolor atrofica. Dermatologia. 1976;20:329–336. [Google Scholar]
- 7.Paradisi M, Constantini G, Muscianese V, Voglino A. Pityriasis versicolor atrophicans. Chron Derm. 1978;5:564–565. [Google Scholar]
- 8.Millaseca ML, Gonzalez-Herrada C, Herranz JM, Corripio F, Jaquetti G. Pitiriasis versicolor atrofica yatrogenica. Gaceta Dermatologica. 1981;2:108–112. [Google Scholar]
- 9.Tatnall FM, Rycroft RJ. Pityriasis versicolor with cutaneous atrophy induced by topical steroid application. Clin Exp Dermatol. 1985;10:258–261. doi: 10.1111/j.1365-2230.1985.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 10.Wagner G, Lubach D. Pityriasis versicolor pseudoatrophicans. A case description. Z Hautkr. 1987;62:321–324. [PubMed] [Google Scholar]
- 11.Vera Castano A, Trasobares Marugan L, Del valle Martin M, Hernanz Hermosa JM, Del Palacio Hernanz A. Pitiriasis versicolor atrofica inducida por corticoides topicos fluorados. Actas Dermo-Sif. 1989;80:69–71. [Google Scholar]
- 12.Mazuecos Blanca J, García-Bravo B, Moreno Giménez JC, Sotillo I, Camacho F. Pseudoatrophic pityriasis versicolor. Med Cutan Ibero Lat Am. 1990;18:101–103. [PubMed] [Google Scholar]
- 13.Fimiani M, Casini L, Simoni S, Andreassi A, Alessandrini C. Un caso di pitiriasi versicolor atrofica: aspetti ultrastrutturali. Micol Dermatol. 1992;6:21–30. [Google Scholar]
- 14.Romano C, Maritati E, Ghilardi A, Miracco C, Mancianti F. A case of pityriasis versicolor atrophicans. Mycoses. 2005;48:439–441. doi: 10.1111/j.1439-0507.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 15.Winter GD, Burton JL. Experimentally induced steroid atrophy in the domestic pig and man. Br J Dermatol. 1976;94(suppl 12):107–109. doi: 10.1111/j.1365-2133.1976.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 16.Tosti A, Villardita S, Fazzini ML. The parasitic colonization of the horny layer in tinea versicolor. J Invest Dermatol. 1972;59:233–237. doi: 10.1111/1523-1747.ep12627257. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann P, Zheng P, Lavker RM, Kligman AM. Corticosteroid atrophy in human skin. A study by light, scanning, and transmission electron microscopy. J Invest Dermatol. 1983;81:169–176. doi: 10.1111/1523-1747.ep12543603. [DOI] [PubMed] [Google Scholar]
- 18.Jablonska S, Groniowska M, Dabroswki J. Comparative evaluation of skin atrophy in man induced by topical corticoids. Br J Dermatol. 1979;100:193–206. doi: 10.1111/j.1365-2133.1979.tb05561.x. [DOI] [PubMed] [Google Scholar]